Abstract
Platelets contribute to antimicrobial host defense against infective endocarditis (IE) by releasing platelet microbicidal proteins (PMPs). We investigated the influence of thrombin-stimulated human platelets on the evolution of simulated IE in the presence and absence of vancomycin or nafcillin. Staphylococcus aureus strains differing in intrinsic susceptibility to PMPs or antibiotics were studied: ISP479C (thrombin-induced PMP-1 [tPMP-1] susceptible; nafcillin and vancomycin susceptible), ISP479R (tPMP-1 resistant; nafcillin and vancomycin susceptible), and GISA-NJ (tPMP-1 intermediate-susceptible; vancomycin intermediate-susceptible). Platelets were introduced and thrombin activated within the in vitro IE model 30 min prior to inoculation with S. aureus. At 0 to 24 h postinoculation, bacterial densities in chamber fluid and simulated endocardial vegetations (SEVs) were quantified and compared among groups. Activated platelets alone, or in combination with antibiotics, inhibited the proliferation of ISP479C in chamber fluid or SEVs over the initial 4-h period (P < 0.05 versus controls). Moreover, nafcillin-containing regimens exerted inhibitory effects beyond 4 h against ISP479C in both model phases. By comparison, activated platelets inhibited GISA-NJ proliferation in SEVs but not in chamber fluid. The combination of platelets plus nafcillin or vancomycin significantly inhibited proliferation of the GISA-NJ strain in SEVs compared to the effect of platelets or antibiotics alone (P < 0.05). In contrast, platelets did not significantly alter the antistaphylococcal efficacies of nafcillin or vancomycin against ISP479R. These data support our hypothesis that a beneficial antimicrobial effect may result from the interaction among platelets, PMPs, and anti-infective agents against antibiotic-susceptible or -resistant staphylococci that exhibit a tPMP-1-susceptible or -intermediate-susceptible phenotype.
The antimicrobial functions of mammalian platelets are associated with their release of a family of small cationic peptides termed platelet microbicidal proteins (PMPs) (28, 33). These peptides have been shown to play a substantial role in antimicrobial host defense, especially against endovascular infections such as infective endocarditis (IE). In vitro, PMPs and thrombin-induced PMPs (tPMPs; e.g., tPMP-1 [33]) exert potent microbicidal activities against pathogens that commonly enter the bloodstream, including Staphylococcus aureus, coagulase-negative staphylococci, viridans group streptococci, and Candida albicans (21, 25, 33). PMPs and tPMPs have also been shown to interfere with platelet adherence and aggregation due to S. aureus and C. albicans in vitro (1, 26, 30-32). In vivo data also support a key role of platelets in antimicrobial host defense. For example, Sullam et al. demonstrated that cardiac vegetations from thrombocytopenic rabbits infected with tPMP-1-susceptible viridans streptococci contained higher bacterial densities than did controls with normal platelet counts (17). Furthermore, tPMP-1-resistant S. aureus or C. albicans strains cause significantly more severe forms of experimental IE than do tPMP-1-susceptible counterpart strains (3, 11, 29). In addition, the enhancement of the antimicrobial activities of conventional antibiotics by tPMPs in vitro was previously demonstrated (8-10).
The ability of PMPs and tPMPs to amplify the efficacies of conventional antibacterial and antifungal agents has also been supported by in vivo findings. For example, Dhawan and colleagues found that tPMP susceptibility favorably influences outcomes of oxacillin prophylaxis and therapy of experimental S. aureus IE (3, 4). Similarly, fluconazole therapy was significantly more efficacious against tPMP-1-susceptible C. albicans than against an orthogenic tPMP-1-resistant strain in experimental IE (M. R. Yeaman, D. Cheng, B. Desai, L. I. Kupferwasser, Y. Q. Xiong, J. E. Edwards, Jr., and A. S. Bayer, Abstr. 98th Gen. Meet. Am. Soc. Microbiol., abstr. V-83, 1998). Collectively, these results provide compelling evidence that platelets play important roles in host defense against endovascular infection and that these effects may be amplified in the presence of antibiotics.
Recently, an established in vitro pharmacologic model was adapted to facilitate dissection of IE pathogenesis and host defense (9, 12). This model has been shown to closely parallel key events in IE pathogenesis and antibiotic efficacy observed in well-characterized in vivo models (3, 4, 6). Like in vivo infections, this in vitro model poses a rigorous challenge to antimicrobial agent efficacy, due to the presence of high organism densities in the setting of a complex biomatrix comprising the simulated vegetation. Additionally, this model minimizes the use of experimental animals and allows control of key experimental variables individually or in combination, including (i) use of microbial inocula and densities within simulated vegetations to reflect early, evolving, and late stages of IE; (ii) the quantity or quality of cellular or soluble host defense components such as platelets, leukocytes, or immunoglobulin; and (iii) the influence of conventional or experimental anti-infective agents (12). Thus, the objective of the present study was to examine the influence of thrombin-activated human platelets on proliferation of S. aureus strains with various susceptibilities to tPMP-1 and antibiotics, within simulated endocardial vegetations and vascular fluid.
(This work was presented in part at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 16 to 19 December 2001.)
MATERIALS AND METHODS
Preparation and assay of purified tPMP-1.
In brief, platelet-rich plasma was obtained from whole rabbit blood by low-speed (75 × g) centrifugation. Platelets (<1% leukocyte contamination) were recovered by centrifugation (10 min at 2,000 × g), washed twice in Tyrode salts solution (Sigma Chemical Co., St. Louis, Mo.), and resuspended in Eagle's minimal essential medium (MEM) to 108 platelets per ml as determined by spectrophotometry (optical density, λ = 600 nm; Spectronic 401, Milton Roy). Platelets (108/ml in MEM) were then stimulated with thrombin (1 U of bovine thrombin [Jones Pharma Inc., St. Louis, Mo.]/ml) and 12.5 μl of 0.2 M CaCl2/ml of washed platelet suspension for 30 min at 37°C. The resulting tPMP-1-rich supernatant was subjected to reversed-phase high-pressure liquid chromatography to purify tPMP-1, followed by verification of purity, mass, and bioactivity as described elsewhere (33). The S. aureus strains were tested for susceptibility to purified tPMP-1 as previously described (22, 28).
Organisms.
Three S. aureus strains were used in these experiments. Two isogenic and laboratory-derived strains, ISP479C (tPMP-1-susceptible parent) and ISP479R (tPMP-1 resistant), were tested. Strain ISP479R is a stable, tPMP-1-resistant mutant generated from parental ISP479C by transposon mutagenesis as detailed previously (3). It has been shown that this transposon disrupts a Na+-H+ antiporter operon, conferring the tPMP-1 resistance phenotype (A. S. Bayer, A. Cheung, L. Kupferwasser, N. Lucindo, P. McNamara, R. Prasad, T. Prasad, R. Proctor, and M. R. Yeaman, Abstr. 103rd Gen. Meet. Am. Soc. Microbiol., abstr. 2482, 2003). ISP479R is resistant to killing by low levels of tPMP-1 in vitro (e.g., ≥90% survival of a 103-CFU/ml inoculum following 2 h of exposure to 2 μg of tPMP-1 at 37°C) compared with tPMP-1-susceptible ISP479C (≤25% survival under identical test conditions) (22, 28). A methicillin-resistant S. aureus clinical strain with reduced susceptibility to vancomycin (termed glycopeptide intermediate-susceptible [GISA]) was also studied (2, 7). This strain, originally isolated from a patient refractory to vancomycin therapy, was obtained from the Centers for Disease Control and Prevention in Atlanta, Ga., and designated GISA-NJ. Of note, this strain exhibited a mean in vitro tPMP-1 susceptibility of 74% ± 12% survival under the conditions described above, intermediate in relation to the ISP479C and ISP479R phenotypes.
For use in the in vitro model (see below), S. aureus colonies harvested from fresh blood agar plates were inoculated into brain heart infusion medium (Difco, Detroit, Mich.) and cultured to mid-logarithmic phase at 37°C. Organisms were then harvested by centrifugation, washed twice in phosphate-buffered saline (pH 7.2), and diluted in normal saline to achieve a 0.5 McFarland density (108 CFU/ml). Inocula were then diluted in normal saline to the desired challenge inoculum. All spectrophotometric approximations were confirmed by quantitative culture.
Antibiotics and susceptibility testing.
Vancomycin and nafcillin were purchased from Sigma Chemical. MICs and minimum bactericidal concentrations (MBCs) were determined by broth microdilution assay, per National Committee for Clinical Laboratory Standards (NCCLS) guidelines, with the use of starting inocula of 5 × 105 CFU/ml and antibiotic ranges of 0.125 to 128 μg/ml (14). MICs and MBCs were considered to be the concentrations of each antibiotic that yielded no visible growth and ≥99.9% killing upon subculture of MIC samples, respectively.
Preparation of SEVs (9, 16).
Fibrin clots representing sterile simulated endocardial vegetations (SEVs) were prepared as previously described (13). Briefly, 0.9 ml of cryoprecipitate from volunteer donors (United Blood Services, Albuquerque, N.Mex.), 0.05 ml of aprotinin solution (Sigma Chemical Co.), and 108 (0.05 ml) washed human platelets in 0.9% NaCl (yielding ∼2.5 × 104 to 3 × 105 platelets per g of SEV mass) were combined in sterile siliconized 1.5-ml Eppendorf tubes. A sterile monofilament line was then introduced, and 0.1 ml of thrombin (5,000 U, reconstituted with 5 ml of sterile water containing 50 mM CaCl2) was added to initiate formation of SEVs. After 30 min at 37°C, the resultant filament-tethered SEV was removed from the Eppendorf tube for use in the IE model described below.
In vitro model of IE (12).
One-compartment infection models (250 ml; Fig. 1) were made in duplicate for each experiment. Each model contained MEM, with continuous supplementation of fresh MEM from sterile reservoirs (simulating the vascular fluid phase compartment). Two SEVs were suspended from each sampling port (total of four SEVs per experiment [Fig. 1]) as previously described (12), and each model was placed in a water bath and maintained at 37°C for the duration of the experiment.
FIG. 1.
In vitro model of IE. PUMP, peristaltic pumps used to introduce fresh or remove exhausted medium.
Control models.
Sterile SEVs were placed in models for each preselected sampling time point. In pilot studies, each model was challenged by inoculation of the chamber fluid with 103 CFU of the individual S. aureus strains/ml to ensure equivalent colonization of SEVs by each strain (data not shown). This inoculum represents S. aureus densities observed in early vegetative IE (4, 5). Moreover, prior studies demonstrated that this inoculum reproducibly infects sterile SEVs in this model (12). A peristaltic pump supplied fresh MEM and removed exhausted medium from the model at a half-life equal to 6 h. At this flow rate, loss of S. aureus from the model is negligible, since the doubling time (∼20 min) far exceeds the loss attributed to model effluent (10). A magnetic stir bar continuously circulated the fluid within the chamber compartment of the model.
Routinely, experiments were conducted over a 24-h time span. Samples were taken more frequently at the early stages of each experiment (e.g., 0.5, 1, 2, and 4 h postinoculation) to optimize detection of the consequences of platelet-bacterial interactions, which are believed to be most extensive early in the evolution of IE (4, 24). At selected time points, two SEVs were removed from each model, individually weighed, and separately placed in 2-ml sterile vials prefilled with 3-mm-diameter glass beads and containing 1.25% trypsin (1:250 powder [Difco]) in normal saline. To homogenize the vegetation, the vial was placed in a mini-bead beater grinder (Biospec Products, Bartlesville, Okla.) for 30 s on ice. Sterile, cold 0.9% saline was used to dilute the homogenized vegetations, 20 μl of each homogenate was plated in triplicate onto brain heart infusion agar and incubated for 24 h at 37°C, and the colonies were counted. Means of the four SEV samples (two from each duplicate model) at each time point were plotted as log10 CFU per gram (± standard deviation) versus time. Likewise, two samples of the chamber fluid from the central compartment of each model were also taken at each time point and quantitatively cultured and served as growth controls. The means of these samples from the two models were plotted as log10 CFU per milliliter versus time.
Influence of platelets and antibiotics on S. aureus proliferation in the IE model.
To model the effect of platelets alone or in combination with antimicrobial agents, 108 platelets/ml were introduced into the chamber fluid and stimulated to release tPMPs by using thrombin, CaCl2, and homologous plasma as described previously (12, 28). This activation procedure reliably liberates ∼15 μg of tPMP-1 activity per 108 washed platelets (33). Thirty minutes after platelet stimulation, S. aureus was introduced at an initial inoculum of 103 CFU/ml. Vancomycin or nafcillin was added to the model as a single dose to achieve initial concentrations equivalent to their MIC against each respective S. aureus strain. When nafcillin was tested against the GISA-NJ strain (nafcillin MIC, ∼64 μg/ml; see Results), a concentration of 8 μg/ml was simulated, as concentrations exceeding this level are not physiologically relevant in terms of achieving and sustaining such levels in human therapy. Two SEVs were removed from each model at each time point (0, 0.5, 1, 2, 4, 8, and 24 h) and processed for quantitative culture as described above. The S. aureus densities (log10 CFU per gram [SEVs] and log10 CFU per milliliter [model fluid phase]) for each time point were compared to those obtained from the respective controls.
Statistical analysis.
Differences in log10 CFU per gram (SEVs) and log10 CFU per milliliter (fluid phase) over the 24-h test period among experimental groups were compared using analysis of variance with Tukey's post hoc analysis for multiple comparisons of significance. P values of ≤0.05 were considered to be significant.
RESULTS
ISP479C.
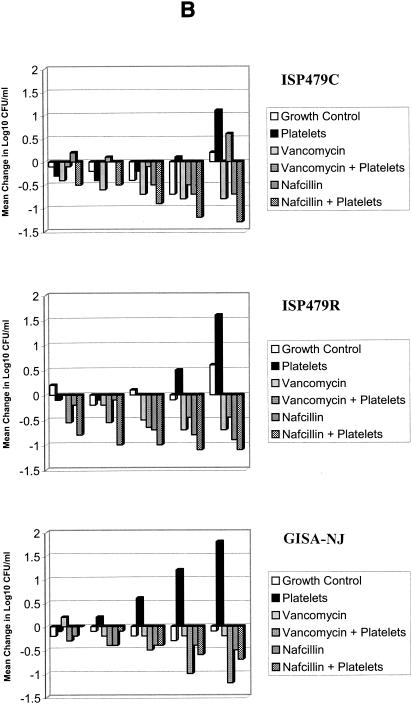
For the S. aureus tPMP-1-susceptible strain ISP479C, vancomycin and nafcillin MICs were 1 and 0.5 μg/ml, respectively. The MBCs of these agents against this strain were 8 and 0.5 μg/ml, respectively. The comparative influences of platelets alone or in combination with vancomycin or nafcillin on ISP479C in the in vitro model of IE are summarized in Fig. 2. Figure 2A depicts only the first 8 h, since most of the substantive effects were observed during this period.
FIG. 2.
S. aureus colonization and proliferation in the IE model in the presence and absence of thrombin-stimulated platelets. Bacterial inocula were introduced at time zero. Simulated vegetations and chamber fluid were quantitatively cultured at the subsequent time points indicated. (A) CFU per gram within the simulated vegetations (SEVs) over time. (B) Change in CFU per milliliter within the chamber fluid over time compared to baseline. The limit of detection in these models was considered to be 101 CFU/g or CFU/ml. Note differences in scale.
(i) Antistaphylococcal effects of platelets or antibiotics alone.
S. aureus ISP479C rapidly seeded SEVs, with a density of at least 102 CFU/g detectable at the 30-min point after inoculation. The ability of this strain to adhere to SEVs was equivalent to those of other strains used in this study (P > 0.05; see below).
Paralleling earlier findings (12), addition of thrombin-stimulated platelets to the chamber produced a sustained reduction in CFU per gram in SEVs compared to that in the control lacking platelets (P < 0.05, Fig. 2A). This effect was similar to that produced by nafcillin, whether alone or in combination with platelets. The bacterial density in chamber fluid was transiently reduced (during the first hour) by addition of stimulated platelets compared to that in control (Fig. 2B). Bacterial densities in the presence of nafcillin alone and in combination with platelets were similar. Beyond the initial 2-h test period, vancomycin alone effected a significant reduction in ISP479C densities within SEVs compared with controls (P < 0.05, Fig. 2A). Likewise, nafcillin and vancomycin alone each significantly inhibited proliferation of this strain in the chamber fluid of the model at each time point examined compared with controls (P < 0.05; exception, 1 h of nafcillin exposure, where the difference did not reach statistical significance).
(ii) Antistaphylococcal effects of platelets and antibiotics in combination.
In SEVs at sample times 0.5 to 4 h, thrombin-stimulated platelets combined with vancomycin achieved significantly greater inhibition of ISP479C proliferation than did vancomycin alone (P < 0.05) but not different from that of platelets alone (P > 0.05) (Fig. 2A). The efficacy of thrombin-stimulated platelets in combination with nafcillin was not different from that of nafcillin alone over the entire experimental period or compared with that of platelets alone from 0.5 to 4 h (P > 0.05). In the fluid phase of the model, ISP479C densities were significantly lower with vancomycin treatment alone than with vancomycin in combination with thrombin-stimulated platelets (P < 0.05, Fig. 2B). As in SEVs, the efficacy of stimulated platelets in combination with nafcillin was not different from that of nafcillin alone throughout the experiment or from that of platelets alone for the first 2-h period (P > 0.05). The regimens that included nafcillin (nafcillin and nafcillin combined with platelets) consistently exhibited a greater sustained response than did those with platelets alone (Fig. 2).
ISP479R.
For the tPMP-1-resistant strain ISP479R, vancomycin and nafcillin MICs were 1 and 0.25 μg/ml, respectively. The MBCs of these antibiotics against this strain were 4 and 0.5 μg/ml, respectively. Thus, the MICs and MBCs for ISP479R were not significantly different from those for ISP479C. The comparative influences of platelets alone or in combination with vancomycin or nafcillin on ISP479R in the in vitro model of IE are summarized in Fig. 2.
(i) Antistaphylococcal effects of platelets or antibiotics alone.
Consistent with prior studies (12), thrombin-stimulated platelets alone failed to inhibit the proliferation of the tPMP-1-resistant strain ISP479R within the context of SEVs compared with that in platelet-free controls across the first 8-h test period (Fig. 2A). This outcome is in contrast with the effect of platelets alone observed against ISP479C (Fig. 2A). Interestingly, the presence of activated platelets was correlated with an increase in the density of this strain in chamber fluid (P < 0.05 versus control at 4 h and beyond, Fig. 2B). Similar to results with ISP479C, nafcillin achieved significant inhibition of ISP479R proliferation within SEVs versus controls at time points beyond 2 h (P < 0.05, Fig. 2A). Vancomycin alone did not inhibit the proliferation of ISP479R within SEVs compared with platelets alone or controls from 0.5 to 4 h (P > 0.05, Fig. 2A). Moreover, nafcillin and vancomycin alone caused significant inhibition of this strain in the chamber fluid phase of the model only at the 8-h time point, compared with untreated controls (P < 0.05, Fig. 2B).
(ii) Antistaphylococcal effects of platelets and antibiotics in combination.
In SEVs, thrombin-stimulated platelets in combination with vancomycin did not achieve reductions in ISP479R densities compared with those achieved by vancomycin alone at any time point or compared with those achieved by platelets alone over the 0.5- to 2-h experiment range (P > 0.05, Fig. 2A). Similarly, the efficacy of thrombin-stimulated platelets in combination with nafcillin was not different from that of nafcillin alone (P > 0.05, Fig. 2A) against this tPMP-1-resistant strain. In the fluid phase of the model, ISP479R densities were similar between the two vancomycin treatment groups (P > 0.05, Fig. 2B) (except at 0.5 h, where vancomycin in combination with platelets achieved greater antibacterial activity; P < 0.05). Interestingly, the addition of activated platelets alone was associated with an increased density of this strain in the fluid phase of the model compared with the that for (i) vancomycin combined with platelets at all time points; (ii) vancomycin alone at 2, 4, or 8 h; and (iii) growth controls at 1, 4, and 8 h (P < 0.05, Fig. 2B). Beyond the 1-h time point in the fluid phase, the efficacy of stimulated platelets in combination with nafcillin was not different from that of nafcillin alone (P > 0.05, Fig. 2B). However, the combination of nafcillin and activated platelets exerted significantly greater anti-ISP479R activity than did platelets alone or nafcillin alone at 0.5- and 1-h time points (P < 0.05, Fig. 2).
GISA-NJ.
As anticipated, the GISA strain exhibited reduced susceptibility to vancomycin and nafcillin, the MICs of these drugs for this strain being 8 and 64 μg/ml, respectively. The MBCs of these antibiotics against this strain were 8 and >128 μg/ml, respectively. The comparative influences of platelets alone or in combination with vancomycin or nafcillin against GISA-NJ in the in vitro model of IE are summarized in Fig. 2.
(i) Antistaphylococcal effects of platelets or antibiotics alone.
The addition of activated platelets significantly limited the early proliferation of GISA-NJ within SEVs (e.g., 2 and 4 h postinoculation; P < 0.05; Fig. 2A). However, beyond 4 h, platelets did not inhibit the proliferation of this strain in SEVs. In contrast, in the liquid phase of the model, the presence of platelets was associated with increased CFU per milliliter subsequent to the 0.5-h time point (P < 0.05 versus control; Fig. 2B). At early time points, vancomycin did not significantly impact GISA-NJ densities within SEVs (P > 0.05; Fig. 2A) compared to that for untreated controls. Likewise, as predicted from MIC results, nafcillin alone did not inhibit the proliferation of this strain within SEVs compared with that for controls (P > 0.05; Fig. 2A). In fact, platelets alone had greater inhibitory effects within SEVs at the 2- and 4-h time points than did nafcillin (P < 0.05; Fig. 2A). No significant differences between vancomycin and nafcillin were observed within the fluid phase of the model compared to the controls over the 24-h time span (P > 0.05; Fig. 2B).
(ii) Antistaphylococcal effects of platelets and antibiotics in combination.
Vancomycin combined with activated platelets significantly inhibited GISA-NJ proliferation in SEVs compared to vancomycin alone over the first 4 h (P < 0.05; Fig. 2A). Moreover, this combination had a greater sustained antistaphylococcal effect than did either regimen alone for 8 h postinoculation. Likewise, nafcillin combined with platelets achieved significantly greater inhibition of this strain within SEVs than did nafcillin alone or controls beyond the 1-h time point (P < 0.05). This antibacterial effect of nafcillin combined with platelets within SEVs was sustained for 8 h (P < 0.05; Fig. 2A). Vancomycin or nafcillin in combination with platelets did not exhibit superior antibacterial effects against GISA-NJ in the fluid phase of the model compared to those of either agent alone (P > 0.05; Fig. 2B).
Overall, results can be summarized as follows. (i) The activity of vancomycin alone is less than that of platelets against the tPMP-1-susceptible strain, at least early on, and it does not add to the antibacterial activity of platelets alone. The interaction between nafcillin and platelets appears to be indifferent against this strain. (ii) For the GISA strain, platelets and antibiotics in combination have a greater inhibition of bacterial proliferation than does either regimen alone. (iii) In contrast, platelets alone appear to have little or no inhibitory effect on proliferation of the tPMP-1-resistant strain and do not enhance the inhibitory effects of antibiotics against this strain. (iv) Platelets appear to have differential antimicrobial effects in the SEV and fluid phases of the model.
DISCUSSION
Prior investigations of the role of PMPs in limiting microbial pathogenesis of endovascular infection have been carried out in vitro and in experimental animal models (12, 17, 21, 24-33). Collectively, these studies indicated that, despite equivalent colonization and proliferation of tPMP-1-susceptible and tPMP-1-resistant strains of S. aureus in SEVs or cardiac vegetations in the absence of platelets, activated platelets limited the proliferation of the tPMP-1-susceptible strains through a mechanism involving localized tPMP release. The direct antistaphylococcal effects of thrombin-activated platelets appeared to be most significant in SEVs or cardiac vegetations, consistent with the concept that PMPs concentrate at sites of vascular infection where platelets accumulate and degranulate. Thus, while the precise mechanisms by which platelets exert these antibacterial effects remain to be fully elucidated, these effects involve direct and indirect bactericidal actions of tPMPs (12). The above investigations provided a basis for the present in vitro study, in which we examined the influence of activated platelets alone or in combination with antistaphylococcal antibiotics versus S. aureus strains exhibiting distinct tPMP-1 or antibiotic susceptibility phenotypes in the in vitro model of IE.
Several interesting findings emerged from these studies. First, the presence of activated platelets in any regimen limited the early proliferation of the ISP479C and the GISA-NJ strains, but not ISP479R, in the SEV phase of the model. These results are consistent with our earlier findings (3, 4) and support the hypothesis that tPMPs directly mediate the early antistaphylococcal effect of platelets in defense against IE. The increases in SEV densities later in the course of these models likely involve multifactorial processes that facilitate repetitive and ongoing seeding of these lesions. For example, it is possible that, after the antistaphylococcal effects of tPMPs have occurred, surviving S. aureus bacteria could conceivably exploit the platelet thrombus as an adhesive surface. To do so, the organism could utilize a number of adhesins targeting activated platelets (1, 24).
Interestingly, the addition of platelets alone was associated with an unanticipated late increase in densities of S. aureus strains within the chamber fluid phase of the models. While the mechanisms accounting for this phenomenon have yet to be defined, several possibilities can be considered. Several studies have reported that platelets interact directly with and internalize microorganisms circulating in the bloodstream (24). This process has been hypothesized to contribute to clearing of hematogenous pathogens, thus preventing the initial or ongoing seeding of vascular lesions with blood-borne organisms. Thus, it is plausible that the presence of thrombin-activated platelets in models prior to S. aureus GISA-NJ or ISP479R inoculation interfered with subsequent colonization of SEVs with these strains. These concepts argue in favor of complementary, indirect roles for platelets in antimicrobial host defense (24). Alternatively, it is possible that staphylococci internalized by activated platelets could serve as a reservoir for the organism (34). Ensuing lysis of platelets could release intraplatelet organisms and contribute to later increases in chamber fluid counts in this model or bacteremia in vivo. We recognize that minor differences in adhesion among the study strains to SEVs or platelets in suspension may modestly influence the observed outcomes. However, we consider this to be a minor factor, as our prior studies indicate that the study strains have equivalent degrees of adherence to SEVs and that any minor differences do not significantly contribute to the antimicrobial effects of platelets or antibiotics, alone or in combination.
Perhaps the most compelling data arising from the present study are related to the impact of activated platelets in combination with antistaphylococcal antibiotics. Platelets alone exerted significant antistaphylococcal effects against the tPMP-1-susceptible strain ISP479C; thus, the addition of vancomycin or nafcillin to platelets in this model yielded no additional inhibition against this strain compared with that with platelets alone. However, in early stages of the infection model, thrombin-stimulated platelets combined with nafcillin or vancomycin yielded significantly greater inhibition of the tPMP-1-intermediate-susceptible GISA-NJ strain in SEVs than did any regimen alone. Further, the addition of platelets to either antibiotic significantly enhanced the antistaphylococcal effect against all S. aureus strains examined, including ISP479R, within 1 h in the fluid phase of the model. Collectively, these results suggest that beneficial antistaphylococcal effects occur when platelets and tPMPs interact with antibiotics against tPMP-susceptible S. aureus. Moreover, these findings may contribute to understanding outcomes in experimental IE in vivo, in which some antibiotics have achieved greater efficacy against resistant organisms than would have otherwise been expected based on the in vitro MIC of that antibiotic (e.g., vancomycin efficacy in IE due to GISA S. aureus).
In summary, the pattern of results presented here suggests that platelets may complement or enhance the effects of certain antibiotics against S. aureus, most predominantly in the complex setting of platelet-fibrin matrices found in IE. In a broader sense, the present findings support a significant and beneficial interaction between innate immune mechanisms and exogenous anti-infective agents, yielding a net synergistic impact (8, 13, 15, 26). It is also probable that certain antimicrobial peptides facilitate the host defense functions of other endogenous mechanisms of innate or adaptive immunity. For example, studies by Tang et al. (18) and Yan and Hancock (23) revealed synergistic interactions among antimicrobial peptides, or between peptides and lysozyme, in vitro. Likewise, Vaara et al. suggested that an outer membrane disorganizing peptide (polymyxin B nonapeptide) sensitizes Escherichia coli to bactericidal activity of serum in vitro, hypothetically by promoting the microbicidal effects of complement fixation (19). Subsequently, this group demonstrated that a polymyxin B nonapeptide acted synergistically with fresh human serum to effect bactericidal activity against gram-negative bacteria in vitro (20). From these perspectives, our present data show that platelets significantly contribute to antimicrobial host defense and potentiate the antimicrobial mechanisms of distinct classes of conventional antistaphylococcal agents. These results support the hypothesis that exogenous antimicrobial agents and endogenous mechanisms of antimicrobial host defense interact to yield mutual potentiation resulting in amplified antimicrobial efficacy. Moreover, as used in the present study, our in vitro model was devoid of neutrophils and serum. However, the potential impact of these additional host defense components should not be underestimated and is amenable to study in this model system.
Acknowledgments
We thank Kimberly Gank and Tiffany Jones for excellent technical support.
These studies were supported in part by grants from the Society of Infectious Disease Pharmacists (SIDP; to R.-C.M.) and the NIH (AI-39001 and AI-48031 to M.R.Y. and AI-39108 to A.S.B.).
REFERENCES
- 1.Bayer, A. S., P. M. Sullam, M. Ramos, C. Li, A. L. Cheung, and M. R. Yeaman. 1995. Staphylococcus aureus induces platelet aggregation via a fibrinogen-dependent mechanism which is independent of principal platelet glycoprotein IIb/IIIa fibrinogen-binding domains. Infect. Immun. 63:3634-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1997. Reduced susceptibility of Staphylococcus aureus to vancomycin—Japan 1996. Morb. Mortal. Wkly. Rep. 46:624-626. [PubMed] [Google Scholar]
- 3.Dhawan, V. K., A. S. Bayer, and M. R. Yeaman. 1998. In vitro resistance to thrombin-induced platelet microbicidal protein is associated with enhanced progression and hematogenous dissemination in experimental Staphylococcus aureus infective endocarditis. Infect. Immun. 66:3476-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhawan, V. K., A. S. Bayer, and M. R. Yeaman. 2000. Thrombin-induced platelet microbicidal protein susceptibility phenotype influences outcomes of oxacillin prophylaxis and therapy of experimental Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 44:3206-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durack, D. T., and P. B. Besson. 1972. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br. J. Exp. Pathol. 53:44-49. [PMC free article] [PubMed] [Google Scholar]
- 6.Hershberger, E., E. A. Coyle, G. W. Kaatz, M. J. Zervos, and M. J. Rybak. 2000. Comparison of a rabbit model of bacterial endocarditis and an in vitro infection model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 44:1921-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 8.Hostacka, A. 1998. Serum sensitivity and cell surface hydrophobicity of Klebsiella pneumoniae treated with gentamicin, tobramycin, and amikacin. J. Basic Microbiol. 38:383-388. [PubMed] [Google Scholar]
- 9.Kang, S. L., and M. J. Rybak. 1995. Pharmacodynamics of RP 59500 alone and in combination with vancomycin against Staphylococcus aureus in an in vitro-infected fibrin clot model. Antimicrob. Agents Chemother. 39:1505-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keil, S., and B. Wiedemann. 1995. Mathematical corrections for bacterial loss in pharmacodynamic in vitro dilution models. Antimicrob. Agents Chemother. 39:1054-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kupferwasser, L. I., M. R. Yeaman, S. Shapiro, C. C. Nast, and A. S. Bayer. 2002. In vitro susceptibility to thrombin-induced platelet microbicidal protein is associated with reduced disease progression and complication rates in experimental Staphylococcus aureus endocarditis: microbiologic, histologic, and echocardiographic analyses. Circulation 105:746-752. [DOI] [PubMed] [Google Scholar]
- 12.Mercier, R. C., M. J. Rybak, A. S. Bayer, and M. R. Yeaman. 2000. Influence of platelets and platelet microbicidal protein susceptibility on the fate of Staphylococcus aureus in an in vitro model of infective endocarditis. Infect. Immun. 68:4699-4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miglioli, P. A., U. Schoffel, and L. Gianfranceschi. 1996. The in vitro synergistic inhibitory effect of human amniotic fluid and gentamicin on growth of Escherichia coli. Chemotherapy 42:206-209. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.Pruul, H., and P. J. McDonald. 1992. Potentiation of antibacterial activity of azithromycin and other macrolides by normal human serum. Antimicrob. Agents Chemother. 36:10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rybak, M. J., H. H. Houlihan, R. C. Mercier, and G. W. Kaatz. 1997. Pharmacodynamics of RP59500 (quinupristin/dalfopristin) administered by intermittent versus continuous infusion against Staphylococcus aureus-infected fibrin-platelet clots in an in vitro infection model. Antimicrob. Agents Chemother. 41:1359-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullam, P. M., U. Frank, M. R. Yeaman, M. G. Tauber, A. S. Bayer, and H. F. Chambers. 1993. Effect of thrombocytopenia on the early course of streptococcal endocarditis. J. Infect. Dis. 168:910-914. [DOI] [PubMed] [Google Scholar]
- 18.Tang, Y. Q., M. R. Yeaman, and M. E. Selsted. 2002. Antimicrobial peptides from human platelets. Infect. Immun. 70:6524-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaara, M., P. Viljanen, T. Vaara, and P. H. Makela. 1984. An outer membrane-disorganizing peptide PMBN sensitizes Escherichia coli to serum bactericidal action. J. Immunol. 132:2582-2589. [PubMed] [Google Scholar]
- 20.Viljanen, P., H. Kayhty, M. Vaara, and T. Vaara. 1986. Susceptibility of Gram-negative bacteria to the synergistic bactericidal action of serum and polymyxin B nonapeptide. Can. J. Microbiol. 32:66-69. [DOI] [PubMed] [Google Scholar]
- 21.Wu, T., M. R. Yeaman, and A. S. Bayer. 1994. In vitro resistance to platelet microbicidal protein correlates with endocarditis source among bacteremic staphylococcal and streptococcal isolates. Antimicrob. Agents Chemother. 38:729-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong, Y. Q., A. S. Bayer, and M. R. Yeaman. 2002. Inhibition of Staphylococcus aureus intracellular macromolecular synthesis by thrombin-induced platelet microbicidal proteins. J. Infect. Dis. 185:348-356. [DOI] [PubMed] [Google Scholar]
- 23.Yan, H., and R. E. W. Hancock. 2001. Synergistic interactions between mammalian antimicrobial defense peptides. Antimicrob. Agents Chemother. 45:1558-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeaman, M. R. 1997. The role of platelets in antimicrobial host defense. Clin. Infect. Dis. 25:951-970. [DOI] [PubMed] [Google Scholar]
- 25.Yeaman, M. R., A. S. Ibrahim, J. E. Edwards, A. S. Bayer, and M. A. Ghannoum. 1993. Thrombin-induced rabbit platelet microbicidal protein is fungicidal in vitro. Antimicrob. Agents Chemother. 37:546-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeaman, M. R., D. C. Norman, and A. S. Bayer. 1992. Platelet microbicidal protein enhances antibiotic-induced killing of and postantibiotic effect in Staphylococcus aureus. Antimicrob. Agents Chemother. 36:1665-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeaman, M. R., D. C. Norman, and A. S. Bayer. 1992. Staphylococcus aureus susceptibility to thrombin-induced platelet microbicidal protein is independent of platelet adherence and aggregation in vitro. Infect. Immun. 60:2368-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeaman, M. R., S. M. Puentes, D. C. Norman, and A. S. Bayer. 1992. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect. Immun. 60:1202-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeaman, M. R., S. S. Soldan, M. A. Ghannoum, J. E. Edwards, S. G. Filler, and A. S. Bayer. 1996. Resistance to platelet microbicidal protein results in increased severity of experimental Candida albicans endocarditis. Infect. Immun. 64:1379-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeaman, M. R., P. M. Sullam, P. F. Dazin, and A. S. Bayer. 1994. Platelet microbicidal protein alone and in combination with antibiotics reduces Staphylococcus aureus adherence to platelets in vitro. Infect. Immun. 62:3416-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeaman, M. R., P. M. Sullam, P. F. Dazin, M. A. Ghannoum, J. E. Edwards, and A. S. Bayer. 1994. Fluconazole and platelet microbicidal protein inhibit Candida adherence to platelets in vitro. Antimicrob. Agents Chemother. 38:1460-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeaman, M. R., P. M. Sullam, P. F. Dazin, D. C. Norman, and A. S. Bayer. 1992. Characterization of Staphylococcus aureus-platelet binding by quantitative flow cytometric analysis. J. Infect. Dis. 166:65-73. [DOI] [PubMed] [Google Scholar]
- 33.Yeaman, M. R., Y. O. Tang, A. J. Shen, A. S. Bayer, and M. E. Selsted. 1997. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect. Immun. 65:1023-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youssefian, T., A. Drouin, J. J. Masse, J. Guichard, and E. M. Cramer. 2002. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood 99:4021-4029. [DOI] [PubMed] [Google Scholar]