Abstract
Four T4-like coliphages with broad host ranges for diarrhea-associated Escherichia coli serotypes were isolated from stool specimens from pediatric diarrhea patients and from environmental water samples. All four phages showed a highly efficient gastrointestinal passage in adult mice when added to drinking water. Viable phages were recovered from the feces in a dose-dependent way. The minimal oral dose for consistent fecal recovery was as low as 103 PFU of phage per ml of drinking water. In conventional mice, the orally applied phage remained restricted to the gut lumen, and as expected for a noninvasive phage, no histopathological changes of the gut mucosa were detected in the phage-exposed animals. E. coli strains recently introduced into the intestines of conventional mice and traced as ampicillin-resistant colonies were efficiently lysed in vivo by phage added to the drinking water. Likewise, an in vitro phage-susceptible E. coli strain freshly inoculated into axenic mice was lysed in vivo by an orally applied phage, while an in vitro-resistant E. coli strain was not lysed. In contrast, the normal E. coli gut flora of conventional mice was only minimally affected by oral phage application despite the fact that in vitro the majority of the murine intestinal E. coli colonies were susceptible to the given phage cocktail. Apparently, the resident E. coli gut flora is physically or physiologically protected against phage infection.
Diarrhea is the second most common cause of morbidity and mortality among infants and children in developing countries, exceeded only by respiratory diseases (47). Diarrhea has a complex etiology (22). However, Escherichia coli and rotavirus account for up to 50% of childhood diarrhea cases in developing countries (2, 3, 16, 23, 26). Enterotoxigenic E. coli (ETEC) is also a leading cause of traveler's diarrhea (9). Although the use of oral rehydration solutions has substantially reduced mortality from dehydration, it has little or no effect on the diarrhea itself and no effect on the transmission of the disease (7). Vaccines against diarrhea-causing E. coli are still in an early developmental stage (42, 43). A potentially low-cost treatment option for bacterial diarrhea was proposed 80 years ago by Félix d'Hérelle in the form of bacterial viruses (bacteriophages). Indeed, in the 1930s American physicians used pharmaceutical phage preparations for the treatment of both diarrheal diseases and staphylococcal infections (reviewed in references 15 and 50). The development of antibiotics in the 1940s replaced phages as therapeutic agents in the West, although enteric diseases, nosocomial infections, burns and wound infections continued to be treated with phage preparations in the Soviet Union on a very large scale (49). Western scientists were either unaware of this work or remained dubious about the reported high success rate despite the positive results obtained with the phage treatment of E. coli infections in a number of farm animals (calves, piglets, lambs, and chickens) (6, 45, 46).
An ideal candidate for phage therapy of E. coli infections is the coliphage T4 family. T4 is arguably the best-characterized biological system (25). This phage family is a natural component of the mammalian gut and can be easily isolated from the environment (stool and sewage) (1, 20, 21, 29). The richest sources of T4-like phages are apparently stools of diarrhea patients (20, 21). T4-like phages can be grown to high titer on laboratory E. coli strains. Early during the infection cycle T4 degrades the host DNA to the nucleotide level, preventing any integration of phage DNA into the bacterial chromosome (lysogeny) (34). T4 and a number of related phages have been completely sequenced (http://phage.bioc.tulane.edu/) (33), and no phage-associated bacterial virulence factors have been detected in these phages.
Despite these assets of the T4 phage system, the potential of bacteriophage therapy in human infections has not yet been carefully documented in scientific publications. Part of the academic community is therefore still “phage skeptic.” They point to the experience that the tremendous in vitro lytic activities of coliphages was rarely, if ever, demonstrated in carefully documented in vivo situations. In fact, not much is known about the factors governing the phage-bacterium interaction in the context of the complex microbial environment of the mammalian gut. To help fill this gap, we report here on the gastrointestinal passage of a set of orally applied T4-like phages in mice. In addition, we studied their in vivo bacteriolytic activities on the resident gut E. coli flora and towards E. coli strains introduced into the gut.
MATERIALS AND METHODS
Phage isolation.
Phage JS4 and JS94.1 were isolated from stool samples of pediatric patients with undifferentiated diarrhea hospitalized at the International Center for Diarrheal Disease Research in Dhaka, Bangladesh. The stool sample (∼10g) was resuspended in TS (NaCl [8.5 g/liter], tryptone [1 g/liter]) to a final volume of 30 ml and centrifuged for 15 min at 14,500 × g in 50-ml Falcon tubes. One milliliter of each stool preparation was filtered through a Millex AP20 prefilter followed by a 0.45-μm-pore-size Minisart filter. Subsequently, the samples were stored at 4°C. Phage JSD.1 was isolated from environmental water in Dhaka, Bangladesh, and phage JSL.6 was isolated from a sewage station in Vidy (Lausanne), Switzerland. Fifty milliliters of water samples was centrifuged at 10,000 rpm for 15 min and filtered through a 0.45-μm-pore-size Minisart filter.
The presence of phages was screened on the laboratory strain K803 (a K-12 derivative lacking prophage lambda, described in reference 5). The strain lacks restriction-modification systems and prophage lambda. K-12 is one of the major strains that have been widely used for phage studies as well as for recombinant DNA work. It is susceptible, for example, to nearly all of the over 100 T4-like phages in the Evergreen collection, most of which were isolated on E. coli B or on some pathogenic strain. The K803 strain was propagated in Hershey broth (prepared according to the recipe in reference 26) at 37°C with agitation (240 rpm). After overnight growth, the strains were streaked on a Hershey agar petri dish. Each time needed, a new culture was grown from a single colony. The stock cultures were kept as stab cultures at 4°C.
Spot testing was done on Hershey plates (15 g/liter agar) overlaid with 3.5 ml of Hershey top agar (7.5 g/liter). Ten microliters of filtered samples was put as eight spots in clockwise distribution around the plate after the top agar with plating bacteria solidified. For phage plaque assays, top agar (7.5 g/liter) was inoculated with 200 μl of a fresh overnight culture and 100 μl of positive sample and incubated overnight at 37°C
One well-separated phage plaque was chosen for amplification from each positive stool sample, picked with a sterile toothpick, and inoculated into 5 ml of Hershey broth together with 1% of an overnight culture of the E. coli strain K803. Incubation was performed with agitation at 240 rpm at 37°C. When lysis occurred, 3 drops of chloroform was added. The lysate was left overnight at room temperature followed by centrifugation at 14,500 × g for 10 min. The supernatant was transferred into a screw-cap glass tube. Three drops of fresh chloroform was added, and the phage stock was stored at 4°C. The phage lysate was at least diluted 1,000-fold into mineral water for the mouse feeding experiments.
Lysis in tube.
The lysis test was done as follows. Five milliliters of Hershey broth (23) was inoculated with 1% of a freshly grown culture (109 CFU/ml) and 1% phage lysate (108 PFU/ml). Incubation at 37°C was continued under aerobic conditions in a shaking incubator (240 rpm) for 3 to 5 h until the uninfected control cells reached the stationary growth phase. The optical density (OD) in the phage-inoculated cell was compared to that of mock-infected control cells. For anaerobic conditions, tubes were held in an anaerobic jar at 37°C for 5 to 10 h. The media were not prereduced; there was thus significant oxygen present during the early hours of the experiment.
Broth culture of phages.
E. coli was inoculated 1:100 in 200 ml of Hershey medium and incubated at 37°C in a shaking incubator (240 rpm). When an OD at 600 nm of 0.1 was reached, stock phages were inoculated with 107 PFU/ml. Each 15 min, samples were taken and OD readings of infected and uninfected cells were done at 600 nm. Samples were then centrifuged (10,000 × g, 5 min, 20°C), and chloroform-treated phage was titrated in the supernatant.
Pathogenic E. coli strains.
The tested collection of pathogenic E. coli strains included 12 enteropathogenic E. coli (EPEC) strains, representing the major serotypes isolated worldwide from pediatric diarrhea patients (41). This set of strains covered 10 different somatic O antigens and 10 different capsular K antigens (Table 1). In addition, the collection contained 12 major ETEC serotypes isolated from either pediatric gastroenteritis patients or adults suffering from traveler's diarrhea (Table 1). The ETEC strains represented 11 further O antigens, 10 distinct H antigens, and various combinations of heat-stable (ST) and heat-labile (LT) toxin producers (Table 1). These 22 pathogenic E. coli strains were obtained from B. Rowe (Central Public Health Laboratory, London, United Kingdom). Twelve further distinct ETEC and six EPEC strains were obtained from the microbiology laboratory of the International Center for Diarrheal Disease Research. They represent predominant E. coli isolates from their hospitalized pediatric diarrhea patients. The pathogenic E. coli strains from Dhaka were typed by DNA probes for the presence of ST and LT enterotoxin, ST, colonization factor antigen (CFA), E. coli surface antigens (CS), and the attaching-effacing genes (A/E) (Table 1) according to published methods (5, 17, 24).
TABLE 1.
Susceptibilities of E. coli strains to infection with T4-like phages
| Strain | Lysisb caused by:
|
|||
|---|---|---|---|---|
| JS4 | JSD.1 | JSL.6 | JS94.1 | |
| EPEC O18:K77 | X | |||
| EPEC O20:K84 | X | X | ||
| EPEC O26:K60 | X | |||
| EPEC O55:K59 | X | X | ||
| EPEC O86:K61 | ||||
| EPEC O111:K58 | X | |||
| EPEC O112:K66 | X | |||
| EPEC O119:K69 | X | |||
| EPEC O124:K72 | X | |||
| EPEC O125:K70 | ||||
| EPEC A/E+a | X | (X) | ||
| EPEC A/E+a | X | |||
| EPEC A/E+a | X | X | ||
| EPEC A/E+a | ||||
| EPECa | (X) | X | ||
| EPECa | ||||
| EPECa | (X) | X | ||
| EPECa | ||||
| ETEC O6:H16 | ||||
| ETEC O8:H9 | X | |||
| ETEC O15:H11 | ||||
| ETEC O25:H42 | X | |||
| ETEC O78:H12 | ||||
| ETEC O115:H51 | ||||
| ETEC O20:H11 | ||||
| ETEC O27:H7 | ||||
| ETEC O128:H18 | ||||
| ETEC O63:H− | ||||
| ETEC O148:H28 | X | |||
| ETEC O153:H12 | ||||
| ETEC LT+/ST+; CFA 1a | ||||
| ETEC LT−/ST+; CS6a | ||||
| ETEC LT−/ST+; PCF O166a | ||||
| ETEC LT−/ST+; CFA1a | X | |||
| ETEC LT−/ST+; CS4; CS6a | ||||
| ETEC LT+/ST+; CS1; CS3a | X | (X) | ||
| ETEC LT+/ST+; CS5; CS6a | ||||
| ETEC LT+/ST+a | ||||
| ETEC LT+/ST+a | ||||
| ETEC LT+/ST−a | X | |||
| ETEC LT+/ST−a | ||||
| ETEC LT+/ST−a | X | |||
| ECOR | ||||
| 1 | ||||
| 2 | ||||
| 4 | X | X | ||
| 5 | ||||
| 6 | ||||
| 8 | X | |||
| 9 | ||||
| 11 | ||||
| 12 | ||||
| 13 | X | |||
| 14 | X | |||
| 15 | X | X | ||
| 24 | X | X | ||
| 26 | X | |||
| 28 | ||||
| 35 | X | |||
| 36 | X | |||
| 38 | X | |||
| 39 | X | |||
| 40 | X | |||
| 41 | ||||
| 42 | ||||
| 43 | ||||
| 48 | X | |||
| 49 | (X) | |||
| 50 | ||||
| 51 | ||||
| 53 | ||||
| 54 | ||||
| 55 | ||||
| 56 | X | |||
| 59 | ||||
| 60 | (X) | X | ||
| 61 | (X) | |||
| 62 | ||||
| 63 | X | |||
| 64 | ||||
| 71 | ||||
| 72 | ||||
Isolated from the Dhaka hospital laboratory.
X, lysis; (X), partial lysis.
Phage purification.
One liter of Hershey medium was inoculated with a bacterial colony, grown to an OD of 0.1, and then infected at a multiplicity of infection of 5. NaCl was added to the lysate to a final concentration of 0.5 M and incubated 1 h at 4°C. After centrifugation at 10,000 rpm (16 min at 4°C) in a Sorvall RC5B centrifuge, polyethylene glycol 6000 was added to the supernatant to a final concentration of 10%. The lysate was incubated overnight at 4°C with gentle stirring. Polyethylene glycol-precipitated phages were collected by centrifugation at 14,500 × g for 16 min. The resulting pellets were resuspended in 3 ml of phage buffer (20 mM Tris-HCl [pH 7.4], 100 mM NaCl, 10 mM MgSO4), loaded on a discontinuous CsCl gradient (CsCl at 1.35, 1.53, and 1.65 g/ml), and centrifuged at 4°C in an SW55 rotor at 40,000 rpm for 3 h in a Beckman L8-60 M ultracentrifuge. Purified phage were recovered with a syringe and dialyzed against phage buffer.
Electron microscopy.
A drop of the phage suspension was applied to a Formvar carbon-coated copper grid for 5 min; the suspension was removed with a pipette and immediately replaced by a mixture of solutions A and B (solution A, 2% ammonium molybdate at pH 7.0 or 2% PTA; solution B, 11% bacitracin in distilled water) or a solution of 3% uranyl acetate. After 1 min the liquid was removed with a filter paper. The grids were examined in a Philips CM12 transmission electron microscope at 80 kV (magnification, ×176,000 or ×224,000). The dimensions of the phage were calibrated with T4 phage particles (25).
DNA purification.
Purified phages were treated with proteinase K at a final concentration of 1 mg/ml for 2 h at 37°C, and 3 M sodium acetate (pH 4.3) was added. DNA was extracted twice by phenol-chloroform and precipitated with 2 volumes of ethanol. After centrifugation, pellets were washed with 70% ethanol and resuspended in 50 μl of Tris-EDTA. DNA was digested with restriction enzymes according to the instructions provided by the manufacturer.
Experimental animals.
Eight-week-old C3H male mice (Charles River, St. Germain sur l'Arbre, France) were held under standard animal house conditions and fed irradiated 03-40 chow from Usine d’Alimentation Rationelle (Villemoissin-Orge, France). The drinking water, which did or did not contain phage at the specified titer, consisted of Vittel mineral water. We initially used this water since it contained bicarbonate at 258 mg/liter, reasoning that bicarbonate would buffer the stomach acidity and allow more efficient stomach passage of the phage. However, in later experiments we observed that mineral waters containing less bicarbonate (65 mg/liter) allowed an equally efficient gut passage of the phage (data not shown). The mineral water was changed every two days. Feces were sampled once a day directly from the hand-held animal into a sterile tube by gently pressing the abdomen of the animals to avoid contamination of the stool with bedding material or dripping from the water bottles. For each experimental series, five mice were used. Each mouse was held in a Makrolon type 3 cage with a filtered lid (Indulab, Gams, Switzerland), preventing cross-contamination between the cages. Stool samples were resuspended in 1 ml of phosphate-buffered saline. Tenfold dilutions of the resuspended feces were plated on Drigalski agar (Bio-Rad). This is a medium recommended for coproculture (Diagnostics Pasteur). The medium is not specific for E. coli but allows the differentiation of lactose-fermenting colonies (E. coli, Klebsiella, Enterobacter) yielding yellow colonies from lactose-nonfermenters (Salmonella, Shigella, Proteus, Providencia, Hafnia, Serratia, Levinea, Edwardsiella, Alcaligenes, Pseudomonas) yielding blue-green colonies (Diagnostics Pasteur). Practically all colonies from mouse fecal pellets were lactose positive. Since Klebsiella and Enterobacter species do not belong to the normal mouse fecal flora (52), the yellow colony count is practically an E. coli count. This diagnosis was confirmed by phage susceptibility: practically all colonies were lysed by one of the T4-like E. coli phages from our collection (see Results). We confirmed that the T4-like phages did not lyse a distinct genus of Enterobacteriaceae, for example, the food pathogen Enterobacter sakazaki (P. Breeuwer, unpublished results). The various T4-like phages were added to the drinking water in dilutions as specified in the text. In the experiments four mice received mineral water with phage while one negative mouse control in each experiment received only mineral water. Phages were titrated by the plaque assay in filtered fecal samples on the E. coli indicator cell K803. At the end of the experiment, the mice were sacrificed and standard gross anatomical and histopathological examinations were conducted. Different parts of the gut were rinsed with physiological salt before cells and phages were counted by colony and plaque assay.
Mouse experiments with ampicillin-resistant cells.
E. coli K803 was grown in Hershey broth to an OD (600 nm) of 0.7. Cells were centrifuged for 20 min at 4,500 rpm. The pellet was carefully resuspended in cold H2O (4°C). The cells were then washed twice with cold 10% glycerol. Finally the pellet was resuspended in 1 ml of cold glycerol and kept at −80°C as competent cells.
One hundred microliters of cells was electroporated with 0.5 ng of pUC18 using the following settings: 25 μF, 2.5 kV, and 200 Ω. The cells were incubated for 1 h in SOC (2% tryptone, 0.5% yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCL2, 20 mM Mg SO4, 20 mM glucose) medium at 37°C and plated on Hershey agar containing 20 μg of ampicillin.
The experiments with the Ampr cells were conducted with a total of 21 animals, i.e., three groups of seven mice per experiment. In each experiment, three mice received the Ampr cells without phage, three received Ampr cells and phage in the drinking water, while one mouse received phage orally but no Ampr cells. Six-week-old C3H male mice were taken for the experiment. The three experiments differed with respect to addition of oral ampicillin (experiment 1, no ampicillin; experiment 2, ampicillin was given together with Ampr cells; experiment 3, ampicillin was given first, followed by Ampr cells). Their drinking water consisted of Vittel supplemented with the four-phage cocktail (106 PFU/ml) and ampicillin (20 mg/ml) as specified in the text. The animals were force fed with ampicillin-resistant K803 (5 × 107 CFU) supplemented with 6 mg of ampicillin as specified in the text. Feces were sampled twice a day for the first 4 days and once a day for the rest of the study. Tenfold dilutions of resuspended feces were plated on Drigalski agar containing 20 μg per ml of ampicillin.
Axenic mice.
A total of six 8-week-old C3H axenic male mice from our own animal house breeding colony were allotted to three experiments. Each group consisted of two animals held under sterile conditions in a Makrolon type 2 cage maintained in the same cage without a filtered lid within a positive pressure isolator of the animal house. In experiment 1 mice were force-fed with E. coli K803 (0.5 ml at a concentration of 108 CFU/ml) using intubation. Daily fecal samples were investigated for E. coli cell counts on Drigalski plates. One week after colonization, sterile-filtered phage JS94.1 was given continuously at a concentration of 105 PFU/ml in the drinking water followed by daily fecal cell and phage counts. In the next week a second force-feeding was performed with E. coli ECOR5 followed two weeks later by force-feeding with ECOR56. In experiment 2 mice received cells and phage at the same time, while in experiment 3 the mice received first the phage and then the bacteria.
RESULTS
Isolation and characterization of the phages.
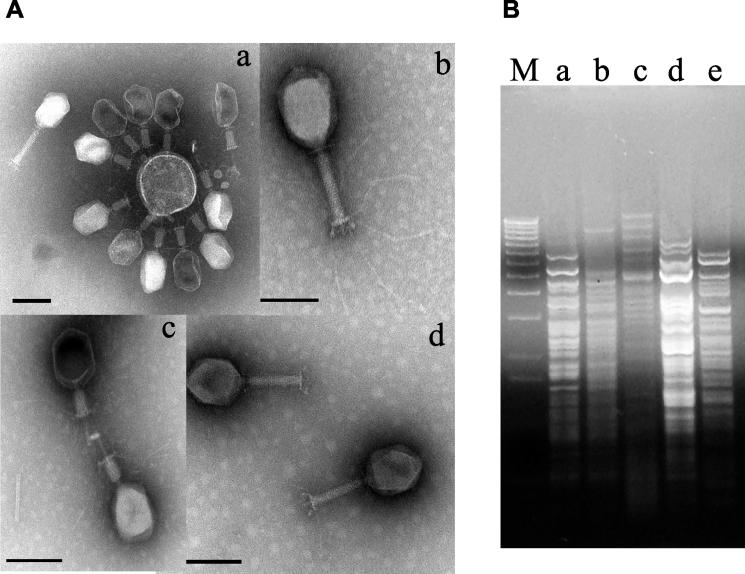
When tested on the E. coli strain K803, stool samples from pediatric diarrhea patients and environmental water samples in Dhaka, Bangladesh, and sewage from Switzerland yielded nearly exclusively Myoviridae (phages with a contractile tail). The elongated heads measured 110 nm by 75 nm. A collar separated the head from the tail sheath, which measured about 95 nm in length and 18 nm in width, with an annular substructure (Fig. 1A). The tail is terminated by a base plate structure to which both short tail spikes and 150-nm-long tail fibers with a central knee joint are attached (Fig. 1A, subpanel b). Phages with a 40-nm contracted tail sheath were also observed (Fig. 1A, subpanels a and c). In these phages, an internal tail tube extended beyond the contracted tail sheath. The morphology suggests T4-like phages. All phage isolates were tested individually in the spot test against a collection of pathogenic E. coli strains associated with diarrhea. From this screening a group of four phages was selected that offered the broadest combined theoretical host range. All four phages showed the typical morphology of T4-like phages (Fig. 1A); showed, like phage T4, a 170-kb genome upon pulsed-field gel electrophoresis; yielded with gp23- and gp32-specific primers the diagnostic PCR products for T4-like phages (36, 53) (data not shown); and showed distinct restriction patterns (Fig. 1B).
FIG. 1.
Four T4-like phages used in the mouse experiments. (A) Transmission electron microscopy picture of CsCl density gradient-purified bacteriophage JS4 (a), JSD.1 (b), JSL.6 (c), and JS94.1 (d). Negative staining was performed with uranyl acetate (c), ammonium molybdate (a and d), or phosphotungstic acid (c). The size bar corresponds to 100 nm. (B) Restriction analysis of phages (for lanes a to d, see corresponding subpanel in panel A; lane e, phage T4) with enzyme DraI. Lane M, DNA size marker (1-kb lambda DNA ladder; Invitrogen).
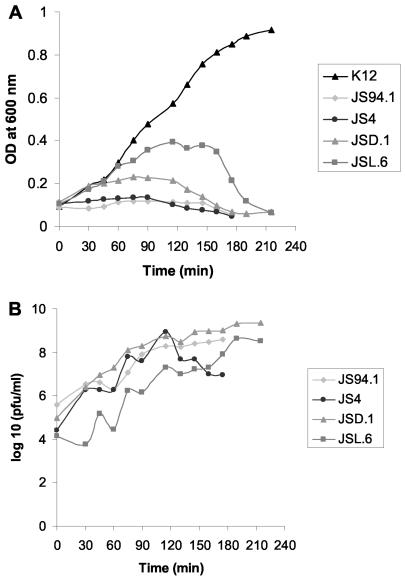
These four selected phages were rescreened for their lytic potential on pathogenic E. coli strains by a tube lysis test. This test is more labor-intensive than the spot test but offers a more rigorous assessment of the bacterial lysis activities of the test phages (Table 1). Also in the tube lysis test, the stool phages JS4 and JS94.1 and the environmental water phages JSD.1 and JSL.6 showed a complementary lytic potential on our pathogenic E. coli strain collection (Table 1). Most notably, the combined theoretical host range determined by adding up the host ranges of the four individual phages was 19 out of 40 (47%) of the pathogenic E. coli strains. When tested on the K803 strain, progeny phage was detected in broth culture infections at about 40 min postinfection and phage titer increased afterwards, sometimes in a biphasic way (Fig. 2B). The OD of phage-infected K803 cultures lagged from the beginning behind the OD development of the uninfected culture (Fig. 2A).
FIG. 2.
Lysis of E. coli K803 strain by the four T4-like phages in broth culture. (A) OD development of an uninfected control culture (K-12) and parallel cultures infected with phages JS94.1, JS4, JSD.1, and JSL.6. (B) Progeny phage release from the four phage-infected cultures depicted in panel A. Phage infectivity was measured by plaque assay.
The fact that E. coli is also a normal constituent of the gut flora of humans could present a peculiar problem for phage therapy of E. coli diarrhea. The four phages constituting the cocktail were therefore also investigated individually for their lytic potential on nonpathogenic E. coli strains from the ECOR collection (30) in the tube lysis test (38). The ECOR collection is a widely used set of 72 reference Escherichia coli strains isolated between 1973 and 1983 from a variety of animal hosts and a variety of geographic locations. In broth culture, JS4, JS94.1, JSL.6, and JSD.1 lysed eight, three, three, and five strains, respectively, from 39 ECOR strains included in the test (Table 1). The combined theoretical host range determined by adding up the host ranges of the four individual phages was 18 (46%) of the nonpathogenic E. coli strains.
In the next step, we explored the in vitro lytic activities of the four phages on the endogenous E. coli gut flora from a group of ten conventional adult mice. Over a 10-day observation period, feces were recovered every day for each mouse and lactose-positive yellow colonies were counted on Drigalski agar plates. The fecal cell counts showed an average of 106 CFU/g (data not shown), which is in agreement with similar results obtained by Poulsen et al. (39). Five random colonies were selected per day for each mouse, and the total of 500 colonies was tested against the four phages in the spot test to reduce the workload. Between 85 and 100% of the tested colonies were lysed by phages JS4 and JSD.1. JSL.6 lysed about 80% of the colonies. More variation was observed with phage JS94.1, which lysed less than 40% of the isolated murine E. coli gut strains in six animals. When the results were combined, practically all cells were lysed by one of the E. coli phages, confirming the attribution of the vast majority of the yellow colonies from the feces of conventional mice to E. coli. One fecal sample of each mouse was tested over 5 days for the presence of phage on the K803 indicator cell in the plaque assay. No phage plaques were detected.
Effect of oral phage on fecal E. coli count in mice.
Next we wanted to determine the threshold for an in vivo lytic effect of orally applied phages on the intestinal E. coli population in laboratory mice. To this end the four phages were added as a cocktail to the drinking water of 10 mice in increasing doses separated by 3 days of phage-free drinking water. Substantial variation was seen for the lactose-positive cell count on the Drigalski plates in all animals, even before phages were added to the drinking water. This variation was also seen during the periods of phage feeding to the animals. Using a two-way analysis of variance with phage dose as a fixed factor and animals as a random factor, we derived the following means and standard errors of the means for fecal colony counts on Drigalski plates: for water only, 106.2 ± 0.04; for 103 phage/ml, 105.9 ± 0.10; for 105 phage/ml, 106.1 ± 0.06; for 107 phage/ml, 105.7 ± 0.06. The effect of the phage dose on the cell count was highly significant (P value < 0.0001) but was in absolute terms very small and thus biologically not significant.
In view of the in vitro phage susceptibility of the most prevalent lactose-positive fecal colonies on Drigalski agar, the lack of a bacteriolytic effect of the oral phage on the fecal cell count was surprising. We considered several hypotheses to explain this observation. First, under the selective pressure of the phages the prevalent phage-susceptible strains might have been replaced by phage-insensitive strains. Second, without protection (antacid or microencapsulation) phages might not survive the gastric passage and thus not be available in the intestine. Third, phages might be present in the gut, but for physiological reasons the endogenous intestinal E. coli cell population resists phage infection.
The first hypothesis was addressed by the isolation of 100 additional lactose-positive colonies from the feces of two animals during the phage treatment period. The colonies showed a comparable phage susceptibility pattern before and during the phage treatment period, leading to the rejection of the hypothesis of an intestinal outgrowth of phage-resistant E. coli or other Enterobacteriaceae under the selective pressure of the oral phages.
Gastrointestinal passage of orally applied phages.
The following experiments demonstrated that unprotected T4-like phages could survive the gastric passage in conventional adult laboratory mice. These experiments refute the second hypothesis.
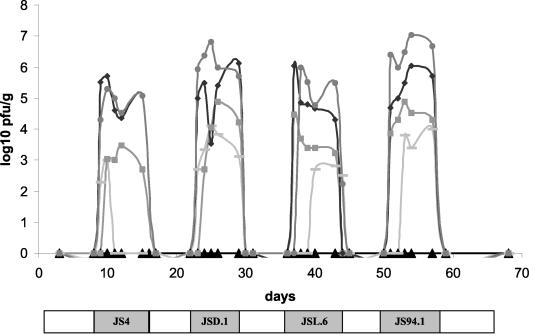
To begin, we determined the lowest phage concentration leading to stable fecal phage excretion. To this end four animals received in the drinking water successively the four individual phages added at 10-fold dilution steps. Fecal phage titers decreased with the titer of the phage in the drinking water in an approximate dose-response pattern (Fig. 3). With the lowest phage concentration of 103 PFU/ml in the drinking water, only low fecal phage titers over short time periods were observed, while exposure to 104 PFU/ml resulted in fecal phage detection nearly over the entire exposure period (Fig. 3).
FIG. 3.
Gastrointestinal passage of the orally added phages in conventional mice. Fecal phage titer after oral addition of the specified phage strain at 106 (circles), 105 (diamonds), 104 (squares), and 103 (bars) PFU/ml, fed to four mice at the times indicated by the shaded bars at the bottom of the figure. The triangles give the phage titers for the control mice. The periods of phage-free drinking water are indicated by white boxes.
In the next experiment, we asked whether the gastrointestinal passage differed between the individual phages or between individual mice. To answer this question, four mice received successively each of the four individual phages at a fixed concentration of 107 PFU/ml in the drinking water. Each phage addition was followed by a phage-free drinking water period before the next phage isolate was added to the drinking water. The fecal phage counts were assessed on a daily basis (data not shown). Three observations were made in this experiment. First, no significant difference was detected between the individual animals with respect to gastrointestinal phage passage. Second, some difference in gastrointestinal passage was detected between the different phage isolates as documented by a 50-fold fecal phage titer difference between mice fed phage JS94.1 and JSL.6 which achieved the highest and the lowest fecal phage titers, respectively (data not shown). Third, in one mouse the identity of the fecally excreted phage with the phage in the drinking water was demonstrated for all four feeding periods by restriction analysis of the fecally reisolated phage.
Next we asked whether the stool phage isolates could not infect their host cells due to the anaerobic atmosphere of the gut environment. This was not the case: during in vitro growth in an anaerobic jar, 11 of 32 T4-like stool phage isolates and one of the four test phages (JSL.6) lysed its target cells under both anaerobic and aerobic conditions.
Finally, we asked whether phages could be given repetitively without interference by an intestinal immune response. Phage JS94.1 was given three times to two mice. Each intervention was followed by a 2-week rest period. In each case infectious phage was detected in the stools samples with titers approximately proportional to concentration of the phage in the drinking water (data not shown).
Phage treatment of axenic mice.
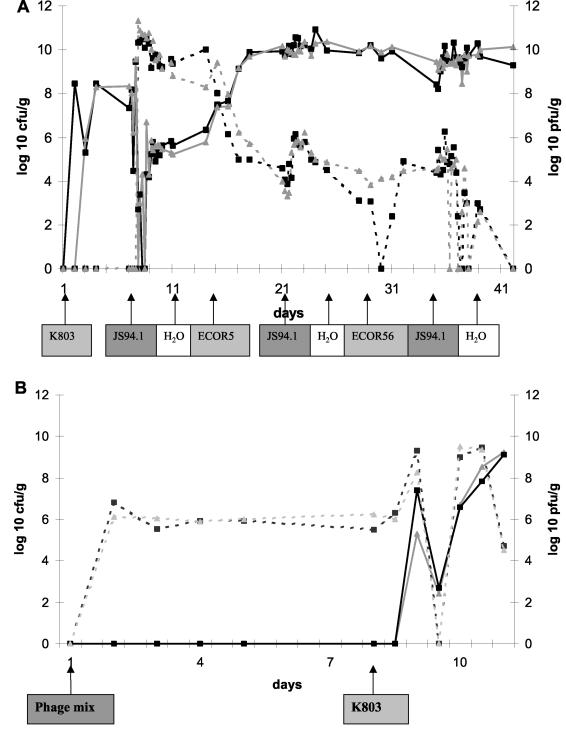
To test the in vivo lytic activities of the isolated stool phages, we inoculated two axenic mice with a single E. coli strain, namely, the indicator cell K803, resulting in a cell concentration of 108 CFU/g of feces (Fig. 4A). One week later, the K803-colonized mice were exposed to phage JS94.1 at 105 PFU/ml in the drinking water. Within a day, the fecal phage titer in the JS94.1-exposed mice rose from undetectable titers to beyond 1010 PFU/ml (Fig. 4A). The 100,000-fold titer increase with respect to the phage concentration in the drinking water documented an active replication of phage JS94.1 in the guts of the experimental animals. Concomitantly, the fecal E. coli cell count dropped from 108 to 104 CFU/ml or even lower, documenting a substantial in vivo bacteriolytic activity of the orally applied phage (Fig. 4A). Despite this serious drop in host cell density, the very high fecal phage titers decreased only slowly over the next four days. Interestingly, over the same time period the fecal cell count increased from undetectable levels to about 105 CFU/ml, which is still 1,000-fold lower than the original fecal cell count. At days 9 and 11, several dozen colonies were picked. All were sensitive to phage JS94.1 suggesting that some of the cells are now in gut sites protected from phage.
FIG. 4.
Effect of oral phage on the inoculated E. coli strain in axenic mice. (A) Fecal E. coli counts (solid line in log CFU per milliliter) and fecal phage counts (dashed line in log PFU per milliliter) in two axenic mice exposed to the specified E. coli strains, phage JS94.1, or water at the specified time points; the start day is indicated with an arrow below the abscissa. A black line with squares or a gray line with triangles identifies values from an individual mouse. (B) 106 PFU/ml was given from day 1 in the drinking water to axenic mice lacking intestinal bacteria. The mice were force-fed with 104 CFU of K803 at day 8. Logarithmic fecal cell (solid line) and phage counts (dashed line) per gram of stool were plotted over 10 days. A black line with squares or a gray line with triangles identifies values from an individual mouse.
Two weeks after the colonization with K803, the mice were force-fed the ECOR5 strain that is insensitive to phage JS94.1 in vitro. Within days, the fecal cell count rose to 1010 CFU/ml, suggesting successful colonization with the new E. coli strain (Fig. 4A). In parallel with the renewed fecal cell count increase, the fecal phage titer dropped by 4 logs or more, consistent with the expected replacement of a phage-sensitive by a phage-insensitive intestinal cell population. Alternatively, the phage-sensitive population did not change, but the ability of the phage to infect was diminished under the circumstances created. The mice were then force-fed with ECOR56 strain (sensitive to phage JS94.1) followed by phage JS94.1 in the drinking water. This operation did not rescue the fecal phage titer (Fig. 4A), suggesting colonization resistance or host immune defenses. At day 42, no phages were detected in four different rinsed gut segments (duodenum, jejunum, ileum, colon) or in the liver or in mesenteric lymph nodes.
In the next experiment, two axenic mice were force-fed with 104 K803 cells and received at the same time the four-phage cocktail at 106 PFU/ml in the drinking water (data not shown). One mouse showed an initial high fecal cell count (109 CFU/g of feces), followed by a precipitous drop to 104/g and lower. Another mouse showed a fecal cell count decrease from 106 to 104 CFU/g (data not shown). Both mice showed a fecal titer 1,000-fold higher than the drinking water phage titer over the first days of the experiment, suggestive of active in vivo phage replication.
Finally, two axenic mice were first exposed to the four-phage cocktail at 106 PFU/ml in the drinking water before receiving cells. Notably, in the absence of intestinal bacteria, 106 PFU of phages were also detected per g of stool (Fig. 4B), demonstrating that the fecal phages are not the result of intestinal replication of phages after a reduction of phage titers in the stomach, but the consequence of a passive transit through the entire gastrointestinal tract including the stomach. One week later the mice were force-fed with 104 K803 cells. Introduction of E. coli into the gut resulted in a transient 1,000- to 10,000-fold fecal phage titer increase (Fig. 4B). During the initial phase of intestinal phage replication, a low and variable fecal cell number was observed. This phase was followed by a steady increase of bacteria to 109 CFU/g stool over the next days (Fig. 4B), and bacteria remained at this level until 2 weeks later (data not shown), while the phage titers dropped to low levels.
Follow-up of Ampr E. coli cells in conventional mice.
The preceding experiments suggested that orally applied phages lysed only E. coli cells that were recently introduced into the intestine. To differentiate newly introduced from resident E. coli strains, 108 CFU of K803 cells transformed with plasmid pUC18 containing an ampicillin resistance marker were force-fed to three conventional mice. Transient peaks of fecal Ampr E. coli cells were detected half a day after the force-feeding, but they were lost from the intestine half a day later (data not shown). No spontaneous fecal phage excretion was seen in these mice or the corresponding control mice of the experiments reported below. Three further mice received in addition 106 PFU of phage per ml in the drinking water. As in the preceding experiment, fecal Ampr E. coli cells were only transiently observed directly after the force-feeding (data not shown). Phage was detected only during the phage feeding period with a 1-day time lag for appearance and 2 days for disappearance.
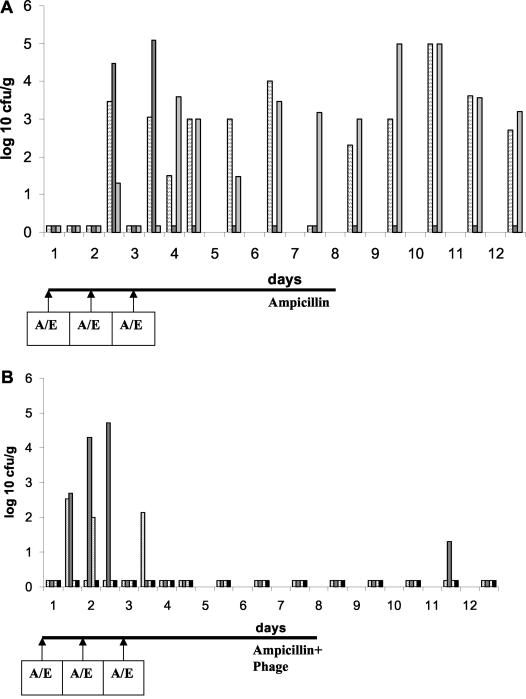
To overcome the colonization resistance of the resident intestinal flora against the introduction of new cells, the Ampr-labeled cells were given together with ampicillin during the force-feeding. During the first week ampicillin was also added to the drinking water (20 μg/ml). Under these conditions, 103 to 105 CFU of the Ampr E. coli cells were detected per g of stool and for at least 5 days maintained after omission of ampicillin from the drinking water (Fig. 5A). When similarly treated mice received phage in addition in the drinking water, Ampr E. coli cells were detected after day 2 only in two fecal samples with low counts, suggesting elimination of Ampr E. coli from the gut (Fig. 5B) by oral phage.
FIG. 5.
Effect of oral phage on the introduction of ampicillin-resistant E. coli in mice. (A) Fecal cell counts in three mice force-fed with 5 × 107 CFU of ampicillin-resistant E. coli and ampicillin (A/E) at the time points marked with an arrow below the time axis. The ordinate shows the logarithm of CFU per gram of stool. Each vertical bar represents the fecal cell count for one animal at the specified time point. (B) The same experiment as depicted in panel A except that in addition to ampicillin the mice also received the phage cocktail at 106 PFU/ml in the drinking water. The rightmost black data points refer to a control mouse not receiving ampicillin-resistant E. coli.
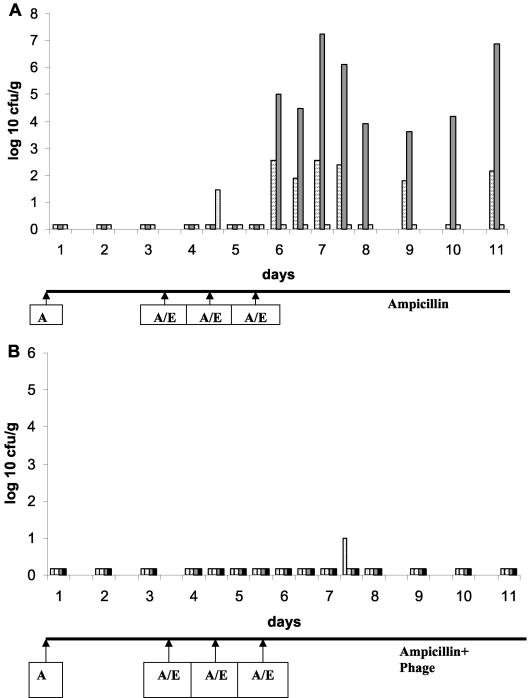
In the next experiment, six mice were pretreated with ampicillin both by force-feeding and in drinking water before being fed with Ampr E. coli cells. In this experiment only one stool sample from three mice receiving phages in the drinking water showed a low fecal cell count of Ampr cells (Fig. 6B) compared to 15 stool samples from the three mice receiving plain water (Fig. 6A).
FIG. 6.
Effect of oral phage on the introduction of ampicillin-resistant E. coli in mice pretreated with ampicillin in the drinking water. (A) Seven mice received ampicillin by force-feeding at day 1 and in the drinking water throughout the experiment. At the time points indicated with arrows marked with A/E, six mice were force-fed with ampicillin and ampicillin-resistant E. coli (a control mouse received only buffer instead of E. coli). Both groups of mice received ampicillin in the drinking water, but some mice were in addition exposed to 106 PFU/ml of the phage cocktail in the drinking water (B). Both panels show the fecal counts of ampicillin-resistant cells. The rightmost black data points in panel B refer to a control mouse not receiving ampicillin-resistant E. coli.
Orally fed T4-like phages remain restricted to the gut.
Four axenic mice were inoculated with K803 cells and then force-fed with 109 PFU of the phage cocktail and maintained on phage at 107 PFU/ml in the drinking water and finally sacrificed. Four different gut segments (once rinsed with buffer to remove the gut content), the liver, and the mesenteric lymph nodes were tested for the presence of K803 cell and phages. Neither cells (<10 CFU/ml) nor phages (<10 PFU/ml) were detected in the mesenteric lymph nodes or the liver. Four further axenic mice were sacrificed 4 days after a change to phage-free drinking water. None of the investigated tissues were associated with phages (data not shown). Tissue samples from eight phage-treated mice and two control mice were processed for standard histology analysis. For the intestinal samples, both longitudinal and cross-sectional histological cuts were analyzed. The tissue morphology was normal in all animals. No behavioral or fur changes were observed in the animals during the treatment period.
DISCUSSION
The scientific literature shows a renewed interest in phage therapy. Part of this interest stems from a series of spectacular experiments conducted with phage lysins (31, 37, 44), another part comes from a number of recent animal experiments with viable phage particles as antibacterial agents (8, 11, 14, 28, 30, 32, 49, 54), and a further part derives from historical reviews of the Soviet experience with phage therapy (4, 10, 12, 18, 48, 49, 50). It is currently difficult to critically assess the potential of the clinical trials conducted in the former Soviet Union. The trials were mainly published in Russian, most of the trials were uncontrolled, and the therapeutic phages were not described in published reports. With the present study, we wanted to address some of the basic preclinical problems of phage therapy in the context of E. coli diarrhea. We have chosen this example because a substantial body of knowledge has been accumulated on E. coli and its phages; the pathogenic target bacteria are located in the gut and are thus principally accessible to orally applied phages; the Soviet experience reported efficiency of phages against dysentery; and we have some experience in the treatment of E. coli diarrhea with biologicals (bovine antibodies [13, 51]) and probiotic lactobacilli (S. Sarker et al., unpublished data).
For the experiments reported here we selected two stool- and two environmental water-derived T4-like phage isolates with a broad host range for diarrhea-associated E. coli serotypes. The chosen T4-like phages survived the gastrointestinal transit in adult mice. More specifically, the fecal phage count corresponded roughly to the orally applied phage titer. This quantitative correlation should on its own not be overinterpreted since it could represent a combination of inactivation of some phage in the stomach and the reproduction of some phage in the endogenous intestinal E. coli population. However, no major inactivation of T4-like phages occurred in the stomachs of adult mice since in axenic mice (lacking any intestinal microbial flora and thus the possibility to amplify phage), a similar oral-fecal phage titer correlation was found. Unprotected T4-like phage thus has the capacity to transit the entire gastrointestinal tract without appreciable infectivity loss. When compared to the timing of a pulse of phage in the drinking water, the appearance and disappearance of the phage in the feces took approximately 1 to 2 days. If coprophagy (the eating of feces, which in contrast to rabbits, was only occasionally observed in mice from our animal house) or water dripping from the bottles into the cage and not the orally applied phage were the source for maintenance of fecal phage titers in mice, one would have expected longer fecal phage disappearance times due to phage recycling. Notably, fecal pellets were not collected from the bedding of the cages excluding passive phage contamination. The murine stomach differs substantially from the human stomach by allowing the thriving of an endogenous Lactobacillus flora in the esophagus-proximal part, showing a mean pH value of 3.8 (52). However, in the pylorus-proximal part of the murine stomach the mean pH value was only 2.2 (52). This acidity killed 95% of acid-sensitive bacteria like Vibrio cholerae and half of the fragile E. coli mutant χ1666, but had little bactericidal effect on E. coli K-12 (19).
In the case of E. coli diarrhea, a peculiar problem for phage therapy is presented by the fact that nonpathogenic E. coli strains are a normal constituent of the gut flora of humans and many animals (52). The in vivo experiments demonstrated that collateral damage on nonpathogenic gut E. coli strains is an unlikely complication of oral application of T4-like phages. In conventional mice we observed no detrimental effect of the phage transit on the physiological E. coli flora. The total count of lactose-positive fecal colonies on the Drigalski plates decreased only slightly during phage treatment. No change from phage-sensitive to phage-resistant E. coli was seen, as if the cells had not experienced a phage infection pressure. In addition, in conventional mice we did not observe evidence for intestinal phage replication. Two basic hypotheses might explain this result. First, the intestinal E. coli cells might be in an altered physiological state (stationary state, starvation, anaerobic growth) that does not permit phage infection. Alternatively, physical factors might prevent the infection of the resident intestinal E. coli flora (reduced phage diffusion in the thickened gut content, difficulty for the phage in finding its target cell in the presence of a large excess of nontarget bacterial cells, and seclusion of the resident E. coli in a nonaccessible niche). Anaerobic growth prevented the in vitro growth of some, but by far not all, T4-like phages. However, residual oxygen was not rigorously excluded in these experiments. Nevertheless, in vivo growth of phages was demonstrated in axenic mice excluding anaerobiosis as a major limiting factor for phage replication.
Data from the literature help to understand the lack of activities of the orally applied T4-like phages on the resident E. coli flora. Fluorescent oligonucleotide probes targeting rRNA were used to localize E. coli cells in the large intestines of mice by the in situ hybridization technique. E. coli cells were seen embedded in the mucosal material overlying the epithelial cells of the large intestine, and no direct attachment to the epithelium was observed (39). Extension of these studies revealed that E. coli consisted in the murine intestine of two populations, one in the mucus which has an apparent generation time of 40 to 80 min and one in the luminal contents which is static (40). Interestingly, a defined E. coli strain when introduced into the murine intestine differentiated into two distinct populations, one that has the characteristics of the laboratory-grown strain and one that appears as a coccoid cell. The authors observed natural selection for the coccus-type cell in the intestine and for the rod-shaped E. coli cell in laboratory medium growth (27).
On the basis of our observations and the literature data, the most conclusive interpretation is the following. Orally applied T4-like phages pass the stomach and intestine as efficiently as E. coli K-12. Transit times of less than a day without amplification or death were reported for radiolabeled K-12 (19) and confirmed by us with Ampr-labeled K-12. The phage meets the viable but nongrowing E. coli in the gut lumen which we counted in the feces. The metabolic state (of starvation?) in this intestinal E. coli population does not permit phage replication. On laboratory media the cells resume growth and become fully susceptible to phage infection. We suspect that the E. coli cells growing as microcolonies in the mucin layer (52) are physically protected against phage infection. However, this cell population might only show up to a limited extent in the fecal flora. It will be interesting to scrape the mucin layer from the large intestines of sacrificed mice and test their E. coli population for T4 phage susceptibility in media supplemented with cecal mucus (35).
Our experiments provide, however, clear evidence that E. coli recently introduced into the murine intestine is susceptible to phage infection. This was demonstrated in both conventional and axenic mice. In conventional mice, the colonization resistance of the resident intestinal flora had to be upset by the feeding of an antibiotic. This treatment permitted an at least temporary foothold to the externally added ampicillin-resistant K-12 E. coli cell even after the cessation of ampicillin feeding. Orally added phage prevented the fecal appearance of ampicillin-resistant K-12 E. coli cells over the observation period, suggesting the in vivo lysis of the added E. coli cells. In axenic mice inoculated with K-12 cells, the orally added phage had a lytic effect on the cells when given before, concomitant with, and after feeding of the cells. However, after a transient lytic phase, E. coli cells were again observed in the fecal samples. It is not clear what factor prevented the phage from permanently clearing the intestine of E. coli. Without the competition of other intestinal bacteria, K-12 might achieve in axenic mice an association with the mucus layer and thus escape phage infection. However, in axenic mice the outgrowth of the cells did not reach the original cell titers. Only when phage-resistant cells were fed in a second wave were the initial high fecal E. coli titers achieved, suggesting that the first E. coli strain remained under phage control.
The success of the phage therapy approach against E. coli diarrhea hinges on the in vivo phage susceptibility of the infecting pathogenic E. coli strains. As pathogenic E. coli targets the small intestine and does not colonize the intestine beyond the acute phase of diarrhea, it is unlikely that EPEC or ETEC strains occupy the phage-resistant niche of the resident E. coli strains in the large intestine. The effectiveness of phages in treating experimental E. coli diarrhea in mice, calves, piglets, and lambs (45, 46) suggests strongly that pathogenic E. coli is susceptible to orally applied phage.
Acknowledgments
We thank the Swiss National Foundation for the financial support of Sandra Chibani-Chennoufi (grant 5002-057832).
We thank Andreas Rytz for statistical advice.
REFERENCES
- 1.Ackermann, H. W., and H. M. Krisch. 1997. A catalogue of T4-type bacteriophages. Arch. Virol. 142:2329-2345. [DOI] [PubMed] [Google Scholar]
- 2.Albert, M. J., A. S. Faruque, S. M. Faruque, R. B. Sack, and D. Mahalanabis. 1999. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J. Clin. Microbiol. 37:3458-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert, M. J., S. M. Faruque, A. S. Faruque, P. K. Neogi, M. Ansaruzzaman, N. A. Bhuiyan, K. Alam, and M. S. Akbar. 1995. Controlled study of Escherichia coli diarrheal infections in Bangladeshi children. J. Clin. Microbiol. 33:973-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alisky, J., K. Iczkowski, A. Rapoport, and N. Troitsky. 1998. Bacteriophages show promise as antimicrobial agents. J. Infect. 36:5-15. [DOI] [PubMed] [Google Scholar]
- 5.Bachmann, B. J. 1996. Derivations and genotypes of some mutant derivatives of Escherichia coli, p. 2460-2488. In F. C. Neidhardt, R. Curtis, J. L. Ingraham, J. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 2. ASM Press, Washington, D.C. [Google Scholar]
- 6.Barrow, P., M. Lovell, and A. Berchieri, Jr. 1998. Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin. Diagn. Lab. Immunol. 5:294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhan, M. K., D. Mahalanabis, O. Fontaine, and N. F. Pierce. 1994. Clinical trials of improved oral rehydration salt formulations: a review. Bull. W. H. O. 72:945-955. [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas, B., S. Adhya, P. Washart, B. Paul, A. N. Trostel, B. Powell, R. Carlton, and C. R. Merril. 2002. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect. Immun. 70:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black, R. E. 1990. Epidemiology of travelers' diarrhea and relative importance of various pathogens. Rev. Infect. Dis. 12(Suppl. 1):S73-S79. [DOI] [PubMed] [Google Scholar]
- 10.Breithaupt, H. 1999. The new antibiotics. Nat. Biotechnol. 17:1165-1169. [DOI] [PubMed] [Google Scholar]
- 11.Broxmeyer, L., D. Sosnowska, E. Miltner, O. Chacon, D. Wagner, J. McGarvey, R. G. Barletta, and L. E. Bermudez. 2002. Killing of Mycobacterium avium and Mycobacterium tuberculosis by a mycobacteriophage delivered by a nonvirulent mycobacterium: a model for phage therapy of intracellular bacterial pathogens. J. Infect. Dis. 186:1155-1160. [DOI] [PubMed] [Google Scholar]
- 12.Carlton, R. M. 1999. Phage therapy: past history and future prospects. Arch. Immunol. Ther. Exp. (Warsaw) 47:267-274. [PubMed] [Google Scholar]
- 13.Casswall, T. H., S. A. Sarker, S. M. Faruque, A. Weintraub, M. J. Albert, G. J. Fuchs, N. H. Alam, A. K. Dahlstrom, H. Link, H. Brüssow, and L. Hammarström. 2000. Treatment of enterotoxigenic and enteropathogenic Escherichia coli-induced diarrhoea in children with bovine immunoglobulin milk concentrate from hyperimmunized cows: a double-blind, placebo-controlled, clinical trial. Scand. J. Gastroenterol. 35:711-718. [DOI] [PubMed] [Google Scholar]
- 14.Cerveny, K. E., A. DePaola, D. H. Duckworth, and P. A. Gulig. 2002. Phage therapy of local and systemic disease caused by Vibrio vulnificus in iron-dextran-treated mice. Infect. Immun. 70:6251-6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duckworth, D. H. 1999. History of virology: bacteriophages, p. 725-730. In A. Granoff and R. G. Webster (ed.), Encyclopedia of virology. Academic Press, Memphis, Tenn.
- 16.Echeverria, P., D. N. Taylor, U. Lexsomboon, M. Bhaibulaya, N. R. Blacklow, K. Tamura, and R. Sakazaki. 1989. Case-control study of endemic diarrheal disease in Thai children. J. Infect. Dis. 159:543-548. [DOI] [PubMed] [Google Scholar]
- 17.Faruque, S. M., K. Haider, M. J. Albert, Q. S. Ahmad, A. N. Alam, S. Nahar, and S. Tzipori. 1992. A comparative study of specific gene probes and standard bioassays to identify diarrhoeagenic Escherichia coli in paediatric patients with diarrhoea in Bangladesh. J. Med. Microbiol. 36:37-40. [DOI] [PubMed] [Google Scholar]
- 18.Fischetti, V. A. 2001. Phage antibacterials make a comeback. Nat. Biotechnol. 19:734-735. [DOI] [PubMed] [Google Scholar]
- 19.Freter, R., H. Brickner, J. Fekete, M. M. Vickerman, and K. E. Carey. 1983. Survival and implantation of Escherichia coli in the intestinal tract. Infect. Immun. 39:686-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furuse, K. 1987. Distribution of coliphages in the general environment: general considerations, p. 87-124. In S. M. Goyal, C. P. Gerba, and G. Bitton (ed.), Phage ecology. John Wiley and Sons, New York, N.Y.
- 21.Furuse, K., S. Osawa, J. Kawashiro, R. Tanaka, A. Ozawa, S. Sawamura, Y. Yanagawa, T. Nagao, and I. Watanabe. 1983. Bacteriophage distribution in human faeces: continuous survey of healthy subjects and patients with internal and leukaemic diseases. J. Gen. Virol. 64:2039-2043. [DOI] [PubMed] [Google Scholar]
- 22.Guerrant, R. L. 1995. Principles and syndromes of enteric infections, p. 945-962. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 4th ed. Churchill Livingstone, New York, N.Y.
- 23.Guerrant, R. L., L. V. Kirchhoff, D. S. Shields, M. K. Nations, J. Leslie, M. A. De Sousa, J. G. Araujo, L. L. Correia, K. T. Sauer, and K. E. Mcclelland. 1983. Prospective study of diarrheal illnesses in northeastern Brazil: patterns of disease, nutritional impact, etiologies, and risk factors. J. Infect. Dis. 148:986-997. [DOI] [PubMed] [Google Scholar]
- 24.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karam, J. D. 1994. Molecular biology of bacteriophage T4. ASM Press, Washington, D.C.
- 26.Kim, K. H., I. S. Suh, J. M. Kim, C. W. Kim, and Y. J. Cho. 1989. Etiology of childhood diarrhea in Korea. J. Clin. Microbiol. 27:1192-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krogfelt, K. A., L. K. Poulsen, and S. Molin. 1993. Identification of coccoid Escherichia coli BJ4 cells in the large intestine of streptomycin-treated mice. Infect. Immun. 61:5029-5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudva, I. T., S. Jelacic, P. I. Tarr, P. Youderian, and C. J. Hovde. 1999. Biocontrol of Escherichia coli O157 with O157-specific bacteriophages. Appl. Environ. Microbiol. 65:3767-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kutter, E., K. Gachechiladze, A. Poglazov, E. Marusich, M. Shneider, P. Aronsson, A. Napuli, D. Porter, and V. Mesyanzhinov. 1995. Evolution of T4-related phages. Virus Genes 11:285-297. [DOI] [PubMed] [Google Scholar]
- 30.Loeffler, J. M., S. Djurkovic, and V. A. Fischetti. 2003. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect. Immun. 71:6199-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loeffler, J. M., D. Nelson, and V. A. Fischetti. 2001. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2170-2172. [DOI] [PubMed] [Google Scholar]
- 32.Merril, C. R., B. Biswas, R. Carlton, N. C. Jensen, G. J. Creed, S. Zullo, and S. Adhya. 1996. Long-circulating bacteriophage as antibacterial agents. Proc. Natl. Acad. Sci. USA 93:3188-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, E. S., J. F. Heidelberg, J. A. Eisen, W. C. Nelson, A. S. Durkin, A. Ciecko, T. V. Feldblyum, O. White, I. T. Paulsen, W. C. Nierman, J. Lee, B. Szczypinski, and C. M. Fraser. 2003. Complete genome sequence of the broad-host-range vibriophage KVP40: comparative genomics of a T4-related bacteriophage. J. Bacteriol. 185:5220-5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, E. S., E. Kutter, G. Mosig, F. Arisaka, T. Kunisawa, and W. Ruger. 2003. Bacteriophage T4 genome. Microbiol. Mol. Biol. Rev. 67:86-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moller, A. K., M. P. Leatham, T. Conway, P. J. Nuijten, L. A. de Haan, K. A. Krogfelt, and P. S. Cohen. 2003. An Escherichia coli MG1655 lipopolysaccharide deep-rough core mutant grows and survives in mouse cecal mucus but fails to colonize the mouse large intestine. Infect. Immun. 71:2142-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monod, C., F. Repoila, M. Kutateladze, F. Tetart, and H. M. Krisch. 1997. The genome of the pseudo T-even bacteriophages, a diverse group that resembles T4. J. Mol. Biol. 267:237-249. [DOI] [PubMed] [Google Scholar]
- 37.Nelson, D., L. Loomis, and V. A. Fischetti. 2001. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 98:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochman, H., and R. K. Selander. 1984. Standard reference strains of Escherichia coli from natural populations. J. Bacteriol. 157:690-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poulsen, L. K., F. Lan, C. S. Kristensen, P. Hobolth, S. Molin, and K. A. Krogfelt. 1994. Spatial distribution of Escherichia coli in the mouse large intestine inferred from rRNA in situ hybridization. Infect. Immun. 62:5191-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poulsen, L. K., T. R. Licht, C. Rang, K. A. Krogfelt, and S. Molin. 1995. Physiological state of Escherichia coli BJ4 growing in the large intestines of streptomycin-treated mice. J. Bacteriol. 177:5840-5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robins-Browne, R. M. 1987. Traditional enteropathogenic Escherichia coli of infantile diarrhea. Rev. Infect. Dis. 9:28-53. [DOI] [PubMed] [Google Scholar]
- 42.Savarino, S. J., E. R. Hall, S. Bassily, F. M. Brown, F. Youssef, T. F. Wierzba, L. Peruski, N. A. El Masry, M. Safwat, M. Rao, H. El Mohamady, R. Abu-Elyazeed, A. Naficy, A. M. Svennerholm, M. Jertborn, Y. J. Lee, J. D. Clemens, et al. 1999. Oral, inactivated, whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine: results of the initial evaluation in children. J. Infect. Dis. 179:107-114. [DOI] [PubMed] [Google Scholar]
- 43.Savarino, S. J., E. R. Hall, S. Bassily, T. F. Wierzba, F. G. Youssef, L. F. Peruski, Jr., R. Abu-Elyazeed, M. Rao, W. M. Francis, H. El Mohamady, M. Safwat, A. B. Naficy, A. M. Svennerholm, M. Jertborn, Y. J. Lee, and J. D. Clemens. 2002. Introductory evaluation of an oral, killed whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Egyptian infants. Pediatr. Infect. Dis. J. 21:322-330. [DOI] [PubMed] [Google Scholar]
- 44.Schuch, R., D. Nelson, and V. A. Fischetti. 2002. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884-889. [DOI] [PubMed] [Google Scholar]
- 45.Smith, H. W., and M. B. Huggins. 1983. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J. Gen. Microbiol. 129:2659-2675. [DOI] [PubMed] [Google Scholar]
- 46.Smith, H. W., M. B. Huggins, and K. M. Shaw. 1987. The control of experimental Escherichia coli diarrhoea in calves by means of bacteriophages. J. Gen. Microbiol. 133:1111-1126. [DOI] [PubMed] [Google Scholar]
- 47.Snyder, J. D., and M. H. Merson. 1982. The magnitude of the global problem of acute diarrhoeal disease: a review of active surveillance data. Bull. W. H. O. 60:605-613. [PMC free article] [PubMed] [Google Scholar]
- 48.Stone, R. 2002. Bacteriophage therapy. Stalin's forgotten cure. Science 298:728-731. [DOI] [PubMed] [Google Scholar]
- 49.Sulakvelidze, A., Z. Alavidze, and J. G. Morris, Jr. 2001. Bacteriophage therapy. Antimicrob. Agents Chemother. 45:649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Summers, W. C. 2001. Bacteriophage therapy. Annu. Rev. Microbiol. 55:437-451. [DOI] [PubMed] [Google Scholar]
- 51.Tacket, C. O., G. Losonsky, H. Link, Y. Hoang, P. Guesry, H. Hilpert, and M. M. Levine. 1988. Protection by milk immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. N. Engl. J. Med. 318:1240-1243. [DOI] [PubMed] [Google Scholar]
- 52.Tannock, G. W. 1997. Normal microbiota of the gastrointestinal tract of rodents, p. 187-215. In R. I. MacKie, B. A. White, and R. E. Isaacson (ed.), Gastrointestinal microbiology. Chapman & Hall, New York, N.Y.
- 53.Tetart, F., C. Desplats, M. Kutateladze, C. Monod, H. W. Ackermann, and H. M. Krisch. 2001. Phylogeny of the major head and tail genes of the wide-ranging T4-type bacteriophages. J. Bacteriol. 183:358-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westwater, C., L. M. Kasman, D. A. Schofield, P. A. Werner, J. W. Dolan, M. G. Schmidt, and J. S. Norris. 2003. Use of genetically engineered phage to deliver antimicrobial agents to bacteria: an alternative therapy for treatment of bacterial infections. Antimicrob. Agents Chemother. 47:1301-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]