Abstract
Aryl hydrocarbon receptor (AhR) is a ligand-dependent transcription factor that plays a critical role in metabolism, cell proliferation, development, carcinogenesis, and xenobiotic response. In general, dioxin-like polychlorinated biphenyls (PCBs) exhibit a ligand-dependent activation of AhR-signaling. Results from this study show that a quinone-derivative (1-(4-Chlorophenyl)-benzo-2,5-quinone; 4-ClBQ) of a non-dioxin like PCB (PCB3) also activates AhR-signaling. Treatments of HaCaT human keratinocytes with 4-ClBQ and dioxin-like PCB126 significantly increased AhR-target gene expression, CYP1A1 mRNA and protein levels. 4-ClBQ-induced increase CYP1A1 expression was associated with an increase in the nuclear translocation of AhR protein as well as an increase in the luciferase-reporter activity of a human CYP1A1 xenobiotic response element (XRE). 6,2′,4′-trimethoxyflavone (TMF), a well-characterized AhR-ligand antagonist significantly suppressed PCB126-induced increase in CYP1A1 expression, while the same treatment did not suppress 4-ClBQ-induced increase in CYP1A1 expression. However, siRNA-mediated down-regulation of AhR significantly inhibited 4-ClBQ-induced increase in CYP1A1 expression, suggesting that AhR mediates 4-ClBQ-induced increase in CYP1A1 expression. Interestingly, treatment with the antioxidant N-acetyl-L-cysteine significantly suppressed 4-ClBQ-induced increase in CYP1A1 expression. Furthermore, CYP1A1 expression also increased in cells treated with hydrogen peroxide. These results demonstrate that a ligand-independent and oxidative stress dependent pathway activates AhR-signaling in 4-ClBQ treated HaCaT cells. Because AhR signaling is believed to mediate xenobiotics response, our results may provide a mechanistic rationale for the use of antioxidants as effective countermeasure to environmental pollutant-induced adverse health effects.
Keywords: AhR, CYP1A1, HaCaT cells, Polychlorinated biphenyls, Quinone
1. Introduction
Aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor and a member of the basic helix-loop-helix (bHLH)/Per-ARNT-Sim (PAS) superfamily of transcription factors (Burbach et al., 1992; Poland et al., 1976). Exogenous ligands of AhR include dioxin-like polychlorinated biphenyls (PCBs), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), and halogenated aromatic hydrocarbons (Puga et al., 2009). AhR in the cytosol is inactive due to its binding to the chaperone proteins (HSP90, p23, and XAP2). Following binding to ligand, AhR undergoes a conformational change resulting in its translocation to the nucleus. In the nucleus, AhR dissociates from the chaperone proteins and binds to another bHLH/PAS family protein, aryl hydrocarbon receptor nuclear translocator (ARNT) (Hoffman et al., 1991; Puga et al., 2009). The AhR-ARNT heterodimer then binds to the xenobiotic response element (XRE, core DNA sequence: 5′-GCGTG-3′), which results in the enhancement of gene transcription (Denison et al., 1988; Puga et al., 2009). An increase in the expression of cytochrome P4501A1 (CYP1A1) that has XRE sequence in its promoter enhancer region is routinely used as an indicator of the activation of the AhR-signaling pathway. In addition to the exogenous ligands, endogenous ligands of AhR (e.g., lipoxin A4, bilirubin, and biliverdin) have also been identified (Phelan et al., 1998; Schaldach et al., 1999). Furthermore, tryptophan and its metabolites are also believed to be the endogenous ligands activating AhR-signaling and enhancing the expression of CYP1A1 in UV-irradiated HaCaT cells (Fritsche et al., 2007). Whereas the majority of the studies report ligand-dependent activation of the AhR-signaling pathways, Chang et al., (2007) have shown a ligand-independent activation of the AhR-signaling pathway. These authors have found that the cellular proliferation was faster in AhR-null mouse embryonic fibroblasts (MEFs) expressing AhR-wt and a ligand-binding domain deleted-AhR gene compared to the proliferation of AhR-null MEFs. These previous reports suggest that both ligand-dependent and ligand-independent pathways can activate AhR-signaling.
Polychlorinated biphenyls (PCBs) are a group of persistent organic pollutants that are ubiquitously found in the environment and they are known to have adverse health effects (Lauby-Secretan et al., 2013). PCBs have 209 possible congeners, among which 12 are dioxin-like PCBs and the remaining 197 are non-dioxin like PCBs (Henry and DeVito, 2003). Dioxin-like PCBs have high affinity for the AhR compared to non-dioxin like PCBs that are believed to have no affinity or very low affinity for binding to AhR (Henry and DeVito, 2003). Exposure to dioxin-like PCBs (3, 3′, 4, 4′-tetrachlorobiphenyl, PCB77; 3, 3′, 4, 4′, 5, 5′-hexachlorobiphenyl, PCB169) increases the enzymatic activity of cyp1a1 that was associated with an inhibition in intracellular communication in mouse Hepa1c1c7 cells. Such an inhibition in intracellular communication was absent in PCB77 and PCB169 treated AhR-mutant cells (De Haan et al., 1994). 3, 3′, 4, 4′, 5-pentachlorobiphenyl (PCB126) is the most potent AhR agonist among PCBs (Kafafi et al., 1993). Treatment with PCB126 significantly stimulates the expression of hepatic CYP1A1 in Sprague–Dawley rats, while treatments with the non-dioxin like PCB 2, 2′, 4, 4′, 5, 5′-hexachlorobiphenyl (PCB153) had no effect on CYP1A1 expression (Vezina et al., 2004). These previous reports suggest that dioxin-like PCBs are ligands for AhR resulting in the activation of the expression of the AhR-target gene, CYP1A1.
Results from this study show that an AhR ligand-independent and oxidative stress-dependent pathway activates AhR-signaling in HaCaT cells treated with PCB3-quinone, a metabolite of non-dioxin like PCB, PCB3.
2. Materials and methods
2.1 Chemicals, reagents, and antibodies
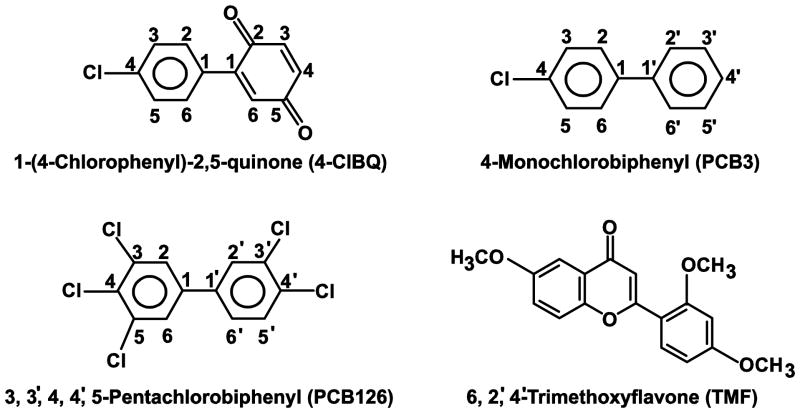
PCBs (4-ClBQ and PCB126, Fig. 1) were provided by the Synthesis Core of the Iowa Superfund Research Project. These compounds were synthesized and purified as described previously (Lehmler and Robertson, 2001). Stealth negative control and human AhR siRNAs were obtained from Invitrogen (Life Technologies, Grand Island, NY). 6,2′,4′-Trimethoxyflavone (TMF) and N-acetyl-L-cysteine (NAC) were purchased from Sigma Chemical Co (St. Louis, MO). Antibodies against human AhR (ab153744) and CYP1A1 (ab3568) were obtained from Abcam (Cambridge, MA); β-actin antibody (sc47778) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA); Lamin A/C antibody (cst4777S; Cell Signaling Technology) was a generous gift from Dr. Jennifer L. Casey, Department of Internal Medicine, University of Iowa.
Fig. 1.
Structure of chemicals used in this study.
2.2 Cell cultures and treatments
Spontaneously immortalized human skin keratinocytes (HaCaT) provided by Dr. Norbert Fusenig (German Cancer Research Center, Heidelberg, Germany) (Boukamp et al., 1988) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum at 37 °C in 5% CO2. Dimethyl sulfoxide (DMSO) was used to prepare stock solutions of PCBs (Venkatesha et al., 2008). Monolayer cultures were treated with 0 - 3.0 μM of PCBs for 24 h in serum-free DMEM. Control cells were treated with the equivalent amount of DMSO (0.1%, v/v) in absence of PCBs.
2.3 cDNA synthesis and quantitative RT-PCR assay
Total RNA of control and PCB treated cells was extracted using TRIzol (Invitrogen, Carlsbad, CA). ND1000 Nanodrop spectrophotometer (Nanodrop, Wilmington, DE) was used to measure the concentration and purity of RNA. One microgram of RNA was reverse transcribed using cDNA Archive Kit (Applied Biosystems, Carlsbad, CA). Eighty nanogram of the cDNA were used to perform real-time PCR amplification using Power SYBR Green PCR Master Mix and StepOnePlus™ System (Applied Biosystems, Carlsbad, CA). The primer-pair sequence of individual genes were: CYP1A1 (NM_000499.3) forward primer: 5′-AGTACCTCAGCCACCTCC-AAG-3′ and reverse primer: 5′-GAGGTCTTGAGGCCCTGATT-3′, amplicon size: 130 bp; AhR (NM_001621.4) forward primer: 5′-AGGGTTTCAGCAGTCTGATGTC-3′ and reverse primer: 5′-ACTACTGTCTGGGGGAGACC-3′, amplicon size: 165 bp; and β-actin (NM_001101.3) forward primer: 5′-TCACCATTGGCAATGAGCGGTT-3′ and reverse primer: 5′-AGTTTCGTGGATGCC-ACAGGACT-3′, amplicon size: 89 bp. Changes in mRNA levels were calculated as follows: ΔΔCt = ΔCt (PCB-treated cells) − ΔCt (control cells); relative expression = 2− ΔΔCt.
2.4 Isolation of nuclear proteins
Nuclear extracts were prepared following a previously published method (He et al., 2013). Control and 24 h of 4-ClBQ treated cells were scraped and pelleted by brief centrifugation, and then incubated in lysis buffer containing 10 mM HEPES, 10 mM KCl, 2 mM MgCl2, and 2 mM EDTA for 15 min on ice followed by the addition of 10% Nonidet P-40. The cell lysates were then centrifuged at 14,000 rpm, 4 °C for 30 sec, and the pellets were re-suspended in 50 mM HEPES, 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, and 10% glycerol for 20 min on ice. Supernatant was collected by centrifugation and protein concentration was determined using the Bradford method.
2.5 Immunoblotting
Equal amounts of total protein lysates or nuclear extracts were separated on 12% SDS-PAGE and electro-transferred to nitrocellulose membrane. Immunoblotting was performed using antibodies to human AhR (1:800; rabbit polyclonal) and CYP1A1 (1:500; rabbit polyclonal). The blots were re-probed with Lamin A/C or β-actin antibody for loading comparison. Horseradish peroxidase conjugated secondary antibodies, Pierce enhanced chemiluminescence Plus reagent (Thermo Scientific, Rockford, IL), and Typhoon FLA 7000 (GE Healthcare, Waukesha, WI) were used for visualization and quantitation of immune-reactive polypeptides.
2.6 Luciferase reporter assay
XRE-luciferase vector was a kind gift from Dr. Linh Chi Bui (French National Institute of Health and Medical Research, UMR-S 747, Paris, France). Human CYP1A1 promoter region (− 1566 ~ +73) was directionally cloned upstream of the Firefly luciferase reporter that was cloned into a pGL3-vector (Morel and Barouki, 1998). Lipofectamine 3000 (Life Technologies, Grand Island, NY) was used to transfect HaCaT cells with plasmid DNA containing XRE-sequence fused to Firefly luciferase cDNA and plasmid DNA containing Renilla luciferase cDNA. A Dual-luciferase Reporter Assay System kit (Promega, Madison, WI) and a Tecan SpectraFluor Plus luminometer was used to measure luciferase activity in control and PCB treated transfected cells. Firefly luciferase activity was normalized to Renilla luciferase activity in individual samples, and fold change was calculated relative to control cells that were not treated with PCBs.
2.7 siRNA knockdown of human AhR
Human AhR-siRNA (Invitrogen) was used to down-regulate AhR mRNA; sense: 5′-CCUUUAAUGGAGAGGUGCUUCAUAU-3′; antisense: 5′-AUAUGAAGCACCUCUCCAUUAAA-GG-3′. Fifty nanogram of negative control siRNA or AhR siRNA were transfected into 70–80% confluent HaCaT cells using Lipofactamine 2000 (Life Technologies, Grand Island, NY). Forty-eight hours post-transfection, control and AhR siRNAs transfected cells were treated with 4-ClBQ for 24 h. AhR and CYP1A1 mRNA expression was measured by q-RT-PCR at the end of the 4-ClBQ treatment.
2.8 Statistical analysis
One-way analysis of variance (ANOVA) followed by Tukey post-test (SPSS 21.0 software) was performed to evaluate statistical significance of results. Results are presented as mean ± standard deviation. Results from at least n = 3 with p < 0.05 were considered significant.
3. Results
3.1 4-ClBQ treatment significantly enhances CYP1A1 expression in HaCaT cells
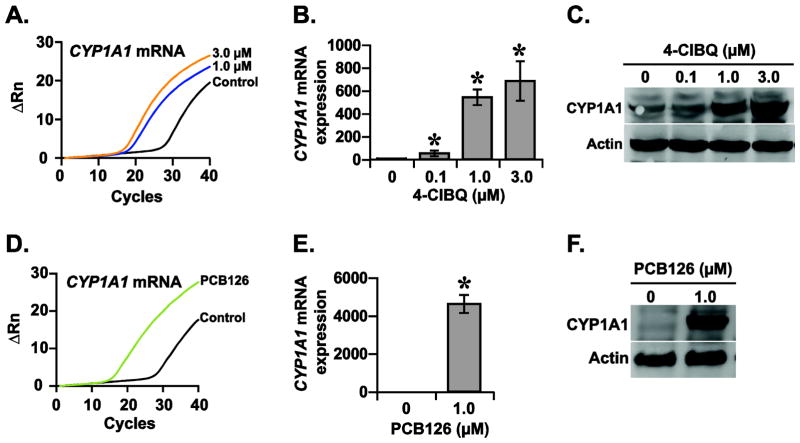
CYP1A1 is a phase I metabolizing enzyme that is induced by xenobiotics including dioxin-like PCBs (Nebert and Dalton, 2006). To determine whether non-dioxin like PCBs, PCB3 and its quinone metabolite 4-ClBQ can also activate CYP1A1 expression, HaCaT cells were treated with 0 – 3.0 μM of 4-ClBQ or PCB3 and CYP1A1 expression was measured using q-RT-PCR and immunoblotting assays. Results from a q-RT-PCR assay showed a dose-dependent increase in CYP1A1 mRNA expression in 4-ClBQ treated cells: approximately 50-fold increase in 0.1 μM 4-ClBQ treated cells and more than 500-fold increase in 1.0 and 3.0 μM 4-ClBQ treated cells (Fig. 2A and 2B). In comparison to 4-ClBQ treatment, PCB3 treatment did not show any significant difference in CYP1A1 mRNA expression (ΔCt = 14 cycles in control and 15 cycles in PCB3 treated cells; Supplemental Fig. 1). These results suggest that while the parental compound PCB3 does not enhance CYP1A1 mRNA expression, its quinone-metabolite significantly increased CYP1A1 mRNA expression. Results from a time-course experiment showed that 4-ClBQ treatment enhanced CYP1A1 mRNA expression as early as 4 h of treatment and more than 1000-fold increase in CYP1A1 mRNA expression was observed at 24 h of treatment (Supplemental Fig. 2). The increase in CYP1A1 mRNA expression in 4-ClBQ treated cells was also consistent with a significant increase in its protein levels (Fig. 2C).
Fig. 2.
4-ClBQ and PCB126 treatments enhance CYP1A1 expression in HaCaT cells. Quantitative RT-PCR and immunoblotting assays were performed to analyze the mRNA and protein levels of CYP1A1 in (A–C) 4-ClBQ and (D–F) PCB126 treated HaCaT cells. Representative PCR amplification curves are shown in panels A and D. β-actin was included for loading correction in individual samples. Asterisks represent statistical significance compared to cells that were not treated with PCB; p < 0.05, n = 3.
To further verify these intriguing results of a quinone-metabolite of a non-dioxin-like PCB activating CYP1A1 mRNA expression, measurements were repeated in HaCaT cells that were treated with a dioxin-like PCB (PCB126). Consistent with a previous report (Vorrink et al., 2014), treatment with PCB126 significantly increased CYP1A1 mRNA expression (Fig. 2D and 2E), which correlated with an increase in its protein levels (Fig. 2F). These results demonstrate that 4-ClBQ, a quinone metabolite of a non-dioxin-like PCB (PCB3) and PCB126 a dioxin-like PCB induces CYP1A1 expression (mRNA and protein) in HaCaT cells.
3.2 4-ClBQ treatment activates AhR-signaling in HaCaT cells
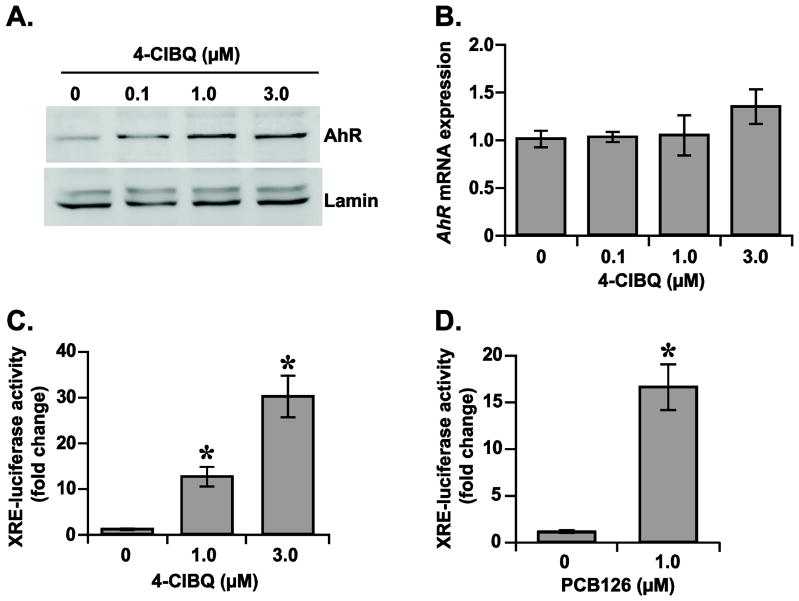
Ligand-activated AhR-signaling enhances the transcription of AhR-target genes that include CYP1A1 expression (Whitlock, 1999). A ligand induced conformational change is believed to facilitate the translocation of AhR to the nucleus followed by the transcriptional activation of the AhR-target genes (Puga et al., 2009; Whitlock, 1999). To determine whether AhR-signaling activates CYP1A1 expression in 4-ClBQ treated cells, initially AhR protein levels were measured in the nuclear extracts isolated from control and 4-ClBQ treated HaCaT cells. Results showed a dose-dependent increase in the protein levels of nuclear AhR in 4-ClBQ treated cells (Fig. 3A). This increase in AhR protein levels is not due to an increase in AhR mRNA levels (Fig. 3B). These results demonstrate that AhR is translocated to the nucleus in 4-ClBQ treated cells, and the mRNA levels of AhR did not change in 4-ClBQ treated cells.
Fig. 3.
4-ClBQ and PCB126 treatments activate AhR-signaling in HaCaT cells. (A) 4-ClBQ treatment increases AhR nuclear accumulation. An immunoblotting assay was used to analyze the protein levels of AhR in nuclear extracts isolated from control and 4-ClBQ treated cells. Protein levels of lamin in the nuclear extracts were used for comparison of results. (B) 4-ClBQ treatment did not alter mRNA levels of AhR. A quantitative RT-PCR assay was used to measure mRNA levels of AhR in control and 4-ClBQ treated cells. (C) 4-ClBQ and (D) PCB126 treatments increase XRE-luciferase reporter activity in HaCaT cells. Cells were co-transfected with plasmid DNAs containing Renilla luciferase reporter gene and human CYP1A1-XRE sequence that was cloned upstream of a Firefly luciferase gene. Luciferase activity was measured in control and PCB-treated cells. Firefly luciferase activity was normalized to Renilla luciferase activity in each sample and fold-change was calculated relative to transfected cells that were not treated with PCBs. Asterisks represent statistical significance compared to transfected cells that were not treated with PCBs; p < 0.05, n = 3.
The hypothesis of 4-ClBQ-induced activation of AhR-signaling and transcription of AhR-target genes was further investigated by performing a XRE-containing luciferase reporter activity assay (Fig. 3C). HaCaT cells were co-transfected with plasmid DNAs containing Renilla luciferase reporter gene and human CYP1A1-XRE sequence that was cloned upstream of a Firefly luciferase gene. Transfected cells were then sub-cultured and treated with 4-ClBQ for 24 h followed by the measurements of luciferase activity. Firefly luciferase activity was first normalized to Renilla luciferase activity in each sample and fold-change was calculated relative to transfected cells that were not treated with 4-ClBQ. A dose-dependent increase in Firefly luciferase activity was observed in 4-ClBQ treated cells: approximately 12-fold increase in 1.0 μM and 30-fold increase in 3.0 μM 4-ClBQ treated cells (Fig. 3C). The activation of the AhR-signaling pathway is also evident from results shown in Figure 3D. The dual-luciferase reporter assay was repeated in cells treated with a dioxin-like PCB (PCB126), a well-known AhR agonist. Results showed approximately 16-fold increase in Firefly luciferase activity in PCB126 treated cells (Fig. 3D). These results (Figs. 2 and 3) demonstrate that both 4-ClBQ (non-dioxin-like) and PCB126 (dioxin-like) treatments activate the AhR-signaling pathway in HaCaT cells resulting in the transcriptional activation of AhR-target gene, CYP1A1.
3.3 4-ClBQ treatment activates an AhR-dependent but ligand-independent signaling pathway in HaCaT cells
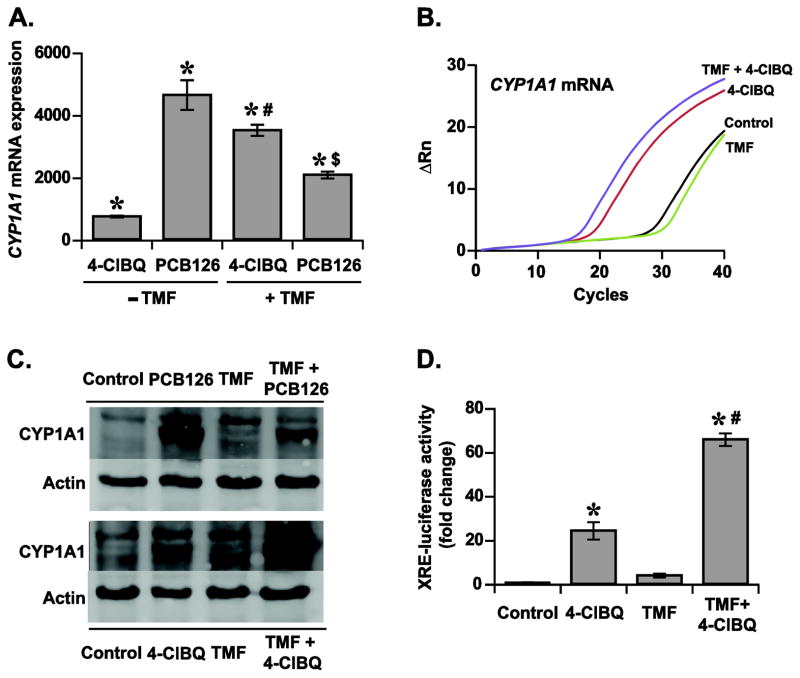
As opposed to dioxin-like PCBs (e.g. PCB126) that are classical AhR-ligands, 4-ClBQ is not considered as a ligand for AhR, yet it activates AhR-signaling and CYP1A1 expression in HaCaT cells. To investigate whether a ligand-independent activation of AhR-signaling could account for the increase of CYP1A1 expression in 4-ClBQ treated cells, experiments were repeated using a well-characterized AhR-ligand antagonist, 6,2′,4′-trimethoxyflavone (TMF) (Murray et al., 2010). TMF competes with exogenous ligands for AhR bioavailability resulting in a decrease in the binding affinity of AhR with its XRE-response element thereby antagonizing AhR-mediated gene transcription (Murray et al., 2010). Control and 5.0 μM TMF treated cells were incubated with 1.0 μM of 4-ClBQ or PCB126 for 24 h and CYP1A1 mRNA levels were measured using a quantitative RT-PCR assay. As shown before (Fig. 2), both 4-ClBQ and PCB126 treatments increased CYP1A1 mRNA levels (Fig. 4A and B). It is interesting to note that while the TMF treatment suppressed PCB126-induced increase in CYP1A1 mRNA levels, the same treatment did not inhibit the increase in CYP1A1 mRNA levels in 4-ClBQ treated cells (Fig. 4A and B). Instead, the TMF treatment further increased CYP1A1 mRNA levels in 4-ClBQ treated cells. Comparable results were also obtained for CYP1A1 protein levels (Fig. 4C). TMF treatment suppressed PCB126-induced increase in CYP1A1 protein levels, while the same treatment did not suppress 4-ClBQ-induced increase in CYP1A1 protein levels (Fig. 4C). AhR-ligand independent activation of CYP1A1 expression in 4-ClBQ treated cells is also evident from the results obtained from the luciferase-reporter assay (Fig. 4D). Firefly and Renilla luciferase activity was measured in control and TMF treated cells that were incubated with 1.0 μM of 4-ClBQ or PCB126. As anticipated, TMF treatment significantly mitigated PCB126 induced increase in Firefly luciferase activity (Supplemental Fig. 3), which is consistent with the CYP1A1 expression results (Fig. 4A and C). Interestingly, as shown before (Fig. 3C), 4-ClBQ treatment increased Firefly luciferase activity approximately 20-fold (Fig. 4D). However, instead of inhibiting luciferase activity pre-treatment with TMF further enhanced Firefly luciferase activity in 4-ClBQ treated cells (Fig. 4D). These results showed that both 4-ClBQ and PCB126 treatments activate AhR-signaling and CYP1A1 expression in HaCaT cells. Whereas the PCB126 induced activation of the AhR-signaling pathway and CYP1A1 expression is AhR-ligand dependent, 4-ClBQ-induced AhR-signaling and CYP1A1 expression appears to be AhR-ligand independent.
Fig. 4.
4-ClBQ treatment induces a ligand-independent activation of AhR-signaling. (A) TMF treatment inhibits PCB126-induced increase in CYP1A1 mRNA expression, while the same treatment did not suppress 4-ClBQ-induced increase in CYP1A1 mRNA expression. Control and 5.0 μM TMF treated cells were incubated with 1.0 μM of 4-ClBQ or PCB126. A quantitative RT-PCR assay was used to measure CYP1A1 mRNA expression. Asterisks represent statistical significance compared to control cells that are not treated with PCBs and TMF; # indicates statistical significance compared to cells treated with 4-ClBQ in absence of TMF; $ indicates statistical significance compared to cells treated with PCB126 in absence of TMF; p < 0.05, n = 3. (B) Representative PCR amplification curves showing CYP1A1 mRNA expression in control and TMF-treated cells that were incubated with and without 4-ClBQ. (C) TMF treatment inhibits PCB-126-induced increase in CYP1A1 protein levels, while the same treatment did not suppress the increase in CYP1A1 protein levels in 4-ClBQ treated cells. Experiments described in panel A were repeated to measure CYP1A1 protein levels; β-actin protein levels were used for comparison. (D) TMF treatment did not suppress 4-ClBQ-induced increase in the reporter activity of XRE-luciferase. Control and TMF treated reporter-plasmid DNA transfected cells were incubated with and without 1.0 μM of 4-ClBQ for 24 h. Luciferase activity was measured as described in Figure 3. Asterisks represent statistical significance compared to cells that were not treated with 4-ClBQ; # indicates statistical significance compared to cells that were treated with 4-ClBQ in absence of TMF; p < 0.05, n = 3.
It is possible that the 4-ClBQ-induced increase in the expression of CYP1A1 could be mediated by an AhR-independent pathway. To investigate this hypothesis, HaCaT cells were transfected with control and AhR siRNAs followed by treatment with 4-ClBQ and measurements of CYP1A1 mRNA expression. Results from a q-RT-PCR assay showed that the treatment with AhR siRNA decreased AhR mRNA levels by approximately 50% compared to AhR mRNA levels in cells transfected with control siRNA (Fig. 5A). Interestingly, 4-ClBQ-induced increase in CYP1A1 mRNA expression was significantly suppressed in cells transfected with AhR siRNA (Fig. 5B). CYP1A1 mRNA expression increased approximately 70-fold in 4-ClBQ treated cells transfected with AhR siRNA compared to approximately 150-fold increase in 4-ClBQ treated cells transfected with control siRNA (Fig. 5B). These results indicate that the 4-ClBQ treatment activates an AhR-dependent but ligand-independent pathway in HaCaT cells.
Fig. 5.
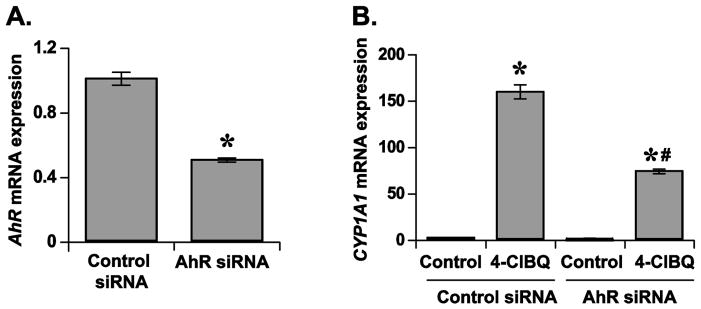
4-ClBQ-induced CYP1A1 mRNA expression is AhR dependent in HaCaT cells. (A) Knockdown of AhR mRNA expression using AhR-siRNA. HaCaT cells were transfected with 50 nM of control or AhR siRNA. After 48 h of transfection, cells were harvested for measurements of AhR mRNA levels by using a quantitative RT-PCR assay. Asterisks represent statistical significance compared to cells that were transfected with control siRNA; p < 0.05, n = 3. (B) AhR knockdown significantly inhibits 4-ClBQ-induced increases in CYP1A1 mRNA expression. Control and AhR siRNA transfected cells were treated with 4-ClBQ for 24 h and CYP1A1 mRNA levels were measured using a quantitative RT-PCR assay. Asterisks represent statistical significance compared to cells that were transfected with control siRNA and not treated with 4-ClBQ; # indicates statistical significance compared to cells that were transfected with control siRNA and treated with 4-ClBQ; p < 0.05, n = 3.
3.4 Oxidative stress mediates AhR-signaling in 4-ClBQ treated HaCaT cells
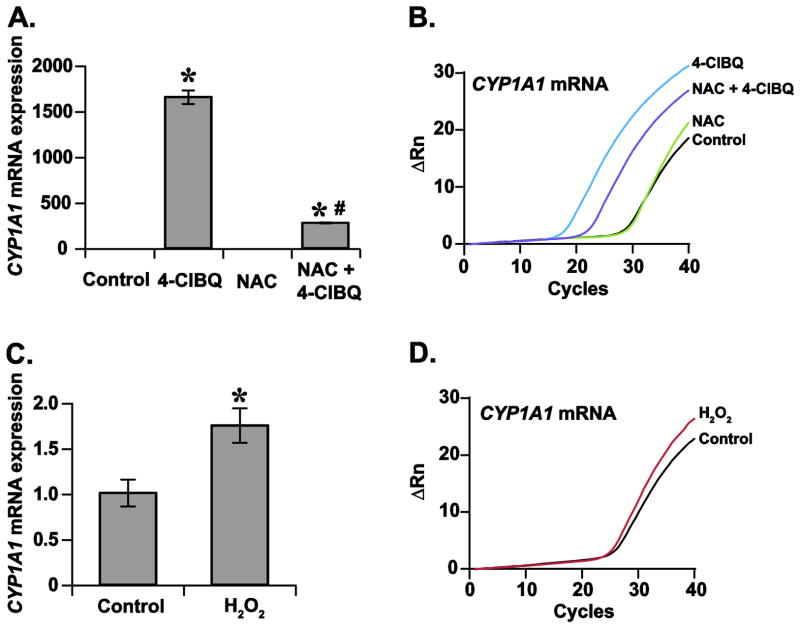
We have shown previously that 4-ClBQ treatment induces oxidative stress in HaCaT cells (Xiao et al., 2014; Xiao et al., 2013). To determine whether oxidative stress regulates the AhR-signaling dependent activation of CYP1A1 mRNA expression, cells were treated with 5.0 mM of antioxidant NAC followed by treatment with 1.0 μM 4-ClBQ. A q-RT-PCR assay was used to measure CYP1A1 mRNA expression. Results showed that the treatment with NAC significantly suppressed 4-ClBQ-induced increase in CYP1A1 mRNA expression (Fig. 6A and B). It is interesting to note that cells treated with hydrogen peroxide also increased CYP1A1 mRNA expression (Fig. 6C and D). These results suggest that oxidative stress mediates the ligand-independent activation of AhR-signaling and CYP1A1 expression in 4-ClBQ treated HaCaT cells.
Fig. 6.
Oxidative stress increases CYP1A1 mRNA expression in HaCaT cells. (A) Treatment with NAC inhibits 4-ClBQ-induced increase in CYP1A1 mRNA expression. Control and 5.0 mM of NAC treated cells were incubated with 3.0 μM of 4-ClBQ for 24 h and CYP1A1 mRNA levels were measured using a quantitative RT-PCR assay. Asterisks represent statistical significance compared to cells that were not treated with 4-ClBQ; # indicates statistical significance compared to cells that were treated with 4-ClBQ in absence of NAC; p < 0.05, n = 3. (B) Representative PCR amplification curves showing CYP1A1 mRNA expression. (C) Treatment with hydrogen peroxide increases CYP1A1 mRNA expression in HaCaT cells. A quantitative RT-PCR assay was used to measure CYP1A1 mRNA expression in control and cells treated with 200 μM of hydrogen peroxide for 6 h. Asterisks represent statistical significance compared to cells that were not treated with hydrogen peroxide; p < 0.05, n = 3. (D) Representative PCR amplification curves showing CYP1A1 mRNA expression in control and cells treated with hydrogen peroxide.
4. Discussion
AhR is a ligand-dependent transcription factor that has been shown to regulate numerous biological processes including cellular response to xenobiotics (Bock and Kohle, 2006). The expression of CYP1A1 is routinely used as an indicator of the activation of the AhR-signaling pathways. Dioxin-like PCBs (e.g. PCB126) are well-known AhR ligands and potent inducer of CYP1A1 expression (Giesy and Kannan, 1998). Results from this study show that a ligand-independent and oxidative stress dependent pathway activates AhR-signaling in non-dioxin-like PCB3-quinone treated HaCaT cells.
PCB3-quinone, 4-ClBQ treatment showed a dose-dependent activation of CYP1A1 mRNA and protein expression in HaCaT cells (Fig. 2 A–C). 4-ClBQ induced activation of CYP1A1 expression was observed as early as 4 h of treatment and a maximal increase of more than 1000-fold was observed at 24 h of treatment (Supplemental Fig. 2). To further examine these intriguing results, HaCaT cells were treated with a dioxin-like PCB (PCB126) and CYP1A1 mRNA expression was measured using a q-RT-PCR assay. As anticipated, PCB126 treatment significantly increased CYP1A1 mRNA and protein expression (Fig. 2 D–F). These results demonstrate that the AhR-signaling pathway is functional in HaCaT cells and both ligand-dependent and ligand-independent activation of AhR-signaling occurs in PCB-treated HaCaT cells. It is interesting to note that while 4-ClBQ treatment activates CYP1A1 mRNA expression, PCB3 (parental compound of 4-ClBQ) treatment did not affect CYP1A1 mRNA expression in HaCaT cells (Supplemental Fig. 1). Our results suggest that the AhR-signaling is functional in HaCaT cells and it gets activated in response to treatments with both dioxin- and non-dioxin-like PCBs.
Literature reports suggest that AhR is a ligand-activated transcription factor that translocates to the nucleus and initiates transcription of AhR-target genes (Puga et al., 2009; Whitlock, 1999). Nuclear translocation of AhR is a prerequisite for the transcriptional activation of AhR-target genes. Dioxin-like PCBs are high-affinity ligands for AhR (Giesy and Kannan, 1998). AhR undergoes a conformational change following binding to its ligand, which facilitates its translocation to the nucleus (Puga et al., 2009; Whitlock, 1999). 4-ClBQ, a quinone metabolite of a non-dioxin-like PCB (PCB3) (McLean et al., 1996) is believed to be not a ligand for AhR, yet treatment with 4-ClBQ significantly increased the expression of AhR-target gene (CYP1A1; Fig. 2 A–C), suggesting that the AhR-signaling pathway is activated in 4-ClBQ treated HaCaT cells. Indeed, nuclear levels of AhR increased in 4-ClBQ treated HaCaT cells (Fig. 3A), whereas the mRNA levels of AhR were not affected by the treatment (Fig. 3B). These results are also consistent with previous reports of TCDD treatment and glucose deprivation enhancing CYP1A1 expression without increasing AhR expression (Terashima et al., 2011; Wanner et al., 1995). Our results suggest that 4-ClBQ treatment may also induce a conformational change in AhR facilitating its nuclear translocation and activation of AhR-target gene expression.
Nuclear localization of AhR is anticipated to enhance transcription of AhR-target genes. In the nucleus, AhR-ARNT heterodimer binds to the XRE sequence (5′-GCGTG-3′) that is present in the promoter region of AhR-target genes (e.g., CYP1A1 that has six XRE in its promoter region, −3000 ~ +100 bp) enhancing their transcription (Denison et al., 1988; Puga et al., 2009). Results from XRE-luciferase activity assay showed that the nuclear accumulation of AhR is associated with a significant increase in Firefly-luciferase activity in 4-ClBQ treated HaCaT cells (Fig. 3C). Measurements of Firefly-luciferase activity in PCB126 treated cells were included as a positive control for the activation of AhR-signaling (Fig. 3D). These results clearly show that treatments with dioxin-like (PCB126) and non-dioxin-like (4-ClBQ) PCBs significantly increased XRE-luciferase activity demonstrating that both types of PCBs activate AhR-signaling in HaCaT cells.
In general, AhR-signaling is activated upon ligand binding (Puga et al., 2009; Whitlock, 1999). To determine whether a ligand-dependent activation of AhR-signaling also occurs in 4-ClBQ treated HaCaT cells, experiments were repeated using TMF, a well-known AhR-ligand antagonist (Murray et al., 2010). As anticipated, treatment with TMF significantly suppressed PCB126-induced increase in CYP1A1 mRNA and protein levels as well as XRE-luciferase activity (Fig. 4 and Supplemental Fig. 3). Surprisingly, pre-treatment with TMF further enhanced 4-ClBQ-induced increase in CYP1A1 expression (Fig. 4). Likewise, pre-treatment with TMF also enhanced XRE-luciferase activity in 4-ClBQ treated cells (Fig. 4D). Although the underlying mechanisms of how TMF treatment enhances CYP1A1 expression and increases XRE-luciferase activity are unknown, it is possible that TMF treatment may enhance 4-ClBQ induced oxidative stress resulting in further enhancement of AhR-signaling and its target gene expression, CYP1A1 (see discussion below). Overall, these results suggest that unlike the canonical AhR-ligand PCB126, a ligand-independent pathway activates AhR-signaling and CYP1A1 expression in 4-ClBQ treated HaCaT cells.
It is possible that an AhR-independent pathway activates CYP1A1 expression in 4-ClBQ treated cells. To investigate this premise, AhR was knocked down by transfecting HaCaT cells with AhR-siRNA (Fig. 5A) and cells were treated with 4-ClBQ. Results show that siRNA-mediated inhibition of AhR expression significantly suppressed 4-ClBQ-induced increase in CYP1A1 mRNA expression (Fig. 5B). These results indicate that the induction in CYP1A1 expression in 4-ClBQ treated HaCaT cells is dependent on AhR. Our observation of PCB3-quinone (4-ClBQ) induced AhR-signaling and activation of CYP1A1 expression is also supported by results from a previous study where the authors showed nuclear translocation of AhR associating with a significant increase in XRE-luciferase activity and CYP1A1 mRNA expression in HepG2 cells treated benzo(a)pyrene-7,8-dione, a quinone metabolite of benzo(a)pyrene (Burczynski and Penning, 2000). Additionally, Chang et al., (2007) have also reported a ligand-independent activation of AhR-signaling. The authors showed that cellular proliferation is enhanced in AhR-null MEFs expressing AhR-wt and a ligand-binding domain deleted-AhR gene compared to the proliferation of AhR-null MEFs. Overall, results from Figures 4 and 5 suggest that 4-ClBQ, a quinone metabolite of a non-dioxin-like PCB (PCB3) activates AhR-signaling via an AhR-dependent but ligand-independent pathway.
Whereas the mechanisms regulating the ligand-independent activation of AhR-signaling are not completely understood, we hypothesize that redox-modification of AhR activates its signaling pathway resulting in its nuclear translocation and transcriptional activation of its target gene expression. Indeed, cellular reactive oxygen species (ROS: superoxide and hydrogen peroxide) levels increased 2- and 10-fold in 1.0 and 3.0 μM 4-ClBQ treated HaCaT cells, suggesting that 4-ClBQ treatment induces oxidative stress in HaCaT cells (Xiao et al., 2014; Xiao et al., 2013). 4-ClBQ induced increase in ROS (hydrogen peroxide) levels may initiate thiol-disulfide exchange reaction in specific cysteines in AhR resulting in a redox-mediated conformational change in AhR that facilitates its nuclear translocation and subsequently transcriptional activation of its target genes. As such, pre-treatment with antioxidants is anticipated to suppress ROS-mediated activation of AhR-signaling in 4-ClBQ treated cells. Pre-treatment with antioxidants (NAC; superoxide dismutase and catalase) has been shown to suppress 4-ClBQ induced increase in ROS levels and toxicity in HaCaT, and human mammary and prostate epithelial cells (Venkatesha et al., 2008; Xiao et al., 2013; Zhu et al., 2009). Pre-treatment with NAC significantly suppressed 4-ClBQ-induced increase in CYP1A1 mRNA expression (Fig. 6A and 6B). Furthermore, cells treated with hydrogen peroxide showed a significant increase in CYP1A1 mRNA expression (Fig. 6C and 6D). Taken together, these results suggest that 4-ClBQ-induced oxidative stress can redox-modify AhR (e.g., cysteine oxidation-reduction reactions) facilitating its translocation to the nucleus and transcriptional-activation of its target genes, e.g. CYP1A1.
In summary, results from this study suggest that 4-ClBQ, a quinone-metabolite of a non-dioxin-like PCB (PCB3) activates an AhR-dependent but ligand-independent signaling pathway presumably via oxidative stress mediated redox-modification of AhR facilitating its nuclear translocation and activation of its target gene expression, CYP1A1. Because AhR signaling is believed to mediate xenobiotics response, our results may provide a mechanistic rationale for the use of antioxidants as an effective countermeasure to environmental pollutant-induced adverse health effects.
Supplementary Material
Highlights.
4-ClBQ, a metabolite of non-dioxin like PCB3 activates AhR signaling in HaCaT cells
4-ClBQ-induced AhR activation is ligand-independent in HaCaT cells
4-ClBQ-induced AhR activation is likely mediated by oxidative stress in HaCaT cells
Acknowledgments
We thank Professors Larry W. Robertson and Hans J. Lehmler at the Occupational & Environmental Health University of Iowa for providing us with PCB compounds. We also thank Dr. Jennifer L. Casey for assistance with the Lamin A/C antiobody and Dr. Kelley R. Salem for technical support with the luminometer. This work was supported by National Institute of Environmental Health and Sciences [P42ES013661] and National Institute of Health [2R01CA111365] to P.C.G.
Abbreviations
- 4-ClBQ
1-(4-Chlorophenyl)-benzo-2,5-quinone
- AhR
aryl hydrocarbon receptor
- ARNT
aryl hydrocarbon receptor nuclear translocator
- CYP1A1
cytochrome P4501A1
- NAC
N-acetyl-L-cysteine
- PCB3
4-monochlorobiphenyl
- PCB77
3, 3′, 4, 4′-tetrachlorobiphenyl
- PCB126
3, 3′, 4, 4′, 5-pentachlorobiphenyl
- PCB169
3, 3′, 4, 4′, 5, 5′-hexachlorobiphenyl
- PCB153
2, 2′, 4, 4′, 5, 5′-hexachlorobiphenyl
- PCBs
polychlorinated biphenyls
- ROS
reactive oxygen species
- XRE
xenobiotic response element
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TMF
6,2′,4′-trimethoxyflavone
Footnotes
Authors disclosure statement
The authors declare they have no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bock KW, Kohle C. Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions. Biochem Pharmacol. 2006;72:393–404. doi: 10.1016/j.bcp.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burczynski ME, Penning TM. Genotoxic polycyclic aromatic hydrocarbon ortho-quinones generated by aldo-keto reductases induce CYP1A1 via nuclear translocation of the aryl hydrocarbon receptor. Cancer Res. 2000;60:908–915. [PubMed] [Google Scholar]
- Chang X, Fan Y, Karyala S, Schwemberger S, Tomlinson CR, Sartor MA, Puga A. Ligand-independent regulation of transforming growth factor beta1 expression and cell cycle progression by the aryl hydrocarbon receptor. Mol Cell Biol. 2007;27:6127–6139. doi: 10.1128/MCB.00323-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan LH, Simons JW, Bos AT, Aarts JM, Denison MS, Brouwer A. Inhibition of intercellular communication by 2,3,7,8-tetrachlorodibenzo-p-dioxin and dioxin-like PCBs in mouse hepatoma cells (Hepa1c1c7): involvement of the Ah receptor. Toxicol Appl Pharmacol. 1994;129:283–293. doi: 10.1006/taap.1994.1253. [DOI] [PubMed] [Google Scholar]
- Denison MS, Fisher JM, Whitlock JP., Jr The DNA recognition site for the dioxin-Ah receptor complex. Nucleotide sequence and functional analysis. J Biol Chem. 1988;263:17221–17224. [PubMed] [Google Scholar]
- Fritsche E, Schafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, Hubenthal U, Cline JE, Hajimiragha H, Schroeder P, et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci U S A. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesy JP, Kannan K. Dioxin-like and non-dioxin-like toxic effects of polychlorinated biphenyls (PCBs): implications for risk assessment. Crit Rev Toxicol. 1998;28:511–569. doi: 10.1080/10408449891344263. [DOI] [PubMed] [Google Scholar]
- He C, Ryan AJ, Murthy S, Carter AB. Accelerated development of pulmonary fibrosis via Cu, Zn-superoxide dismutase-induced alternative activation of macrophages. J Biol Chem. 2013;288:20745–20757. doi: 10.1074/jbc.M112.410720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry TR, DeVito MJ. Non-dioxin-like PCBs: effects and consideration in ecological risk assessment. US Environmental Protection Agency, Experimental Toxicology Division; Cincinnati OH: 2003. [Google Scholar]
- Hoffman EC, Reyes H, Chu FF, Sander F, Conley LH, Brooks BA, Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991;252:954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- Kafafi SA, Afeefy HY, Ali AH, Said HK, Abd-Elazem IS, Kafafi AG. Affinities for the aryl hydrocarbon receptor, potencies as aryl hydrocarbon hydroxylase inducers and relative toxicities of polychlorinated biphenyls. A congener specific approach. Carcinogenesis. 1993;14:2063–2071. doi: 10.1093/carcin/14.10.2063. [DOI] [PubMed] [Google Scholar]
- Lauby-Secretan B, Loomis D, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. The lancet oncology. 2013;14:287–288. doi: 10.1016/S1470-2045(13)70104-9. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ, Robertson LW. Synthesis of polychlorinated biphenyls (PCBs) using the Suzuki-coupling. Chemosphere. 2001;45:137–143. doi: 10.1016/s0045-6535(00)00546-4. [DOI] [PubMed] [Google Scholar]
- McLean MR, Bauer U, Amaro AR, Robertson LW. Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem Res Toxicol. 1996;9:158–164. doi: 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- Morel Y, Barouki R. Down-regulation of cytochrome P450 1A1 gene promoter by oxidative stress. Critical contribution of nuclear factor 1. J Biol Chem. 1998;273:26969–26976. doi: 10.1074/jbc.273.41.26969. [DOI] [PubMed] [Google Scholar]
- Murray IA, Flaveny CA, DiNatale BC, Chairo CR, Schroeder JC, Kusnadi A, Perdew GH. Antagonism of aryl hydrocarbon receptor signaling by 6,2′,4′-trimethoxyflavone. The Journal of pharmacology and experimental therapeutics. 2010;332:135–144. doi: 10.1124/jpet.109.158261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- Phelan D, Winter GM, Rogers WJ, Lam JC, Denison MS. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch Biochem Biophys. 1998;357:155–163. doi: 10.1006/abbi.1998.0814. [DOI] [PubMed] [Google Scholar]
- Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976;251:4936–4946. [PubMed] [Google Scholar]
- Puga A, Ma C, Marlowe JL. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol. 2009;77:713–722. doi: 10.1016/j.bcp.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaldach CM, Riby J, Bjeldanes LF. Lipoxin A4: a new class of ligand for the Ah receptor. Biochemistry. 1999;38:7594–7600. doi: 10.1021/bi982861e. [DOI] [PubMed] [Google Scholar]
- Terashima J, Habano W, Gamou T, Ozawa S. Induction of CYP1 family members under low-glucose conditions requires AhR expression and occurs through the nuclear translocation of AhR. Drug metabolism and pharmacokinetics. 2011;26:577–583. doi: 10.2133/dmpk.DMPK-11-RG-054. [DOI] [PubMed] [Google Scholar]
- Venkatesha VA, Venkataraman S, Sarsour EH, Kalen AL, Buettner GR, Robertson LW, Lehmler HJ, Goswami PC. Catalase ameliorates polychlorinated biphenyl-induced cytotoxicity in nonmalignant human breast epithelial cells. Free Radic Biol Med. 2008;45:1094–1102. doi: 10.1016/j.freeradbiomed.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina CM, Walker NJ, Olson JR. Subchronic exposure to TCDD, PeCDF, PCB126, and PCB153: Effect on hepatic gene expression. Environmental Health Perspectives. 2004;112:1636–1644. doi: 10.1289/txg.7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorrink SU, Severson PL, Kulak MV, Futscher BW, Domann FE. Hypoxia perturbs aryl hydrocarbon receptor signaling and CYP1A1 expression induced by PCB 126 in human skin and liver-derived cell lines. Toxicol Appl Pharmacol. 2014;274:408–416. doi: 10.1016/j.taap.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner R, Brommer S, Czarnetzki BM, Rosenbach T. The differentiation-related upregulation of aryl hydrocarbon receptor transcript levels is suppressed by retinoic acid. Biochem Biophys Res Commun. 1995;209:706–711. doi: 10.1006/bbrc.1995.1556. [DOI] [PubMed] [Google Scholar]
- Whitlock JP., Jr Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- Xiao W, Sarsour EH, Wagner BA, Doskey CM, Buettner GR, Domann FE, Goswami PC. Succinate dehydrogenase activity regulates PCB3-quinone-induced metabolic oxidative stress and toxicity in HaCaT human keratinocytes. Arch Toxicol. 2014 doi: 10.1007/s00204-014-1407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Zhu Y, Sarsour EH, Kalen AL, Aykin-Burns N, Spitz DR, Goswami PC. Selenoprotein P regulates 1-(4-Chlorophenyl)-benzo-2,5-quinone-induced oxidative stress and toxicity in human keratinocytes. Free Radic Biol Med. 2013;65C:70–77. doi: 10.1016/j.freeradbiomed.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Kalen AL, Li L, Lehmler HJ, Robertson LW, Goswami PC, Spitz DR, Aykin-Burns N. Polychlorinated-biphenyl-induced oxidative stress and cytotoxicity can be mitigated by antioxidants after exposure. Free Radic Biol Med. 2009;47:1762–1771. doi: 10.1016/j.freeradbiomed.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.