Abstract
Lysostaphin is an endopeptidase that kills Staphylococcus aureus, a predominant organism in catheter-related infections. Lysostaphin-coated catheters prevented catheter colonization by several strains of S. aureus, and activity was maintained for at least 4 days. Prophylactic use of lysostaphin in catheters may help prevent the occurrence of catheter-related staphylococcal infections.
Catheter-related infections continue to be a significant source of morbidity and mortality in patients requiring catheterization (6, 10, 12, 15) and increase medical expenses by prolonging hospitalization (7). Catheter infections are most commonly caused by staphylococci, either coagulase-negative staphylococci (CoNS) or Staphylococcus aureus. Currently, six types of antiseptic catheters have been tested in clinical trials: cefazolin-, teicoplanin-, vancomycin-, silver-, chlorohexidine-silver sulfadiazine-, and minocycline-rifampin-coated catheters (3, 7). However, only the minocycline-rifampin- and chlorohexidine-silver sulfadiazine-coated catheters have been shown to reduce the incidence of catheter-related bloodstream infections, and long-term efficacy has not been shown (3, 7, 16). Lysostaphin is an endopeptidase that cleaves the cross-linking pentaglycine bridges of the cell wall of staphylococci (2, 5, 8, 11, 14, 17). Lysostaphin is highly active against S. aureus because of the prevalence of pentaglycine cross-linking in the cell wall of S. aureus and has lesser activity against CoNS (2, 4). This study demonstrated that lysostaphin is readily adsorbed onto catheter surfaces while maintaining its staphylolytic activity and may have the potential to decrease the incidence of S. aureus- and CoNS-related catheter infections.
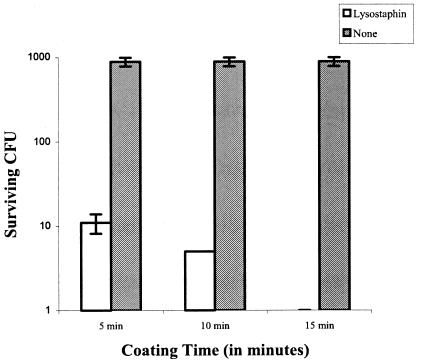
Lysostaphin, obtained from AMBI, Inc., was coated onto two different plastic surfaces, polystyrene and FEP polymer, a Teflon-like material used in Angiocath catheters produced by Becton Dickinson. Twenty-four-well polystyrene plates were coated overnight at 4°C with 300 μl of 10, 1, or 0.1 mg of lysostaphin/ml diluted in phosphate-buffered saline (PBS). The lumenal side of the catheters was coated for 1 h at room temperature with 0.1 mg of lysostaphin/ml. The coated surfaces were washed extensively with 50 ml of PBS, and the last aliquot of wash solution was tested to confirm the absence of unbound lysostaphin. The surfaces were challenged with an inoculum of about 104 S. aureus capsule type 5 organisms (SA5; clinical isolate) in tryptic soy broth, incubated for 1 to 2 h, and then streaked onto blood agar to enumerate surviving colonies (Table 1). On average, 610 CFU was recovered from the polystyrene control wells, whereas only 3 CFU remained in the lysostaphin-coated wells, a 99.5% reduction in bacterial counts. The lysostaphin-coated catheters were completely cleared of bacteria as compared to control catheters, from which an average of 493 CFU was recovered. The killing was not concentration dependent in the range of 10 to 0.1 mg/ml, as all three concentrations reduced bacterial titers to the same level. These results suggest that lysostaphin binds to plastic surfaces and maintains killing activity against S. aureus. Catheter coating times (5, 10, or 15 min with 0.1 mg of lysostaphin/ml) were also examined to determine the minimum time necessary to effectively coat the surface (Fig. 1). Catheters had high levels of killing activity after 5 min of coating with lysostaphin, but the killing efficiency increased with coating time, with complete clearance after 15 min of coating.
TABLE 1.
Efficacy of surface-bound lysostaphin against S. aureus
| Surface | Coating concn (mg/ml) | CFU (n = 2) |
|---|---|---|
| Polystyrene | 10 | 1 |
| 1 | 1 | |
| 0.1 | 3 | |
| 0 | 610 | |
| Angiocath | 0.1 | 0 |
| 0 | 493 |
FIG. 1.
Antimicrobial efficacy of catheters as a function of lysostaphin coating time.
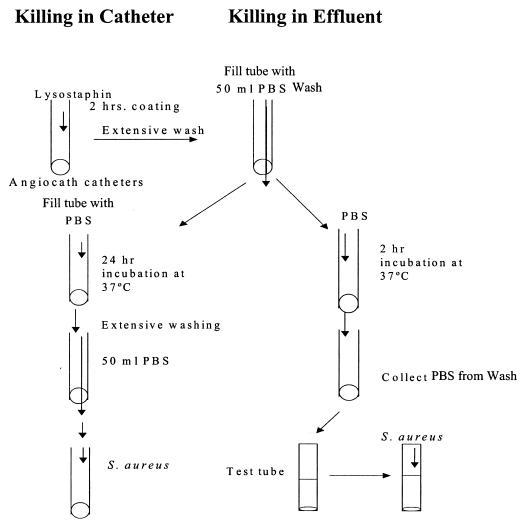
The killing activity on these coated plastics is primarily due to the activity of bound lysostaphin and is not a result of free lysostaphin leaching off of the plastic and thereby killing bacteria in solution. Experiments were performed with catheters to examine both leaching of lysostaphin and the activity of remaining bound lysostaphin (Fig. 2). Catheters were coated with 0.1 mg of lysostaphin/ml for 60 min and then washed extensively with 50 ml of PBS. The catheters were then filled with fresh PBS and incubated for 2 h. The PBS, which would contain any leached lysostaphin, was then collected, and the same inoculum of bacteria that was used to challenge the catheters was added to this wash, incubated for 1 h, and then plated. No bacterial killing was observed, which suggests that even if a small amount of lysostaphin leached off the catheter it was at concentrations that were not effective against this bacterial challenge. Similar results were found when leaching was examined on polystyrene surfaces coated with 10, 1, or 0.1 mg of lysostaphin/ml. However, it was apparent that leaching increased as the coating concentration of lysostaphin increased. Leaching of lysostaphin at these high coating concentrations may be due to formation of multiple protein layers adsorbing onto the plastic surface. As these lysostaphin layers stack further from the surface, they may bind less tightly to the plastic and slough off into solution with time, contributing to the killing activity seen in the effluent.
FIG. 2.
Methods for evaluating leaching of lysostaphin from coated catheters.
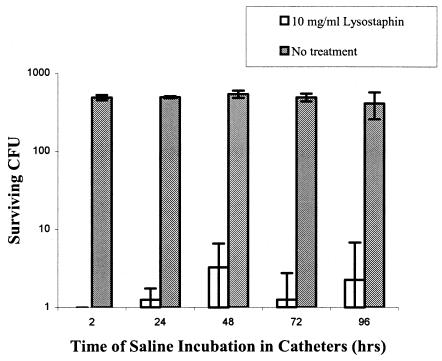
The durability of lysostaphin-coated catheters was determined by incubating them with PBS for up to 96 h, with the PBS being refreshed every 24 h in each catheter (Fig. 3). At each time point, two of the catheters were washed extensively with 50 ml of PBS and then challenged with about 104 CFU of SA5 to determine if they maintained their killing activity. After a 2-h incubation with PBS, there was complete clearance of bacteria from the lysostaphin-coated catheters as compared to the uncoated catheters. There was about a 2.5-log reduction at every time thereafter up to 96 h. These data show that lysostaphin-coated catheters maintain significant killing activity for at least 4 days after coating and may protect catheters from staphylococcal colonization at the time of insertion and for several days thereafter. If necessary, the catheter could be flushed every few days with a concentrated solution of lysostaphin to recoat the surface of the catheter and thereby maintain optimal bactericidal efficacy.
FIG. 3.
Long-term antimicrobial effectiveness of lysostaphin-coated catheters against S. aureus (n = 2).
These results demonstrate that lysostaphin effectively clears bacteria from the catheter lumen solution. To determine whether lysostaphin-coated catheters would prevent the adherence of bacteria to the catheter surface and remain sterile when exposed to bacteria, uncoated catheters were inoculated with bacteria and incubated for 2 or 24 h. The effluent was then discarded, and the catheters were washed extensively with 50 ml of PBS. The last milliliter of the wash was collected and streaked onto a blood agar plate. More bacteria adhered to the catheter surface at 24 h than at 2 h, and after an overnight incubation in medium, the catheters were well colonized and the medium solution was positive for bacterial growth. A second set of catheters were coated on the inside and outside surfaces with 0.1 mg of lysostaphin/ml and washed with 50 ml of PBS. The catheters were incubated with 104 CFU of SA5 for 3 h and then placed in medium to look for bacterial growth. Following an overnight incubation, the medium remained sterile, suggesting that the lysostaphin-coated catheters can both prevent surface colonization and actively kill bacteria in solution.
In a clinical setting, catheters and other implantable devices are exposed to serum proteins, which may either inhibit the activity of lysostaphin or may adsorb over the lysostaphin bound to the plastic and block its accessibility to the bacteria. To test this, catheters were coated with 10, 1, or 0.1 mg of lysostaphin/ml for 60 min, washed extensively with 50 ml of PBS, and then incubated with human serum for 24 h. The catheters were washed again with 50 ml of PBS and inoculated with SA5. The catheters coated with 0.1 mg of lysostaphin/ml showed a 99% reduction in bacterial counts, and those coated with 10 and 1 mg of lysostaphin/ml cleared all bacteria. These results suggest that the presence of serum proteins do not adversely affect the activity of lysostaphin bound to the catheters.
Common organisms of catheter-related bloodstream infection also include CoNS, such as S. epidermidis. Previous studies have shown that lysostaphin is less effective against S. epidermidis strains (4), most likely due to the smaller percentage of pentaglycine cross-bridges in the cell wall. The susceptibility of several S. aureus and S. epidermidis strains were tested in the in vitro catheter model, including a methicillin-resistant S. aureus (MRSA) strain and an archetypical biofilm-producing S. epidermidis strain (Table 2). S. epidermidis 35984 was purchased from the American Type Culture Collection, and all other staphylococcal strains used were clinical isolates. Our results suggest that bound lysostaphin has significant activity against S. epidermidis and is only slightly less active than it is against S. aureus. The three CoNS strains that were tested in the catheter model are all clinical isolates and are known to be biofilm-producing strains. Combined with positive killing activity against other S. aureus strains (including MRSA), these results demonstrate that lysostaphin-coated catheters may be useful in the prevention of most clinically relevant staphylococcal catheter infections.
TABLE 2.
Susceptibility of various staphylococcal strains to lysostaphin-coated catheters
| Coating concn (mg/ml) | Bacterial strain | CFU (n = 2) |
|---|---|---|
| 0.1 | S. epidermidis 380 | 4 |
| 0 | S. epidermidis 380 | 678 |
| 0.1 | S. epidermidis 1175 | 68 |
| 0 | S. epidermidis 1175 | 824 |
| 0.1 | S. epidermidis 35984 | 16 |
| 0 | S. epidermidis 35984 | 757 |
| 0.1 | SA5 | 1 |
| 0 | SA5 | 785 |
| 0.1 | SA8 | 0 |
| 0 | SA8 | 1,593 |
| 0.1 | MRSA | 1 |
| 0 | MRSA | 910 |
Our study supports and extends the findings of other studies suggesting that the coating of catheters with antimicrobial agents may be an effective way to prevent catheter-related bloodstream infections (1, 7, 9, 13). However, lysostaphin-coated catheters may prove to be more suitable than the antimicrobial catheters currently available. The rapid coating time of the catheters allows a health-care worker to quickly and efficiently flush a solution of lysostaphin through the catheter, with minimal on-site catheter preparation. If bacteria were to invade the lysostaphin-coated catheter, the rapidity of kill would eradicate them within a very short amount of time, eliminating the risk of infection. Lysostaphin-coated surfaces may serve as an important advance in the management and prevention of catheter- and implant-related bloodstream infections.
REFERENCES
- 1.Bassetti, S., J. Hu, R. B. D'Agostino, Jr., and R. J. Sherertz. 2001. Prolonged antimicrobial activity of a catheter containing chlorhexidine-silver sulfadiazine extends protection against catheter infections in vivo. Antimicrob. Agents Chemother. 45:1535-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Climo, M. W., R. L. Patron, B. P. Goldstein, and G. L. Archer. 1998. Lysostaphin treatment of experimental methicillin-resistant Staphylococcus aureus aortic valve endocarditis. Antimicrob. Agents Chemother. 42:1355-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farr, B. M. 2001. Preventing vascular catheter-related infections: current controversies. Clin. Infect. Dis. 33:1733-1738. [DOI] [PubMed] [Google Scholar]
- 4.Kiri, N., G. Archer, and M. W. Climo. 2002. Combinations of lysostaphin with β-lactams are synergistic against oxacillin-resistant Staphylococcus epidermidis. Antimicrob. Agents Chemother. 46:2017-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin, R. R., and A. White. 1967. The selective activity of lysostaphin in vivo. J. Lab. Clin. Med. 70:1-8. [PubMed] [Google Scholar]
- 6.O'Grady, N. P., M. Alexander, E. P. Dellinger, J. L. Gerberding, S. O. Heard, D. G. Maki, H. Masur, R. D. McCormick, L. A. Mermel, M. L. Pearson, Raad, I. I., A. Randolph, and R. A. Weinstein. 2002. Guidelines for the prevention of intravascular catheter-related infections. Morb. Mortal. Wkly. Rep. Recomm. Rep. 51:1-29. [PubMed] [Google Scholar]
- 7.Pai, M. P., S. L. Pendland, and L. H. Danziger. 2001. Antimicrobial-coated/bonded and -impregnated intravascular catheters. Ann. Pharmacother. 35:1255-1263. [DOI] [PubMed] [Google Scholar]
- 8.Quickel, K. E., Jr., R. Selden, J. R. Caldwell, N. F. Nora, and W. Schaffner. 1971. Efficacy and safety of topical lysostaphin treatment of persistent nasal carriage of Staphylococcus aureus. Appl. Microbiol. 22:446-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raad, I. 1998. Intravascular-catheter-related infections. Lancet 351:893-898. [DOI] [PubMed] [Google Scholar]
- 10.Raad, I., R. Hachem, R. K. Tcholakian, and R. Sherertz. 2002. Efficacy of minocycline and EDTA lock solution in preventing catheter-related bacteremia, septic phlebitis, and endocarditis in rabbits. Antimicrob. Agents Chemother. 46:327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaffner, W., M. A. Melly, J. H. Hash, and M. G. Koenig. 1967. Lysostaphin: an enzymatic approach to staphylococcal disease. I. In vitro studies. Yale J. Biol. Med. 39:215-229. [PMC free article] [PubMed] [Google Scholar]
- 12.Shah, C. B., M. W. Mittelman, J. W. Costerton, S. Parenteau, M. Pelak, R. Arsenault, and L. A. Mermel. 2002. Antimicrobial activity of a novel catheter lock solution. Antimicrob. Agents Chemother. 46:1674-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherertz, R. J., D. M. Forman, and D. D. Solomon. 1989. Efficacy of dicloxacillin-coated polyurethane catheters in preventing subcutaneous Staphylococcus aureus infection in mice. Antimicrob. Agents Chemother. 33:1174-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stark, F. R., C. Thornsvard, E. P. Flannery, and M. S. Artenstein. 1974. Systemic lysostaphin in man—apparent antimicrobial activity in a neutropenic patient. N. Engl. J. Med. 291:239-240. [DOI] [PubMed] [Google Scholar]
- 15.Thodis, E., P. Passadakis, V. Vargemezis, and D. G. Oreopoulos. 2001. Prevention of catheter related infections in patients on CAPD. Int. J. Artif. Organs 24:671-682. [PubMed] [Google Scholar]
- 16.Veenstra, D. L., S. Saint, and S. D. Sullivan. 1999. Cost-effectiveness of antiseptic-impregnated central venous catheters for the prevention of catheter-related bloodstream infection. JAMA 282:554-560. [DOI] [PubMed] [Google Scholar]
- 17.Zygmunt, W. A., and P. A. Tavormina. 1972. Lysostaphin: model for a specific enzymatic approach to infectious disease. Prog. Drug Res. 16:309-333. [DOI] [PubMed] [Google Scholar]