Abstract
Gemcitabine (difluorodeoxycytidine; dFdCyd) is a potent radiosensitizer, noted for its ability to enhance cytotoxicity with radiation at noncytotoxic concentrations in vitro and subchemotherapeutic doses in patients. Radiosensitization in human tumor cells requires dFdCyd-mediated accumulation of cells in S phase with inhibition of ribonucleotide reductase, resulting in ≥80% deoxyadenosine triphosphate (dATP) depletion and errors of replication in DNA. Less is known of the role of specific DNA replication and repair pathways in the radiosensitization mechanism. Here the role of homologous recombination (HR) in relationship to the metabolic and cell cycle effects of dFdCyd was investigated using a matched pair of CHO cell lines that are either proficient (AA8 cells) or deficient (irs1SF cells) in HR based on expression of the HR protein XRCC3. The results demonstrated that the characteristics of radiosensitization in the rodent AA8 cells differed significantly from those in human tumor cells. In the AA8 cells, radiosensitization was achieved only under short (≤4 h) cytotoxic incubations, and S-phase accumulation did not appear to be required for radiosensitization. In contrast, human tumor cell lines were radiosensitized using noncytotoxic concentrations of dFdCyd and required early S-phase accumulation. Studies of the metabolic effects of dFdCyd demonstrated low dFdCyd concentrations did not deplete dATP by ≥80% in AA8 and irs1SF cells. However, at higher concentrations of dFdCyd, failure to radiosensitize the HR-deficient irs1SF cells could not be explained by a lack of dATP depletion or lack of S-phase accumulation. Thus, these parameters did not correspond to dFdCyd radiosensitization in the CHO cells. To evaluate directly the role of HR in radiosensitization, XRCC3 expression was suppressed in the AA8 cells with a lentiviral-delivered shRNA. Partial XRCC3 suppression significantly decreased radiosensitization [radiation enhancement ratio (RER) = 1.6 ± 0.15], compared to nontransduced (RER = 2.7 ± 0.27; P = 0.012), and a substantial decrease compared to nonspecific shRNA-transduced (RER =2.5 ± 0.42; P =0.056) AA8 cells. Although the results support a role for HR in radiosensitization with dFdCyd in CHO cells, the differences in the underlying metabolic and cell cycle characteristics suggest that dFdCyd radiosensitization in the nontumor-derived CHO cells is mechanistically distinct from that in human tumor cells.
INTRODUCTION
Gemcitabine [2′,2′-difluoro-2′-deoxycytidine (dFdCyd)] is a nucleoside analog commonly used to treat a wide variety of solid tumors. To achieve its antitumor activity, dFdCyd requires phosphorylation within the tumor cell to reach its active diphosphate (dFdCDP) and triphosphate (dFdCTP) forms. Of these metabolites, dFdCTP accumulates to the highest levels within tumor cells and its incorporation into DNA correlates with cytotoxicity (1). The other active metabolite, dFdCDP, is a mechanism-based inhibitor of ribonucleotide reductase (2, 3), an enzyme that converts ribonucleoside diphosphates to their corresponding deoxyribonucleoside diphosphates, to supply the cell with the deoxynucleoside triphosphates (dNTPs) necessary for DNA synthesis. Inhibition of this enzyme results in decreased dNTPs and inhibition of DNA synthesis (4). In solid tumor cells, the largest decrease is observed in dATP (5).
In addition to its activity as a chemotherapeutic, dFdCyd also produces a synergistic enhancement in tumor cell killing when combined with ionizing radiation (IR) (6). Mechanistic studies in many human tumor cell lines demonstrate that radiosensitization is strongly dependent on the dFdCyd-mediated inhibition of ribonucleotide reductase resulting in ≥80% depletion of dATP, DNA synthesis inhibition and consequent accumulation of cells in S phase (5, 7–9). Limited replication of DNA with decreased dATP results in replication errors in DNA, which also correlates with radiosensitization (10).
Exposure to radiation produces a variety of types of DNA damage, with DNA double-strand breaks (DSBs) representing the most detrimental lesion. Two mechanisms that have been shown to increase radiosensitization, are either to increase the number of DSBs or to decrease the rate or extent of the repair [reviewed in ref. (6)]. However, neither of these mechanisms accounted for radiosensitization by dFdCyd (11, 12). Studies in cells proficient or deficient in DSB repair pathways provided some insight into the repair mechanisms involved in radiosensitization with dFdCyd. There are two major pathways that repair DSBs in mammalian cells: 1. nonhomologous end joining (NHEJ), an error-prone pathway that involves ligation of blunt ends resulting in DSB resolution with loss of information; and 2. homologous recombination (HR), which utilizes a homologous template, with preference for a sister chromatid, resulting in virtually error-free DSB repair (13). Studies of Chinese hamster ovary (CHO) cells that were NHEJ deficient showed that radiosensitization by dFdCyd was still achieved, suggesting NHEJ to be dispensable for radiosensitization by dFdCyd (14). In contrast, CHO cells that were HR deficient were not radiosensitized, suggesting that HR is important for radiosensitization by dFdCyd in CHO cells (15). However, radiosensitization was evaluated at only two cytotoxic concentrations of dFdCyd, and effects on dNTPs and cell cycle were not reported. Thus, it is not known whether radiosensitization by dFdCyd in CHO cells is mechanistically similar to that in human tumor cells. The availability of matched HR-proficient and deficient CHO cell lines (versus human cells) makes the rodent lines very useful for studying the role of HR (15–20). These cell lines are used routinely to elucidate the mechanism of HR and its role in the sensitivity of cells to drugs or radiation. Here, we have further evaluated the role of HR in radiosensitization of CHO cells by dFdCyd over a broad range of concentrations, with corresponding studies of dFdCyd metabolism and effects on cell cycle distribution. Furthermore, the role of HR was directly evaluated by shRNA suppression of an essential HR protein, XRCC3. The results confirm a role for HR in radiosensitization by dFdCyd in CHO cells, but demonstrate significant mechanistic differences in radiosensitization in CHO cells compared to human tumor cells.
MATERIALS AND METHODS
Cell Lines and Cell Cycle Analysis
The CHO AA8 (HR-proficient) and irs1SF (HR-deficient) cells were grown in MEMα medium (Invitrogen™, Carlsbad, CA) with 10% fetal bovine serum (FBS) (Invitrogen), L-glutamine (Fisher Scientific, Boston, MA), and penicillin/streptomycin at 37°C, 5% CO2. CHO cell doubling time was measured over 72 h. For evaluation of cell cycle progression, CHO cells were harvested, fixed and stained with propidium iodide. Cells were processed and analyzed by flow cytometry as described previously (9).
Cytotoxicity and Radiosensitization
Cell survival was measured using a standard colony formation assay (5). Colonies were allowed to form for 7 days in the AA8 cells and for 12–14 days in the irs1SF cells to accommodate differences in doubling times. Each experiment was performed in triplicate and repeated at least three times. IC10 and IC50 values were determined by extrapolation from the best-fit analysis of the dose-response graphs using Prism software (GraphPad, San Diego, CA). For measurement of radiosensitization, cells were incubated with dFdCyd prior to irradiation (60Co Theratron 80, 1–2 Gy/min at room temperature), and then assessed for survival using a colony formation assay. The dose-response data were fit to a linear-quadratic equation after correction for survival of cells with drug alone. The areas under the resulting survival curves (AUC) were used to calculate the radiation enhancement ratio (RER), defined as the AUC (radiation alone) divided by the AUC (dFdCyd + IR) (21). Dose enhancement ratios (DERs) were calculated as the radiation dose producing 50% cell survival with no drug divided by the radiation dose producing 50% cell survival with dFdCyd. The survival curves were also used to determine the surviving fraction at 2 Gy (SF2) and the D-bar values in the absence of drug, both of which are measures of the sensitivity to radiation for each cell line in the absence of drug.
HPLC Analysis of Deoxynucleotides
Cells were harvested and nucleotides extracted with perchloric acid, neutralized and analyzed by anion exchange HPLC (5). The endogenous deoxynucleoside triphosphates (dNTPs) and dFdCTP were detected by ultraviolet (UV) absorbance at 254 and 281 nm, and quantitated by comparison of peak areas to those of known amounts of standards.
shRNA Suppression of XRCC3
Lentiviral plasmids (pLKO-1.puro) containing XRCC3 shRNA target sequences were used to transduce AA8 cells followed by selection with puromycin, as described previously (22). Cells surviving selection were then transferred to medium without puromycin and expanded. The extent and duration of XRCC3 suppression was measured by Western blot analysis.
Western Blot Analysis
Proteins in cell lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis using a 10% polyacrylamide gel. Separated proteins were transferred to an Immobilon-P membrane and probed with antibodies to XRCC3 (kindly provided by Dr. Patrick Sung, Yale University) or actin (Calbiochem) (22). Protein bands were visualized using an enhanced chemiluminescence kit (Pierce, Rockford, IL) and quantified using Image-J software (NIH).
RESULTS
Cytotoxicity and Radiosensitization with dFdCyd in CHO Cells
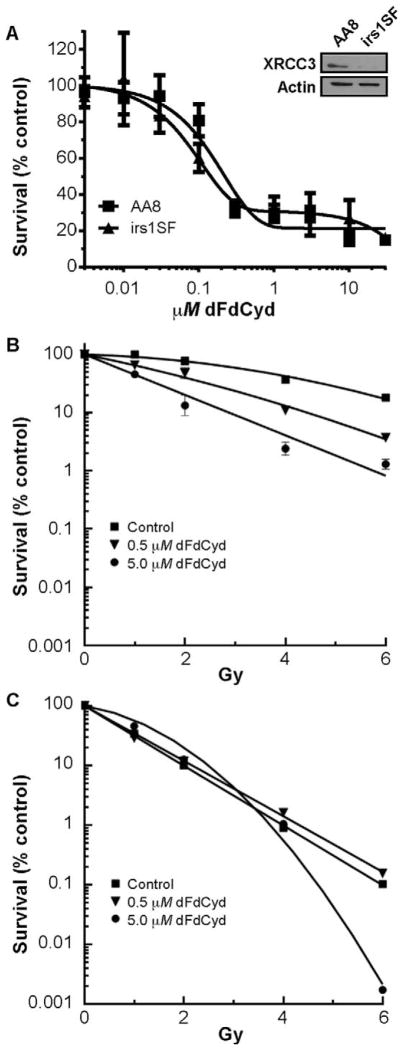
The HR proficient AA8 and HR deficient irs1SF CHO cells displayed some similarity in sensitivity to dFdCyd at several concentrations over a wide range of drug concentrations when incubated for 4 h (Fig. 1A; Table 1). The irs1SF cells were as expected more sensitive to ionizing radiation than the AA8 cells when radiation sensitivity was measured by SF2 and D-bar values, consistent with their deficiency in the HR required XRCC3 protein (Fig. 1A; Table 1). Initially we evaluated radiosensitization of AA8 and irs1SF CHO cells with 0.5 and 5 μM dFdCyd, as reported in a previously published article (15), which decreased cell survival by approximately 70% in both cell lines (Fig. 1A). When the two cell lines were incubated with these concentrations of dFdCyd for 4 h and then irradiated, radiosensitization was only observed in the HR proficient AA8 cells (Fig. 1B and C), with excellent RERs and DERs ranging from 1.6–2.7 (Table 2). In contrast, RERs and DERs were <1.0 in irs1SF cells at the same concentrations of dFdCyd, indicating a lack of radiosensitization. RERs in AA8 cells were also significantly greater than those in irs1SF cells (P ≤ 0.04).
FIG. 1.
Cytotoxicity and radiosensitization of AA8 and irs1SF CHO cells with dFdCyd. Panel A: Sensitivity of AA8 (■) and irs1SF (▲) cells to dFdCyd was determined after a 4 h drug incubation, and survival was measured by a colony formation assay. Points, means of triplicate determinations ± SEM. Western blot demonstrates that irs1SF cells were deficient in XRCC3 conferring HR deficiency, whereas XRCC3 is present in AA8 cells. Radiosensitization with dFdCyd was determined in AA8 (panel B) and irs1SF (panel C) cells after incubation with either no drug (■), 0.5 μM (▼) or 5 μM (●) dFdCyd for 4 h, followed by irradiation at the indicated doses. Radiosensitization was measured using a colony formation assay with correction for the cytotoxicity of dFdCyd alone, as described in Materials and Methods.
TABLE 1.
Sensitivity of CHO Cells to dFdCyd and Ionizing Radiation
| Cell line | Time (h) | IC10 | IC50 |
|---|---|---|---|
| Sensitivity to dFdCyd
|
|||
| AA8 | 4 | 44 ± 16.8 | 200 ± 24 |
| 10 | 15 ± 2.5 | 55 ± 4.3 | |
| irs1SF | 4 | 20 ± 10.2 | 146 ± 36.5 |
| 10 | 40 ± 9.2 | 100 ± 8.8 | |
| 20 | 1 ± 0.2 | 17 ± 1.1 | |
|
|
|||
| Sensitivity to ionizing radiation
|
|||
| Surviving fraction 2 Gy | D-bar | ||
| AA8 | 0.57 ± 0.1 | 3.1 ± 0.4 | |
| irs1SF | 0.14 ± 0.05 | 1.0 ± 0.1 | |
| AA8 mock infected | 0.44 ± 0.07 | 2.5 ± 0.3 | |
| AA8 NS | 0.46 ± 0.03 | 2.4 ± 0.1 | |
| AA8 shRNA1 | 0.37 ± 0.04 | 1.8 ± 0.3 | |
Notes. IC10 and IC50 values were measured from the dFdCyd survival curves with drug incubation times of 4, 10 or 20 h. Values represent the mean ± SE. Surviving fraction at 2 Gy and D-bar values were measured from the radiation dose-response curves in the absence of dFdCyd. Values were determined as described in Materials and Methods.
TABLE 2.
Effect of dFdCyd Concentration, Length of Incubation and XRCC3 Suppression on Radiosensitivity in CHO Cells
| Cell line | Drug incubation (h) | dFdCyd (μM) | RER | DER |
|---|---|---|---|---|
| AA8 | 2 | 0.5 | 1.5 ± 0.1 | 1.7 ± 0.2 |
| 5.0 | 2.1 ± 0.2 | 2.6 ± 0.5 | ||
| 4 | 0.5 | 1.6 ± 0.3 | 1.7 ± 0.4 | |
| 5.0 | 2.4 ± 0.5 | 2.7 ± 0.8 | ||
| irs1SF | 2 | 0.5 | 1.0 ± 0.1 | 1.0 ± 0.2 |
| 5.0 | 0.7 ± 0.1 | 0.6 ± 0.1 | ||
| 4 | 0.5 | 0.9 ± 0.1 | 0.9 ± 0.2 | |
| 5.0 | 0.9 ± 0.3 | 0.8 ± 0.4 | ||
| AA8 (mock infected) | 4 | 5.0 | 2.7 ± 0.3 | 2.7 ± 0.3 |
| AA8 NS shRNA | 4 | 5.0 | 2.5 ± 0.4 | 2.6 ± 0.6 |
| AA8 shRNA #1 | 4 | 5.0 | 1.6 ± 0.2 | 1.5 ± 0.1 |
Notes. RERs and DERs were determined in AA8 cells and irs1SF cells incubated with either 0.5 or 5 μM dFdCyd for 2 or 4 h as indicated, followed by irradiation. XRCC3 was suppressed by lentivirus-delivered shRNA as described in Materials and Methods, and the effects compared to cells that were either mock infected or transduced with a nonspecific shRNA. Cell survival was measured by a colony formation assay, with RERs and DERs calculated as described in Materials and Methods after correction for the effect of gemcitabine alone on cell survival.
The AA8 cells grew at twice the rate of the irs1SF cells (doubling times of 10 h and 20 h, respectively), and thus the AA8 cells were exposed to dFdCyd for a longer percentage of their cell cycle. Because dFdCyd radiosensitization in human tumor cells is dependent on time and extent of dATP depletion (5, 7), we equalized drug exposure according to the percentage of cell doubling time, and thus radiosensitization was also evaluated after a 2 h exposure to 0.5 μM and 5 μM dFdCyd. Significant radiosensitization was still achieved in the AA8 cells, with similar RERs and DERs compared to those obtained after 4 h of incubation (Table 2). In contrast, radiosensitization was not observed in irs1SF cells under any of these conditions.
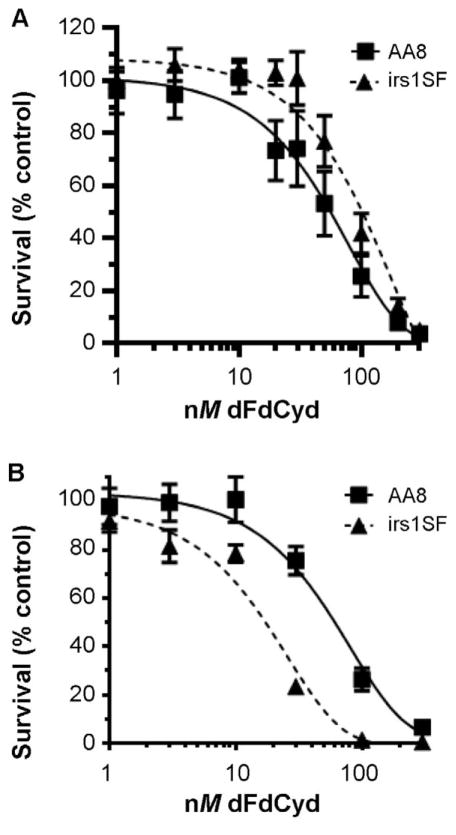
While these incubations produced excellent radiosensitization in the AA8 cells, they also resulted in significant cell killing with dFdCyd alone in both cell lines, making interpretation of radiosensitization difficult. Previous studies in many different human tumor cell lines have demonstrated excellent radiosensitization with noncytotoxic (IC10) or moderately cytotoxic (IC50) incubations with dFdCyd during one cell doubling time (5, 7, 8, 23, 24). To perform a similar analysis in the CHO cells, we incubated each cell line with dFdCyd for 10 h or 20 h and constructed dose-response curves. After a 10 h exposure of AA8 cells to dFdCyd, the cells showed an increased sensitivity to dFdCyd compared to the irs1SF cells (Fig. 2A and Table 3; IC50 values of 55 ± 4.3 nM and 100 ± 8.8 nM for AA8 cells and irs1SF cells, respectively). However, when cells were incubated with dFdCyd for one doubling time, the irs1SF cells exhibited greater drug sensitivity than AA8 cells (Fig. 2B and Table 3; IC50 values of 55 ± 4.3 nM and 17 ± 1.1 nM for AA8 and irs1SF cells, respectively).
FIG. 2.
Effect of equal dFdCyd exposure on CHO cell survival. Sensitivity to dFdCyd was determined in AA8 (■) and irs1SF (▲) CHO cells incubated with the indicated concentrations of dFdCyd for varying lengths of time. Drug incubation time was 10 h in both cell lines (panel A), or 10 h in AA8 cells and 20 h in irs1SF cells (panel B) (equivalent to corresponding doubling time for each cell line).
TABLE 3.
Short or Long Incubation with Gemcitabine at ≤IC50 Does Not Radiosensitize CHO Cells
| Cell line | Drug incubation (h) | dFdCyd | RER | DER |
|---|---|---|---|---|
| AA8 | 10 | IC10 | 1.2 ± 0.1 | 1.3 ± 0.1 |
| IC50 | 1.2 ± 0.1 | 1.2 ± 0.2 | ||
| 4 | IC10 | 1.1 ± 0.1 | 1.0 ± 0.2 | |
| IC50 | 1.3 ± 0.2 | 1.1 ± 0.1 | ||
| irs1SF | 20 | IC10 | 1.2 ± 0.1 | 1.1 ± 0.1 |
| IC50 | 1.1 ± 0.2 | 1.0 ± 0.2 | ||
| 4 | IC10 | 1.1 ± 0.1 | 1.1 ± 0.1 | |
| IC50 | 1.0 ± 0.1 | 0.9 ± 0.004 |
Notes. AA8 cells and irs1SF cells were incubated with IC10 or IC50 dFdCyd, derived from a drug incubation time of either 4 h or equivalent to one doubling time (10 h and 20 h in AA8 and irs1SF cells, respectively), followed by irradiation. RERs and DERs were calculated as described in Materials and Methods, with correction for the effect of dFdCyd alone on cell survival.
Radiosensitization was then determined by incubating the AA8 and irs1SF cells with dFdCyd for one cell doubling time at the corresponding IC10 and IC50 (15 ± 2.5 and 55 ± 4.3 nM for AA8 cells, and 1 ± 0.2 and 17 ± 1.1 nM for irs1SF cells, respectively) prior to irradiation. Under these conditions, no radiosensitization was observed in either cell line (Table 3). In a further attempt to evaluate radiosensitization under conditions of low cytotoxicity, we evaluated IC10 and IC50 for dFdCyd derived from the 4 h drug exposure (44 ± 16.8 and 200 ± 24 nM for AA8 cells and 20 ± 10.2 and 146 ± 36.5 nM for irs1SF cells, respectively) (Fig. 1). These conditions also did not radiosensitize the CHO cells (Table 3). Thus, of the conditions tested, radiosensitization was achieved only in the AA8 cells and only when they were incubated with dFdCyd at concentrations ≥0.5 μM.
Effect of dFdCyd on dFdCTP and dNTPs
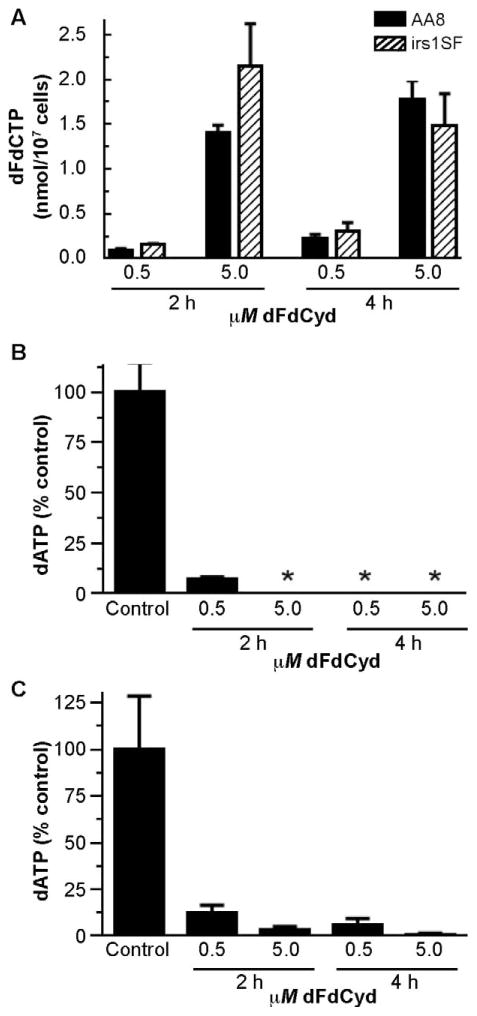
Previously we demonstrated that, in human tumor cells, radiosensitization with dFdCyd correlates strongly with depletion of dATP and accumulation of cells in S phase, but not with accumulation of dFdCTP (5, 7). We evaluated these relationships in the CHO cell lines to determine whether the lack of radiosensitization in the irs1SF cells could be attributed to altered metabolic effects of dFdCyd. To inhibit ribonucleotide reductase or cause cytotoxicity through its incorporation into DNA, dFdCyd must be phosphorylated, therefore, we measured the predominant phosphorylated metabolite, dFdCTP. After incubation with 0.5 and 5 μM dFdCyd for 2 and 4 h, dFdCTP accumulated to similar levels in both AA8 and irs1SF cells (Fig. 3A). These results are consistent with an earlier study that reported comparable dFdCTP levels in AA8 and irs1SF cells after a 4 h exposure to a single concentration of dFdCyd (5 μM) (15). In the studies presented here, at nearly every condition measured dFdCTP was at least as high in the irs1SF cells as in the AA8 cells. Thus, insufficient activation of dFdCyd does not account for the lack of radiosensitization in the irs1SF cells.
FIG. 3.
Effect of radiosensitizing concentrations of dFdCyd on dFdCTP and dATP levels in CHO cells. AA8 and irs1SF cells were incubated with 0.5 or 5 μM dFdCyd for 2 or 4 h as indicated. At the conclusion of the drug incubation, intracellular dFdCTP and dATP were measured by HPLC analysis. Panel A: dFdCTP levels in AA8 (solid bars) and irs1SF (hatched bars) cells. Panel B: dATP levels in AA8 cells. Panel C: dATP levels in irs1SF cells. Control dATP: AA8 cells, 0.103 ± 0.015 nmol/107 cells; irs1SF cells, 0.0645 ± 0.0185 nmol/107 cells. *dATP was below the limit of detection (<0.0015 nmol/107 cells).
Although similar levels of dFdCTP accumulated in AA8 and irs1SF cells, we considered the possibility that dFdCyd might have a greater effect on ribonucleotide reductase and hence dNTPs in the AA8 compared to the irs1SF cells, resulting in sufficient depletion of dATP in the AA8 but not irs1SF cells to mediate radiosensitization. Thus we evaluated the effect of dFdCyd on dNTPs in the CHO cells to determine whether dATP depletion was greater in AA8 than irs1SF cells. The results demonstrated that, in both AA8 and irs1SF cells, concentrations of dFdCyd that radiosensitized AA8 cells produced a >90% and >85% decrease in dATP within 2 h in the AA8 and irs1SF cell lines, respectively, and remained at or below those low levels 4 h after the addition of the drug (Fig. 3B and C). This degree of dATP depletion produces excellent radiosensitization in human tumor cells (5, 7, 11). No significant changes occurred in other endogenous dNTPs in either cell line at these concentrations (data not shown). Thus, differences in dATP depletion cannot account for the lack of radiosensitization in the irs1SF cells under these conditions.
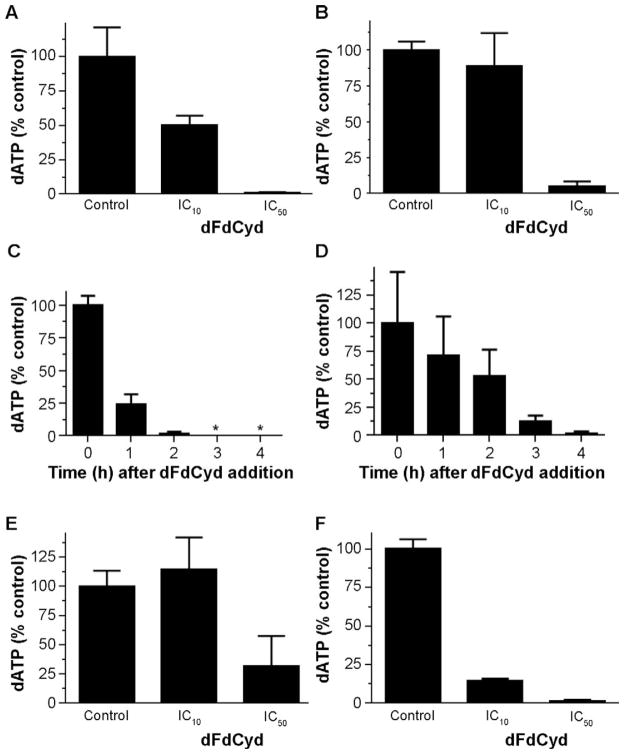
We then evaluated whether the lack of radiosensitization at lower dFdCyd concentrations observed in the AA8 and irs1SF cells (Table 3) could be explained by an insufficient depletion of dATP. Incubation with the 4 h IC10 for dFdCyd resulted in modest depletion of dATP (≤50%) in both the AA8 (Fig. 4A) and irs1SF (Fig. 4B) cells. In contrast, >90% dATP depletion was observed with the 4 h IC50 for dFdCyd in the AA8 and irs1SF cells, however these conditions also did not radiosensitize either cell line. Further analysis demonstrated that, in the AA8 cells, an incubation of 4 h IC50 dFdCyd depleted dATP by >98% of control by 2 h, and thereafter was undetectable for at least 4 h (Fig. 4C). Although this depletion is similar to that observed with 0.5 μM dFdCyd (Fig. 3B) and it mimics the extent and duration of dATP depletion that produces excellent radiosensitization in human tumor cells, no radiosensitization was observed with the 4 h IC50 for dFdCyd in the AA8 cells. The irs1SF cells also achieved >98% dATP depletion with the 4 h IC50 for dFdCyd, but they were slower than the AA8 cells to reach this degree of depletion (Fig. 4D).
FIG. 4.
Effect of nonradiosensitizing concentrations of dFdCyd on dATP levels in CHO cells. AA8 and irs1SF cells were incubated with IC10 or IC50 for dFdCyd based on either a 4 or 10 h incubation. At the conclusion of the incubation, dNTPs were extracted and analyzed by HPLC. After exposure to 4 h IC10 or IC50, dATP was measured in AA8 cells (panel A) and irs1SF cells (panel B). A time course was performed to determine the kinetics of dATP depletion in response to the 4 h IC50 dFdCyd in AA8 cells (panel C) and irs1SF cells (panel D). In addition, dATP was measured after exposure to the 10 h IC10 and IC50 in AA8 cells (panel E) and irs1SF cells (panel F). Values represent the amount of dATP in dFdCyd-treated cells expressed as a percentage of that in untreated control cells.
Next, dATP depletion was evaluated under nonradiosensitizing conditions using longer incubation periods (10 h) with the corresponding IC10 and IC50 for dFdCyd. These conditions produced <70% decrease in dATP in AA8 cells at the conclusion of the incubations (Fig. 4E). However, irs1SF cells showed >85% dATP depletion at the 10 h IC10 and IC50 (Fig. 4F). Thus, under the conditions tested, there was no clear association between dATP depletion and radiosensitization.
Effect of dFdCyd on Cell Cycle
Radiosensitization with dFdCyd in human tumor cells is also associated with accumulation of cells in S phase after dFdCyd and prior to irradiation (5, 8). Incubation of AA8 cells with radiosensitizing concentrations of 0.5 or 5 μM dFdCyd for 4 h resulted in ≤27% increase in S phase relative to controls (Table 4). Larger increases in S phase (69–100%) occurred with a 10 h incubation with IC10 or IC50, although this did not result in radiosensitization. In the irs1SF cells, 0.5 or 5 μM dFdCyd for 4 h resulted in ~40% increase in S phase. For 10 h incubations, S-phase irs1SF cells increased to 70% with IC10 but only to 47% with IC50 dFdCyd. Thus, S-phase accumulation did not appear to be associated with radiosensitization in the CHO cells.
TABLE 4.
Effect of dFdCyd on Cell Cycle Distribution
| Cell line | dFdCyd (μM) | Time (h) | G1 phase (%) | S phase (%) | G2/M phase (%) |
|---|---|---|---|---|---|
| AA8 | 0 | 4 | 37.0 | 52.0 | 11.0 |
| 0.5 | 4 | 33.3 | 65.9 | 0.8 | |
| 5.0 | 4 | 40.7 | 57.8 | 1.5 | |
| irs1SF | 0 | 4 | 40.0 | 31.0 | 29.0 |
| 0.5 | 4 | 39.0 | 44.3 | 16.7 | |
| 5.0 | 4 | 41.8 | 43.7 | 14.5 | |
| AA8 | IC10 | 10 | 6.4 | 69.0 | 24.6 |
| IC50 | 10 | 0 | 100 | 0 | |
| irs1SF | IC10 | 10 | 15.0 | 70.1 | 14.9 |
| IC50 | 10 | 43.9 | 47.3 | 8.8 |
Notes. CHO cells were incubated with dFdCyd for 4 or 10 h as indicated, then harvested and stained with propidium iodide. Cell cycle distribution was measured by flow cytometry analysis.
Effect of XRCC3 Suppression in AA8 Cells
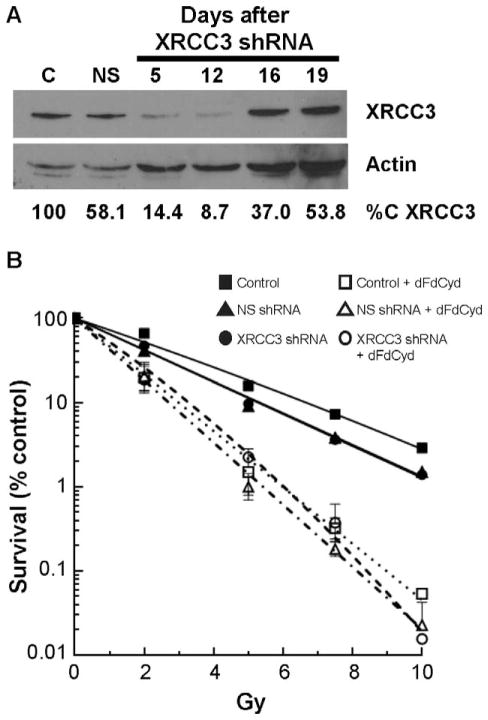
The irs1SF cells were derived from the AA8 cells more than 25 years ago, thus it is possible that the irs1SF cells have acquired alterations in addition to XRCC3 deficiency that affects radiosensitization with dFdCyd. To directly evaluate the role of HR in radiosensitization in the CHO cells, we suppressed expression of XRCC3 in the AA8 cells to mimic the defect responsible for the HR deficiency in irs1SF cells. Suppression of XRCC3 with a lentivirus-delivered shRNA in the AA8 cells decreased XRCC3 expression by 46–91% from day 5–19 after transduction (Fig. 5A). A nonspecific (NS) shRNA had a more modest effect on XRCC3 expression. XRCC3 suppression resulted in a marked slowing of cell growth, consistent with the slow growth of the XRCC3 deficient irs1SF cells. Cell growth returned to normal approximately one week after transduction while maintaining excellent XRCC3 suppression. Another XRCC3 shRNA produced higher suppression of XRCC3 but did not allow recovery of cell growth after transduction, thus it could not be used in the radiosensitization studies (data not shown).
FIG. 5.
Effect of XRCC3 suppression on radiosensitization with dFdCyd in AA8 cells. AA8 cells were transduced with lentivirus-delivered shRNA for XRCC3, followed by selection of transduced cells with puromycin. Panel A: Extent of XRCC3 suppression by Western blot analysis. Panel B: AA8 cells transduced with XRCC3 shRNA were treated with radiation alone or after a 4 h incubation with 5 μM dFdCyd as described in Materials and Methods. Survival was measured by a colony formation assay. XRCC3 suppressed AA8 cells were treated with radiation alone (■), IR + NS shRNA (▲), XRCC3 shRNA followed by irradiation (●), dFdCyd followed by irradiation (□), NS shRNA followed by dFdCyd followed by irradiation (△), or XRCC3 shRNA followed by dFdCyd followed by irradiation (○). Shown is a representative graph of XRCC3 suppression on radiosensitization with dFdCyd; the experiment was performed three times.
To evaluate the effect of XRCC3 suppression on radiosensitization of AA8 cells with dFdCyd, cells were transduced with the lentivirus delivered shRNA for XRCC3 or the NS shRNA. Cell growth returned to normal after approximately one week, when suppression of XRCC3 was approximately 85–90% (Fig. 5A). At that time cells were treated with either no drug, radiation alone or incubated for 4 h with 5 μM dFdCyd followed by irradiation. Treatment of the AA8 cells with the NS shRNA did not alter sensitivity to radiation (Fig. 5B), as there was no significant difference in the D-bar values between control or NS shRNA treated cells (2.5 ± 0.3 and 2.4 ± 0.1, respectively; P = 0.4) (Table 2). Although the XRCC3 shRNA appeared to enhance sensitivity to radiation (D-bar = 1.8 ± 0.3) compared to the NS shRNA, it did not reach statistical significance, nor did the SF2 values differ significantly. However, suppression of XRCC3 in AA8 cells with a subsequent 4 h incubation with 5 μM dFdCyd followed by irradiation resulted in a significant decrease in radiosensitization (RER = 1.6 ± 0.2) compared to nontransduced cells (RER = 2.7 ± 0.3; P = 0.012), and a substantial decrease compared to NS shRNA-transduced (RER =2.5 ± 0.4; P = 0.056) cells (Fig. 5B). The NS shRNA did not decrease radiosensitization with dFdCyd compared to the control cells (P = 0.35). Thus, decreased expression of XRCC3 resulted in a significant decrease in radiosensitization with dFdCyd, directly validating a role for HR in radiosensitization with dFdCyd in CHO AA8 cells.
DISCUSSION
While dFdCyd treatment in combination with exposure to ionizing radiation has been used safely in a variety of solid malignancies including pancreatic cancer and in some tumors such as head and neck, its use is limited by toxicity (6). Greater understanding of the mechanism by which dFdCyd produces radiosensitization may allow for the use of this drug and radiation combination therapy in a more selective manner. Metabolism studies in many different human tumor cell lines have demonstrated that radiosensitization with dFdCyd requires depletion of dATP by ≥80% for ≥4 h with accumulation of cells in S phase (6), as cells in this phase are most sensitive to the combination of dFdCyd and ionizing radiation (8). In addition, evaluation of DNA repair pathways has implicated a role for mismatch repair and HR. Human tumor cells deficient in mismatch repair exhibit enhanced radiosensitization with dFdCyd (25, 26), due to their reduced ability to correct the nucleotide misincorporations that occur in DNA as a result of low-dATP levels (10, 23). Wachters et al. implicated HR in radiosensitization of CHO cells with dFdCyd at cytotoxic concentrations (15). However, whether the underlying mechanistic features associated with dFdCyd radiosensitization in CHO cells were similar to those established in human tumor cells was not reported. Here we evaluated the mechanistic characteristics for radiosensitization with dFdCyd by means of the commonly used model of matched HR proficient and HR deficient CHO cells. Our results confirm the previous report that only the HR proficient AA8 cells were radiosensitized by dFdCyd. However, the parameters associated with radiosensitization in the AA8 cells were distinctly different from those in human tumor cells. Radiosensitization in the AA8 cells was achieved only with highly cytotoxic concentrations of dFdCyd independent of S-phase accumulation, and high dATP depletion did not correspond to radiosensitization. This contrasts with human tumor cells that can be well radiosensitized with noncytotoxic concentrations of dFdCyd, with dATP depletion and S-phase accumulation as predictors for radiosensitization. The results presented here suggest that there are significant differences in the mechanisms of radiosensitization with dFdCyd among the CHO and human tumor cells that must be considered when extrapolating results from CHO to human tumor cells.
Available CHO cell lines that are deficient in specific DNA repair pathways have been utilized by many investigators studying DNA damage and its repair. Studies of such cells demonstrated that deficiencies in NHEJ and base excision repair did not alter radiosensitization with dFdCyd (14, 15). In contrast, HR deficiency prevented radiosensitization with cytotoxic concentrations of dFdCyd (15). Our results confirm the latter report. To ensure that the effect on radiosensitization was due to the HR deficiency and not other unrelated alterations that may have accumulated in the two CHO cell lines after years in culture (27), we also used lentivirus-delivered shRNA to suppress expression of the HR required protein XRCC3 in the AA8 cells. Suppression of XRCC3 resulted in an increased sensitivity to radiation alone as expected based on previously reported results in the irs1SF cells. Furthermore, suppression of XRCC3 resulted in a significant decrease in radiosensitization with 5 μM dFdCyd. These results directly demonstrate that HR is a necessary pathway to achieve radiosensitization with dFdCyd in the AA8 cells.
Gemcitabine is noted for its ability to produce radiosensitization at noncytotoxic concentrations in most human tumor cell lines in vitro and at sub-chemotherapeutic doses in vivo [reviewed in (6)]. However, in the CHO cells, radiosensitization was not achieved under noncytotoxic conditions. Despite a comprehensive analysis of many different drug concentrations and durations of incubation, dFdCyd at ≤IC50 did not produce radiosensitization in either the AA8 or irs1SF cells, even under conditions in which there was strong dATP depletion and accumulation of cells in S phase. In our experience, in the few instances in which dFdCyd did not radiosensitize human tumor cell lines at noncytotoxic concentrations, the cells either displayed an inability to accumulate in S phase after drug exposure, suggesting intact cell cycle checkpoints (9), or exhibited high expression of mismatch repair proteins (10, 25) which could correct errors of replication produced by the dNTP imbalance. As cell lines derived from normal tissue, CHO cells may have higher mismatch repair capability, compared to human tumor cells, which commonly exhibit deficiencies in mismatch repair proteins (28, 29). Nevertheless, the AA8 cells did not require high accumulation of cells in S phase under radiosensitizing conditions, suggesting that replication of DNA during the time of dATP depletion is not required for radiosensitization with dFdCyd in this cell line.
To the best of our knowledge this is the first report of the effects of dFdCyd on all four dNTPs in CHO cells. Both the AA8 and irs1SF cells exhibited greater sensitivity to dFdCyd-mediated depletion of dATP compared to the other three endogenous dNTPs. A previous publication reported a 50% decrease in dCTP in CHO cells but only at an excessively high concentration of 100 μM (30). The AA8 and irs1SF cells readily accumulated dFdCTP when incubated with dFdCyd at 0.5 or 5 μM, similar to previous reports (15, 31), although at the nM concentrations of dFdCyd used here dFdCTP levels were below the level of detection (data not shown). Overall, the CHO cells exhibited dFdCyd-mediated effects on dNTPs similar to those in solid tumor cells, but different from CEM lymphoblastic leukemia cells in which decreases in dCTP and dTTP are more prominent (4, 5, 7).
CHO cells have been used to understand the mechanisms of radiosensitization with other antimetabolites, such as bromodeoxyuridine and hydroxyurea (15, 32, 33). The majority of these studies have focused on the relationship between radiosensitization and cell cycle specific effects, production of DNA double-strand breaks and inhibition of their repair [reviewed in (34)]. Although the ability to radiosensitize CHO cells with antimetabolites has generally corresponded to an ability to radiosensitize human tumor cells, specific differences between CHO and human tumor cells have been noted with respect to mechanism (35). This may be due to differences in the rodent cells versus human cells, or reflect differences in nontumor-derived cells compared to tumor cells. Recently we have demonstrated that radiosensitization by dFdCyd and 5-fluorodeoxyuridine is mediated primarily through their effects on dNTP depletion (10, 36), an area that is relatively unexplored in most studies of antimetabolite radiosensitizers. The balance of dNTPs in the cell alters the fidelity of DNA replication and the number of errors in DNA, and these in turn can determine which DNA repair pathways may be involved in cytotoxicity and/or radiosensitization (37, 38). Indeed, all of the known antimetabolite radiosensitizers alter dNTPs in the cell (6), albeit through different pathways, and thus exploration of the relationship between metabolic effects and radiosensitization as we have done here is warranted.
Our results validate a role for HR in radiosensitization with dFdCyd in CHO AA8 cells. In view of the further finding that the mechanism by which this radiosensitization occurs differs substantially in the CHO compared to human tumor cells, a similar role for HR in human tumor cells remains to be proven. This is important because, if a role for HR in radiosensitization with dFdCyd is established in human tumor cells, it would suggest that treatment with dFdCyd and concurrent radiotherapy would be most beneficial, and perhaps most selective, in patients with HR-overexpressing tumors.
Acknowledgments
This work was supported in part by National Institutes of Health grant CA083081 (National Cancer Institute). We are grateful to Dr. Patrick Sung for the kind gift of the XRCC3 antibody.
References
- 1.Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W. Action of 2′,2′-difluorodeoxycytidine on DNA synthesis. Cancer Res. 1991;51:6110–7. [PubMed] [Google Scholar]
- 2.Baker CH, Banzon J, Bollinger JM, Stubbe J, Samano V, Robins MJ, et al. 2′-Deoxy-2′-methylenecytidine and 2′-deoxy-2′,2′-difluorocytidine 5′-diphosphates: potent mechanism-based inhibitors of ribonucleotide reductase. J Med Chem. 1991;34:1879–84. doi: 10.1021/jm00110a019. [DOI] [PubMed] [Google Scholar]
- 3.van der Donk WA, Yu G, Silva DJ, Stubbe J, McCarthy JR, Jarvi ET, et al. Inactivation of ribonucleotide reductase by (E)-2′-fluoromethylene-2′-deoxycytidine 5′-diphosphate: a paradigm for nucleotide mechanism-based inhibitors. Biochemistry. 1996;35:8381–91. doi: 10.1021/bi960190j. [DOI] [PubMed] [Google Scholar]
- 4.Heinemann V, Xu YZ, Chubb S, Sen A, Hertel LW, Grindey GB, et al. Inhibition of ribonucleotide reduction in CCRF-CEM cells by 2′,2′-difluorodeoxycytidine. Mol Pharmacol. 1990;38:567–72. [PubMed] [Google Scholar]
- 5.Shewach DS, Hahn TM, Chang E, Hertel LW, Lawrence TS. Metabolism of 2′,2′-difluoro-2′-deoxycytidine and radiation sensitization of human colon carcinoma cells. Cancer Res. 1994;54:3218–23. [PubMed] [Google Scholar]
- 6.Shewach DS, Lawrence TS. Antimetabolite radiosensitizers. J Clin Oncol. 2007;25:4043–50. doi: 10.1200/JCO.2007.11.5287. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence TS, Chang EY, Hahn TM, Hertel LW, Shewach DS. Radiosensitization of pancreatic cancer cells by 2′,2′-difluoro-2′-deoxycytidine. Int J Radiat Oncol Biol Phys. 1996;34:867–72. doi: 10.1016/0360-3016(95)02134-5. [DOI] [PubMed] [Google Scholar]
- 8.Latz D, Fleckenstein K, Eble M, Blatter J, Wannenmacher M, Weber KJ. Radiosensitizing potential of gemcitabine (2′,2′-difluoro-2′-deoxycytidine) within the cell cycle in vitro. Int J Radiat Oncol Biol Phys. 1998;41:875–82. doi: 10.1016/s0360-3016(98)00105-9. [DOI] [PubMed] [Google Scholar]
- 9.Ostruszka LJ, Shewach DS. The role of cell cycle progression in radiosensitization by 2′,2′-difluoro-2′-deoxycytidine. Cancer Res. 2000;60:6080–8. [PubMed] [Google Scholar]
- 10.Flanagan SA, Robinson BW, Krokosky CM, Shewach DS. Mismatched nucleotides as the lesions responsible for radiosensitization with gemcitabine: a new paradigm for antimetabolite radiosensitizers. Mol Cancer Ther. 2007;6:1858–68. doi: 10.1158/1535-7163.MCT-07-0068. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence TS, Chang EY, Hahn TM, Shewach DS. Delayed radiosensitization of human colon carcinoma cells after a brief exposure to 2′,2′-difluoro-2′-deoxycytidine (Gemcitabine) Clin Cancer Res. 1997;3:777–82. [PubMed] [Google Scholar]
- 12.Gregoire V, Beauduin M, Bruniaux M, De Coster B, Octave PM, Scalliet P. Radiosensitization of mouse sarcoma cells by fludarabine (F-ara-A) or gemcitabine (dFdC), two nucleoside analogues, is not mediated by an increased induction or a repair inhibition of DNA double-strand breaks as measured by pulsed-field gel electrophoresis. Int J Radiat Biol. 1998;73:511–20. doi: 10.1080/095530098142059. [DOI] [PubMed] [Google Scholar]
- 13.Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell. 2012;47:497–510. doi: 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 14.van Putten JWG, Groen HJM, Smid K, Peters GJ, Kampinga HH. End-joining deficiency and radiosensitization induced by gemcitabine. Cancer Res. 2001;61:1585–91. [PubMed] [Google Scholar]
- 15.Wachters FM, van Putten JW, Maring JG, Zdzienicka MZ, Groen HJ, Kampinga HH. Selective targeting of homologous DNA recombination repair by gemcitabine. Int J Radiat Oncol Biol Phys. 2003;57:553–62. doi: 10.1016/s0360-3016(03)00503-0. [DOI] [PubMed] [Google Scholar]
- 16.Rothkamm K, Kruger I, Thompson LH, Lobrich M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol. 2003;23:5706–15. doi: 10.1128/MCB.23.16.5706-5715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Somaiah N, Yarnold J, Lagerqvist A, Rothkamm K, Helleday T. Homologous recombination mediates cellular resistance and fraction size sensitivity to radiation therapy. Radiother Oncol. 2013;108:155–61. doi: 10.1016/j.radonc.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Holmes AL, Joyce K, Xie H, Falank C, Hinz JM, Wise JP., Sr The impact of homologous recombination repair deficiency on depleted uranium clastogenicity in Chinese hamster ovary cells: XRCC3 protects cells from chromosome aberrations, but increases chromosome fragmentation. Mutat Res Fundam Mol Mech Mutagen. 2014;762:1–9. doi: 10.1016/j.mrfmmm.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Bajinskis A, Olsson G, Harms-Ringdahl M. The indirect effect of radiation reduces the repair fidelity of NHEJ as verified in repair deficient CHO cell lines exposed to different radiation qualities and potassium bromate. Mutat Res. 2012;731:125–32. doi: 10.1016/j.mrfmmm.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 20.El-Awady RA, Saleh EM, Dahm-Daphi J. Targeting DNA double-strand break repair: is it the right way for sensitizing cells to 5-fluorouracil? Anticancer Drugs. 2010;21:277–87. doi: 10.1097/CAD.0b013e328334b0ae. [DOI] [PubMed] [Google Scholar]
- 21.Fertil B, Dertinger H, Courdi A, Malaise EP. Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res. 1984;99:73–84. [PubMed] [Google Scholar]
- 22.Flanagan SA, Cooper KS, Mannava S, Nikiforov MA, Shewach DS. Short hairpin RNA suppression of thymidylate synthase produces DNA mismatches and results in excellent radiosensitization. Int J Radiat Oncol Biol Phys. 2012;84:e613–20. doi: 10.1016/j.ijrobp.2012.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson BW, Shewach DS. Radiosensitization by gemcitabine in p53 wild-type and mutant MCF-7 breast carcinoma cell lines. Clin Cancer Res. 2001;7:2581–9. [PubMed] [Google Scholar]
- 24.Rosier JF, Beauduin M, Bruniaux M, De Bast M, De Coster B, Octave-Prignot M, et al. The effect of 2′-2′ difluorodeoxycytidine (dFdC, gemcitabine) on radiation-induced cell lethality in two human head and neck squamous carcinoma cell lines differing in intrinsic radiosensitivity. Int J Radiat Biol. 1999;75:245–51. doi: 10.1080/095530099140708. [DOI] [PubMed] [Google Scholar]
- 25.Robinson BW, Im MM, Ljungman M, Praz F, Shewach DS. Enhanced radiosensitization with gemcitabine in mismatch repair-deficient HCT116 cells. Cancer Res. 2003;63:6935–41. [PubMed] [Google Scholar]
- 26.van Bree C, Rodermond HM, de Vos J, Haveman J, Franken NA. Mismatch repair proficiency is not required for radioenhancement by gemcitabine. Int J Radiat Oncol Biol Phys. 2005;62:1504–9. doi: 10.1016/j.ijrobp.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Fuller LF, Painter RB. A Chinese hamster ovary cell line hypersensitive to ionizing radiation and deficient in repair replication. Mutat Res. 1988;193:109–21. doi: 10.1016/0167-8817(88)90041-7. [DOI] [PubMed] [Google Scholar]
- 28.Taverna P, Liu L, Hanson AJ, Monks A, Gerson SL. Characterization of MLH1 and MSH2 DNA mismatch repair proteins in cell lines of the NCI anticancer drug screen. Cancer Chemother Pharmacol. 2000;46:507–16. doi: 10.1007/s002800000186. [DOI] [PubMed] [Google Scholar]
- 29.Peltomaki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol. 2003;21:1174–9. doi: 10.1200/JCO.2003.04.060. [DOI] [PubMed] [Google Scholar]
- 30.Heinemann V, Schulz L, Issels RD, Plunkett W. Gemcitabine: a modulator of intracellular nucleotide and deoxynucleotide metabolism. Semin Oncol. 1995;22 (4 Suppl 11):11–8. [PubMed] [Google Scholar]
- 31.Heinemann V, Hertel LW, Grindey GB, Plunkett W. Comparison of the cellular pharmacokinetics and toxicity of 2′,2′-difluoro-deoxycytidine and 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1988;48:4024–31. [PubMed] [Google Scholar]
- 32.Dewey WC, Humphrey RM. Increase in radiosensitivity to ionizing radiation related to replacement of thymidine in mammalian cells with 5-bromodeoxyuridine. Radiat Res. 1965;26:538–53. [PubMed] [Google Scholar]
- 33.Sinclair WK. The combined effect of hydroxyurea and x-rays on Chinese hamster cells in vitro. Cancer Res. 1968;28:198–206. [PubMed] [Google Scholar]
- 34.Shewach DS, Lawrence TS. Nucleoside radiosensitizers. In: Peters GJ, editor. Deoxynucleoside analogs in cancer therapy. Totowa, NJ: Humana Press; 2006. pp. 289–329. [Google Scholar]
- 35.Tang H, Davis MA, Strickfaden SM, Maybaum J, Lawrence TS. Influence of cell cycle phase on radiation-induced cytotoxicity and DNA damage in human colon cancer (HT29) and Chinese hamster ovary cells. Radiat Res. 1994;138 (1 Suppl):S109–S12. [PubMed] [Google Scholar]
- 36.Flanagan SA, Krokosky CM, Mannava S, Nikiforov MA, Shewach DS. MLH1 deficiency enhances radiosensitization with 5-fluo-rodeoxyuridine by increasing DNA mismatches. Mol Pharmacol. 2008;74:863–71. doi: 10.1124/mol.107.043349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martomo SA, Mathews CK. Effects of biological DNA precursor pool asymmetry upon accuracy of DNA replication in vitro. Mutat Res. 2002;499:197–211. doi: 10.1016/s0027-5107(01)00283-4. [DOI] [PubMed] [Google Scholar]
- 38.Bebenek K, Roberts JD, Kunkel TA. The effects of dNTP pool imbalances on frameshift fidelity during DNA replication. J Biol Chem. 1992;267:3589–96. [PubMed] [Google Scholar]