Abstract
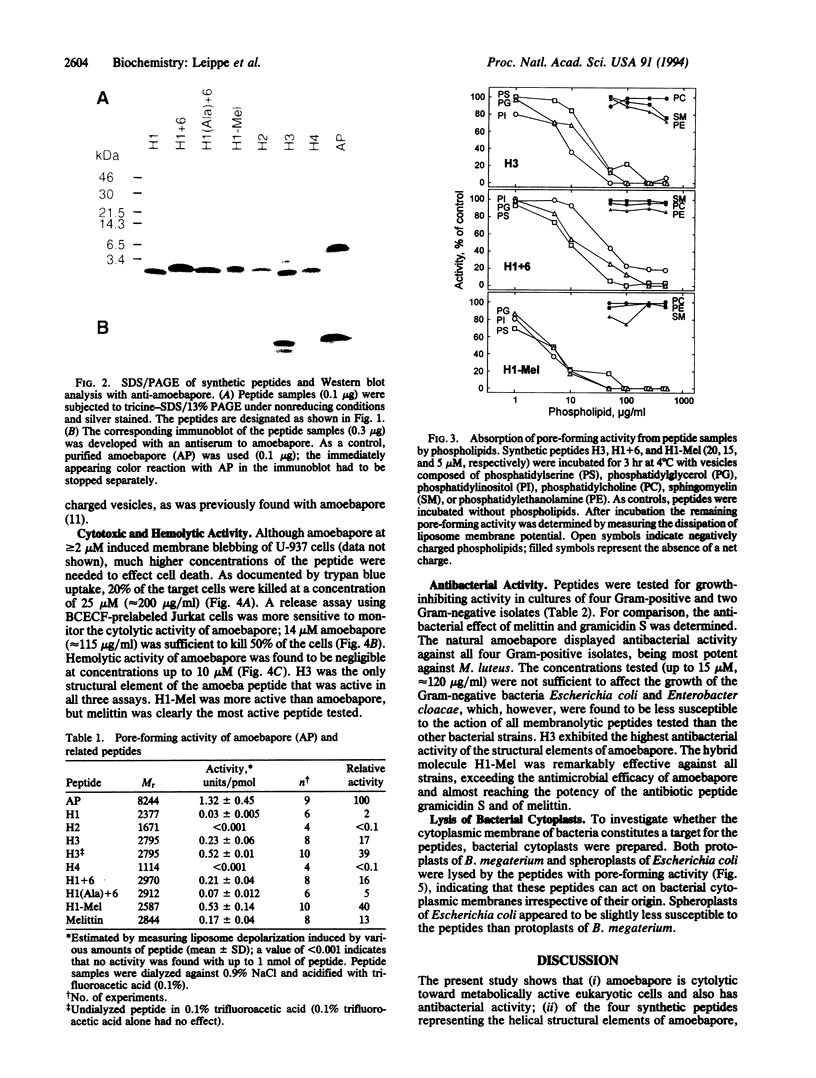
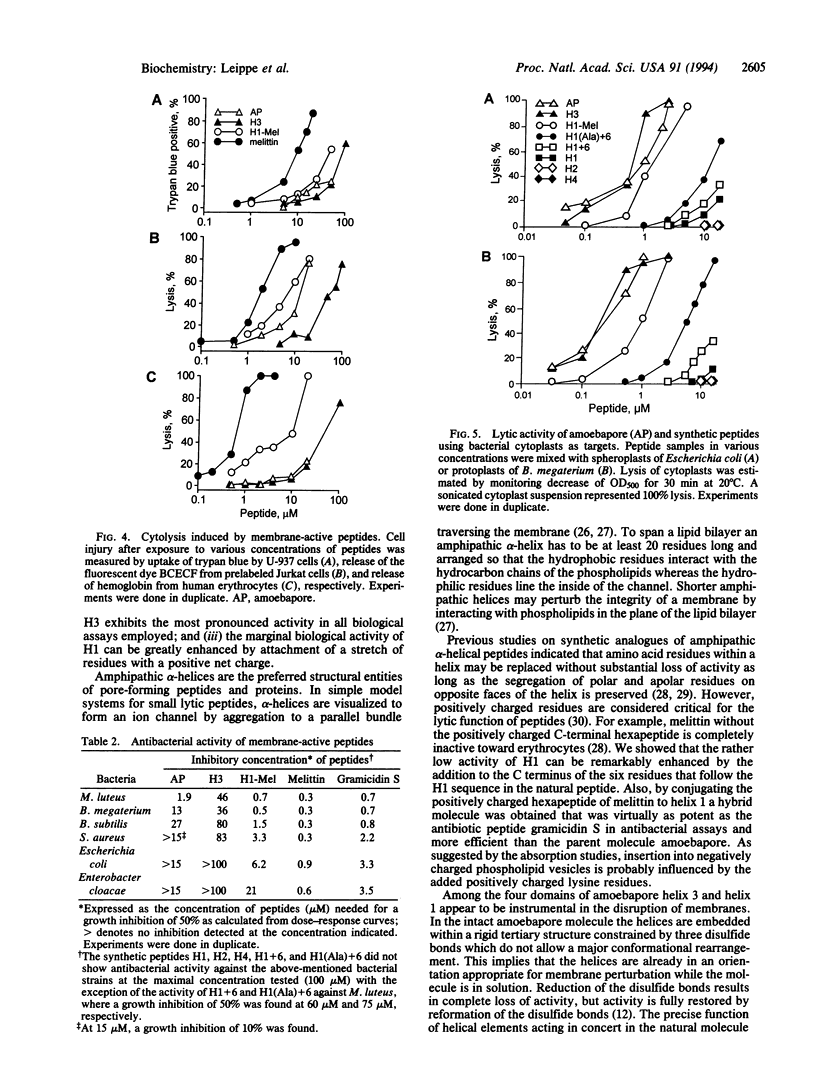
The pore-forming peptide amoebapore is considered part of the cytolytic armament of pathogenic Entamoeba histolytica. Amoebapore is composed of 77 amino acid residues arranged in four alpha-helical domains. For structure-function analysis, synthetic peptides were constructed corresponding to these four domains: H1 (residues 1-22), H2 (25-39), H3 (40-64), and H4 (67-77). The peptides H1 and H3, representing two highly amphipathic alpha-helical regions of amoebapore, possessed pore-forming activity. Peptide H3 displayed cytolytic and antibacterial functions similar to those of natural amoebapore. The most potent antibacterial activity and the broadest activity spectrum were expressed by H1-Mel, a hybrid molecule composed of the N-terminal alpha-helix of amoebapore and the C-terminal hexapeptide of melittin from the venom of Apis mellifera.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHEIMER A. W., SCHWARTZ L. L. LYSIS OF BACTERIAL PROTOPLASTS AND SPHEROPLASTS BY STAPHYLOCOCCAL ALPHA-TOXIN AND STREPTOLYSIN S. J Bacteriol. 1965 May;89:1387–1392. doi: 10.1128/jb.89.5.1387-1392.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechinger B., Kim Y., Chirlian L. E., Gesell J., Neumann J. M., Montal M., Tomich J., Zasloff M., Opella S. J. Orientations of amphipathic helical peptides in membrane bilayers determined by solid-state NMR spectroscopy. J Biomol NMR. 1991 Jul;1(2):167–173. doi: 10.1007/BF01877228. [DOI] [PubMed] [Google Scholar]
- Boman H. G. Antibacterial peptides: key components needed in immunity. Cell. 1991 Apr 19;65(2):205–207. doi: 10.1016/0092-8674(91)90154-q. [DOI] [PubMed] [Google Scholar]
- Buck M. A., Olah T. A., Weitzmann C. J., Cooperman B. S. Protein estimation by the product of integrated peak area and flow rate. Anal Biochem. 1989 Nov 1;182(2):295–299. doi: 10.1016/0003-2697(89)90597-6. [DOI] [PubMed] [Google Scholar]
- Cammue B. P., De Bolle M. F., Terras F. R., Proost P., Van Damme J., Rees S. B., Vanderleyden J., Broekaert W. F. Isolation and characterization of a novel class of plant antimicrobial peptides form Mirabilis jalapa L. seeds. J Biol Chem. 1992 Feb 5;267(4):2228–2233. [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Cruciani R. A., Barker J. L., Zasloff M., Chen H. C., Colamonici O. Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3792–3796. doi: 10.1073/pnas.88.9.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey C. E. The actions of melittin on membranes. Biochim Biophys Acta. 1990 May 7;1031(2):143–161. doi: 10.1016/0304-4157(90)90006-x. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Fields G. B., Noble R. L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990 Mar;35(3):161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Ganz T., Oren A., Lehrer R. I. Defensins: microbicidal and cytotoxic peptides of mammalian host defense cells. Med Microbiol Immunol. 1992;181(2):99–105. doi: 10.1007/BF00189428. [DOI] [PubMed] [Google Scholar]
- Gennaro R., Skerlavaj B., Romeo D. Purification, composition, and activity of two bactenecins, antibacterial peptides of bovine neutrophils. Infect Immun. 1989 Oct;57(10):3142–3146. doi: 10.1128/iai.57.10.3142-3146.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler C., Calef E., Rosenberg I. Cytopathogenicity of Entamoeba histolytica. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):73–85. doi: 10.1098/rstb.1984.0110. [DOI] [PubMed] [Google Scholar]
- Keller F., Hanke W., Trissl D., Bakker-Grunwald T. Pore-forming protein from Entamoeba histolytica forms voltage- and pH-controlled multi-state channels with properties similar to those of the barrel-stave aggregates. Biochim Biophys Acta. 1989 Jun 26;982(1):89–93. doi: 10.1016/0005-2736(89)90178-8. [DOI] [PubMed] [Google Scholar]
- Kini R. M., Evans H. J. A common cytolytic region in myotoxins, hemolysins, cardiotoxins and antibacterial peptides. Int J Pept Protein Res. 1989 Oct;34(4):277–286. doi: 10.1111/j.1399-3011.1989.tb01575.x. [DOI] [PubMed] [Google Scholar]
- Kolber M. A., Quinones R. R., Gress R. E., Henkart P. A. Measurement of cytotoxicity by target cell release and retention of the fluorescent dye bis-carboxyethyl-carboxyfluorescein (BCECF). J Immunol Methods. 1988 Apr 6;108(1-2):255–264. doi: 10.1016/0022-1759(88)90427-9. [DOI] [PubMed] [Google Scholar]
- Lear J. D., Wasserman Z. R., DeGrado W. F. Synthetic amphiphilic peptide models for protein ion channels. Science. 1988 May 27;240(4856):1177–1181. doi: 10.1126/science.2453923. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Selsted M. E. Defensins: endogenous antibiotic peptides of animal cells. Cell. 1991 Jan 25;64(2):229–230. doi: 10.1016/0092-8674(91)90632-9. [DOI] [PubMed] [Google Scholar]
- Leippe M., Ebel S., Schoenberger O. L., Horstmann R. D., Müller-Eberhard H. J. Pore-forming peptide of pathogenic Entamoeba histolytica. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7659–7663. doi: 10.1073/pnas.88.17.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leippe M. Membrane perforation by Entamoeba histolytica: structural implications derived from the sequence of the pore-forming peptide. Arch Med Res. 1992;23(2):35–37. [PubMed] [Google Scholar]
- Leippe M., Tannich E., Nickel R., van der Goot G., Pattus F., Horstmann R. D., Müller-Eberhard H. J. Primary and secondary structure of the pore-forming peptide of pathogenic Entamoeba histolytica. EMBO J. 1992 Oct;11(10):3501–3506. doi: 10.1002/j.1460-2075.1992.tb05432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. D., Carroll J., Ellar D. J. Crystal structure of insecticidal delta-endotoxin from Bacillus thuringiensis at 2.5 A resolution. Nature. 1991 Oct 31;353(6347):815–821. doi: 10.1038/353815a0. [DOI] [PubMed] [Google Scholar]
- Lichtenstein A. Mechanism of mammalian cell lysis mediated by peptide defensins. Evidence for an initial alteration of the plasma membrane. J Clin Invest. 1991 Jul;88(1):93–100. doi: 10.1172/JCI115310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch E. C., Rosenberg I. M., Gitler C. An ion-channel forming protein produced by Entamoeba histolytica. EMBO J. 1982;1(7):801–804. doi: 10.1002/j.1460-2075.1982.tb01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattus F., Massotte D., Wilmsen H. U., Lakey J., Tsernoglou D., Tucker A., Parker M. W. Colicins: prokaryotic killer-pores. Experientia. 1990 Feb 15;46(2):180–192. [PubMed] [Google Scholar]
- Peitsch M. C., Amiguet P., Guy R., Brunner J., Maizel J. V., Jr, Tschopp J. Localization and molecular modelling of the membrane-inserted domain of the ninth component of human complement and perforin. Mol Immunol. 1990 Jul;27(7):589–602. doi: 10.1016/0161-5890(90)90001-g. [DOI] [PubMed] [Google Scholar]
- Ravdin J. I. Immunobiology of human infection by Entamoeba histolytica. Pathol Immunopathol Res. 1989;8(3-4):179–205. doi: 10.1159/000157148. [DOI] [PubMed] [Google Scholar]
- Rosenberg I., Bach D., Loew L. M., Gitler C. Isolation, characterization and partial purification of a transferable membrane channel (amoebapore) produced by Entamoeba histolytica. Mol Biochem Parasitol. 1989 Mar 15;33(3):237–247. doi: 10.1016/0166-6851(89)90085-6. [DOI] [PubMed] [Google Scholar]
- Sansom M. S. The biophysics of peptide models of ion channels. Prog Biophys Mol Biol. 1991;55(3):139–235. doi: 10.1016/0079-6107(91)90004-c. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., De Loof H., Dohlman J. G., Brouillette C. G., Anantharamaiah G. M. Amphipathic helix motif: classes and properties. Proteins. 1990;8(2):103–117. doi: 10.1002/prot.340080202. [DOI] [PubMed] [Google Scholar]
- Stüber W., Knolle J., Breipohl G. Synthesis of peptide amides by Fmoc-solid-phase peptide synthesis and acid labile anchor groups. Int J Pept Protein Res. 1989 Sep;34(3):215–221. doi: 10.1111/j.1399-3011.1989.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Nabholz M. Perforin-mediated target cell lysis by cytolytic T lymphocytes. Annu Rev Immunol. 1990;8:279–302. doi: 10.1146/annurev.iy.08.040190.001431. [DOI] [PubMed] [Google Scholar]
- Young J. D., Cohn Z. A. Molecular mechanisms of cytotoxicity mediated by Entamoeba histolytica: characterization of a pore-forming protein (PFP). J Cell Biochem. 1985;29(4):299–308. doi: 10.1002/jcb.240290404. [DOI] [PubMed] [Google Scholar]
- Young J. D., Young T. M., Lu L. P., Unkeless J. C., Cohn Z. A. Characterization of a membrane pore-forming protein from Entamoeba histolytica. J Exp Med. 1982 Dec 1;156(6):1677–1690. doi: 10.1084/jem.156.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Antibiotic peptides as mediators of innate immunity. Curr Opin Immunol. 1992 Feb;4(1):3–7. doi: 10.1016/0952-7915(92)90115-u. [DOI] [PubMed] [Google Scholar]




