Abstract
Our aim was to evaluate the antiviral effect of a combination of two nucleoside reverse transcriptase inhibitors, emtricitabine (FTC) and clevudine (L-FMAU), with the addition of an adenovirus-driven delivery of recombinant gamma interferon (IFN-γ) in the woodchuck model of hepatitis B virus infection. Six woodchuck hepatitis virus (WHV)-infected woodchucks received L-FMAU (10 mg/kg) plus FTC (30 mg/kg) intraperitoneally for 8 weeks; six other animals received in addition an intravenous injection of a recombinant adenovirus vector expressing woodchuck IFN-γ (Ad-IFN) at weeks 4 and 8. In the control group, two animals received Ad-IFN alone, two received adenovirus vector expressing the green fluorescent protein reporter gene, and one remained untreated. In less than 2 weeks, all woodchucks that received L-FMAU plus FTC showed a rapid and marked inhibition of viral replication, with a 4-log10 drop in serum WHV DNA. In two animals, viremia remained suppressed for several months after the end of treatment. Similarly, a dramatic decrease in intrahepatic replicative intermediates of viral DNA was observed in the L-FMAU/FTC-treated groups. The additional administration of Ad-IFN led to increased inflammation in the liver but did not enhance the antiviral effect of the L-FMAU/FTC combination. In conclusion, therapies combining L-FMAU and FTC in WHV-infected woodchucks resulted in a potent and sustained antihepadnaviral effect both in the liver and in the blood circulation. However, no extra benefit of adding IFN-γ gene transduction to the L-FMAU/FTC combination could be detected.
Chronic hepatitis B virus (HBV) infection remains a worldwide major public health problem that is responsible for more than 500,000 deaths each year. Despite the availability of an effective vaccine, there are 350 million HBV chronic carriers with a significant risk to develop liver disease, including cirrhosis and hepatocellular carcinoma (20).
At present, only a few treatment options are available for chronic hepatitis B. Recently, the nucleoside analogs lamivudine and adefovir have been shown to be potent inhibitors of HBV replication (17, 25). However, due to the long half-life of infected hepatocytes and the persistence of viral covalently closed circular DNA (cccDNA) in infected hepatocytes, long-term administration would be required to eradicate viral infection (44). Fifty percent of the patients receiving lamivudine therapy develop drug-resistant mutants after 3 years of therapy (21, 28). Very recently, the selection of resistant viruses has also been reported after treatment with adefovir (39, 40). Therefore, new treatment strategies for chronic hepatitis B based on a combination of nucleoside inhibitors and/or immune modulators are being investigated (19, 36). Due to their high level of antiviral activity and very good selectivity indices, compounds which belong to the β-l-nucleoside analog family may represent potential candidates. Preliminary results obtained in experimental studies showed that two of these analogs, L-FMAU [1-(2-fluoro-5-methyl-β,l-arabinofuranosyl) or clevudine] and FTC [(−)-β-2′,3′-dideoxy-5-fluoro-3′-thiacytidine or emtricitabine], are promising candidates for the inhibition of viral replication. FTC is a potent inhibitor of both the duck hepatitis B virus (DHBV) and HBV reverse transcriptase (RT) activities, whereas L-FMAU inhibits the DNA-dependent DNA polymerase activity (34). In chronically infected woodchucks, FTC inhibits viral replication but does not induce viral clearance (8). In the same model, L-FMAU strongly inhibited viral replication and decreased the number of infected hepatocytes (29, 43). However, clearance of viral cccDNA was never observed with either FTC or L-FMAU (8, 29, 43). Furthermore, the combination of L-FMAU and FTC (L-FMAU/FTC) was shown to be additive in vitro in tissue culture and in vivo in the DHBV model (33). Both compounds are currently under evaluation in human clinical trials (phase II for L-FMAU and phase III for FTC) for their efficacy in the treatment of chronic hepatitis B (11).
Interestingly, it has been shown that in patients treated with lamivudine the decrease in viral DNA titers is associated with a restoration of the CD4 response to HBV, followed by a CD8-specific response, suggesting that lamivudine therapy may overcome T-cell hyporesponsiveness to HBV (2, 3). Moreover, in HBV-transgenic mice the intrahepatic expression of TH1 cytokines, including gamma interferon (IFN-γ), tumor necrosis factor alpha, and interleukin-12, can inhibit viral replication via a noncytolytic pathway (4, 10, 11). More recently, similar noncytopathic antiviral events were shown to occur in the liver of chimpanzees acutely infected with HBV (12, 37). IFN-γ inhibited DHBV replication in primary duck hepatocytes but did not prevent cccDNA formation (31, 32). In acute resolving woodchuck hepatitis virus (WHV) infection, intrahepatic expression of woodchuck IFN-γ (wIFN-γ) correlated with the appearance of CD8+ cells, which peaked at the time of recovery (13). However, a causative role for wIFN-γ in the clearance of WHV is not yet established. More recently, biologically active wIFN-γ was recombinantly expressed in Escherichia coli and mammalian cells (22). In permanent woodchuck cell lines, the recombinant protein caused a strong upregulation of major histocompatibility complex (MHC) class I heavy-chain expression and a specific inhibition of reporter genes under control of viral promoters. Similarly, MHC class I expression was upregulated in primary woodchuck hepatocytes from both naive and persistently WHV-infected animals. However, treatment did not reduce the amounts of WHV replication intermediates in infected cells (23). This may be explained by the fact that the activity of the recombinant IFN-γ protein is rapidly lost in the cell culture media and the chronic infection renders the cells unresponsive to the cytokine.
The WHV in vivo infection model is extremely useful for the evaluation of antiviral agents. Several studies have shown the relevance of this animal model in terms of antiviral efficacy and toxicity for evaluating new nucleoside analogs (6, 7, 16, 18, 27). In addition, a preliminary study with a limited number of animals had shown that superinfection of chronic carrier woodchucks with a very high dose (5 × 1011 PFU) of a β-galactosidase-expressing adenovirus vector could lead to a moderate transient suppression of WHV replication but not to the clearance of infection (42). We therefore reasoned that a potential benefit of intrahepatic wIFN-γ expression would manifest itself more clearly under conditions of reduced viral burden.
The aim of our study was to examine the effect of L-FMAU/FTC bitherapy in chronically WHV-infected woodchucks and to determine whether, under these conditions, adenovirus-mediated delivery of a wIFN-γ gene into the liver would allow for a more sustained reduction of viral replication or, possibly, for the clearance of infection altogether.
MATERIALS AND METHODS
IFN-γ gene delivery vectors.
Details of the adenovirus vector construction will be published elsewhere (M. Nassal, unpublished data). In brief, a gene encoding woodchuck IFN-γ, including the 23-amino-acid signal sequence, was chemically synthesized on the basis of an mRNA sequence published in GenBank (accession number AF081502). The gene was cloned into a modified pAdTrack shuttle plasmid (14) under the control of the cytomegalovirus (CMV) promoter. The enhanced green fluorescent protein (eGFP) gene driven by original CMV immediate-early promoter was replaced by a red fluorescent protein (RFP) with a nuclear localization signal under the control of a simian virus 40 promoter. The shuttle plasmid was recombined, in E. coli, with the AdEasy vector backbone plasmid in which the E1 and E3 regions are deleted. The recombinant replication-defective adenovirus vector genome was excised by using the restriction enzyme PacI, and recombinant vector particles were obtained by transfection into HEK 293 cells. Titers were determined via plaque assays on HEK 293 cells. Generation of biologically active woodchuck IFN-γ was assessed by protection of the woodchuck cell line WCH17 from vesicular stomatitis virus infection. Supernatants from HEK 293 cells transfected with the shuttle plasmid or infected with a recombinant adenovirus vector expressing woodchuck IFN-γ (Ad-IFN) were protective at dilutions between 1:1,024 and 1:4,096. In addition, in the WCH17 cell line, the supernatants induced expression of an RNA that hybridized with a probe derived from a mixture of mouse and human guanylate-binding protein 1 (GBP-1), a member of a family of large GTPases known to be induced in response to IFN-γ (24). No induction was seen when human IFN-γ was used instead. A similar adenovirus vector containing a CMV promoter-eGFP cassette (Ad-GFP) was used as a control (30). The recombinant adenovirus vectors were administered by intravenous injection at a previously determined optimal dose to target the liver (3 × 1010 PFU/animal) in a dose-finding pilot study (data not shown).
Antiviral treatment.
Seventeen woodchucks (Marmota monax) chronically infected with WHV were purchased from Northeastern Wildlife (South Plymouth, N.Y) and distributed in different treatment arms. Six WHV-infected woodchucks received L-FMAU (10 mg/kg of body weight) plus FTC (30 mg/kg) intraperitoneally for 8 weeks, and six other animals received, in addition, an intravenous injection of Ad-IFN at weeks 4 and 8. In the control group, two animals received Ad-IFN alone, two received Ad-GFP, and one remained untreated. The treatment protocol was performed from 8 September 2001 to 8 November 2001 (2 months). L-FMAU and FTC were provided by Triangle Pharmaceuticals and administered intraperitoneally. Daily injections of the drugs were performed during the first week and then repeated every other day for the next 7 weeks. At weeks 4 and 8, intravenous inoculations of 3 × 1010 PFU of Ad-IFN/animal were performed; this dose was chosen, based on a prior dose-finding study, to give the highest possible IFN-γ expression without leading to acute toxicity. During therapy, blood and liver samples were collected after each inoculation of Ad-IFN. After drug withdrawal, blood samples were collected every other day until the rise in viremia, followed by sampling once a week for 24 weeks and then sampling twice a month until the end of the study.
Analysis of viremia.
Viremia levels were assessed by quantitative detection of WHV DNA in serum. WHV DNA was extracted from serum by using the High-Pure PCR template DNA extraction kit (Roche Diagnostics), and 50-μl extracted samples were spotted onto nitrocellulose filters (Sartorius, Göttingen, Germany) in parallel with serial dilutions of a WHV DNA standard by using the Hybri-Dot Manifold apparatus (Life Technologies, Cergy Pontoise, France). After fixation, filters were hybridized with a 32P-labeled full-length WHV DNA probe. A quantitative analysis was performed by phosphorimager scanning (Amersham Pharmacia Biotech) with ImageQuant software. The detection limit of the dot blot assay was determined to be 6 × 107 copies/ml (200 pg/ml). When virus titers dropped further, a real-time PCR assay was used as described earlier (9). The same WHV DNA standard was used for both the dot blot and the real-time PCR assays. A good correlation was found between the two assays (data not shown). The lower limit of detection of the PCR assay was determined as 2.5 × 102 copies/ml.
Sequencing of the WHV polymerase gene.
At the end of therapy or at the last time point before death, a sequence of the RT domain of the polymerase gene from circulating WHV was performed after PCR amplification to identify the presence of escape mutants in the viral pool. DNA was extracted from woodchuck virus-infected serum, and 35 cycles (94°C for 1 min, 50°C for 1 min, and 72°C for 1 min) of amplification were performed with Taq polymerase and a specific primer pair (5′-AGATTGGTTGGTGCACTTCT-3′ [nucleotides 385 to 403] and 5′-AATTGTCAGTGCCCAACA-3′ [nucleotides 1468 to 1461]) matching the B and C domains of the RT gene (15). A nested PCR was performed on samples that were negative after the first round of amplification with the specific primers 5′-GGATGTATCTGCGGCGTTT-3′ (nucleotides 510 to 528) and 5′-CCCAAATCAAGAAAAACAGAACA-3′ (nucleotides 953 to 931) (18). The nucleotide sequence and the derived amino acid sequence of the RT obtained for each sample were then compared to published sequences (15, 26).
Liver biopsy.
Surgical liver biopsies were performed after laparotomy under general anesthesia prior to therapy (M0), twice during treatment at months 1 (M1) and 2 (M2) and then 4 months after drug withdrawal (M6). The on-treatment biopsies were performed 4 days after each recombinant adenovirus vector inoculation. A first piece was stored at −80°C for subsequent viral DNA analysis. A second piece was fixed in formalin and embedded in paraffin for liver histology and immunostaining analyses. The remainder of liver biopsies was fixed in 1% osmium tetroxide for the electron microscopy study (M0 and M2). A macroscopic liver examination was performed at the time of surgery.
Analysis of intrahepatic viral DNA synthesis.
After homogenization of the frozen liver fragments in 10 mM Tris-HCl (pH 7.5)-10 mM EDTA, each liver lysate was divided into two aliquots: one for isolation of total viral DNA (proteinase K digestion and phenol-chloroform extraction, followed by ethanol precipitation) and the other for isolation of non-protein-bound cccDNA (sodium dodecyl sulfate-KCl precipitation of protein bound DNA and phenol-chloroform extraction, followed by ethanol precipitation) as described previously (18).
DNA concentration was determined by UV densitometric analysis of agarose gel purified DNA fragments by comparison with a standard to normalize the cellular DNA amount of each sample. Southern blot analysis was performed with 5 μg of the total DNA or cccDNA preparations. Samples were subjected to electrophoresis through 1.2% agarose gels and alkaline transfer onto nylon membranes. The viral DNAs were detected by specific hybridization and quantified (18).
Analysis of liver histology.
Formalin-fixed liver biopsy tissue sections were stained with hematoxylin-eosin-safran stain and examined under a light microscope. The degree of hepatocyte necrosis (acidophilic bodies), the level of inflammation of the portal tract and the intralobular space, and the fibrosis stage were semiquantitatively assessed by using the Metavir score (1). Liver biopsy sections were also assessed for steatosis, ductular proliferation, hepatocyte dysplasia, and hepatocellular carcinoma.
Examination of liver mitochondria by electron microscopy.
Liver biopsy sections were fixed in 1% osmium tetroxide in 150 mM sodium cacodylate-HCl (pH 7.4), dehydrated in graded ethanol, and embedded in epoxy resin (Cipec, Paris, France). Ultrathin sections (75 μm) were obtained on an LKB Ultratome V (Leica, Nanterre, France), contrasted with methanolic uranyl acetate and lead citrate, and observed with a transmission electron microscope 1200EX (JEOL, Kyoto, Japan) to rule out mitochondrial toxicity of the combination of L-FMAU and FTC as described earlier (18).
Visualization of the GFP or RFP reporter gene expression in the liver by confocal microscopy.
The number of liver cells infected by the defective recombinant adenovirus vector and expressing the reporter gene was monitored by direct observation of coded samples corresponding to 5-μm-thick deparaffinized liver tissue sections using confocal microscopy with appropriate filters.
Immunostaining of liver sections for WHV core antigen and PCNA.
The number of WHV-infected cells was monitored by immunostaining for capsid proteins. Deparaffinized liver tissue sections 5 μm thick were incubated overnight with rabbit serum containing polyclonal antibody directed against the WHV core antigen (1/2,000 dilution). This step was followed by incubation with a biotinylated goat anti-rabbit immunoglobulin G (Dako, Trappes, France). The antigen-antibody complex was then revealed with streptadivin-horseradish peroxidase (Dako). Visualization was performed with the 3,3′-diaminobenzidine tetrahydrochloride chromogen substrate according to the manufacturer's instructions (Dako) (38). All specimens were evaluated without knowledge of the antiviral protocol. Quantification of the number of infected hepatocytes expressing viral proteins was performed in representative areas of the liver section corresponding to ca. 1,000 cells (18).
The number of hepatocytes progressing in the cell cycle was determined by immunoperoxidase staining for PCNA as previously described (13, 27, 43).
RESULTS
The combination of L-FMAU and FTC induced a rapid, pronounced, and long-term decrease of WHV replication.
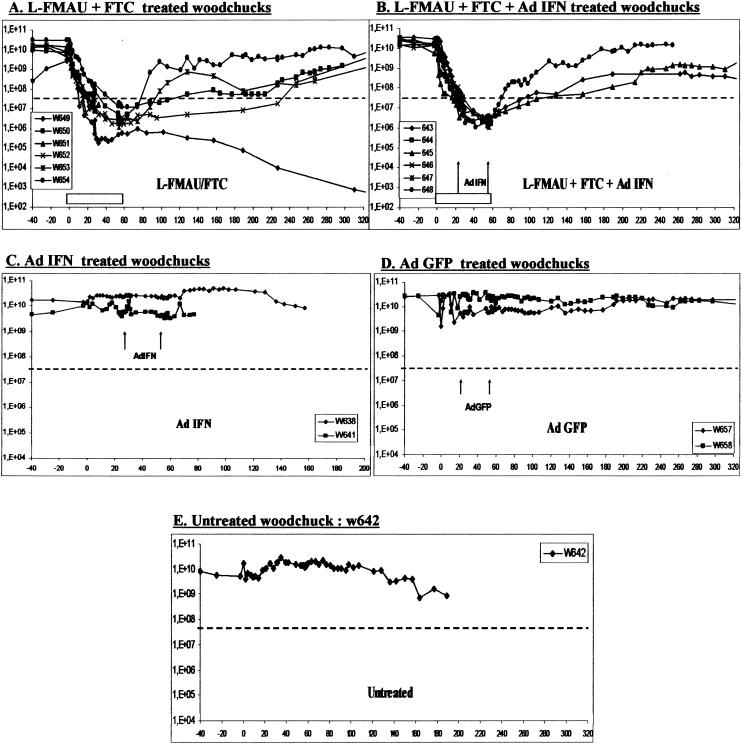
In the present study, L-FMAU and FTC were administered simultaneously and intraperitoneally to assess the antiviral efficacy of this combination over an 8-week treatment period. Our results showed a significant reduction of the levels of viremia. During treatment, a 4-log10 viral load drop from baseline, down to the limit of detection of the dot blot assay, was observed in all of the animals treated by L-FMAU/FTC bitherapy (Fig. 1A and B). However, untreated woodchucks, woodchucks that received Ad-GFP, and woodchucks treated with Ad-IFN alone showed no significant variations in viremia (Fig. 1C, D, and E). The reduction of viremia after L-FMAU/FTC bitherapy was remarkably rapid. Viremia decreased to undetectable levels by dot blot assay in less than 1 or 2 weeks of treatment. To further analyze the levels of viremia, we performed a real-time PCR assay on the samples below the limit of detection of the dot blot assay. The results showed a continued and progressive decrease of viremia in treated animals to minimal values of 3 × 102 to 4 × 106 copies/ml, which represent a 1- to 4-log10 additional decline compared to pretreatment values. The lowest value of 3.1 × 102 copies/ml was observed at week 52 in woodchuck w649; this value represents a maximal 8-log10 decrease in WHV titers. Viremia in untreated animals remained stable with viral load values comprised between 1 × 1010 and 4 × 1010 copies/ml.
FIG. 1.
Combination therapy decreases viremia in chronically infected woodchucks. The viremia level for each woodchuck is plotted on the graph. The gray horizontal bar indicates the antiviral treatment period, and the black arrows indicate adenoviral injections at weeks 4 and 8. The black dotted line indicates the cutoff of the dot blot assay (6 × 107 WHV copies/ml). All viremia levels under this cutoff were analyzed and followed up by a specific real-time PCR assay.
It is noteworthy that the combination of L-FMAU and FTC induced a long-term inhibition of WHV replication after drug withdrawal. In animals treated with the L-FMAU/FTC bitherapy, viremia remained suppressed for various lengths of time after the withdrawal of treatment. The evolution of viremia after the end of the treatment followed three profiles (1). In the first group, the viremia followed a typical rebound as WHV titers increased early after the end of the therapy and reached dot blot detection limit in less than 1 to 2 weeks with a 3-log10 increase in 8 weeks (see the viremia curves of animals w653 and w654 [Fig. 1A] and w648 [Fig. 1B]) (2). In the second group, viremia did not start to rebound until 3 to 4 weeks after drug withdrawal, with a slower increase of 1 to 2 log10 over the same delay of 8 weeks (see the viremia curves of animals w650 [Fig. 1A] and w643 and w645 [Fig. 1B]) (3). In the last and most interesting group, the suppression of viremia was maintained for several months after the end of treatment (see the viremia curves of animals w649 and w652 [Fig. 1A]). In this last group, viremia started to rise to a level detectable by the dot blot assay only at week 24 for woodchuck w652, whereas the viremia level of woodchuck w649 was still undetectable by dot blot assay 1 year after drug withdrawal but remained positive by real-time PCR at 8 × 104 copies/ml.
To determine whether antiviral treatment was associated with the selection of drug-resistant virus, direct sequencing of PCR products spanning the RT domain, including the motif comprising the pyrimidine analog-resistant mutation (41, 42), was carried out at the end of the therapy or at the last time point before animal death. Sequence analysis did not show any changes in the amino acid sequence of the viral polymerase in any woodchucks during the study period. Therefore, 8 weeks of treatment with L-FMAU/FTC bitherapy did not trigger the emergence of WHV-resistant variants.
The L-FMAU/FTC bitherapy inhibited WHV DNA replicative intermediate and cccDNA intrahepatic synthesis but was not sufficient to clear cccDNA.
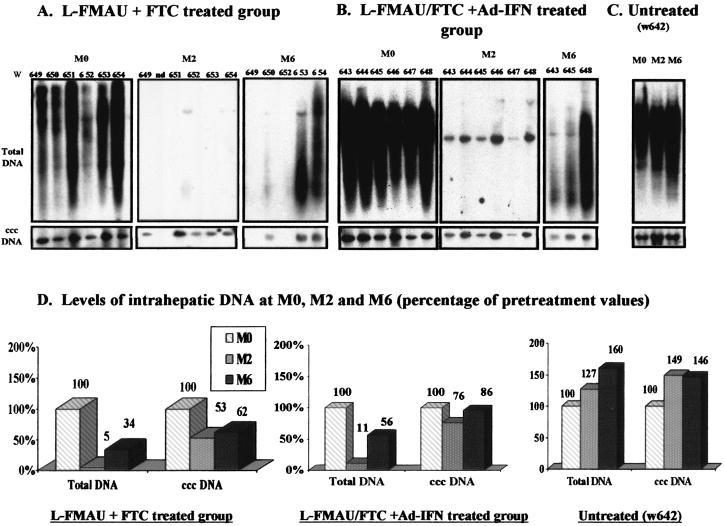
To determine the effect of antiviral therapy on the accumulation of viral DNA in the liver, total DNA and cccDNA were extracted from liver biopsies during the course of therapy. Southern blot analysis of intrahepatic viral DNA (Fig. 2) demonstrated changes in the levels of intrahepatic WHV replication intermediates that were generally consistent with viremia patterns. Intrahepatic WHV DNA replicative intermediates levels dropped rapidly by ca. 89 to 95% compared to baseline value at M0 in both of the L-FMAU/FTC-treated groups. In comparison, cccDNA levels in the liver decreased more slowly with a 24 to 47% decrease compared to the pretreatment value (compare M0 and M2, Fig. 2A, B, and D). For most of the treated animals, the inhibition of WHV replication by L-FMAU/FTC bitherapy was not followed by the complete clearance of intrahepatic viral cccDNA (Fig. 2A and B). However, at M6 in woodchucks w649 and w652, the cccDNA decreased to undetectable levels by Southern blot analysis (see Fig. 2A at M6). This was associated with a prolonged suppression of viremia in these animals (Fig. 1A). In contrast, in the untreated woodchuck, the total DNA and cccDNA levels increased over time by 27 and 49%, respectively.
FIG. 2.
Combination of therapy leads to a pronounced decrease in intrahepatic WHV replication. (A to C) Southern blot analysis of total liver DNA (10 μg of total DNA/lane) and cccDNA (10 μg of total DNA/lane) in woodchuck livers for L-FMAU/FTC-treated animals (A), L-FMAU/FTC+Ad-IFN-γ-treated animals (B), and untreated animals (C). (D) The relative band intensity for total WHV DNA and cccDNA was quantified by phosphorimager scanning for each sample, and the mean results for each animal group are indicated in graphs showing the percentages of the pretreatment value. M0, beginning of the treatment; M1, month 1, M2, month 2 (the end of the treatment); M6, month 6 (4 months after drug withdrawal). Woodchuck identification numbers w638 to w654 are indicated above the lane.
After cessation of the therapy, all woodchucks that presented a rebound of viral load in the circulation (w643, w645, and w648 in the L-FMAU/FTC+Ad-IFN-treated group and w650, w653, and w654 in the L-FMAU/FTC-treated group) showed similar increases in DNA replicative intermediates and the cccDNA in the liver. The replicative intermediate levels increased by an average of 37% between M2 and M6 compared to 9.5% for the cccDNA during the same time frame. In contrast, woodchucks w649 and w652 that showed a continuous suppression of viremia demonstrated long-lasting inhibition of WHV DNA synthesis in the liver, with viral loads remaining under the limit of detection of our Southern blot assay.
A slow decrease in the number of WHV-infected cells in the liver was associated with an increase of hepatocyte turnover.
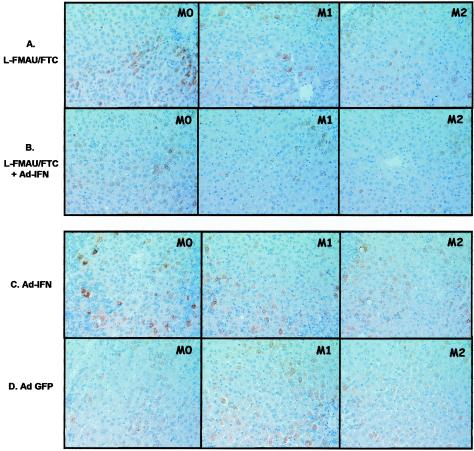
Immunostaining with specific antibodies directed against WHV core antigen was performed to determine whether the absence of WHV DNA replicative intermediates in the liver was correlated with a clearance of WHV-infected hepatocytes during and after the bitherapy. Infected hepatocytes expressing viral antigen were observed in the parenchyma and were preferentially clustered in perivascular regions of the hepatic lobule (Fig. 3). After 8 weeks of bitherapy, the mean number of viral core antigen positive hepatocytes was ca. 7 to 8% lower than the baseline compared to a mean increase of 4.5% in the control groups (Ad-IFN-treated, Ad-GFP-treated, and untreated woodchucks) (Table 1). Interestingly, the two animals (w649 and w652) in which WHV DNA remained below the detection limit of the Southern blot assay after treatment showed 51 and 44% reductions in the number of infected cells during the course of therapy (data not shown). At M6, a dramatic 79% decrease in the number of hepatocytes expressing WHV core antigen was noted for woodchuck w652, whereas no hepatocytes expressing viral antigens were found in liver sections for woodchuck w649 (data not shown). These results are consistent with the Southern blot analysis, indicating that the decline of cccDNA to below the detection limit coincided with a drastic loss of infected cells in the liver. Nevertheless, infection was not fully resolved since 8 × 104 copies/ml of WHV DNA were detected in the serum of w649 at M6 by real-time PCR analysis, suggesting that low copy numbers of cccDNA may have persisted in the liver.
FIG. 3.
Combination treatment produced a decline in viral core antigen in the liver. Three biopsies per woodchuck are illustrated for each group, one collected before treatment (M0) and the others collected 1 and 2 months later (4 days after adenovirus injections) (M1 and M2). Observation of WHV core antigen staining for L-FMAU/FTC-treated groups (L-FMAU/FTC administration [A] and L-FMAU/FTC+Ad-IFN-γ administration [B]) shows a decline in the number of WHV core antigen-positive hepatocytes and, particularly. in the number of hepatocytes with a strong staining reaction compared to control groups (Ad-IFN-γ administration [C] and Ad-GFP administration [D]). One animal per group is illustrated as follows: A, w650; B, w645; C, w641; and D, w642.
TABLE 1.
Summary of analysis of viral load, antigen staining, and histology of the liver
| Group and time | No. of animals analyzed | Reporter gene (GFP or RFP) fluorescence in livera | Viremia (mean no. of copies/ml ± SD) | Mean % intrahepatic WHV DNA ± SD
|
Antigen core staining (mean % WHc virus-positive hepatocytes ± SD) | PCNA staining (mean % PCNA-positive hepatocytes ± SD) | Inflammatory activity (mean Metavir score ± SD) | |
|---|---|---|---|---|---|---|---|---|
| Total DNA (initial total DNA) | cccDNA (initial cccDNA) | |||||||
| L-FMAU-FTC | ||||||||
| T0 | 6 | − (0/6) | 1.7 × 1010 ± 9.3 × 109 | 100 | 100 | 45 ± 10 | 0.38 ± 0.27 | 1.5 ± 0.58 |
| M1 | 6 | 1.1 × 106 ± 1.0 × 106 | 12 ± 4 | 63 ± 19 | 38 ± 5 | 0.67 ± 0.46 | 1.7 ± 0.51 | |
| M2 | 5 | − (0/5) | 5.3 × 106 ± 5.0 × 106 | 5 ± 3 | 53 ± 37 | 37 ± 11 | 1.37 ± 0.51 | 1.6 ± 0.54 |
| M6 | 5 | 5.6 × 107 ± 9.4 × 107 | 34 ± 13 | 62 ± 58 | 23 ± 18 | 1.32 ± 1.12 | 1.5 ± 0.57 | |
| L-FMAU-FTC+Ad-IFN | ||||||||
| T0 | 6 | − (0/6) | 2.5 × 1010 ± 7.6 × 109 | 100 | 100 | 52 ± 6 | 0.58 ± 0.25 | 1.2 ± 0.45 |
| M1 | 6 | 8.6 × 106 ± 2.5 × 106 | 19 ± 25 | 88 ± 41 | 41 ± 10 | 0.84 ± 0.73 | 2.4 ± 0.89 | |
| M2 | 5 | + (5/5) | 4.7 × 106 ± 9.0 × 105 | 11 ± 5 | 76 ± 71 | 45 ± 7 | 1.06 ± 0.61 | 2.5 ± 0.58 |
| M6 | 3 | 2.5 × 109 ± 3.7 × 109 | 56 ± 60 | 86 ± 100 | 43 ± 24 | 1.49 ± 0.66 | 2.4 ± 0.50 | |
| Ad-IFN | ||||||||
| T0 | 2 | − (0/2) | 1.4 × 1010 ± 1.6 × 109 | 100 | 100 | 50 ± 3 | 0.43 ± 0.22 | 2 ± 0 |
| M1 | 2 | 2.2 × 1010 ± 9.5 × 109 | 112 ± 18 | 85 ± 16 | 56 ± 5 | 0.98 ± 0.42 | 2 ± 0 | |
| M2 | 2 | + (2/2) | 1.4 × 1010 ± 1.5 × 1010 | 113 ± 14 | 80 ± 21 | 55 ± 4 | 1.36 ± 0.04 | 2 ± 0 |
| M6 | 1 | 93 | 101 | 59 | 1.06 | 2 ± 0 | ||
| Ad-GFP | ||||||||
| T0 | 2 | − (0/2) | 1.8 × 1010 ± 1.1 × 1010 | 100 | 100 | 42 ± 3 | 0.95 ± 0.82 | 1.5 ± 0.5 |
| M1 | 2 | 1.1 × 1010 ± 9.9 × 109 | 136 ± 85 | 119 ± 51 | 58 ± 9 | 1.2 ± 0.65 | 1.5 ± 0.5 | |
| M2 | 2 | + (2/2) | 1.1 × 1010 ± 3.9 × 109 | ND | ND | 49 ± 15 | 1.48 ± 0.74 | 1.5 ± 0.5 |
| M6 | 2 | 1.8 × 1010 ± 8.3 × 109 | 166 ± 88 | 126 ± 63 | 49 ± 10 | 0.665 ± 0.83 | 1.5 ± 0.5 | |
| Untreated | ||||||||
| T0 | 1 | − (0/1) | 1.5 × 1010 | 100 | 100 | 57 | 0.78 | 2 |
| M1 | 1 | 1.9 × 1010 | 121 | 85 | 57 | 0.55 | 1.5 | |
| M2 | 1 | − (0/1) | 2.8 × 1010 | 127 | 149 | 55 | 0.83 | 2 |
| M6 | 1 | 4.0 × 1010 | 160 | 146 | 50 | 0.978 | 2 | |
A “+” indicates that specific fluorescence was detected by using confocal microscopy in liver sections. The ratio in parentheses indicates the number of animals that presented a positive fluorescence versus the total liver sections observed per group. All results mentioned here (viremia, intrahepatic DNA, anticore, PCNA staining and Metavir score) are means of the different results obtained for all animals in the same group. For intrahepatic viral DNA, baseline levels were considered arbitrarily as 100%. For WHV core antigen and PCNA staining, the values indicate the mean numbers of hepatocytes expressing these antigens. For liver histology analysis, the mean inflammatory activity score for each animal group is presented.
Hepatocyte turnover was analyzed to determine whether hepatocyte regeneration coincided with the decrease of the number of infected cells in the liver. PCNA immunostaining was performed on liver sections at M0, M1, M2, and M6. The results showed that the number of PCNA-positive hepatocytes increased significantly by two- to fourfold in the L-FMAU/FTC bitherapy groups during the course of therapy compared to the baseline value at M0 (Table 1).
The addition of Ad-IFN-γ did not increase the antiviral activity of the L-FMAU/FTC combination.
Experiments were carried out to determine whether IFN-γ can induce a direct, noncytolytic loss of intrahepatic viral nucleic acids and whether, in addition to the potent L-FMAU/FTC bitherapy, it could lead to recovery from chronic infection or at least delay the rebound in virus titers. In a preliminary study, the infectivity of the adenoviral vectors was measured in vitro in cultured HuH7 cells by quantitation of the expression of the reporter gene (RFP or eGFP) using confocal microscopy. The results showed that the majority of the cells were transduced by the adenovirus at 24 h postinoculation. To determine the efficiency of hepatocyte transduction by the recombinant adenovirus vector in vivo, confocal microscopy was performed on the liver sections at M1 and M2 (just after the Ad-IFN inoculations) and compared to liver sections at M0 (before the Ad-IFN injections). These observations showed expression of the fluorescent reporter gene in the liver, with up to 50% of the hepatocytes infected 4 days after inoculation with the adenovirus vector (Table 1).
The analysis of viremia titers of animals inoculated with Ad-IFN or Ad-GFP indicated that there was no significant variation in viremia levels during the course of the therapy (Table 1). In addition, viremia levels in the group treated with Ad-IFN in combination with the L-FMAU/FTC bitherapy were not significantly different from the group treated with L-FMAU/FTC bitherapy alone. Thus, wIFN-γ gene delivery did not show any additive effect in combination with L-FMAU and FTC on the level of viremia in the serum. Further analysis demonstrated that Ad-IFN alone did not affect total viral DNA and cccDNA levels in the liver and that the addition of Ad-IFN did not increase the antiviral activity of the L-FMAU/FTC bitherapy. In conclusion, the magnitude of the decline in total viral DNA and cccDNA in the liver was not significantly different in L-FMAU/FTC-treated animals whether or not they received Ad-IFN-γ (Fig. 2 and Table 1).
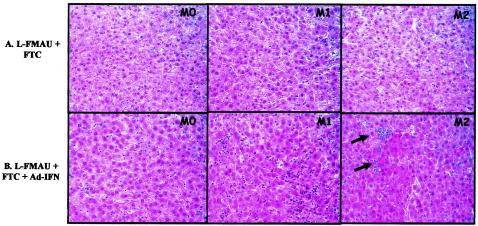
In an attempt to demonstrate wIFN-γ production in the Ad-IFN-treated animals, liver biopsies from several animals, obtained before and after Ad-IFN administration, were tested for the induction of GBP-specific mRNAs. Because of the limited material available, the signals were too faint for quantitation. However, induction of a specific RNA was clearly detectable in four out of six animals (w643, w644, w645, and w646) from the L-FMAU/FTC+Ad-IFN group (data not shown). Increased GBP expression was substantiated by comparison with GAPDH (glyceraldehyde-3-phosphate dehydrogenase) signals. Some evidence for GBP induction was also obtained for w641 from the Ad-IFN only group. Further evidence for successful adenovirus vector infection was obtained by histological examination which revealed an intrahepatic inflammatory response in animals that received L-FMAU/FTC+Ad-IFN (Table 1 and Fig. 4). In these animals, a significant infiltration of inflammatory cells with lobular lesions in the liver was observed, a finding similar to the picture usually seen in acute lobular hepatitis. All of the Ad-IFN-treated woodchucks from this group showed a maximal inflammatory activity. In this same group, the woodchucks that showed a stronger elevation in Metavir score between M0 and M6 also showed the greatest elevation in PCNA-positive hepatocytes within the same time frame (data not shown), probably reflecting cell division after enhanced cell killing. Animals treated with L-FMAU/FTC alone did not show similar profiles. In this group, the maximal inflammatory activity was lower than in the L-FMAU/FTC+Ad-IFN-treated group, and only three of the six woodchucks showed an increase of the inflammatory activity over time. In contrast, animals in control groups or treated with Ad-IFN alone showed stable Metavir scores over time.
FIG. 4.
Adenovirus infection induced hepatic inflammation in liver. Histological examinations of liver biopsies before treatment (M0) and after each Ad-IFN-γ injection (M1 and M2) were compared. Two woodchucks with typical histological profiles are presented: one for the L-FMAU/FTC-treated group (w650 [A]); and one for the L-FMAU/FTC+Ad-IFN-γ-treated group (w645 [B]). Black arrows show areas in the liver section with increased necrosis and inflammation.
Analysis of animal tolerance to antiviral and gene therapy.
No clinical signs of toxicity were seen during the treatment or recovery periods in treated animals. Neither control nor bitherapy-treated animals showed a significant weight change during or after dosing. Liver biopsy specimens were examined for each animal prior to dosing and at the end of the study. Electron microscopic examination showed the absence of ultrastructural modification of hepatocyte mitochondria, biliary canaliculi, and bile duct epithelial cells in treated animals, as well as in control group (data not shown).
DISCUSSION
We assessed here the antiviral activity of a novel treatment strategy for hepatitis B in chronically infected woodchucks by using L-FMAU/FTC combination therapy with or without the additional delivery of a IFN-γ gene via a recombinant adenovirus vector. The combination of L-FMAU and FTC significantly inhibited WHV replication in vivo. A rapid and significant decrease in viremia level by a mean 4 to 5 log10 was observed within 2 weeks and a maximal final drop of 9 log10 was observed for woodchuck w649. A >3-log10 reduction in virus titers within 2 weeks has been reported by Peek et al. in the sera of chronically infected WHV carriers after treatment with L-FMAU at a daily dose of 10 mg per kg of body weight (29). Zhu et al. confirmed these results in a more recent study in WHV-infected woodchucks after 4 weeks of L-FMAU treatment (43). In a long-term L-FMAU treatment study, the minimum WHV titers reached 106 copies/ml between weeks 20 and 30 (43). Cullen et al. demonstrated that FTC at an intraperitoneal dose of 30 mg/kg induced a 56-fold (1.75-log10) reduction in the WHV DNA level in serum over a 4-week treatment period (8). Based on these reproducible historical controls for L-FMAU and FTC monotherapy, our observations suggest that the antiviral activity of FTC and L-FMAU was improved when the drugs were combined. This is consistent with the results obtained in vitro with the same compounds in the DHBV model (33) and therefore deserves further evaluation.
The antiviral effect of L-FMAU/FTC bitherapy persisted over time and was characterized by a delayed viral rebound in four of eight animals with a long-term follow-up. Indeed, viremia remained undetectable by dot blot assay (<6.107 copies/ml) in serum samples 1 to >12 months after treatment cessation. Inhibition of viral replication was especially sustained in two animals (w649 and w652) which showed the lowest viremia levels at baseline. In these woodchucks, the intrahepatic levels of WHV DNA and serum viral load remained suppressed for several months. This prolonged antiviral effect was consistent with a dramatic decrease in cccDNA levels. The delay observed in rebound of viral replication suggests that the combination of L-FMAU and FTC may have induced, at least in some animals, a reduction of WHV DNA synthesis below a threshold level necessary to maintain steady-state levels of cccDNA. This latency could be a reflection of the time required to reestablish cccDNA pools through reinfection of new hepatocytes and/or reamplification of existing intracellular pools of cccDNA in infected cells.
Although there was no control group with single drug administration because of the limited access to this animal model, the antiviral activity of L-FMAU and FTC in combination was greater than that previously reported for other single antiviral drugs administered to WHV chronic carriers (6, 7, 16, 18, 27). The sustained antiviral effect on WHV replication after drug withdrawal observed in the present study suggests that life-long therapy may not be required for long-term control of chronic HBV infection. This new concept should be confirmed by further experimental evidence in a larger number of animals.
Interesting findings also came from our analysis of the evolution of intrahepatic viral load. Southern blot analysis of intrahepatic DNA showed a significant loss in the level of cccDNA and DNA replicative forms throughout the bitherapy, with a much lower rate of decline for the cccDNA than the replicative DNA intermediates (18, 27, 43). This effect may be due to the antiviral activity of L-FMAU, as previously suggested by Zhu et al. (43). However, in the latter study, long-term treatment with L-FMAU alone allowed the emergence of WHV drug-resistant strains (41, 43). The results of our study suggests that the potency of short-term antiviral combination therapy with L-FMAU and FTC may significantly decrease the viral cccDNA pool in less than 4 weeks, thus delaying the rebound of viral replication after treatment cessation and the selection of drug-resistant mutants.
Two different mechanisms may account for the loss of cccDNA in the liver: (i) a reduction of cccDNA in individual infected cells (12) and/or (ii) a replacement of infected cells by newly divided virus-free hepatocytes (13, 35). The results of the analysis of PCNA expression in the livers of infected animals are consistent with the hypothesis that a fraction of cccDNA may survive through mitosis, being distributed to daughter cells. Thus, the cccDNA copy number in infected cells should decline through dilution as infected cells divide. In that case, most hepatocytes that remain infected would have fewer copies of cccDNA. This in turn may explain, in some animals, the sustainability of viral suppression after drug withdrawal and the decrease of viral antigens.
The results of experiments performed with HBV-transgenic mice supports the possibility that hepadnavirus replication intermediates may be cleared by noncytolytic processes induced by the expression of TH1 cytokines in the liver (5). Zhou et al. assessed, in chronically infected woodchucks, the effect of lamivudine treatment combined with superinfection with a β-galactosidase-expressing adenovirus. These authors observed a short-term suppression of the WHV replication that coincided with the time of recovery from the adenovirus infection (42). One possible explanation for this pattern of response is that the virus replication was temporarily suppressed by the activation of the host immune response to WHV. The transient reduction in cccDNA and replicative intermediates may be explained by cytotoxic-T-lymphocyte killing of the adenovirus-infected hepatocytes and/or by a bystander effect as described for HBV-transgenic mice infected with adenovirus (4).
We failed to obtain evidence that Ad-IFN, directly expressing wIFN-γ rather than indirectly inducing its production, can deplete the cccDNA or decrease the number of infected cells in the liver. No significant antiviral effect of wIFN-γ delivery was observed after detailed analysis of serum and intrahepatic viral loads. For lack of suitable tools, the actual levels of woodchuck IFN-γ expression cannot currently be quantified; formally, this negative result might therefore be attributed to the failure of the Ad-IFN vector to deliver a biologically active wIFN-γ gene. However, several lines of evidence argue against this interpretation. First, the Ad-IFN vector induced, upon transduction into HEK 293 cells, secretion of an activity into the supernatants that protected the woodchuck WCH17 cell line from VSV infection, at similar if not higher dilutions than those previously reported (23). Second, this activity was, as expected for IFN, species specific because the 293 supernatants, but not human IFN-γ, induced expression in the WCH17 cell line of a bona fide GBP mRNA. In at least five of the Ad-IFN-treated animals a band of similar mobility on Northern blots was induced (data not shown). More directly, animals that received L-FMAU/FTC+Ad-IFN showed clear signs of enhanced liver inflammation. All of these findings are indicative of the generation of biologically active wIFN-γ.
A second potential problem is ineffective delivery of the Ad-IFN vector into the woodchuck livers. However, consistent with previously reported data from studies using a homologous adenovirus (42), specific RFP fluorescence could be detected in the majority of the hepatocytes 4 days postinoculation in the livers of the woodchucks that received the Ad-IFN vector (Table 1). This finding therefore suggests that the Ad-IFN vector indeed reached the liver and was transcriptionally active in the hepatocytes.
Very recently, Summers et al. provided evidence that hepatocyte killing and regeneration play an important role in eliminating cccDNA during clearance of acute WHV infection (35). Either the enhanced hepatocyte turnover associated with Ad-IFN transduction in our experiments was therefore insufficient in quantity and/or duration to cause a detectable antiviral effect, or chronically WHV-infected hepatocytes may qualitatively differ from acutely infected cells in their abilities to respond to IFN-γ even when viral replication is drastically reduced by chemotherapy. Notably, our negative results are consistent with a recent in vitro study by Lu et al., who showed that at a concentration of up to 1,000 U/ml, wIFN-γ was unable to inhibit WHV replication in primary hepatocytes from persistently WHV-infected woodchucks (23).
In conclusion, we showed here that a combination of the two polymerase inhibitors, L-FMAU and FTC, may be able to induce a prolonged and sustained inhibition of viral replication in WHV-infected woodchucks. The delivery of wIFN-γ after the initial drop in viral load by the nucleoside bitherapy induced an inflammatory cell infiltration in the liver and an increase of cell turnover, which were, however, insufficient to enhance the antiviral effect of the polymerase inhibitor administration.
Acknowledgments
This study was supported by grants from the INSERM and the German Bundes ministerium für Bildung und Forschung (BMBF 01KV98041 to M.N.).
REFERENCES
- 1.Bedossa, P., T. Poynard, et al. 1996. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 24:289-293. [DOI] [PubMed] [Google Scholar]
- 2.Boni, C., A. Bertoletti, A. Penna, A. Cavalli, M. Pilli, S. Urbani, P. Scognamiglio, R. Boehme, R. Panebianco, F. Fiaccadori, and C. Ferrari. 1998. Lamivudine treatment can restore T-cell responsiveness in chronic hepatitis B. J. Clin. Investig. 102:968-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boni, C., A. Penna, G. S. Ogg, A. Bertoletti, M. Pilli, C. Cavallo, A. Cavalli, S. Urbani, R. Boehme, R. Panebianco, F. Fiaccadori, and C. Ferrari. 2001. Lamivudine treatment can overcome cytotoxic T-cell hyporesponsiveness in chronic hepatitis B: new perspectives for immune therapy. Hepatology 33:963-971. [DOI] [PubMed] [Google Scholar]
- 4.Cavanaugh, V. J., L. Guidotti, and F. V. Chisari. 1997. Interleukine-12 inhibits hepatitis B virus replication in transgenic mice. J. Virol. 71:3236-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chisari, F. 1995. Hepatitis B virus transgenic mice: insights into the virus and the disease. Hepatology 22:1316-1325. [DOI] [PubMed] [Google Scholar]
- 6.Colonno, R. J., E. V. Genovesi, I. Medina, L. Lamb, S. K. Durham, M. L. Huang, L. Corey, M. Littlejohn, S. Locarnini, B. C. Tennant, B. Rose, and J. M. Clark. 2001. Long-term entecavir treatment results in sustained antiviral efficacy and prolonged life span in the woodchuck model of chronic hepatitis infection. J. Infect. Dis. 184:1236-1245. [DOI] [PubMed] [Google Scholar]
- 7.Cullen, J. M., D. H. Li, C. Brown, E. J. Eisenberg, K. C. Cundy, J. Wolfe, J. Toole, and C. Gibbs. 2001. Antiviral efficacy and pharmacokinetics of oral adefovir dipivoxil in chronically woodchuck hepatitis virus-infected woodchucks. Antimicrob. Agents Chemother. 45:2740-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullen, J. M., S. L. Smith, M. G. Davis, S. E. Dunn, C. Botteron, A. Cecchi, D. Linsey, D. Linzey, L. Frick, M. T. Paff, A. Goulding, and K. Biron. 1997. In vivo antiviral activity and pharmacokinetics of (−)-cis-5-fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)cytosine in woodchuck hepatitis virus-infected woodchucks. Antimicrob. Agents Chemother. 41:2076-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dandri, M., M. R. Burda, H. Will, and J. Petersen. 2000. Increased hepatocyte turnover and inhibition of woodchuck hepatitis B virus replication by adefovir in vitro do not lead to reduction of the closed circular DNA. Hepatology 32:139-146. [DOI] [PubMed] [Google Scholar]
- 10.Gilles, P., G. Fey, and F. Chisari. 1992. Tumor necrosis factor alpha negatively regulates hepatitis B virus gene expression in transgenic mice. J. Virol. 66:3955-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gish, R. G., N. W. Leung, T. L. Wright, H. Trinh, W. Lang, H. A. Kessler, L. Fang, L. H. Wang, J. Delehanty, A. Rigney, E. Mondou, A. Snow, and F. Rousseau. 2002. Dose range study of pharmacokinetics, safety, and preliminary antiviral activity of emtricitabine in adults with hepatitis B virus infection. Antimicrob. Agents Chemother. 46:1734-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidotti, L. G., R. Rochford, J. Chung, M. Shapiro, R. Purcell, and F. V. Chisari. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284:825-829. [DOI] [PubMed] [Google Scholar]
- 13.Guo, J. T., H. Zhou, C. Liu, C. Aldrich, J. Saputelli, T. Whitaker, M. I. Barrasa, W. S. Mason, and C. Seeger. 2000. Apoptosis and regeneration of hepatocytes during recovery from transient hepadnavirus infections. J. Virol. 74:1495-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodama, K., N. Ogasawara, H. Yoshikawa, and S. Murakami. 1985. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: evolutional relationship between hepadnaviruses. J. Virol. 56:978-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korba, B. E., P. Cote, W. Hornbuckle, B. C. Tennant, and J. L. Gerin. 2000. Treatment of chronic woodchuck hepatitis virus infection in the Eastern woodchuck (Marmota monax) with nucleoside analogues is predictive of therapy for chronic hepatitis B virus infection in humans. Hepatology 31:1165-1175. [DOI] [PubMed] [Google Scholar]
- 17.Lai, C. L., R. W. Chine, N. W. Y. Leung, T. T. Chang, R. Guan, D. I. Tai, K. Y. Ng, P. C. Wu, J. C. Dent, J. Barber, S. L. Stephenson, and F. Gray. 1998. A one year trial of lamivudine for chronic hepatitis B. N. Engl. J. Med. 339:61-68. [DOI] [PubMed] [Google Scholar]
- 18.Le Guerhier, F., C. Pichoud, C. Jamard, S. Guerret, M. Chevallier, S. Peyrol, O. Hantz, I. King, C. Trepo, Y. C. Cheng, and F. Zoulim. 2001. Antiviral activity of β-l-2′,3′-dideoxy-2′,3′-didehydro-5-fluorocytidine in woodchucks chronically infected with woodchuck hepatitis virus. Antimicrob. Agents Chemother. 45:1065-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Guerhier, F., A. Thermet, S. Guerret, M. Chevallier, C. Jamard, C. S. Gibbs, C. Trepo, L. Cova, and F. Zoulim. 2003. Antiviral effect of adefovir in combination with a DNA vaccine in the duck hepatitis B virus infection model. J. Hepatol. 38:328-334. [DOI] [PubMed] [Google Scholar]
- 20.Lee, W. M. 1997. Hepatitis B virus infection. N. Engl. J. Med. 337:1733-1745. [DOI] [PubMed] [Google Scholar]
- 21.Leung, N. W., C. L. Lai, T. T. Chang, R. Guan, C. M. Lee, K. Y. Ng, S. G. Lim, P. C. Wu, J. C. Dent, S. Edmundson, L. D. Condreay, and R. N. Chien. 2001. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 33:1527-1532. [DOI] [PubMed] [Google Scholar]
- 22.Lohrengel, B., M. Lu, and M. Roggendorf. 1998. Molecular cloning of the woodchuck cytokines: TNF-α, IFN-γ, and IL-6. Immunogenetics 47:332-335. [DOI] [PubMed] [Google Scholar]
- 23.Lu, M., B. Lohrengel, G. Hilken, T. Kemper, and M. Roggendorf. 2002. Woodchuck gamma interferon upregulates major histocompatibility complex class I transcription but is unable to deplete woodchuck hepatitis virus replication intermediates and RNAs in persistently infected woodchuck primary hepatocytes. J. Virol. 76:58-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubeseder-Martellato, C., E. Guenzi, A. Jorg, K. Topolt, E. Naschberger, E. Kremmer, C. Zietz, E. Tschachler, P. Hutzler, M. Schwemmle, K. Matzen, T. Grimm, B. Ensoli, and M. Sturzl. 2002. Guanylate-binding protein-1 expression is selectively induced by inflammatory cytokines and is an activation marker of endothelial cells during inflammatory diseases. Am. J. Pathol. 161:1749-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcellin, P., T. T. Chang, S. G. Lim, M. J. Tong, W. Sievert, M. L. Shiffman, L. Jeffers, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, and C. L. Brosgart. 2003. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N. Engl. J. Med. 348:808-816. [DOI] [PubMed] [Google Scholar]
- 26.Mason, A. L., L. Xu, L. Guo, M. Kuhns, and R. P. Perillo. 1998. Molecular basis for persistent hepatitis B virus infection in the liver after clearance of serum hepatitis B surface antigen. Hepatology 27:1736-1742. [DOI] [PubMed] [Google Scholar]
- 27.Mason, W. S., J. Cullen, G. Moraleda, J. Saputelli, C. E. Aldrich, D. S. Miller, B. Tennant, L. Frick, D. Averett, L. D. Condreay, and A. R. Jilbert. 1998. Lamivudine therapy of WHV-infected woodchucks. Virology 245:18-32. [DOI] [PubMed] [Google Scholar]
- 28.Nafa, S., S. Ahmed, D. Tavan, C. Pichoud, F. Berby, L. Stuyver, M. Johnson, P. Merle, H. Abidi, C. Trepo, and F. Zoulim. 2000. Early Detection of viral resistance by determination of hepatitis B virus polymerase mutations in patients treated by lamivudine for chronic hepatitis B. Hepatology 32:1078-1088. [DOI] [PubMed] [Google Scholar]
- 29.Peek, S. F., P. J. Cote, J. R. Jacob, I. A. Toshkov, W. E. Hornbuckle, B. H. Baldwin, F. V. Wells, C. K. Chu, J. L. Gerin, B. C. Tennant, and B. E. Korba. 2001. Antiviral activity of clevudine [L-FMAU,(1-(2-fluoro-5-methyl-β,l-arabinofuranosyl)uracil)] against woodchuck hepatitis virus replication and gene expression in chronically infected woodchucks (Marmota monax). Hepatology 33:254-266. [DOI] [PubMed] [Google Scholar]
- 30.Ren, S., and M. Nassal. 2001. Hepatitis B virus (HBV) virion and covalently closed circular DNA formation in primary tupaia hepatocytes and human hepatoma cell lines upon HBV genome transduction with replication-defective adenovirus vectors. J. Virol. 75:1104-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz, U., and F. V. Chisari. 1999. Recombinant duck interferon gamma inhibits duck hepatitis B virus replication in primary hepatocytes. J. Virol. 73:3162-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz, U., J. Summers, P. Staeheli, and F. V. Chisari. 1999. Elimination of duck hepatitis B virus RNA-containing capsids in duck interferon-alpha-treated hepatocytes. J. Virol. 73:5459-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seigneres, B., P. Martin, B. Werle, O. Schorr, C. Jamard, L. Rimsky, C. Trepo, and F. Zoulim. 2003. Effects of pyrimidine and purine analog combinations in the duck hepatitis B virus infection model. Antimicrob. Agents Chemother. 47:1842-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seigneres, B., C. Pichoud, P. Martin, P. Furman, C. Trepo, and F. Zoulim. 2002. Inhibitory activity of dioxolane purine analogs on wild-type and lamivudine-resistant mutants of hepadnaviruses. Hepatology 36:710-722. [DOI] [PubMed] [Google Scholar]
- 35.Summers, J., A. R. Jilbert, W. Yang, C. E. Aldrich, J. Saputelli, S. Litwin, E. Toll, and W. S. Mason. 2003. Hepatocyte turnover during resolution of a transient hepadnaviral infection. Proc. Natl. Acad. Sci. USA 100:11652-11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thermet, A., C. Rollier, F. Zoulim, C. Trepo, and L. Cova. 2003. Progress in DNA vaccine for prophylaxis and therapy of hepatitis B. Vaccine 21:659-662. [DOI] [PubMed] [Google Scholar]
- 37.Thimme, R., S. Wieland, C. Steiger, J. Ghrayeb, K. A. Reimann, R. H. Purcell, and F. V. Chisari. 2003. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77:68-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trigueiro de Araujo, M. S., S. Guerret, F. Gérard, P. Chossegros, M. Chevallier, and J. A. Grimaud. 1997. Quantitative studies on liver fibrosis and alpha-smooth muscle actin expression in heroin abusers. Cell. Mol. Biol. 43:589-596. [PubMed] [Google Scholar]
- 39.Villeneuve, J. P., D. Durantel, S. Durantel, C. Westland, S. Xiong, C. L. Brosgart, C. S. Gibbs, P. Parvaz, B. Werle, C. Trepo, and F. Zoulim. 2003. Selection of a hepatitis B virus strain resistant to adefovir in a liver transplantation patient. J. Hepatol. 39:1085-1089. [DOI] [PubMed] [Google Scholar]
- 40.Xiong, S., H. Yang, C. Westland, I. W. Delaney, D. Colledge, A. Bartholomeusz, V. Thibault, Y. Benhamou, P. Angus, G. Kitis, M. Wulfsohn, C. Gibbs, J. Fry, C. Brosgar, and S. Locarnini. 2003. Resistance surveillance of HBeAg-chronic hepatitis B patients treated for two years with adefovir dipivoxil. J. Hepatol. 38:A4543. (Abstract.)
- 41.Yamamoto, T., S. Litwin, T. Zhou, Y. Zhu, L. Condreay, P. Furman, and W. S. Mason. 2002. Mutations of the woodchuck hepatitis virus polymerase gene that confer resistance to lamivudine and 2′-fluoro-5-methyl-β-l-arabinofuranosyluracil. J. Virol. 76:1213-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou, T., J. T. Guo, F. A. Nunes, K. L. Molnar-Kimber, J. M. Wilson, C. E. Aldrich, J. Saputelli, S. Litwin, L. D. Condreay, C. Seeger, and W. S. Mason. 2000. Combination therapy with lamivudine and adenovirus causes transient suppression of chronic woodchuck hepatitis virus infections. J. Virol. 74:11754-11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu, Y., T. Yamamoto, J. Cullen, J. Saputelli, C. E. Aldrich, D. S. Miller, S. Litwin, P. A. Furman, A. R. Jilbert, and W. S. Mason. 2001. Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis. J. Virol. 75:311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zoulim, F., and C. Trépo. 1998. Drug therapy for chronic hepatitis B: antiviral efficacy and influence of hepatitis B virus polymerase mutations on the outcome of therapy. J. Hepatol. 29:151-168. [DOI] [PubMed] [Google Scholar]