Abstract
BAL5788 is the water-soluble prodrug of BAL9141, a novel broad-spectrum cephalosporin with potent bactericidal activity against methicillin-resistant Staphylococcus aureus (MRSA) and penicillin-resistant Streptococcus pneumoniae. Safety and pharmacokinetic data from a multiple-dose study with 16 healthy male volunteers are reported. Subjects were randomized to receive BAL5788 at 500 or 750 mg (as BAL9141 equivalents; n = 6 subjects per dose) or placebo (n = 2 subjects per dose). The doses were given as 200-ml infusions over 30 min once daily on days 1 and 8 and twice daily on days 2 to 7. BAL5788 was well tolerated, with no severe or serious adverse events (AEs) or dosing-related changes in laboratory parameters, electrocardiographic findings, or vital signs. Drug accumulation in plasma was negligible during the dosing period. The results of pharmacokinetic analyses agreed well with data reported from a previous single-ascending-dose study. The elimination half-life of BAL9141 was about 3 h. The volume of distribution at steady state was equal to the volume of the adult extracellular water compartment. BAL9141 was predominantly eliminated in urine, and renal clearance of the free drug corresponded to the normal glomerular filtration rate in adults. After multiple infusions of 750 mg, the mean concentrations of BAL9141 in plasma exceeded the MIC at which 100% of MRSA isolates are inhibited (4 μg/ml) for approximately 7 to 9 h, corresponding to 58 to 75% of a 12-h dosing interval.
BAL5788 is the water-soluble prodrug of BAL9141 (Fig. 1), a novel cephalosporin with potent activity in vitro against multiresistant pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus epidermidis, and penicillin-resistant Streptococcus pneumoniae (7). The range of BAL9141 MICs is 0.12 to 4 μg/ml for MRSA clinical isolates from the United States, Europe, South America, and Japan (7, 8). The prodrug BAL5788 was effective in animal models of abscess, pneumonia, and aortic valve endocarditis caused by multiresistant pathogens, including MRSA and penicillin-resistant S. pneumoniae (5, 7; D. R. Andes and W. A. Craig, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1079, 2000; E. Azoulay-Dupuis, J. Mohler, J. Bedos, A. Schmitt-Hoffmann, and S. Shapiro, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F335, 2002).
FIG. 1.
Structures of BAL5788 (prodrug) and BAL9141 (active drug).
The clinical efficacies of β-lactam antibiotics are generally correlated to T > MIC of at least 40 to 60%, with T > MIC defined as the time (as a percentage of the dosing interval) that the concentration of active drug in plasma exceeds the MICs for target pathogens (2, 4, 10). A close relationship between efficacy and values of T > MIC of 14 to 28% has been confirmed for BAL9141 in a model of thigh infection in neutropenic mice caused by MRSA (Andes and Craig, 40th ICAAC). A recent report has described the pharmacokinetics and safety of single BAL5788 doses from 125 to 1,000 mg (BAL9141 equivalents) (14). Single infusions of 750 mg of BAL5788 (as BAL9141 equivalents) led to mean plasma BAL9141 concentrations above 4 μg/ml (the MIC at which 100% of MRSA isolates are inhibited) for approximately 7 h, or 58% of a 12-h dosing interval, and infusions of BAL5788 at 750 mg twice a day (b.i.d.) were predicted to be adequate for the treatment of infections caused by MRSA (14).
In view of the promising activity of BAL9141 in vitro and in animal models and the favorable pharmacokinetic profile of BAL5788 when it is given to human subjects as single doses, we have investigated the safety and pharmacokinetic characteristics of BAL5788 in a multiple-dose clinical study. This trial was designed to test whether the T > MICs predicted from data from single-dose studies could be confirmed in studies with multiple-dose administration and to evaluate the safety and tolerability of a b.i.d. dosing regimen.
MATERIALS AND METHODS
Subjects and study procedures.
The present study was a single-center, double-blind, randomized, placebo-controlled, multiple-dose study carried out with 16 healthy male volunteer subjects. The BAL5788 doses administered were 500 and 750 mg (equivalents of the active drug BAL9141 corresponding to 667 and 1,000 mg of the BAL5788 prodrug, respectively). The study was performed at the Quintiles Clinical Trials Unit in Uppsala, Sweden, in accordance with the principles of the Declaration of Helsinki and all of its amendments. All volunteer subjects gave written, informed consent to participate in the study.
Cohorts of healthy male subjects were randomly assigned to receive BAL5788 (six subjects per dosing group) or placebo (two subjects per dosing group). A prestudy screening was done within 2 weeks before the first study day and included a medical history; s physical examination, including electorcardiography (ECG); and clinical laboratory tests, including tests for creatinine clearance. The doses were given for 8 days. Subjects were housed at the pharmacology unit from the evening before the first infusion until the final samples for pharmacokinetic analysis were taken on day 9, 24 h after the last infusion. On days 1 and 8, the subjects received a single infusion in the morning in order to allow 24-h sampling for pharmacokinetic analysis. On days 2 to 7, subjects received two infusions 12 h apart. Infusions of BAL5788 (diluted to 200 ml in 5% dextrose) or placebo (200 ml of 5% dextrose) were given over 30 min with a continuous-rate infusion pump.
On days 1 and 8 (the days for 24-h sampling for pharmacokinetic analysis), the morning infusions were given after an overnight fast, and no food was given for 4 h after the infusion. ECGs were obtained before, during, and 24 h after each infusion. Vital signs were measured before and at the end of dosing and afterwards at frequent intervals. Blood samples for laboratory safety tests were obtained before dosing and 24 h after dosing. On days 2 to 7, vital signs were measured before and after each infusion. Adverse events (AEs) were recorded throughout the study. Before escalation of the BAL5788 dose from 500 to 750 mg, all safety data from the 500-mg cohort were reviewed. All subjects were given a follow-up examination, including ECG and laboratory tests, 1 week after the last infusion (15 ± 3 days).
Pharmacokinetic sampling.
On study days 1 and 8, blood samples for pharmacokinetic analysis were collected and treated with EDTA and citric acid immediately before the start of infusion and at 5, 15, 30, and 45 min and 1, 1.25, 1.5, 2, 3, 4, 6, 8, 10, 12, 14, 16, and 24 h after the start of infusion. Blood samples were treated with EDTA and citric acid to stabilize them and to prevent the ex vivo hydrolysis of BAL5788. Subjects were asked to void urine a few minutes before the infusion. Subsequently, all urine samples were collected in containers pretreated with citric acid for collection periods of 2 h initially and, later, 4 to 12 h and up to 24 h after the start of the infusion. On days 3, 5, and 7, blood was collected immediately before the start of the morning infusion for determination of the trough BAL9141 levels.
Sample analysis.
BAL9141 and BAL5788 were quantified by gradient reversed-phase liquid chromatography (LC) in the back-flush mode coupled online with a tandem mass spectrometer (LC/LC-mass spectrometry [MS]/MS). Monitoring of selected reactions was performed on a SCIEX API365 mass spectrometer operated in the positive ionization detection mode. The applied transitions were m/z 535 to >308 for BAL9141 and m/z 691 to >535 for BAL5788 at a dwell time of 250 ms.
Sample workup was carried out with 100 μl of plasma or 200 μl of urine. Plasma samples were prepared by precipitation of plasma proteins with 20% perchloric acid, addition of internal standards, and centrifugation of the mixtures. Aliquots of supernatant (75 μl into a 250-μl loop) were injected for analysis. Urine samples were prepared by dilution with 0.1 M ammonium acetate buffer and internal standard solution and centrifugation. Aliquots of the supernatant (30 μl into a 250-μl loop) were injected for analysis.
The limit of quantification (LOQ) was the lowest concentration of analyte in plasma or urine, which can be measured with an interassay precision and an accuracy of 100% ± 20%. For plasma, this limit was set to 20.0 ng/ml for both BAL9141 and BAL5788, and all concentrations above 16.0 ng/ml detected are reported. For urine, the LOQ was set to 100 ng/ml for both BAL9141 and BAL5788, and all concentrations above 80.0 ng/ml detected are reported. Lower concentrations detected are reported as below the LOQ.
When mean drug levels were calculated for a subject cohort, if <50% of the samples had concentrations below the LOQ, the drug levels were set equal to zero. If ≥50% of the samples had concentrations below the LOQ, no mean value was calculated.
Pharmacokinetic analysis.
The values of the following pharmacokinetic parameters were derived by noncompartmental and two-compartmental methods with WinNonlin (version 2.1; Scientific Consulting Inc.): maximum concentration in plasma (Cmax), half-life (t1/2) in the distribution phase (t1/2α), t1/2 in the postdistribution phase (t1/2β), the area under the concentration-time curve (AUC) from time zero to the last sampling time (AUC0-t), the AUC from time zero extrapolated to infinity (AUC0-∞), total systemic clearance (CLS), total renal clearance (CLR), and the volume of distribution at steady state (VSS). For these calculations, CLR was estimated by the equation CLS × fu, where fu is the fraction of dose excreted in urine within 24 h on day 1 or within 12 h on day 8. CLR of the free (unbound) fraction of BAL9141 was calculated as CLR/0.62, where 0.62 is the fraction of unbound drug in plasma, since the level of protein binding of BAL9141 in plasma is 38% (A. Schmitt-Hoffmann, unpublished data). VSS was estimated by the equation [dose × (AUMC/AUC2)] − [dose × (T/2AUC)], where AUMC is the area under the first moment curve and T is the infusion time.
Drug accumulation was estimated from the AUC from 0 to 12 h (AUC0-12) on day 8 divided by the AUC0-12 on day 1. The predicted level of drug accumulation (R) was calculated by the equation 1/[1 − e−(βτ)], where τ is the dosing interval and β is the elimination rate constant. Urinary excretion was calculated from the concentrations of BAL9141 or BAL5788 in urine and the volumes of urine collected during the 24 h after the start of infusion.
The primary parameters for the assessment of dose proportionality were AUC0-∞ on day 1, AUC0-12 on day 8, and the BAL9141 Cmaxs after b.i.d. dosing with 500 or 750 mg. The effect of time on drug exposure was estimated by the AUC0-12 on day 8 divided by the AUC0-∞ on day 1. Statistical analysis was performed with S-Plus 2000 software. In order to test the dose proportionality and the time dependency of exposure, a three-way analysis of variance (ANOVA) model was applied to the logarithmically transformed and dose-normalized values of AUC and Cmax. The statistical test of dose proportionality for AUC and Cmax was based on the hypothesis (HA) of a difference between group means of dose-normalized exposure values versus the null hypothesis (H0) of no difference between group means, at a significance of alpha equal to 0.05. The test for time dependency was based on the intraindividual differences in the dose-normalized AUC0-∞ values on study day 1 and the AUC0-12 values on study day 8, in which the difference in the logarithms of the AUCs on days 1 and 8 was the dependent variable and dose was the independent variable in the ANOVA model. A possible effect of time was tested as an intercept value different from zero, at a significance of alpha equal to 0.05. Although the protocol did not specify the use of ANOVA to compare the values of VSS and CLS for the different doses, these analyses were also carried out and are regarded as exploratory rather than prospective.
RESULTS
A total of 16 male subjects ages 19 to 38 years were enrolled in the study. All 16 subjects completed the dosings and the scheduled assessments, and their data were evaluable for safety analyses. The data for all 12 subjects who received BAL5788 were also evaluable for pharmacokinetic analyses.
Conversion of the prodrug BAL5788 was rapid; plasma BAL5788 concentrations were only sporadically measurable in eight subjects during the infusion period, and the levels at the end of infusion were less than 2% of those determined for the active drug, BAL9141. No plasma BAL5788 levels were measurable after the completion of infusion, and a pharmacokinetic characterization of the prodrug BAL5788 was therefore not possible.
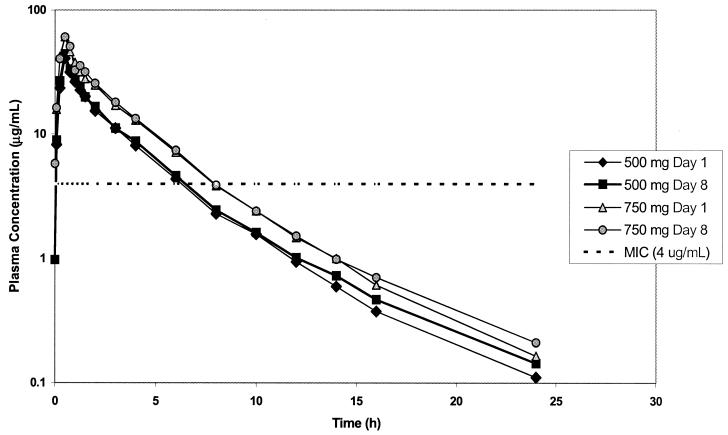
Figure 2 shows the profiles of the mean concentrations of the active drug BAL9141 in plasma for each dose level on day 1 and day 8. Table 1 presents the corresponding pharmacokinetic parameter values. Intersubject variabilities were low on both days; coefficients of variation for Cmax and AUC0-∞ were 18.2 and 9.0%, respectively, for the 500-mg dose and 7.5 and 12.3%, respectively, for the 750-mg dose on day 1 and were 24.4 and 20.5%, respectively, for the 500-mg dose and 16.5 and 7.8%, respectively, for the 750-mg dose on day 8. The levels of drug accumulation (mean ± standard deviation) on day 8, after the administration of multiple doses, amounted to 1.06 ± 0.18 for the 500-mg dose and 1.04 ± 0.09 for the 750-mg dose. ANOVA revealed linear dose proportionality for AUC and Cmax, and no significant effect of dose on CLS or VSS was found. The influence of time on the values of the pharmacokinetic parameters for BAL9141 was not statistically significant, and equality can be assumed for AUC and Cmax values on day 1 and day 8. The mean trough plasma BAL9141 concentrations were in the range of 0.9 to 1.3 μg/ml (500-mg group) and 1.5 to 1.7 μg/ml (750-mg group) throughout the dosing interval, with the concentrations having low variabilities and no tendency to increase with longer exposure times.
FIG. 2.
Profiles of mean concentrations of BAL5788 in plasma on study day 1 and after infusions b.i.d. on study day 8.
TABLE 1.
Values of pharmacokinetic parameters for BAL9141 on study days 1 and 8 after intravenous infusion of 500 and 750 mga
| Dose (mg) and study day | AUC0-∞ (μg · h/ml) | AUC0-12 (μg · h/ml) | Cmax (μg/ml) | t1/2α (h)b | t1/2β (h) | VSS (liters) | CLS (liters/h) | CLR (liters/h) | Recovery in urine (% of dose)c |
|---|---|---|---|---|---|---|---|---|---|
| 500 | |||||||||
| 1 | 101 (9.04) | 95.9 (7.59) | 40.6 (7.38) | 1.06 (0.46) | 3.63 (0.48) | 16.4 (2.11) | 4.99 (0.46) | 4.12 (0.75) | 82.2 (9.11) |
| 8 | 108 (22.2) | 102 (20.0) | 44.2 (10.8) | 1.03 (0.42) | 4.04 (0.31) | 16.7 (3.58) | 5.05 (0.95) | 4.47 (1.07) | 87.8 (9.30) |
| 750 | |||||||||
| 1 | 156 (19.3) | 148 (17.6) | 60.7 (4.55) | 0.96 (0.59) | 3.64 (0.32) | 16.3 (1.82) | 4.85 (0.57) | 4.05 (0.47) | 83.7 (3.91) |
| 8 | 165 (12.8) | 156 (11.1) | 60.6 (9.99) | 0.99 (0.50) | 4.11 (0.41) | 16.1 (2.20) | 4.84 (0.34) | 4.09 (0.60) | 84.4 (9.23) |
The values were determined by noncompartmental analysis, unless indicated otherwise, and are presented as the arithmetic means (standard deviations) for six samples.
The values were obtained by two-compartment analysis.
Recoveries from 0 to 24 h on day 1 and 0 to 12 h on day 8.
BAL9141 was predominantly eliminated in urine. As shown in Table 2, the mean cumulative recovery of BAL9141 in urine within 24 h after the start of infusion accounted for 82 to 88% of the dose administered, and the cumulative recoveries were comparable on days 1 and 8. The mean CLR of BAL9141 ranged from 4.0 to 4.5 liters/h (67 to 75 ml/min). Because the free (unbound) fraction of BAL9141 in plasma amounts to 62% (Schmitt-Hoffmann, unpublished), the CLR of the free fraction reached 108 to 121 ml/min, close to the normal glomerular filtration rate of 125 ml/min in adults.
TABLE 2.
Recovery and concentrations of BAL9141 in urine
| BAL5788 dosea (mg) and study day | Urine collection interval (h) | Maximum BAL9141 concn in urine (μg/ml) | Cumulative 24-h recovery of BAL9141 in urine (% of dose)b |
|---|---|---|---|
| 500 | |||
| 1 | 0-2 | 1,406 | 82.2 (70.5-94.8) |
| 2-4 | 220 | ||
| 8 | 0-2 | 800 | 87.8 (76.9-104.2) |
| 2-4 | 293 | ||
| 750 | |||
| 1 | 0-2 | 2,540 | 83.7 (76.0-87.1) |
| 2-4 | 169 | ||
| 8 | 0-2 | 2,060 | 84.4 (73.1-100.2) |
| 2-4 | 331 |
Doses are expressed as equivalent milligrams of BAL9141.
The values are arithmetic means (ranges).
Table 3 shows the number of hours that the total and unbound concentrations of BAL9141 in plasma exceeded the MIC of 4 μg/ml for each dose on days 1 and 8. The total concentrations of BAL9141 were above this MIC for approximately 5 to 7 h after infusion of the 500-mg dose (corresponding to values of the T > MIC of 40 to 58% of the 12-h dosing interval) and 7 to 9 h after infusion of the 750-mg dose (values of T > MIC of 58 to 75% of the 12-h dosing interval).
TABLE 3.
Doses, times that plasma drug concentrations exceeded the MIC, and T > MIC on day 1 and day 8
| BAL5788 dosea (mg) | Time (h) the plasma BAL9141 concn exceeded the MICb
|
T > MIC (% of 12-h dosing interval)
|
||
|---|---|---|---|---|
| Total | Unbound | Total | Unbound | |
| 500 | 6 | 5 | 50 | 42 |
| 750 | 7 | 6 | 58 | 50 |
Doses are expressed as equivalent milligrams of BAL9141.
The MIC is 4 μg/ml.
No severe or serious AEs were reported, and most AEs in each dose group were mild (Table 4). The most frequently reported events were transient nausea, headache, and a taste disturbance described as caramel-like during the infusion period. No significant reactions at the peripheral infusion site were observed. No ECG abnormalities or adverse changes in vital signs were reported. Minor changes in laboratory parameters in some subjects receiving the active treatment and placebo were not considered clinically significant.
TABLE 4.
AEs as number of events by dose and intensity
| Characteristic or AE | 500-mg dose (n = 6)
|
750-mg dose (n = 6)
|
Placebo group (n = 4)
|
|||
|---|---|---|---|---|---|---|
| Mild | Moderate | Mild | Moderate | Mild | Moderate | |
| Total no. of subjects with at least one AEa | 3 | 2 | 1 | 5 | 1 | 2 |
| Total number of AEs | 12 | 2 | 22 | 5 | 3 | 2 |
| No. of subjects with the following AE: | ||||||
| Abdominal pain lower | 1 | 1 | ||||
| Diarrhea | 1 | |||||
| Nausea | 1 | 1 | 3 | 3 | ||
| Fatigue | 1 | |||||
| Feeling hot | 1 | |||||
| Injection-site reaction | 1 | |||||
| Pharyngitis | 1 | |||||
| Increased alanine aminotransferase levels | 2 | 1 | 1 | |||
| Increased alpha-1 globulin levels | 1 | |||||
| Increased blood creatinine levels | 1 | |||||
| Dizziness (excluding vertigo) | 1 | |||||
| Headache | 3 | 1 | 5 | 1 | 1 | |
| Somnolence | 1 | 1 | ||||
| Taste disturbance | 4 | 6 | ||||
| Urine abnormal | 1 | |||||
Subjects were classified according to the most intense AE; a subject with a mild and a moderate AE is counted as having a moderate AE only.
DISCUSSION
The results of this multiple-dose study indicate that BAL5788 has stable pharmacokinetic properties over an 8-day course of dosing, with low intersubject variabilities. The results of this trial are in good agreement with those of a previous trial with single doses (14). The prodrug BAL5788 was rapidly converted to the active cephalosporin BAL9141. Peak plasma BAL9141 levels were observed at the end of the 30-min infusion, with a subsequent biphasic decrease reflecting the rapid distribution of BAL9141 from the systemic circulation into other body compartments. The volume of distribution was similar to those of other β-lactam antibiotics (9, 16), which is about equal to the volume of the extracellular water compartment in adults (11). BAL9141 was predominantly excreted in urine, apparently through normal glomerular filtration. Drug accumulation was negligible over an 8-day dosing period, with no evidence of an induction of metabolism or increased clearance.
The clinical efficacies of β-lactam antibiotics are correlated to values of T > MIC of at least 40 to 60% (2, 4, 15), although the findings from studies with some animal models support a lower T > MIC range (14 to 28%) for BAL9141 for S. aureus and S. pneumoniae species (7; Andes and Craig, 40th ICAAC; Azoulay-Dupuis et al., 42nd ICAAC). The t1/2β of 3 to 4 h for BAL9141 in plasma supports a b.i.d. treatment regimen, and the results obtained in this study indicate that b.i.d. administration of BAL5788 at 750 mg (as BAL9141 equivalents) led to total plasma BAL9141 concentrations above the MIC at which 100% of MRSA isolates are inhibited (4 μg/ml) for 7 to 9 h, corresponding to 58 to 75% of a 12-h dosing interval.
Multiple doses of BAL5788 were well tolerated, with no severe or serious local or systemic AEs and with no ECG abnormalities or adverse changes in vital signs. One characteristic AE, reported in all subjects treated with the higher dose, was a transient caramel-like taste disturbance attributable to the release of diacetyl during conversion of the prodrug to active cephalosporin (3, 7, 13, 14). Diacetyl is further metabolized to form acetoin and 2,3-butanedione (1).
Clinical cure of severe infections with β-lactam antibiotics, with eradication of the causative pathogens, typically requires treatment for at least 7 to 10 days. The results of this study demonstrate that BAL5788 has a predictable and stable pharmacokinetic profile when it is given over 8 days at doses leading to high T > MICs. The microbiological and pharmacokinetic profiles of BAL5788 indicate that it may represent a welcome advance in antimicrobial therapy, especially in view of its potent activity against MRSA, the rising incidence of MRSA infections worldwide (12), and the development of MRSA strains resistant to existing antibiotics (6, 15, 17). Therapeutic studies are warranted to evaluate its efficacy and safety in patients.
Acknowledgments
We are grateful to S. Shapiro and M. Heep for discussions and critical review of the manuscript. P. H. Joubert provided valuable assistance in preparing the manuscript.
REFERENCES
- 1.Alexander, J., D. S. Bindra, J. D. Glass, M. A. Holahan, M. L. Renyer, G. S. Rork, et al. 1996. Investigation of (oxodioxolenyl) methyl carbamates as nonchiral bioreversible prodrug moieties for chiral amines. J. Med. Chem. 39:480-486. [DOI] [PubMed] [Google Scholar]
- 2.Cars, O. 1997. Efficacy of beta-lactam antibiotics: integration of pharmacokinetics and pharmacodynamics. Diagn. Microbiol. Infect. Dis. 27:29-33. [DOI] [PubMed] [Google Scholar]
- 3.Chew, T. A., and J. M. Smith. 1992. Detection of diacetyl (caramel odor) in presumptive identification of the “Streptococcus milleri” group. J. Clin. Microbiol. 30:3028-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig, W. A. 2001. Does the dose matter? Clin. Infect. Dis. 33(Suppl. 2):233-237. [DOI] [PubMed] [Google Scholar]
- 5.Entenza, J. M., P. Hohl, I. Heinze-Krauss, M. P. Glauser, and P. Moreillon 2002. BAL9141, a novel extended-spectrum cephalosporin active against methicillin-resistant Staphylococcus aureus in treatment of experimental endocarditis. Antimicob. Agents Chemother. 46:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs, P. C., A. L. Barry, and S. D. Brown. 2000. Bactericidal activity of quinupristin-dalfopristin against Staphylococcus aureus: clindamycin susceptibility as a surrogate indicator. Antimicrob. Agents Chemother. 44:2880-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. P. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63-1941, a novel broad-spectrum cephalosporin with activity agains methicillin-resistant staphylococci. Antimicob. Agents Chemother. 45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones, R. N., L. M. Deshpande, A. H. Mutnick, and D. J. Biedenbach. 2002. In vitro evaluation of BAL9141, a novel parenteral cephalosporin active against oxacillin-resistant staphylococci. J. Antimicrob. Ther. 50:915-932. [DOI] [PubMed] [Google Scholar]
- 9.Klepser, M. E., M. N. Marangos, K. B. Patel, D. P. Nicolau, R. Quintiliani, and C. H. Nightingale. 1995. Clinical pharmacokinetics of newer cephalosporins. Clin. Pharmacokinet. 28:361-384. [DOI] [PubMed] [Google Scholar]
- 10.Mouton, J. W., and N. Punt. 2001. Use of the t>MIC to choose between different dosing regimens of beta-lactam antibiotics. J. Antimicrob. Chemother. 47:500-501. [DOI] [PubMed] [Google Scholar]
- 11.Nix, D., S. Goodwin, C. Peloquin, D. Rotella, and J. Schentag. 1991. Antibiotic tissue distribution and its relevance: models of tissue penetration and their meaning. Antimicrob. Agents Chemother. 35:1947-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Nosocomial Infections Surveillance System. 2001. NNIS special article. National Nosocomial Infections Surveillance System Report, data summary from January 1992-June 2001, issued August 2001. Am. J. Infect. Control 29:404-421. [DOI] [PubMed] [Google Scholar]
- 13.Rogerson, F. S., H. Castro, N. Fortunato, Z. Azevedo, A. Macedo, and V. A. De Freitas. 2001. Chemicals with sweet aroma descriptors found in Portuguese wines from the Douro region: 2,6,6-trimethylcyclohex-2-ene-1,4-dione and diacetyl. J. Agric. Food Chem. 49:263-269. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt-Hoffmann, A., B. Roos, M. Schleimer, J. Sauer, A. Man, N. Nashed, T. Brown, A. Perez, E. Weidekamm, and P. Kovács. 2004. Single-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicob. Agents Chemother. 48:2570-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sievert, D. M., M. L. Boulton, G. Stoltman, D. Johnson, M. G. Stobierski, F. P. Downes, P. A. Somsel, J. T. Rudrik, W. Brown, W. Hafeez, T. Lundstrom, E. Flanagan, R. Johnson, J. Mitchell, and S. Chang. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 16.Sörgel, F., and M. Kinzig. 1993. The chemistry, pharmacokinetics and tissue distribution of piperazillin/tazobactam. J. Antimicrob. Chemother. 31:39-60. [DOI] [PubMed] [Google Scholar]
- 17.Tsiodras, S., H. S. Gold, G. Sakoulas, G. M. Eliopoulos, C. Wennersten, L. Venkataraman, R. C. Moellering, and M. J Ferraro. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]