Abstract
The role of oxygen limitation in protecting Pseudomonas aeruginosa strains growing in biofilms from killing by antibiotics was investigated in vitro. Bacteria in mature (48-h-old) colony biofilms were poorly killed when they were exposed to tobramycin, ciprofloxacin, carbenicillin, ceftazidime, chloramphenicol, or tetracycline for 12 h. It was shown with oxygen microelectrodes that these biofilms contain large anoxic regions. Oxygen penetrated about 50 μm into the biofilms, which averaged 210 μm thick. The region of active protein synthesis was visualized by using an inducible green fluorescent protein. This zone was also limited to a narrow band , approximately 30 μm wide, adjacent to the air interface of the biofilm. The bacteria in mature biofilms exhibited a specific growth rate of only 0.02 h−1. These results show that 48-h-old colony biofilms are physiologically heterogeneous and that most of the cells in the biofilm occupy an oxygen-limited, stationary-phase state. In contrast, bacteria in 4-h-old colony biofilms were still growing, active, and susceptible to antibiotics when they were challenged in air. When 4-h-old colony biofilms were challenged under anaerobic conditions, the level of killing by antibiotics was reduced compared to that for the controls grown aerobically. Oxygen limitation could explain 70% or more of the protection afforded to 48-h-old colony biofilms for all antibiotics tested. Nitrate amendment stimulated the growth of untreated control P. aeruginosa isolates grown under anaerobic conditions but decreased the susceptibilities of the organisms to antibiotics. Local oxygen limitation and the presence of nitrate may contribute to the reduced susceptibilities of P. aeruginosa biofilms causing infections in vivo.
When bacteria attach to surfaces and grow as biofilms, they are able to survive antibiotic treatments that readily eradicate free-floating cells (17). One straightforward mechanism that explains this tolerance is that bacteria in a biofilm experience nutrient limitation that leads to localized slow growth or starvation (3, 23). Many antibiotics are known to be less effective against nongrowing or stationary-phase cells than against rapidly growing cells. This protective mechanism is plausible for aerobic microorganisms, as oxygen limitation is a common feature of biofilms.
Pseudomonas aeruginosa is a convenient model organism with which to investigate the roles of oxygen limitation and anaerobiosis in protecting biofilm cells from antibiotics. This bacterium is commonly isolated from infections thought to involve biofilm formation, including those associated with burn wounds, keratitis, urinary catheters, otitis media, and pneumonia in patients with cystic fibrosis (6). It has recently been demonstrated that oxygen is locally depleted in the infected mucus of individuals with cystic fibrosis, underscoring the potential relevance of anaerobiosis in vivo (22). Oxygen gradients and local anaerobic conditions have also been measured in P. aeruginosa biofilms grown in vitro (20, 24).
In addition to slow growth, some of the other factors that have been implicated in P. aeruginosa biofilm protection from killing by antibiotics include specific genetic determinants (2, 13, 21), phenotypic variation (8), the formation of specialized persister cells (16), quorum sensing (15), and overproduction of the matrix polysaccharide alginate (11). Several earlier studies have also investigated aspects of biofilm susceptibility as a function of growth rate (9, 14, 18). None of those studies, however, focused on the role of oxygen limitation in particular in the protection of P. aeruginosa biofilms.
There are two pathways through which P. aeruginosa is known to be able to grow anaerobically. The first pathway is denitrification, which requires nitrate or nitrite as an alternative electron acceptor (5). Nitrate and nitrite are present in the mucus of individuals with cystic fibrosis (10). Yoon et al. (25) have shown that P. aeruginosa forms robust biofilms under anaerobic denitrifying conditions. The same group of investigators also showed that the OprF gene is upregulated during anaerobiosis in the presence of nitrate and that its protein can be detected in the sputum of patients with cystic fibrosis. These results suggest that denitrification by P. aeruginosa could be important in vivo. The second pathway of anaerobic growth is the fermentation of arginine (19). Thus, in an anaerobic environment, in the absence of nitrate and arginine, the growth and metabolism of P. aeruginosa are completely arrested (5, 19).
There is a very limited literature that addresses the antibiotic sensitivity of P. aeruginosa under anaerobic conditions. Davey et al. (7) measured the MICs and minimal bactericidal concentrations (MBCs) of ciprofloxacin, gentamicin, and imipenem for P. aeruginosa in vitro under aerobic and anaerobic conditions. Under anaerobic conditions, the MIC and MBC of ciprofloxacin were slightly reduced, those of gentamicin were increased 8-fold, while those of imipenem were increased 1.5-fold. The fact that MICs were measured under anaerobic conditions indicates that some anaerobic growth occurred in the Mueller-Hinton broth used. These data suggest that gentamicin may be less effective against P. aeruginosa under anaerobic conditions. Bryant and Mazza (4) measured the bactericidal activities of ciprofloxacin at 5 μg/ml and imipenem at 10 μg/ml against P. aeruginosa incubated for 18 h at 37°C in human pus under both aerobic and anaerobic conditions. Neither antibiotic had much effect—log reductions were less than 1 in all cases—so it was not possible to detect differences due to oxygen availability. We have not been able to locate any published data on the role of nitrates or denitrification in modulating the antibiotic susceptibility of P. aeruginosa.
The goal of the present study was to determine how oxygen and nitrate availability affect antibiotic killing in P. aeruginosa biofilms.
MATERIALS AND METHODS
Bacterial strains, media, and antibiotics.
Pure cultures of P. aeruginosa PAO1 were used throughout the study. Bacteria were grown on tryptic soy agar (TSA) or tryptic soy broth (TSB), supplied by Difco (Detroit, Mich.). To provide a substrate for anaerobic respiration, 1% KNO3 (Fisher Scientific, Denver, Colo.) was added to TSA. Ciprofloxacin hydrochloride powder was donated by Bayer Corporation (Leverkusen, Germany). Tobramycin sulfate, chloramphenicol, and tetracycline hydrochloride were purchased from Sigma Chemical Company (St. Louis, Mo.). Carbenicillin disodium salt was supplied by Anatrace (Maumee, Ohio). Ceftazidime pentahydrate was a gift from GlaxoSmithKline (Research Triangle Park, N.C.). Powered antibiotics were dissolved in sterile water and were added to molten culture medium (∼50°C) to create antibiotic-supplemented agar plates.
MICs were determined on Mueller-Hinton agar (Difco) with Etest strips (AB Biodisk, Solna, Sweden). The following antibiotics were used at the indicated concentrations: carbenicillin, 250 μg/ml; ceftazidime, 10 μg/ml; chloramphenicol, 250 μg/ml; ciprofloxacin, 1 μg/ml; tetracycline, 250 μg/ml; tobramycin, 10 μg/ml. These concentrations were selected because they are 10 to 20 times the MIC, the minimum concentration typically needed to measure some effect against a mature biofilm.
Biofilm preparation.
Colony biofilms were grown on polycarbonate membrane filters (diameter, 25 mm; Poretics, Livermore, Calif.) resting on TSA plates, as described by Walters et al. (20). Membranes were sterilized by exposure to UV light (15 min per side) and then inoculated with a 25-μl spot of an overnight culture of planktonic P. aeruginosa PAO1 previously grown in TSB and diluted with the same medium to an optical density at 600 nm of 0.05. The bacteria were incubated at 37°C and grown for either 4 h (young biofilms) or 48 h (mature biofilms). For the growth of mature colony biofilms, the membranes were transferred to fresh TSA plates every 24 h.
Anaerobic environment.
Anaerobic growth conditions were obtained by incubating the bacteria in anaerobic bags (BBL GasPak pouch system; Becton Dickinson, Cockeysville, Md.). All the agar plates used in the anaerobic system were previously placed in an anaerobic bag for at least 24 h to deplete the agar of the oxygen naturally present.
Biofilm susceptibility.
Antibiotic efficacy was tested by transferring either young or mature colony biofilms previously grown on TSA to antibiotic-supplemented agar plates. For each antibiotic, the concentration used for susceptibility testing was 10 to 20 times the MIC. For all antibiotic treatments, the bacteria were incubated at 37°C for 12 h. Each membrane-supported biofilm was sampled after the antibiotic challenge, as described by Anderl et al. (1). The cultures were diluted in phosphate-buffered water (0.31 mM phosphate, 2.0 mM magnesium), and the viable cell numbers were enumerated by plating serially diluted samples on TSA plates by the drop plating method (12). Killing was reported as the log reduction calculated relative to the cell count at the time of initiation of antibiotic exposure. Experiments were performed in triplicate. The standard error of the mean is reported for each value.
Specific growth rate.
Specific growth rates were calculated from growth curves as the slope of the linear regression of the natural log cell number-versus-time data. The error indicated is the standard deviation.
Oxygen penetration.
The profiles of the oxygen concentrations in colony biofilms were measured with a dissolved oxygen microelectrode. The oxygen microelectrode is based on the principle of the common amperometric Clark oxygen electrode. This system is described in detail elsewhere (20).
Spatial pattern of protein synthesis.
The location of active protein synthesis inside the biofilms was determined by using an inducible green fluorescent protein (GFP) construct that has been described previously (20; E. Werner, F. Roe, A. Bugnicourt, M. Franklin, A. Heydorn, S. Molin, B. Pitts, and P. S. Stewart, submitted for publication). Briefly, colony biofilms of P. aeruginosa PAO1 carrying plasmid pAB1 were transferred to TSA plates containing 1mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h at 37°C. The colonies were counterstained with rhodamine B, cryoembedded, sectioned, and examined with a Leica NT confocal scanning laser microscope.
RESULTS
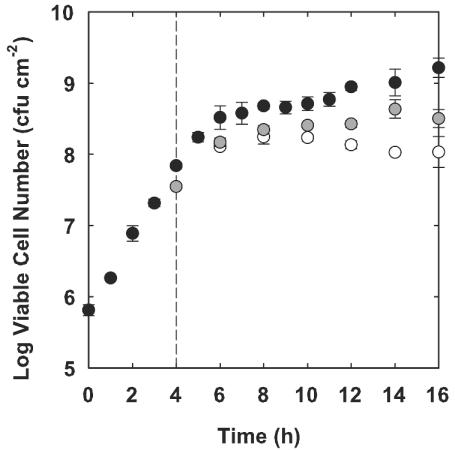
Most of the antibiotic susceptibility tests performed in this study were performed with P. aeruginosa PAO1 colony biofilms that were grown for either 4 or 48 h prior to antibiotic exposure. The polycarbonate membranes that supported colony biofilms were initially seeded with approximately 106 CFU of cells. Bacteria began to grow exponentially and remained in exponential phase for approximately 6 h (Fig. 1). The specific growth rate calculated between 0 and 5 h was 1.14 ± 0.03 h−1. Growth slowed after 6 h, and the colony appeared to enter stationary phase. The average specific growth rate between 24 and 48 h was 0.02 ± 0.01 h−1.
FIG. 1.
Growth of colony biofilms under aerobic conditions (solid circles), anaerobic conditions (empty circles), and anaerobic conditions with nitrate (gray circles). All colony biofilms were grown under aerobic conditions for the first 4 h and were then transferred to alternative conditions at that time, indicated on the graph by the dashed vertical line. The error bars represent the standard error of the mean for each value.
The antibiotic susceptibilities of mature (48-h-old) colony biofilms were investigated in vitro. Bacteria were challenged with approximately 10 to 20 times the MIC measured under aerobic conditions (Table 1) for 12 h. The antibiotics killed the P. aeruginosa organisms growing in 48-h-old colony biofilms poorly (Table 2). The largest log reduction was recorded with ciprofloxacin (log reduction, 1.13). All of the other antibiotics generated log reductions of 0.82 or less for mature biofilms.
TABLE 1.
MICs of antibiotics for P. aeruginosa under various conditions of oxygen and nitrate availability
| Antibiotic | MIC (μg/ml) |
||
|---|---|---|---|
| Air | Air + nitrate | No O2 + nitrate | |
| Carbenicillin | 20 | 50 | 20 |
| Ceftazidime | 0.5 | 0.75 | 0.38 |
| Chloramphenicol | 24 | 48 | 8 |
| Ciprofloxacin | 0.064 | 0.094 | 0.064 |
| Tetracycline | 6 | 8 | 3 |
| Tobramycin | 0.5 | 1 | 0.75 |
TABLE 2.
Susceptibilities of mature (48-h-old) colony biofilms to antibiotics
| Antibiotic | LRa (mean ± SEM) | % Protection explained byb: |
|
|---|---|---|---|
| Lack of oxygen | Lack of oxygen for 24 h | ||
| None | 0.14 ± 0.07 | ||
| Ciprofloxacin | 1.13 ± 0.14 | 62 | 84 |
| Tobramycin | 0.52 ± 0.12 | 69 | 70 |
| Ceftazidime | 0.82 ± 0.02 | >110 | >110 |
| Chloramphenicol | 0.05 ± 0.03 | 95 | 109 |
| Carbenicillin | 0.53 ± 0.12 | NC | NC |
| Tetracycline | 0.21 ± 0.05 | 80 | 105 |
LR, log reduction.
The maximum percentage of biofilm protection that can potentially be explained by oxygen deprivation was calculated as [(log reduction for aerobic 4-h-old biofilm − log reduction for anaerobic 4-h-old biofilm)/(log reduction for aerobic 4-h-old biofilm − log reduction for 48-h-old biofilm)] × 100. NC, not calculated because killing in 48-h-old biofilms was greater than that in 4-h-old biofilms.
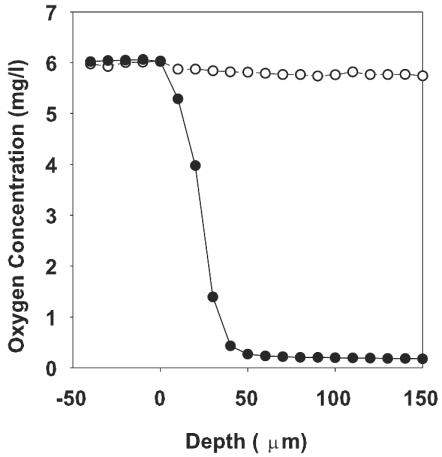
A direct measurement of the oxygen concentration profile in 48-h-old P. aeruginosa colony biofilms was obtained with oxygen microelectrodes (Fig. 2). Mature biofilms were mostly anaerobic. These measurements show that oxygen penetrated approximately 50 μm into the colony from the air interface. As the colony biofilms were approximately 210 μm thick, the oxygen-replete portion can be estimated to be about one-quarter of the entire colony. Most of the mature colony biofilm can therefore be assumed to inhabit a state of anoxia or a state with low dissolved oxygen concentrations.
FIG. 2.
Oxygen concentrations in mature (48-h-old) P. aeruginosa colony biofilms (solid circles) versus sterile agar (empty circles). The result shown is the average for three oxygen concentration profiles.
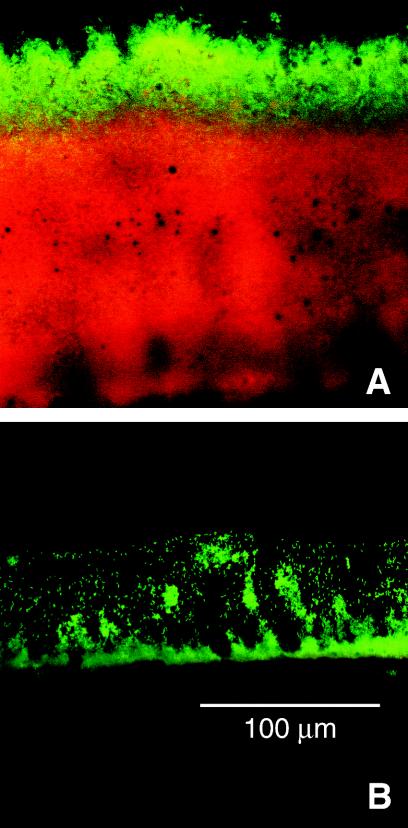
When 48-h-old colony biofilms formed by a strain of P. aeruginosa carrying an inducible, stable GFP were exposed to the inducing agent IPTG for 4 h, only those bacteria in a narrow band adjacent to the air interface were able to express GFP (Fig. 3A). In Fig. 3, green defines the region of the biofilm in which active protein synthesis is taking place, while red indicates biomass that is inactive. It can be deduced that IPTG penetrates the biofilm because the zone of active GFP induction is at the edge of the biofilm opposite from that to which the inducer was delivered. In other work (Werner et al., submitted), it has been shown that the zone of GFP induction corresponds to the region of bacterial growth, as mapped by other techniques. Because the activation of GFP fluorescence is oxygen dependent, the bright zone could reveal only the region of aerobic protein synthesis. In this experiment, however, in which neither an alternative electron acceptor nor arginine was present, the zone of aerobic metabolism is predicted to be the only zone of active protein synthesis. The dimension of this zone of GFP synthesis was determined by image analysis to be 32 ± 3 μm. These results show that 48-h-old P. aeruginosa colony biofilms are physiologically heterogeneous. The majority of the biofilm occupied an inactive or anaerobic state.
FIG. 3.
Pattern of protein synthesis in P. aeruginosa colony biofilms that were 48 h (A) or 4 h (B) old. Green corresponds to induced GFP and red derives from a counterstain for all biomass. The supporting membrane and agar were at the bottom and the air was at the top in this frozen section of the colony.
Oxygen availability influenced the growth of young (4-h-old) colony biofilms. At this early stage of development, the bacteria in colony biofilms incubated in air still had access to oxygen and all of the cells were metabolically active. When a biofilm formed by the strain carrying the inducible GFP construct was grown for 4 h and then induced with IPTG for 4 h, the cells did not yet appear to be fully aggregated and they all expressed GFP (Fig. 3B). There were no red zones in the biofilm, as there were in the 48-h-old biofilms (Fig. 3A), which would have indicated inactive or anaerobic biomass. The bacteria in the 4-h-old biofilms were still growing. The average specific growth rate calculated between 6 and 16 h for colonies grown under aerobic conditions was 0.16 ± 0.01 h−1 (Fig. 1). When 4-h-old colony biofilms were transferred to anaerobic growth conditions, growth was arrested within 2 h. The average specific growth rate under anaerobic conditions over the same time interval (4 to 16 h) was −0.04 ± 0.02 h−1. This shows that oxygen is important for colony biofilm growth from 4 to 16 h under the conditions tested.
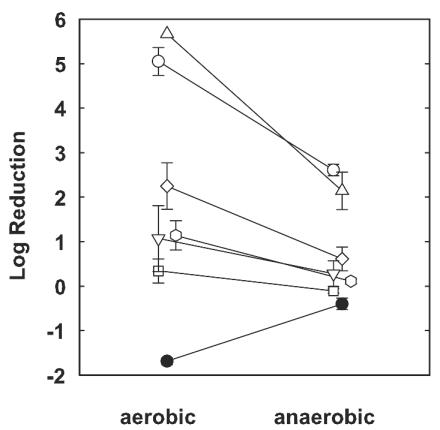
Young (4-h-old) colony biofilms challenged under aerobic growth conditions were susceptible to antibiotics, especially ciprofloxacin and tobramycin (Fig. 4). When the same assays were performed under anaerobic growth conditions, antibiotic efficacies were reduced in all cases (Fig. 4). For example, 4-h-old colony biofilms challenged with ciprofloxacin and tobramycin on TSA in air experienced almost complete killing (log reductions, 5.05 ± 0.31 and 5.67 ± 0.00 [killing to the detection limit], respectively), while the log reductions resulting from the same two agents were much less (2.61 ± 0.13 and 2.14 ± 0.42, respectively) in the absence of oxygen. These differences were statistically significant (P < 0.001 in both cases). It was also determined that the other three antibiotics tested, carbenicillin, chloramphenicol, and tetracycline, had reduced efficacies under anaerobic growth conditions. These results show that oxygen availability is an important determinant of antibiotic susceptibility in P. aeruginosa.
FIG. 4.
Effect of oxygen availability on antibiotic susceptibilities of young (4-h-old) P. aeruginosa colony biofilms. The error bars represent the standard errors of the means for each value. •, control; ○, ciprofloxacin; ▵, tobramycin; □, carbenicillin; ▿, ceftazidime; , chloramphenicol; ◊, tetracycline).
Mature (48-h-old) colony biofilms may contain bacterial cells that have been deprived of oxygen for a prolonged period. To simulate the state of low metabolic activity that bacteria may occupy in mature biofilms, 4-h-old colony biofilms were incubated in an anaerobic environment for 24 h prior to antibiotic challenge. The subsequent 12-h antibiotic treatment was also delivered under anaerobic conditions. There was little difference between the log reductions measured in these experiments and those determined under anaerobic conditions without prolonged anoxia. The only exception was for ciprofloxacin, in which case the log reduction was reduced to 1.75 ± 0.08 under conditions of prolonged anaerobic incubation.
To assess the effects of nitrates on P. aeruginosa growth, 4-h-old colony biofilms were transferred to plates supplemented with 1% KNO3 and incubated both aerobically and anaerobically. The log increase in viable cell numbers over the 12-h test period in an aerobic environment was 1.38 ± 0.08, slightly less than that when no nitrate was present. On the other hand, colony biofilms that were incubated anaerobically grew more in the presence of nitrate than when nitrate was absent. The log increase for biofilms incubated anaerobically in the presence of nitrate was 0.87 ± 0.08 (Fig. 1), which is statistically significantly larger than that for the anaerobic control without nitrate (P = 0.004). The specific growth rate in the presence of nitrates under anaerobic growth conditions calculated between 6 and 16 h was 0.36 ± 0.07 h−1. These results show that nitrate facilitates the growth of colony biofilms under anaerobic conditions but has a slight detrimental effect on aerobically growing biofilms.
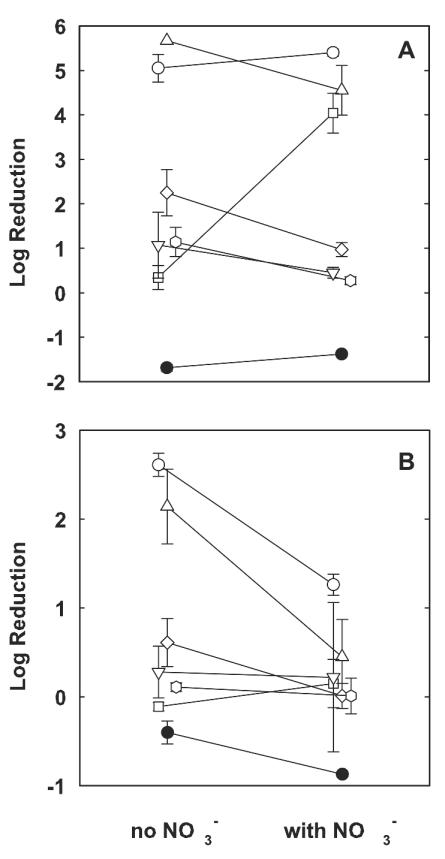
The effect of nitrate supplementation on the antibiotic susceptibilities of 4-h-old colony biofilms was evaluated under both aerobic and anaerobic growth conditions. Nitrate slightly increased the MICs under aerobic conditions (Table 1). The susceptibilities of young colony biofilms under aerobic conditions in the presence of nitrate were similar to the susceptibilities of these biofilms under aerobic conditions in the absence of nitrate for most antibiotics (Fig. 5A). For example, in the presence of oxygen, nitrate supplementation had no statistically significant effect on killing by ciprofloxacin (P = 0.42). The efficacy of killing by tobramycin decreased from a log reduction of 5.67 ± 0.00 (killing to the detection limit) in the absence of nitrate to a log reduction of 4.55 ± 0.56 in the presence of nitrate. The only antibiotic whose efficacy was notably altered by the addition of nitrate under aerobic conditions was carbenicillin (Fig. 5A). Carbenicillin had almost no effect on colony biofilm bacteria in the standard test in the presence of air and the absence of nitrate (log reduction, 0.34 ± 0.27). When nitrate was added, killing by this antibiotic rose to a log reduction of 4.04 ± 0.31. In summary, under aerobic conditions nitrate supplementation had little effect on the efficacies of most antibiotics, with the exception of carbenicillin, whose efficacy was substantially increased by the presence of nitrate.
FIG. 5.
Effect of nitrate availability on antibiotic susceptibilities of young (4-h-old) P. aeruginosa colony biofilms under aerobic (A) and anaerobic (B) conditions. The error bars represent the standard errors of the means for each value. •, control; ○, ciprofloxacin; ▵, tobramycin; □, carbenicillin; ▿, ceftazidime; , chloramphenicol; ◊, tetracycline.
Under anaerobic conditions, nitrate supplementation decreased the levels of killing by ciprofloxacin and tobramycin (Fig. 5B). The log reductions obtained with both agents were more than halved in the presence of nitrate and the absence of oxygen, and these effects were statistically significant (P = 0.003 and P < 0.001 for ciprofloxacin and tobramycin, respectively). Nitrate had no statistically significant effect on the efficacies of the other three antibiotics under anaerobic conditions. The enhancement of the action of carbenicillin that was so striking under aerobic conditions was abolished under anaerobic conditions. These data show that nitrate can reduce the efficacies of clinically important antibiotics used for the treatment of P. aeruginosa infections when anoxia prevails.
DISCUSSION
Anaerobic conditions reduced the efficacies of all six antibiotics tested against P. aeruginosa. These diminished susceptibilities were statistically highly significant (P < 0.001) in the cases of ciprofloxacin, tobramycin, and carbenicillin. While oxygen deprivation protected P. aeruginosa, it did not reduce susceptibility in short-term cultures to the same low levels measured for 48-h-old colony biofilms in most cases. When the log reductions for mature colony biofilms are compared with the log reductions for young biofilms under anaerobic conditions, oxygen limitation could account for as much as 62 to 100% of the protection, depending on the agent (Table 2). This suggests that oxygen limitation is one of the important factors in the tolerance of P. aeruginosa biofilms to killing by antibiotics, but it is probably not the only factor.
The protection from bacterial killing by ciprofloxacin and tobramycin afforded by an anaerobic environment was enhanced by the addition of nitrate. This result was surprising to us, because nitrate supplementation under anaerobic conditions promoted the growth of the untreated control. This shows that antibiotic susceptibility is not strictly a function of the growth state but is determined by the specific nutrients present. The reduced efficacies of antibiotics for the killing of P. aeruginosa in the presence of nitrate and the absence of oxygen could help to explain the failure of antibiotic chemotherapy to control many infections associated with this microorganism.
Unlike conventional planktonic cultures, microbial biofilms are heterogeneous with respect to the physiological state of the cells that they harbor. Key nutrients and electron acceptors may be locally depleted inside a biofilm cell cluster. Biofilms probably contain cells occupying a spectrum of states from rapidly growing to not growing at all. The possibility that antibiotics target only one slice of this spectrum could help explain why biofilm infections respond poorly to antimicrobial chemotherapy. The data presented in this article support this protective mechanism, showing that, in P. aeruginosa the availability of the electron acceptors oxygen and nitrate modulates antibiotic susceptibility.
Acknowledgments
This work was supported by NIH grant R01DC04173-01A1 and by an award from the W. M. Keck Foundation.
Betsey Pitts assisted with microscopy, and Martin Hamilton assisted with the statistical analyses.
REFERENCES
- 1.Anderl, J. N., M. J. Franklin, and P. S. Stewart. 2000. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 44:1818-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagge, N., O. Ciofu, L. T. Skovgaard, and N. Hoiby. 2000. Rapid development in vitro and in vivo of resistance to ceftazidime in biofilm-growing Pseudomonas aeruginosa due to chromosomal beta-lactamase. APMIS 108:589-600. [DOI] [PubMed] [Google Scholar]
- 3.Brown, M. R. W., D. G. Allison, and P. Gilbert. 1988. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J. Antimicrob. Chemother. 22:777-783. [DOI] [PubMed] [Google Scholar]
- 4.Bryant, R. E., and J. A. Mazza. 1989. Effect of the abscess environment on the antimicrobial activity of ciprofloxacin. Am. J. Med. 87(Suppl. 5A):23S-27S. [DOI] [PubMed] [Google Scholar]
- 5.Carlson, C. A., and J. L. Ingraham. 1983. Comparison of denitrification by Pseudomonas stutzeri, Pseudomonas aeruginosa, and Paracoccus denitrificans. Appl. Environ. Microbiol. 45:1247-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 7.Davey, P., M. Barza, and M. Stuart. 1988. Tolerance of Pseudomonas aeruginosa to killing by ciprofloxacin, gentamicin and imipenem in vitro and in vivo. J. Antimicrob. Chemother. 21:395-404. [DOI] [PubMed] [Google Scholar]
- 8.Drenkard, E., and F. M. Ausubel. 2002. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416:740-743. [DOI] [PubMed] [Google Scholar]
- 9.Evans, D. J., D. G. Allison, M. R. Brown, and P. Gilbert. 1991. Susceptibility of Pseudomonas aeruginosa and Escherichia coli biofilms towards ciprofloxacin: effect of specific growth rate. J. Antimicrob. Chemother. 27:177-184. [DOI] [PubMed] [Google Scholar]
- 10.Grasemann, H., I. Ioannidis, R. P. Tokiewicz, H. de Groot, B. K. Rubin, and F. Ratjen. 1998. Nitric oxide metabolites in cystic fibrosis lung disease. Arch. Dis. Child. 78:49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hentzer, M., G. M. Teitzel, G. J. Balzer, A. Heydorn, S. Molin, M. Givskov, and M. R. Parsek. 2001. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 183:5395-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herigstad, B., M. Hamilton, and J. Heersink. 2001. How to optimize the drop plate methods for enumerating bacteria. J. Microbiol. Methods 44:121-129. [DOI] [PubMed] [Google Scholar]
- 13.Mah, T. F., B. Pitts, B. Pellock, G. C. Walker, P. S. Stewart, and G. A. O'Toole. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306-310. [DOI] [PubMed] [Google Scholar]
- 14.Shigeta, M., H. Komatsuzawa, M. Sugai, H. Suginaka, and T. Usui. 1997. Effect of the growth rate of Pseudomonas aeruginosa biofilms on the susceptibility to antimicrobial agents. Chemotherapy (Basel) 43:137-141. [DOI] [PubMed] [Google Scholar]
- 15.Shih, P. C., and C. T. Huang. 2002. Effects of quorum-sensing deficiency on Pseudomonas aeruginosa biofilm formation and antibiotic resistance. J. Antimicrob. Chemother. 49:309-314. [DOI] [PubMed] [Google Scholar]
- 16.Spoering, A. L., and K. Lewis. 2001. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 183:6746-6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka, G., M. Shigeta, H. Komatsuzawa, M. Sugai, H. Suginaka, and T. Usui. 1999. Effect of growth rate of Pseudomonas aeruginosa biofilms on the susceptibility to antimicrobial agents: beta-lactams and fluoroquinolones. Chemotherapy (Basel) 45:28-36. [DOI] [PubMed] [Google Scholar]
- 19.Vander Wauven, C., A. Pierard, M. Kley-Raymann, and D. Haas. 1984. Pseudomonas aeruginosa mutants affected in anaerobic growth on arginine: evidence for a four-gene cluster encoding the arginine deiminase pathway. J. Bacteriol. 160:928-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walters, M. C., F. Roe, A. Bougnicourt, M. J. Franklin, and P. S. Stewart. 2003. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 47:317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whiteley, M., M. G. Bangera, R. E. Bumgarner, M. R. Parsek, G. M. Teitzel, S. Lory, and E. P. Greenberg. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860-864. [DOI] [PubMed] [Google Scholar]
- 22.Worlitzsch, D., R. Tarran, M. Ulrich, U. Schwab, A. Cekici, K. C. Meyer, P. Birrer, G. Bellon, J. Berger, T. Weiss, K. Botzenhart, J. R. Yankaskas, S. Randell, R. C. Boucher, and G. Döring. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 109:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu, K. D., G. A. McFeters, and P. S. Stewart. 2000. Biofilm resistance to antimicrobial agents. Microbiology 146:547-549. [DOI] [PubMed] [Google Scholar]
- 24.Xu, K. D., P. S. Stewart, F. Xia, C.-T. Huang, and G. A. McFeters. 1998. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl. Environ. Microbiol. 64:4035-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon, S. S., R. F. Hennigan, G. M. Hilliard, U. A. Ochsner, K. Parvatiyar, M. C. Kamani, H. L. Allen, T. R. DeKievit, P. R. Gardner, U. Schwab, J. J. Rowe, B. H. Iglewski, T. R. McDermott, R. P. Mason, D. J. Wozniak, R. E. W. Hancock, M. R. Parsek, T. L. Noah, R. C. Boucher, and D. J. Hassett. 2002. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell 3:593-603. [DOI] [PubMed] [Google Scholar]