Abstract
The clinical potential of mefloquine has been compromised by reports of adverse neurological effects. A series of 4-quinolinecarbinolamines were compared in terms of neurotoxicity and antimalarial activity in an attempt to identify replacement drugs. Neurotoxicity (MTT [thiazolyl blue reduction] assay) was assessed by exposure of cultured embryonic rat neurons to graded concentrations of the drugs for 20 min. The 50% inhibitory concentration (IC50) of mefloquine was 25 μM, while those of the analogs were 19 to 200 μM. The relative (to mefloquine) therapeutic indices of the analogs were determined after using the tritiated hypoxanthine assay for assessment of the antimalarial activity of the analogs against mefloquine-sensitive (W2) and -resistant (D6 and TM91C235) Plasmodium falciparum strains. Five analogs, WR157801, WR073892, WR007930, WR007333, and WR226253, were less neurotoxic than mefloquine and exhibited higher relative therapeutic indices (RTIs) against TM91C235 (2.9 to 12.2). Conventional quinoline antimalarials were generally less neurotoxic (IC50s of 400, 600, and 900 for amodiaquine, chloroquine, and quinine) or had higher RTIs (e.g., 30 for halofantrine against TM91C235). The neurotoxicity data for the 4-quinolinecarbinolamines were used to develop a three-dimensional (3D), function-based pharmacophore. The crucial molecular features correlated with neurotoxicity were a hydrogen bond acceptor (lipid) function, an aliphatic hydrophobic function, and a ring aromatic function specifically distributed in the 3D surface of the molecule. Mapping of the 3D structures of a series of structurally diverse quinolines to the pharmacophore allowed accurate qualitative predictions of neurotoxicity (or not) to be made. Extension of this in silico screening approach may aid in the identification of less-neurotoxic quinoline analogs.
Malaria remains a global public health problem, with approximately 300 million clinical cases and as many as 2.7 million deaths a year (30), most of which occur in sub-Saharan Africa. Malaria also poses a significant risk to travelers and military personnel deployed for long periods of time to countries where malaria is endemic. There are no effective malaria vaccines, and the efficacy of the available antimalarial drugs continues to decline as a consequence of the emergence of drug-resistant parasites (11). The list of available drugs for malaria prophylaxis in the United States includes doxycycline, mefloquine (Lariam), atovaquone-proguanil (Malarone), chloroquine, and hydroxychloroquine sulfate (18). Doxycycline, Lariam, and Malarone are used in countries where malaria is endemic and where chloroquine resistance has been reported (18). Mefloquine remains the drug of choice for U.S. military deployments in such regions, primarily because its longer half-life (compared to those of Malarone or doxycycline [22]) allows weekly administration, thereby making compliance less problematic. However, compliance will inevitably be affected when a drug causes—or is suspected to cause—adverse effects.
Adverse central nervous system (CNS) events have been associated with mefloquine use. Severe CNS events requiring hospitalization (e.g., seizures and hallucinations) occur in 1:10,000 patients taking mefloquine for chemoprophylaxis (22). However, milder CNS events (e.g., dizziness, headache, insomnia, and vivid dreams) are more frequently observed, occurring in up to 25% of patients (22). The rate of adverse neurological events associated with mefloquine is higher than for Malarone (20), and subjects receiving mefloquine in clinical trials are more likely to withdraw from the trial than those receiving placebo (8). The higher incidence of adverse events observed when the drug is used at the higher doses needed for malaria treatment (22, 23) implies a dose effect. There is no accepted biochemical basis for the neurotoxicity of the drug; however, we recently showed that mefloquine severely disrupts calcium homeostasis in rat neurons in vitro at concentrations in excess of 20 μM, an effect closely related to the acute neurotoxicity of the drug in terms of dose effect and kinetics (10). Peak plasma levels of mefloquine are 3.8 and 2.1 to 23 μM after prophylaxis and treatment, respectively (16, 25). However, the drug crosses the blood-brain barrier and accumulates as much as 30-fold in the central nervous system and mefloquine brain concentrations as high as 50 μM have been reported in human postmortem cases (14, 21). Mefloquine brain concentrations as high as 90 μM have been reported in rats given a therapy-equivalent dose rate, with concentrations in subcompartments in the brain exceeding 100 μM (2). Since it has long been known that a prolonged disruption of neuronal calcium homeostasis may lead to neuronal cell death and injury (6, 13), it is reasonable to suppose that such events may contribute to the clinical neuropathy of the drug.
Mefloquine remains a useful antimalarial drug for many patients who are able to tolerate the drug or are unable or unwilling to take doxycycline or Malarone. However, the neurotoxicity associated with mefloquine is such that some have questioned its clinical utility as a prophylactic drug (7). There are several approaches to the amelioration of this problem, including (i) administration of neuroprotective drugs such as physostigmine (26), (ii) reformulation of mefloquine as a pure isomer (24), and (iii) reengineering of the mefloquine molecule to yield derivatives that are less neurotoxic but retain their antimalarial activity. Bhattacharjee and Karle (3) earlier showed that the in vivo potency of 4-quinolinecarbinolamines was correlated with key stereoelectronic features, including electrostatic potential and lipophilicity. However, the issues of neurotoxicity and drug resistance were not addressed. In the present report, we show that the antimalarial potential of 4-quinolinecarbinolamines may be limited by their neurotoxicity and cross-resistance of mefloquine-resistant parasites. We also describe the generation of a reliable function-based three-dimensional (3D) quantitative structure activity relationship (QSAR) pharmacophore model for neurotoxicity of this class of compounds which may be useful for selecting new quinoline analog candidates devoid of such toxicity.
MATERIALS AND METHODS
Mefloquine analogs
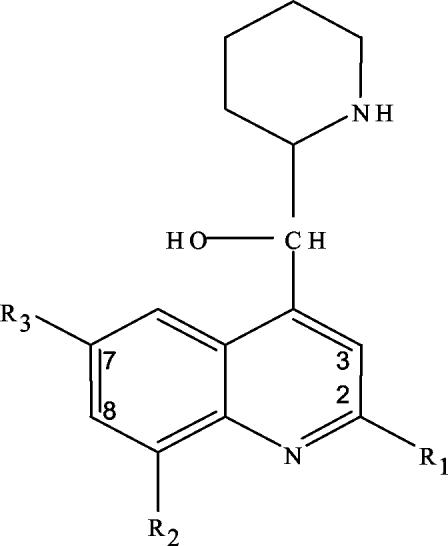
All of the mefloquine analogs tested were 4-quinolinecarbinolamines and were obtained through the Walter Reed Army Institute of Research chemical inventory system. Their structures have been described in earlier work (3) and are presented in Table 1 and Fig. 1. Related drugs also investigated in the present study were quinine, chloroquine, amodiaquine, and halofantrine. All of these drugs were obtained from the Walter Reed Army Institute of Research chemical inventories except for chloroquine, which was purchased from Sigma. Stock solutions (8 × 10−3 to 40 × 10−3 M in dimethyl sulfoxide [DMSO]) were prepared, and aliquots were frozen at −20°C. Prior to each experiment, aliquots were thawed and diluted appropriately in Locke's neuronal culture medium as previously described (10).
TABLE 1.
Molecular structures, neurotoxicity, and in vitro and in vivo antimalarial activity of 4-quinolinecarbinolamines analogs
| Category and analog | Molecular structure of:
|
In vivo antimalarial activity (MfI)a | Neurotoxicity (IC50 in μM) | Antimalarial activity [(IC50 against P. falciparum strain in nM)/(therapeutic index relative to mefloquine)]e
|
||||
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | W2 | D6 | TM91C235 | |||
| Training set | ||||||||
| WR187044 | -(CH2)3-b | Me | Me | NC | 200 | 130 (0.61) | 190 (1.4) | 750 (0.87) |
| WR073872 | CF3 | Me | Me | NC | 110 | 15 (2.9) | 37 (3.9) | 59 (6.1) |
| WR228974 | CF3 | Cl | H | 0.03 | 70 | 14 (2.0) | 45 (2.1) | 110 (2.1) |
| WR157801 | Ph-3′-CF3 | CF3 | H | 1.2 | 63 | <5.4 (4.6)d | 9.7 (8.6) | 17 (12.2) |
| WR073892 | Ph-4′-Cl | Me | H | 0.07 | 58 | <6.6 (3.5)d | 14 (5.5) | 27 (7.1) |
| WR007333 | Ph | Phc | H | 0.1 | 35 | 6.3 (2.2) | 16 (2.9) | 26 (4.4) |
| WR007573 | Ph-4′-Cl | Phc | H | NC | 33 | 5.7 (2.3) | 14 (3.1) | 34 (3.2) |
| WR007552 | Ph | Me | H | NC | 33 | 14 (0.93) | 25 (1.7) | 42 (2.6) |
| WR226253 | CF3 | Cl | Cl | 0.17 | 30 | 5.7 (2.1) | 18 (2.2) | 34 (2.9) |
| WR007930 | Ph | Cl | Cl | 0.04 | 28 | 3.2 (3.5) | 7.8 (4.7) | 18 (5.1) |
| WR073879 | CF3 | Me | H | NC | 28 | 8 (0.6) | 42 (0.88) | 78 (1.2) |
| Mefloquine | CF3 | CF3 | H | 1 | 25 | 9.9 (1.0) | 33 (1.0) | 82 (1.0) |
| WR122950 | OPh-4′-Cl | Me | Me | 0.03 | 23 | 14 (0.65) | 30 (1.0) | 65 (1.2) |
| WR006006 | Ph | Cl | H | 0.05 | 18 | 8.4 (0.85) | 24 (0.99) | 31 (1.9) |
| Test set | ||||||||
| WR159314 | CF3 | CF3 | OCH3 | 0.81 | 35 | NT | NT | NT |
| WR007936 | Ph-4′-Cl | Cl | Cl | 1.29 | 19 | NT | NT | NT |
| WR073898 | Ph-4′-Cl | Me | Me | 0.03 | 10 | NT | NT | NT |
| WR062175 | Ph | CF3 | H | 0.11 | 10 | NT | NT | NT |
| Conventional quinolines | ||||||||
| Amodiaquine | NAf | NA | NA | Active | 400 | 25 (6.3) | 6.2 (85) | 73 (180) |
| Halofantrine | NA | NA | NA | Active | 55 | 1.5 (15) | 5.0 (15) | 60 (30) |
| Chloroquine | NA | NA | NA | Active | 600 | 500 (0.47) | 11 (72) | 190 (10) |
| Quinine | NA | NA | NA | Active | 900 | 320 (1.1) | 66 (18) | 300 (10) |
The in vivo activity of the test compound is expressed in terms of the molar ratio of the 50% curative dose of mefloquine hydrochloride to that of the test compound after a single subcutaneous dose administration in the P. berghei mouse model (29). NC, not curative.
This cyclopentyl substituent incorporates positions 2 and 3 of the quinoline ring.
This phenyl substituent incorporates the 7 and 8 positions of the quinoline ring.
The lowest concentration tested in the malaria screening assay was 2.44 ng/ml, at which inhibition was greater than 50%. Therefore, IC50s of these compounds were <2.44 ng/ml. They have been expressed in nanomolar units to facilitate ease of comparison across the table.
Actual therapeutic indices of mefloquine against W2, D6, and TM91C235 were 2,525, 758, and 305, respectively. Numbers in parentheses represent therapeutic indices relative to mefloquine. NT, not tested against malaria parasites.
NA, not applicable.
FIG. 1.
Core structure of the 4-quinolinecarbinolamines. Substituents at positions R1, R2, and R3 for each analog are listed in Table 1.
Neurotoxicity assay.
The effects of mefloquine analogs on the viability of rat neurons in primary culture were investigated. Neurons were isolated and cultured as previously described (15). Animal care and use was approved by an institutional animal ethics committee in accordance with national guidelines. Neurons were exposed to graded concentrations of the mefloquine analogs for 20 min as previously described (10). Effects of the analogs on neuronal viability were assessed using the colorimetric MTT (thiazolyl blue reduction) assay as previously described (10). Results were expressed as percentages of change in viability compared to appropriate DMSO control results. For most analogs the final DMSO concentration in the cultures was 1 or 2% at the highest concentration tested (usually 200 μM). DMSO concentrations were lower at lower drug concentrations, as dilutions were performed in culture medium with no added DMSO. For quinine and WR187044, the highest starting concentrations were 3.2 and 1.6 mM, respectively, at final DMSO concentrations of 8% (necessary to ensure solubility). In control experiments (data not shown), neuronal viability was unaffected by 20 min of exposure to 8% (or less) DMSO. Viability data were used to plot concentration-effect curves, from which 50% inhibitory concentrations (IC50s) were estimated. Each drug was tested at least in duplicate (triplicate in most cases).
Antimalarial activity.
The susceptibility of different malaria strains to the mefloquine analogs was determined using the tritiated hypoxanthine incorporation assay of Desjardins et al. (9), as modified by Milhous et al. (17), except that the drug exposure period was 48 h. IC50s of the drugs were determined using a nonlinear logistic dose response program. The P. falciparum clones used were W2, D6, and TM91C235 (19). W2 is a mefloquine-sensitive strain resistant to chloroquine and pyrimethamine. Strains D6 and TM91C235 are both resistant to mefloquine. TM91C235 is a strain from Southeast Asia that is highly resistant to mefloquine and a number of other antimalarials. Therapeutic indices were calculated using the following formula: neurotoxicity of drug (IC50 in micromoles)/antimalarial activity of drug (IC50 in micromoles). From these data, therapeutic indices relative to mefloquine were calculated using the following formula: therapeutic index of drug/therapeutic index of mefloquine. In vivo efficacy data are expressed as a mefloquine index (MfI). These values were determined using the Plasmodium berghei mouse model with a single subcutaneous dose at 640 mg/kg of body weight as the highest dose (29). MfI is defined as the ratio of the molar 50% curative dose of mefloquine to the 50% curative dose of the test compound. The 50% curative dose is that which cures 50% of test animals. These values are considered approximate because of the relatively few animals used in testing (5 mice/dose; six dosing levels).
Confocal microscopy.
The effects of some of the analogs on neuronal calcium homeostasis were investigated as previously described (10, 15). The neurons were loaded with the calcium-sensitive dye Fluo 3-AM (5 μM for 1 h), rinsed, and returned to an incubator for 15 min prior to the imaging experiment. Changes in neuronal calcium homeostasis were monitored using a Bio-Rad Radiance 2000 confocal imaging system. Changes in cytoplasmic calcium were recorded as fluctuations in the emitted fluorescence of Fluo-3-complexed calcium at 530 nm (excitation was 488 nM). Sequential image scans of fields containing 5 to 25 neurons were used to construct temporal profiles of the effects of the different analogs. Scans were made at 10-s intervals. To compare the fluorescence levels in different neurons (which were often in slightly different focal planes) on different days, readings at each time point were normalized to the first value measured for each neuron. Drugs (at concentrations of 100 μM or 4× the drug's IC50 in 1% DMSO) were added after four scans, and their effects on calcium homeostasis were monitored for 6 min. Each drug was tested at least in triplicate. After subtraction of baseline values (1% DMSO control), the effects of the drugs are expressed as the percentage of increase in Fluo-3 fluorescence over time. For the chloroquine experiments, the drug was prepared in Locke's buffer, which was also used as the baseline control.
Generation of a neurotoxicity pharmacophore.
The 3D neurotoxicity pharmacophore model was developed using the HypoGen algorithm of the CATALYST methodology (1). Structures of the 4-quiniolinecarbinolamines were imported into CATALYST to create a training set, and energy was minimized to the closest local minimum with the generalized CHARMM-like force field as implemented in the program. The CATALYST model treats molecular structures as templates comprised of chemical functions localized in space that will bind effectively with complementary functions on the respective binding proteins. The most relevant chemical features are extracted from a small set of compounds that cover a broad range of activity (28). Molecular flexibility is taken into account by considering each compound as an ensemble of conformers representing different accessible areas in 3D space. The “best searching procedure” was applied to select representative conformers within 10 kcal/mol of the global minimum (12). CATALYST allows the use of structure and activity data for a set of lead compounds to create a hypothesis characterizing the activity of the lead set. HypoGen generates 10 hypotheses for the training set with various costs. The hypotheses are described by a set of functional features such as hydrophobicity, hydrogen bond donor, hydrogen bond acceptor, and positively and negatively ionizable sites distributed over a 3D space. The hydrogen bonding features are vectors, whereas all other functions are points. The statistical relevance of the obtained hypothesis is assessed on the basis of their cost relative to the null hypothesis and their correlation coefficient. The difference between the fixed and null costs in the present study was found to be 68 bits, and the cost range between the first and the 10th hypotheses is about 8 bits. Therefore, it can be expected that for all these hypotheses there is a 75 to 90% chance of representing a true correlation of the data. The validation of statistical significance of a hypothesis is based on Fischer's randomization test as implemented in CATALYST (1). However, the main goal of performing this type of validation is to check whether there is a strong correlation between the chemical structures and biological activity.
Prediction of neurotoxicity of test set members.
The neurotoxicity pharmacophore was converted into a 3D-shape-based template. This template was used to predict the neurotoxicity of a test set of compounds including four 4-quinolinecarbinolamines, amodiaquine, chloroquine, halofantrine and quinine. The IC50s of the compounds were estimated by fast-fitting their 3D structures to the template. The analogs were predicted to be neurotoxic when their estimated IC50s were less than 300 μM. This criterion was based on a toxicity threshold of 100 μM (the maximum level to which mefloquine accumulates across the blood-brain barrier) multiplied threefold to account for the error inherent in the pharmacophore model. These criteria are conservative, because we have assumed that mefloquine analogs may accumulate in the CNS to the same degree as mefloquine and that estimates of neurotoxicity will be lower by a factor of three in every case. The actual IC50s of the test set members were then determined as described above.
RESULTS
Neurotoxicity and antimalarial activity.
All the 4-quinolinecarbinolamines tested exhibited a neurotoxic effect on primary rat neurons, with 16 of 18 analogs exhibiting IC50s of less than 100 μM after 20 min of exposure (Table 1). Two analogs, WR073872 and WR0187044, possessed IC50s that were higher than 100 μM for neurons; however, neither of these has a curative antimalarial effect in vivo (Table 1). Twelve analogs were less neurotoxic than mefloquine. Five of these, WR157801, WR073892, WR007333, WR226253, and WR007930, have curative antimalarial activity in vivo, lower IC50s than mefloquine for all the P. falciparum strains tested, and higher therapeutic indices than mefloquine against TM91C235 and therefore represent the best candidates for further development (Table 1). Both mefloquine-resistant P. falciparum strains appeared to exhibit a degree of cross-resistance to the 4-quinolinecarbinolamines, since in every case IC50s for these strains were higher than for W2 (Table 1). This was not necessarily the case for the other quinolines tested. Among the conventional quinolines, only halofantrine possessed an IC50 of less than 100 μM for neurons. The IC50s of halofantrine against the three P. falciparum strains were always lower, and the relative therapeutic indices were always higher, than those of the five most promising 4-quinolinecarbinolamines.
Effects of quinolines on neuronal calcium homeostasis.
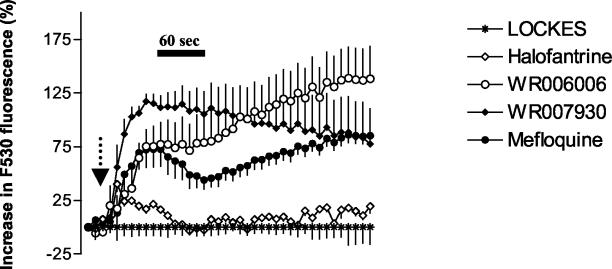
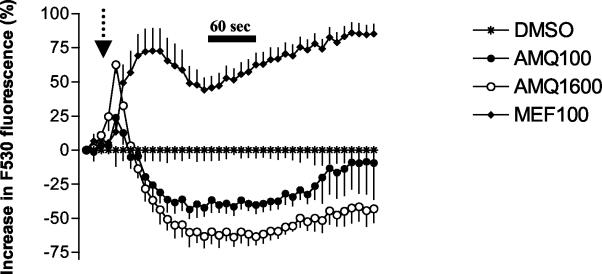
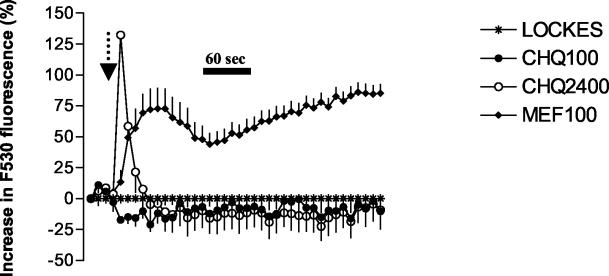
The effects of a number of different quinolines at a concentration of 100 μM on neuronal calcium homeostasis were investigated using confocal microscopy. Treatment of neurons with mefloquine, WR006006, WR007930, halofantrine, and amodiaquine but not with chloroquine increased cytoplasmic calcium concentrations (Fig. 2, 3, and 4). This effect was much more pronounced with the 4-quinolinecarbinolamines (Fig. 2). Halofantrine induced a transient increase in cytosolic calcium concentrations (Fig. 2). This effect was similar in duration and magnitude to that observed at lower mefloquine concentrations (data not shown). At concentrations approximately four times higher than their IC50s, chloroquine and amodiaquine treatment induced a sharp initial increase in intracellular calcium concentration followed by a sharp decline relative to baseline values (Fig. 3 and 4). The effects of chloroquine and amodiaquine were qualitatively different from that of mefloquine at an equivalent concentration (100 μM), as the latter drug induces a more sustained elevation in cytoplasmic calcium concentrations (Fig. 3 and 4).
FIG. 2.
Effects of 4-quinolinecarbinolamines and halofantrine on neuronal calcium homeostasis. Drugs were added after 30 s as indicated by the arrow, and their effects on neuronal cytoplasmic calcium levels were monitored using confocal microscopy. Data are expressed as the percentages of change (± standard errors of the means [SEM]) in Fluo 3-AM (F530) fluorescence after subtraction of appropriate baseline values (1% DMSO). Mefloquine, WR006006, and WR007930 at concentrations of 100 μM induced sustained elevations in cytoplasmic calcium levels. Halofantrine (100 μM) exhibited a more modest and transient increase in cytoplasmic calcium levels. The concentration of mefloquine used is four times higher than the compound's IC50 against embryonic rat neurons and represents the maximum level of accumulation of the drug in the brain after transport across the blood-brain barrier.
FIG. 3.
Effect of amodiaquine on neuronal calcium homeostasis. Drugs were added after 30 s as indicated by the arrow, and their effects on neuronal cytoplasmic calcium levels were monitored using confocal microscopy. Data are expressed as the percentages of change (± SEM) in Fluo 3-AM (F530) fluorescence after subtraction of appropriate baseline values (1% DMSO). Amodiaquine at a concentration of 100 μM (AMQ100) induced a more modest and transient increase in the cytoplasmic calcium concentration than mefloquine (MEF100). At a concentration (1,600 μM [AMQ1600]) equivalent to that used for mefloquine, amodiaquine also induced a sharp, albeit brief, increase in the cytoplasmic calcium concentration that was followed by a decline below the baseline level.
FIG. 4.
Effect of chloroquine on neuronal calcium homeostasis. Drugs were added after 30 s as indicated by the arrow, and their effects on neuronal cytoplasmic calcium levels were monitored using confocal microscopy. Data are expressed as the percentages of change (± SEM) in Fluo 3-AM (F530) fluorescence after subtraction of appropriate baseline values (1% DMSO for mefloquine and Locke's buffer for chloroquine). In comparison to mefloquine (100 μM [MEF100]), chloroquine (100 μM [CHQ100]) did not alter calcium homeostasis. At concentrations (2,400 μM [CHQ2400]) equivalent to that used for mefloquine, chloroquine induced a sharp but brief increase in the cytoplasmic calcium concentration that was followed by a decline below the baseline level.
Development of mefloquine neurotoxicity pharmacophore.
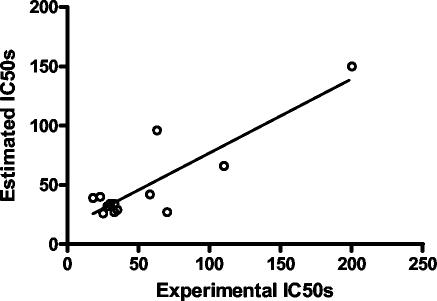
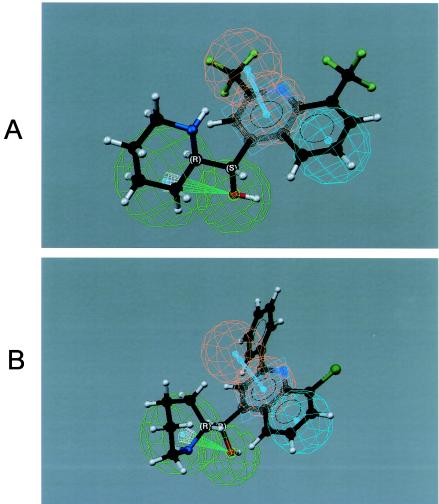
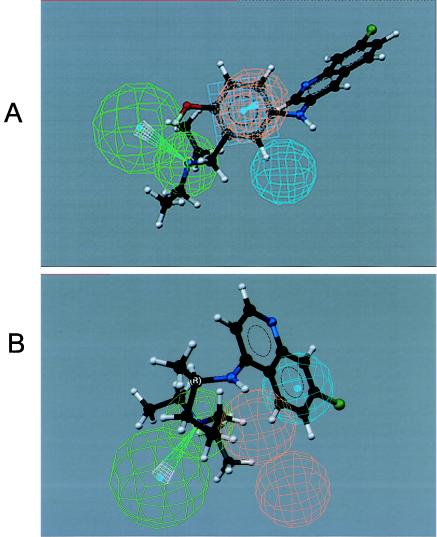
The 3D-QSAR pharmacophore model for the neurotoxicity of 4-quinolinecarbinolamines was found to contain one hydrogen bond acceptor (lipid) function, one aliphatic hydrophobic function, and a ring aromatic function at specific geometric orientation in the molecule (Fig. 5). It was developed from a set of 14 structurally diverse 4-quinolinecarbinolamines that included the parent compound mefloquine shown in Table 1. The experimental neurotoxicity data of the 14 analogs covers a range from 18 to 200 μM. The pharmacophore was developed using CATALYST methodology (1) by placing suitable constraints on the number of available features such as aromatic hydrophobic or aliphatic hydrophobic interactions, hydrogen bond donors, hydrogen bond acceptors, hydrogen bond acceptors (lipid), and ring aromatic sites to describe the neurotoxicity of the 4-quinolinecarbinolamines. Earlier reported quantum chemical calculations of the stereoelectronic properties for a few of these compounds (3) provided guidance for selection of these physicochemical features. During pharmacophore development, the molecules were mapped to the features, with their predetermined conformations generated using the fast-fit techniques in the CATALYST methodology. The procedure resulted in the generation of 10 alternative pharmacophores for antimalarial activity of the compounds and appeared to perform quite well for the training set. Significantly, the best pharmacophore (Fig. 5) is also statistically the most relevant pharmacophore. The estimated activity values, along with the experimentally determined neurotoxicity values of the compounds, are presented in Fig. 6. Experimentally determined IC50 values were well correlated with estimated values within a range of uncertainty of 3 (r = 0.86; P < 0.0001 [Pearson correlation]). The more neurotoxic analogues of the series such as mefloquine and WR006006 map all the functional features of the pharmacophore (Fig. 7), whereas the nonneurotoxic compounds such as amodiaquine and chloroquine do not map all the features (Fig. 8).
FIG. 5.
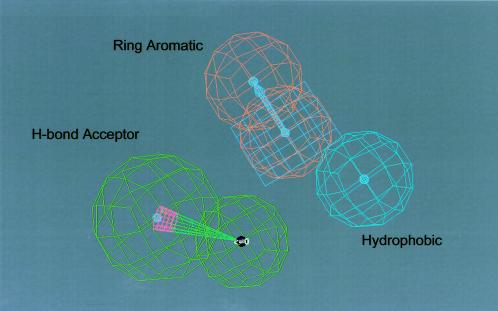
Neurotoxicity pharmacophore for 4-quinolinecarbinolamines: The pharmacophore for the neurotoxicity of 4-quinolinecarbinolamines is depicted. The key functional features required for neurotoxicity include (i) one lipid type H-bond acceptor function (shown in green with a direction vector), (ii) one ring aromatic function (shown in light magenta), and (iii) one aliphatic hydrophobic function (shown as a blue sphere).
FIG. 6.
Correlation (r = 0.86; P < 0.0001 [Pearson correlation]) of experimental and estimated neurotoxicity (IC50) data for the training set.
FIG. 7.
Mapping of the pharmacophore on two known neurotoxic compounds, mefloquine (A) and WR006006 (B), showing how all the features of the pharmacophore map onto them.
FIG. 8.
Mapping of the pharmacophore on two nonneurotoxic compounds, amodiaquine (A) and chloroquine (B), showing how not all of the features of the pharmacophore map onto them.
Assessment of predictive value of the pharmacophore using the test set
To cross-validate the reliability of the pharmacophore, we searched the in-house chemical inventory system database with the pharmacophore as the template and identified several 4-quinolinecarbinolamines. The test set contains four 4-quinolinecarbinolamines together with the four conventional antimalarials halofantrine, chloroquine, amodiaquine, and quinine. The four 4-quinolinecarbinolamines and halofantrine were predicted to be neurotoxic, whereas quinine, chloroquine, and amodiaquine were not (Table 2). In all cases, the qualitative predictions made about neurotoxicity were accurate (Table 2).
TABLE 2.
Actual and predicted neurotoxicity of test set compounds
| Analog | Predicted valuea | Neurotoxic?b | Good prediction?c |
|---|---|---|---|
| Amodiaquine | 620 | No | Yes |
| Halofantrine | 50 | Yes | Yes |
| Chloroquine | 550 | No | Yes |
| Quinine | 540 | No | Yes |
| WR062175 | 110 | Yes | Yes |
| WR159314 | 140 | Yes | Yes |
| WR007936 | 64 | Yes | Yes |
| WR073898 | 54 | Yes | Yes |
Predicted value for the IC50 of the compounds against rat neurons after fast-fit mapping of the compound's structure to the neurotoxicity pharmacophore.
The analog was predicted to be neurotoxic if the predicted IC50 was <300 μM (100 μM threshold multiplied threefold for the error constraints of the pharmacophore model).
“Yes” or “No” indicates whether the compound was or was not correctly categorized as being neurotoxic on the basis of the experimental values in Table 1.
DISCUSSION
The clinical utility of mefloquine or a mefloquine-like drug would be enhanced if measures could be employed to negate the toxicity of the drug. This could be achieved through the use of an intrinsically less neurotoxic analog or by lowering the required dose of a similarly neurotoxic analog. Of the 18 mefloquine analogs tested here, 2, WR187044 and WR073872, possessed IC50s greater than 100 μM. This is a physiologically relevant threshold, since mefloquine crosses the blood-brain barrier and accumulates in the CNS at a level 10- to 30-fold higher relative to plasma levels at therapeutic dose rates, reaching concentrations as high as 100 μM (2, 14, 20). While it is possible that 4-quinolinecarbinolamines do not cross the blood-brain barrier to the same degree as mefloquine, in the absence of specific information to the contrary it is prudent to take a conservative approach and assume that they do. In any case, WR187044 and WR073872 do not represent viable alternative drugs to mefloquine, since they display little in vivo antimalarial activity (Table 1).
The utility of a mefloquine replacement drug could also be improved if the relative dose rate could be reduced. This might be possible if a mefloquine analog were at worst equivalent to mefloquine in terms of neurotoxicity but exhibited a greater relative therapeutic index against mefloquine-resistant strains of malaria. Selection of a particular relative therapeutic index as a threshold is necessarily problematic, because the reduction of dose that would be possible and the degree to which CNS accumulation would be consequently reduced are difficult to predict. Therefore, an empirically derived benchmark is probably the most appropriate. Halofantrine is a conventional quinoline antimalarial that displays some cross-resistance to mefloquine both in vitro (high relative IC50s against D6 and TM91C235; Table 1) and in vivo (5). Halofantrine was the only one of the conventional antimalarials to exhibit neurotoxicity in the same concentration range as the 4-quinolinecarbinolamines, with some mechanistic attributes in common.
Therefore, we propose that the threshold therapeutic index relative to mefloquine should be approximately 30 against TM91C235, the same as that of halofantrine. On this basis, the 4-quinolinecarbinolamines tested here do not exhibit sufficient selective antimalarial activity.
However, this does not mean that other quinolines would not be suitable replacement drugs (11, 27). Three of the conventional antimalarial antimalarials tested here were much less neurotoxic than mefloquine and exhibited qualitatively different mechanisms of action against neurons. Further, not all quinoline antimalarials exhibit the same inherent cross-resistance to mefloquine as halofantrine and the 4-quinolinecarbinolamines (Table 1). Therefore, there are reasonable grounds to propose that there may be other, as-yet-undiscovered quinolines that exhibit much greater selective toxicity than those tested here. One might be able to identify such compounds by developing a reliable 3D pharmacophore and using it for virtual screening of compound databases, since these techniques not only enable predictions of the biological activity of unknown compounds but also provide a basis for custom-designed synthesis of compounds with optimum efficacy that have both the necessary chemical functions and the requisite stereoelectronic properties (4). As a first step in the development of such an in silico screening method for quinoline antimalarials, we have developed a pharmacophore on the basis of the neurotoxicity data for the 4-quinolinecarbinolamines.
The crucial molecular features that appear to correlate with the neurotoxic properties of the 4-quinolinecarbinolamines include (i) one hydrogen bond acceptor (lipid) function, (ii) one aliphatic hydrophobic function, and (iii) a ring aromatic function at specific geometric locations distributed over the 3D space of the molecule. When the pharmacophore was employed as a qualitative in silico screening tool, we observed that the approach was able to correctly predict whether a series of quinolines were neurotoxic (or not) on the basis of the mapping of their 3D structures to the pharmacophore (Table 2 and Fig. 7 and 8). These preliminary data suggest that the approach has merit. The next step in the process is obviously to develop an appropriate pharmacophore on the basis of the antimalarial activity of quinolines. This approach is presently under investigation in our laboratory.
Acknowledgments
The opinions asserted herein are solely those of the authors and should not be construed as reflecting the official position or policy of the United States Army or Department of Defense.
This project was funded through a grant from the U.S. Military Infectious Diseases Research Program.
REFERENCES
- 1.Accelrys Inc. 2001. CATALYST, version 4.6. Accelrys Inc., San Diego, Calif.
- 2.Baudry, S., Y. T. Pham, B. Baune, S. Vidrequin, C. Crevoisier, F. Gimenez, and R. Farinotti. 1997. Stereoselective passage of mefloquine through the blood-brain barrier in the rat. J. Pharm. Pharmacol. 49:1086-1090. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharjee, A. K., and J. M. Karle. 1996. Molecular electronic properties of a series of 4-quinolinecarbinolamines define antimalarial activity profile. J. Med. Chem. 39:4622-4629. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharjee, A. K., D. E. Kyle, J. L. Vennerstrom, and W. K. Milhous. 2002. A 3D QSAR pharmacophore model and quantum chemical structure activity analysis of chloroquine (CQ)-resistance reversal. J. Chem. Inf. Comput. Sci. 42:1212-1220. [DOI] [PubMed] [Google Scholar]
- 5.Canfield, C. J. 1980. Antimalarial aminoalcohol alternatives to mefloquine. Acta Trop. 37:232-237. [PubMed] [Google Scholar]
- 6.Choi, D. W. 1988. Calcium-mediated neurotoxicity: relationship to specific channel types and roles in ischemic damage. Trends Neurosci. 11:465-469. [DOI] [PubMed] [Google Scholar]
- 7.Cook, G. C. 1995. Mefloquine prophylaxis. Mefloquine toxicity should limit its use to treatment alone. BMJ 311:190-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croft, A. M., and P. Garner. 2000. Mefloquine for preventing malaria in non-immune adult travelers. Cochrane Database Syst. Rev. 3:CD000138. [DOI] [PubMed] [Google Scholar]
- 9.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dow, G. S., T. H. Hudson, M. Vahey, and M. L. Koenig. 2003. The acute neurotoxicity of mefloquine may be mediated through a disruption of calcium homeostasis and ER function in vitro. Malaria J. 2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foley, M., and L. Tilley. 1998. Quinoline antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacol. Ther. 79:55-87. [DOI] [PubMed] [Google Scholar]
- 12.Grigorov, M., J. Weber, J. M. Tronchet, C. W. Jefford, W. K. Milhous, and D. Maric. 1997. A QSAR study of the antimalarial activity of some synthetic 1,2,4-trioxanes. J. Chem. Inf. Comput. Sci. 37:124-130. [DOI] [PubMed] [Google Scholar]
- 13.Hartley, D. M., M. C. Kurth, L. Bjerkness, J. H. Weiss, and D. W. Choi. 1993. Glutamate receptor-induced 45Ca2+ accumulation in cortical cell cultures correlates with subsequent neuronal degeneration. J. Neurosci. 13:1993-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, R., G. Kunsman, B. Levine, M. Smith, and C. Stahl. 1994. Mefloquine distribution in postmortem cases. Forensic Sci. Int. 68:29-32. [DOI] [PubMed] [Google Scholar]
- 15.Koenig, M. L., C. M. Sgarlat, D. L. Yourick, J. B. Long, and J. L. Meyerhoff. 2001. In vitro neuroprotection against glutamate-induced toxicity by pGlu-Glu-Pro-NH(2) (EEP). Peptides 22:2091-2097. [DOI] [PubMed] [Google Scholar]
- 16.Kollaritsch, H., J. Karbwang, G. Weidermann, A. Mikolasek, K. Na-Bangchang, and W. H. Wernsdorfer. 2000. Mefloquine concentration profiles during prophylactic dose regimens. Wein. Klin. Wochenschr. 112:441-447. [PubMed] [Google Scholar]
- 17.Milhous, W. K., N. F. Weatherly, J. H. Bowdre, and R. E. Desjardins. 1985. In vitro activities of and mechanisms of resistance to antifol antimalarial drugs. Antimicrob. Agents Chemother. 27:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Center for Infectious Diseases. 2003. Accession date,z 19 February 2004. Prescription drugs for preventing malaria. [Online.] Centers for Disease Control and Prevention. http://www.cdc.gov/travel/malariadrugs.htm.
- 19.Oduola, A. M., W. K. Milhous, N. F. Weatherly, J. H. Bowdre, and R. E. Desjardins. 1988. Plasmodium falciparum: induction of resistance to mefloquine in cloned strains by continuous drug exposure in vitro. Exp. Parasitol. 67:354-360. [DOI] [PubMed] [Google Scholar]
- 20.Overbosch, D., H. Schilthuis, U. Bienzle, R. H. Behrens, K. C. Kain, P. D. Clarke, S. Toovey, J. Knobloch, H. D. Nothdurft, D. Shaw, N. S. Roskell, and J. D. Chulay. 2001. Atovaquone-proguanil versus mefloquine for malaria prophylaxis in non-immune travelers: results from a randomized, double-blind study. Clin. Infect. Dis. 33:1015-1021. [DOI] [PubMed] [Google Scholar]
- 21.Pham, Y. T., F. Nosten, R. Farinotti, N. J. White, and F. Gimenez. 1999. Cerebral uptake of mefloquine enantiomers in fatal cerebral malaria. Int. J. Clin. Pharmacol. Ther. 37:58-61. [PubMed] [Google Scholar]
- 22.Phillips-Howard, P. A., and F. O. ter Kuile. 1995. CNS adverse events associated with antimalarial agents: fact or fiction? Drug Saf. a370-383. [DOI] [PubMed] [Google Scholar]
- 23.Schlagenhauf, P. 1999. Mefloquine for malaria chemoprophylaxis 1992-1998. J. Travel Med. 6:122-133. [DOI] [PubMed] [Google Scholar]
- 24.Shepherd, J. September1998. Use of (+) mefloquine for the treatment of malaria. International patent WO98/39003.
- 25.Simpson, J., A. Price, R. ter Kuile, F. Teja-Isavatharm, P. Nosten, F. Chongsuphajaisiddhi, T. Looareesuwan, S. L. Aarons, and N. J. White. 1999. Population pharmacokinetics of mefloquine in patients with acute falciparum malaria. Clin. Pharmacol. Ther. 66:472-484. [DOI] [PubMed] [Google Scholar]
- 26.Speich, R., and A. Haller. 1994. Central anticholinergic syndrome with the antimalarial drug mefloquine. N. Engl. J. Med. 331:57-58. [DOI] [PubMed] [Google Scholar]
- 27.Stocks, P. A., K. J. Raynes, and S. A. Ward. 2001. Novel quinoline antimalarials, p 235-253. In P. Rosenthal (ed.), Antimalarial chemotherapy: mechanism of action, resistance and new directions in drug discovery. Humana Press Inc., Totowa, N.J.
- 28.Sutter, J., O. Guner, R. Hoffman, H. Li, and M. Waldman. 2000. Effect of variable weights and tolerances on predictive model generation, p. 501-511. In O. F. Guner (ed.), Pharmacophore, perception, development, and use in drug design. International University Line, La Jolla, Calif.
- 29.Sweeney, T. R. 1991. A survey of compounds from the antimalarial drug development program of the U.S. Army Medical Research and Development Command, vol. 1. Walter Reed Army Institute of Research, Washington, D.C.
- 30.World Health Organization. 1998. The World Health Report 1998. World Health Organization, Geneva, Switzerland.