Abstract
We describe a method for deriving the linear cortical magnification factor from positional error across the visual field. We compared magnification obtained from this method between normally sighted individuals and amblyopic individuals, who receive atypical visual input during development. The cortical magnification factor was derived for each subject from positional error at 32 locations in the visual field, using an established model of conformal mapping between retinal and cortical coordinates. Magnification of the normally sighted group matched estimates from previous physiological and neuroimaging studies in humans, confirming the validity of the approach. The estimate of magnification for the amblyopic group was significantly lower than the normal group: by 4.4 mm deg−1 at 1° eccentricity, assuming a constant scaling factor for both groups. These estimates, if correct, suggest a role for early visual experience in establishing retinotopic mapping in cortex. We discuss the implications of altered cortical magnification for cortical size, and consider other neural changes that may account for the amblyopic results.
Keywords: alignment, amblyopia, bisection, E2, localization, strabismus, distortion, retinotopy
Introduction
In normally sighted humans, the center of gaze is oversampled relative to the periphery at all stages in visual processing. In cortex, an object at the fovea activates a larger area than does the same-sized object a few degrees into the periphery. This change in cortical sampling across the visual field is quantified by the cortical magnification factor (M), the amount of visual cortex (mm) devoted to one degree of visual angle (Daniel & Whitteridge, 1961; Cowey & Rolls, 1974). Inverse cortical magnification, M−1, is correlated with the decline in resolution and positional acuity from the central to the peripheral visual field (Cowey & Rolls, 1974; Drasdo, 1977; Levi, Klein, & Aitsebaomo, 1985; Levi & Klein, 1990; Duncan & Boynton, 2003). Hence, positional error across the visual field may be used to infer the cortical magnification factor in individuals. We used a two-dimensional model of the mapping between retinal and cortical space (Schwartz, 1980) to calculate the cortical magnification factor from positional error at 32 locations in the visual field. We assessed whether the method produces realistic estimates of magnification for normally sighted subjects, and compared magnification between normally sighted and amblyopic subjects.
In amblyopia, monocular input is disrupted in childhood due to misalignment of the ocular axes of the eyes (strabismus), chronic blur in one eye (anisometropia), or a combination of the two. Disruption of monocular input during the critical period alters cortical architecture, and has numerous consequences for visual function, including poor visual acuity in the affected eye, loss of stereopsis, and positional distortions in one or more parts of the visual field. The neural changes thus far investigated as the substrate for amblyopic deficits are primarily of a local nature, such as changes in receptive field size, number, responsiveness, and wiring (Levi, Klein, & Yap, 1987; Hess & Field, 1994; Kiorpes, Kiper, O'Keefe, Cavanaugh, & Movshon, 1998; Lu & Constantine-Paton, 2004; Li, Fitzpatrick, & White, 2006), or changes in the columnar organization of eye-specific input (Hubel & Wiesel, 1962; Adams, Sincich, & Horton, 2007). However, the strong interdependence in cortex between response properties of local units (e.g., single cells) and more global cortical organization (e.g., retinotopic mapping) (Blasdel & Campbell, 2001; Yu, Farley, Jin, & Sur, 2005), suggests that alterations in retinotopic mapping may accompany other cortical anomalies in amblyopic individuals.
The approach taken in this paper was to use the gradient in performance across the visual field to detect potential alterations in retinotopic mapping in amblyopia. Positional judgements such as spatial interval discrimination (bisection), and spatial offset discrimination (alignment), at separations exceeding half a degree of visual angle, and outside the immediate foveal region, are limited primarily by cortical sampling, or are M-scaled (Klein & Levi, 1987; Levi & Klein, 1990). We used a task that falls squarely in the M-scaling regime for normal subjects, and assumed that cortical sampling constrained performance on the task for both groups. A direct interpretation of any group difference in magnification measured using this method is that retinotopic mapping (and cortical magnification) is altered in amblyopia. Such a change in magnification has been suggested previously on the basis of orientation discrimination performance in the fovea and periphery of amblyopes (Vandenbussche, Vogels, & Orban, 1986). Alternatively, other cortical changes such as disruptions in neuronal connectivity (Hess & Field, 1994; Li, Mullen, Thompson, & Hess, 2011) or changes in receptive field attributes (Levi et al., 1987; Kiorpes et al., 1998) may influence performance in tasks normally limited by cortical sampling, or may accompany changes in retinotopic mapping in this group. The method presented here provides a starting point to examine whether retinotopic mapping is different than normal in amblyopic subjects.
Materials and methods
Observers
Forty-two amblyopic (34 strabismic and eight anisometropic; mean age = 37.6 years, SD = 12.89 years) and 20 normally sighted observers were tested (mean age = 25.8 years, SD = 7.32 years). Two observers were tested later in a control condition and were not included in the main experiment (see Table 1 and Results). Clinical details of the amblyopic observers are given in Table 1. The criterion for inclusion in the study was 0.2 logMAR difference (or greater) in visual acuity between the amblyopic and the fellow (strong) eye. Normally sighted observers were undergraduate students and research staff at the University of Nottingham. All observers were informed of the purpose and procedure of the study, and gave written consent to participate. Amblyopic observers provided a detailed ophthalmic history and were refracted by a registered optometrist prior to testing. Ocular movements, ocular alignment for distance and near, and binocular functions were also examined. LogMAR acuity was measured using the Bailey-Lovie chart.
Table 1.
Participant clinical details. Notes: *Observers excluded from dichoptic fits. **Monocular analyses only. SOT: Esotropia; XOT: Exotropia; Micro: Microtropia; BD: Base-down.
| ID |
Age, sex |
Eye, type |
Patching, operation |
Refractive error |
Alignment |
LogMAR |
| AA | 40 M | L Aniso | Yes, No | OD +1.50/−0.50 × 150 | — | 0.02 |
| OS +4.50/−0.25 × 15 | 0.38 | |||||
| AB | 31 M | L Aniso | Yes, No | OD +0.25DS | — | −0.06 |
| OS +3.50/−1.50 × 10 | 0.40 | |||||
| AF | 19 F | L Aniso | Yes, No | OD −0.25/−0.75 × 165 | 0.06 | |
| OS +2.50/−0.75 × 20 | — | 0.52 | ||||
| AP | 45 M | L Strab | Yes, Yes | OD −0.50/−0.75 × 175 | 0.00 | |
| OS +0.00/−0.50 × 175 | SOT | 0.96 | ||||
| BC* | 32 M | R Strab | Yes, Yes | OD +3.25/−2.00 × 165 | 16 SOT | 0.34 |
| OS +3.75/−2.25 × 180 | −0.04 | |||||
| BM | 35 F | R Mixed | No, No | OD +0.75/−0.50 × 105 | Micro | 0.50 |
| OS −4.00/−0.50 × 120 | 0.06 | |||||
| BS* | 32 M | R Strab | Yes, No | OD −1.25/−1.00 × 120 | 4 SOT | 0.68 |
| OS −1.75/−0.50 × 85 | −0.06 | |||||
| CA | 19 M | L Mixed | Yes, Yes | OD +0.25DS | 0.02 | |
| OS +7.00DS | 10 XOT | 1.58 | ||||
| CB | 57 M | L Mixed | Yes, No | OD +3.00/−0.25 × 75 | −0.08 | |
| OS +6.00DS | 8 SOT | 0.64 | ||||
| CC* | 43 M | L Strab | Yes, No | OD +0.75/−0.50 × 180 | −0.08 | |
| OS +6.75/−2.75 × 12.5 | 12 XOT | 1.00 | ||||
| CG | 70 M | L Strab | Yes, Yes | OD +5.25/−0.50 × 175 | −0.08 | |
| OS +8.00/−1.25 × 65 | 10 SOT | 0.30 | ||||
| CM1* | 53 F | L Strab | Yes, No | OD +2.25/−1.00 × 10 | −0.10 | |
| OS +2.50/−1.50 × 160 | Micro | 0.64 | ||||
| CM2 | 57 F | L Strab | No, No | OD +1.25/−0.50 × 60 | 0.02 | |
| OS +1.00/−0.50 × 175 | Micro | 0.60 | ||||
| CR | 41 F | R Aniso | Yes, Yes | OD +4.00/−3.00 × 170 | — | 0.54 |
| OS +0.50/−0.75 × 140 | −0.06 | |||||
| CS1 | 56 F | R Aniso | Yes, No | OD +4.00/−1.00 × 15 | — | 0.60 |
| OS −1.75/−1.00 × 150 | 0.06 | |||||
| CS2* | 38 M | R Strab | Yes, No | OD +0.75/−1.00 × 170 | 10 BD | 0.10 |
| OS +0.75/−0.75 × 10 | −0.10 | |||||
| DJ | 47 M | R Strab | Yes, No | OD +4.75/−3.25 × 15 | Micro | 0.70 |
| OS +0.25/−0.50 × 160 | 0.00 | |||||
| DR | 33 F | L Strab | Yes, No | OD +4.50DS | 0.00 | |
| OS +6.50/−0.50 × 180 | 20 SOT | 0.60 | ||||
| EF | 46 M | R Aniso | Yes, No | OD +5.50/−2.00 × 130 | — | 0.60 |
| OS −0.25/−0.75 × 45 | −0.22 | |||||
| GJ | 29 M | R Mixed | Yes, Yes | OD +4.75/−2.50 × 10 | 4 XOT | 1.06 |
| OS +0.75/−0.25 × 10 | −0.06 | |||||
| GL | 48 F | L Strab | Yes, No | OD −0.25/−0.25 × 135 | −0.10 | |
| OS +0.50DS | Micro | 0.20 | ||||
| HM1 | 21 F | L Strab | Yes, Yes | OD plano/−2.00 × 180 | 0.00 | |
| OS −0.75/−2.25 × 180 | 15 XOT | 0.12 | ||||
| HM2 | 27 F | L Mixed | Yes, No | OD +4.50/−0.50 × 15 | 6 SOT | −0.14 |
| OS +3.00/−0.25 × 35 | 0.20 | |||||
| JA | 21 F | L Strab | Yes, Yes | OD +3.50/−0.50 × 145 | −0.02 | |
| OS +4.50/−0.75 × 45 | 8 SOT | 0.54 | ||||
| JC1 | 33 M | L Strab | Yes, Yes | OD +1.00/−0.25 × 70 | 0.04 | |
| OS +8.50/−4.50 × 65 | 3 XOT | 1.00 | ||||
| JC2 | 45 M | L Strab | Yes, Yes | OD +0.25/−1.00 × 175 | 0.00 | |
| OS +0.75/−0.25 × 50 | 6 SOT | 0.62 | ||||
| JN* | 20 M | L Mixed | Yes, No | OD plano | −0.02 | |
| OS +5.00/−3.50 × 20 | Micro | 0.80 | ||||
| JO** | 20 M | L Mixed | Yes, No | OD −2.50/−0.50 × 30 | 0.06 | |
| OS plano/−3.50 × 160 | 12 XOT | 1.04 | ||||
| JP | 35 M | R Mixed | Yes, No | OD +2.50/−0.50 × 130 | 6 SOT | 0.32 |
| OS plano | 0.02 | |||||
| KA* | 45 M | L Strab | Yes, No | OD plano | −0.04 | |
| OS plano/−0.75 × 40 | 16 SOT | 0.62 | ||||
| KE | 23 F | L Mixed | No, No | OD plano | −0.16 | |
| OS +2.25DS | Micro | 0.84 | ||||
| LA | 21 F | R Mixed | Yes, No | OD +4.25/−1.00 × 150 | Micro | 0.56 |
| OS +2.25/−0.50 × 35 | −0.08 | |||||
| LS* | 48 F | L Mixed | No, No | OD +0.75/−0.50 × 15 | 0.02 | |
| OS +1.75/−2.00 × 150 | 4 SOT | 1.12 | ||||
| MB | 22 M | R Mixed | No, No | OD +6.50/−1.75 × 5 | 20 SOT | 0.38 |
| OS +4.50/−1.50 × 5 | 0.08 | |||||
| RB** | 28 F | R Mixed | No, No | OD +3.50/−5.25 × 10 | 6 XOT | 0.34 |
| OS +0.50DS | −0.20 | |||||
| RC1 | 49 M | R Aniso | Yes, No | OD +0.25/−5.00 × 12.5 | — | 0.18 |
| OS plano/−0.25 × 65 | −0.08 | |||||
| RC2 | 46 M | R Mixed | Yes, No | OD +6.25/−1.75 × 10 | Micro | 0.42 |
| OS +6.00/−2.50 × 170 | −0.06 | |||||
| RM* | 44 M | L Mixed | No, No | OD −0.50/−0.50 × 120 | −0.16 | |
| OS +6.50/−6.25 × 85 | Micro | 0.16 | ||||
| SE | 17 M | R Strab | Yes, No | OD +4.50/−0.75 × 105 | 12 SOT | 0.48 |
| OS +3.00/−0.75 × 095 | −0.08 | |||||
| SM | 34 M | R Strab | Yes, No | OD −0.50DS | 12 SOT | 1.02 |
| OS −0.50/−0.50 × 160 | 0.00 | |||||
| ST* | 37 M | R Strab | Yes, Yes | OD +3.50/−1.50 × 170 | 14 RSOT | 0.56 |
| OS −3.25/−0.25 × 30 | −0.06 | |||||
| TT | 54 F | L Aniso | No, No | OD +1.50/−0.50 × 60 | — | −0.04 |
| OS +5.50/−0.75 × 15 | 0.30 |
Apparatus and stimuli
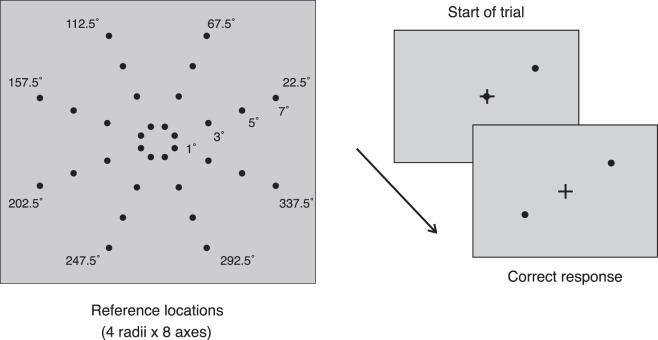
The experiment was performed with an Apple G5 iMac computer, running PsychoPy (Peirce, 2007). The monitor was a Trinitron Dell P1130 with a screen width of 40 cm and resolution of 1280 × 1024 pixels. Mean background luminance was 41 cd/m2. The monitor was positioned straight ahead of observers at a viewing distance of 114 cm. The fixation mark was a black cross subtending 0.38° of visual angle, and the reference and response probes were broadband dots subtending 0.28° of visual angle. The probes were made to flicker (8 Hz) to counteract suppression of the amblyopic eye by the fellow eye. Kodak Wratten filters (numbers 58 and 29) were used for dichoptic viewing. The red filter (no. 29) transmits only wavelengths above 600 nm, and green filter (no. 58) transmission is between 470–610 nm. The response and reference probe spectral content were matched to each of the filters respectively, and the background color was adjusted to ensure no bleed between filters. Two experimenters confirmed that only one probe could be seen through each filter (or through each eye). All observers also confirmed that they could only see one probe with each eye. We tested whether the task could be performed when both probes were set to one color (red or green), while viewing through the opposite filter. Under these conditions, no probes were visible on the screen, and the task could not be performed. Therefore, aside from the fixation cross, viewing of the probes was fully dichoptic. The background when viewed dichoptically appeared grey. Reference locations were sampled from one of eight polar angles (22.5°, 67.5°, 112.5°, 157.5°, 202.5°, 247.5°, 292.5°, and 337.5°), and four eccentricities (1°, 3°, 5°, and 7° of visual angle), yielding 32 stimulus locations. The stimulus locations are shown in Figure 1.
Figure 1.
Stimulus and schematic of tasks. Thirty-two locations were probed (4 eccentricities × 8 polar angles). The observer's task was to position the response probe diagonally across the reference probe, while maintaining fixation on the fixation cross. The reference and response probes were viewed through red–green filters matched to their spectral profile; thus, each probe was viewed by a separate eye. Stimuli were flickered to counter interocular suppression in amblyopic observers. Viewed through the filters, the background and stimuli appeared gray.
Procedure and task
All observers were fitted with their best optical correction, seated in a darkened room and viewing was stabilized by a chin-rest. Several practice trials were given after the instructions and before the session began.
Each trial comprised a fixation cross in the center of the screen, a reference probe positioned randomly at one of the 32 predetermined locations, and a response probe positioned on the fixation cross. The reference probe was always the green probe, and for the amblyopic group, always viewed by the fellow eye; the response probe was always viewed by the amblyopic eye. Only the fixation cross was viewed by both eyes. The observer's task was to move the response probe, using the mouse, to a position diagonally across the reference in the opposite hemifield (i.e., across both vertical and horizontal meridians), such that the fixation cross bisected the two points and the three stimuli fell in a straight line. Hence, the task comprised a dual bisection- and alignment judgement, made between the two eyes. The response was registered by pressing the space bar, and the intertrial interval was 200 ms. Response time was unlimited and observers were instructed to maintain fixation at all times. Fixating on the cross while responding ensured that both the reference probe and (the correct position of) the response probe were equally eccentric, reducing the advantage from eye movements to the probes or to other parts of the screen. For amblyopic observers, the experimenter ensured that both dots could be seen and that observers did not report double vision (diplopia) for the fixation cross. Seven trials were run at each of the 32 points, yielding a total of 224 trials per session. A schematic of the task sequence is shown in Figure 1.
Calculation of positional error
Each positional judgement comprised bisection (radial) error and alignment (tangential) error, which was calculated by taking the magnitude of the difference between the radius and polar angle of the correct response from the radius and polar angle of the actual response:
 |
 |
Both types of error were expressed in degrees of visual angle (rerr, θerr), calculated from the errors in pixel units (r̃err, θ̃err). At a screen resolution of 1280 × 1024 pixels and viewing distance of 114 cm, one degree of visual angle corresponded to 64 pixels (i.e., 1.067 arc minutes/pixel), therefore rerr in degrees of visual angle is given by r̃err/64, and θerr in degrees of visual angle is given by θ̃err/64.
Rms error combines radial and tangential error to give an estimate of the overall positional error in each response:
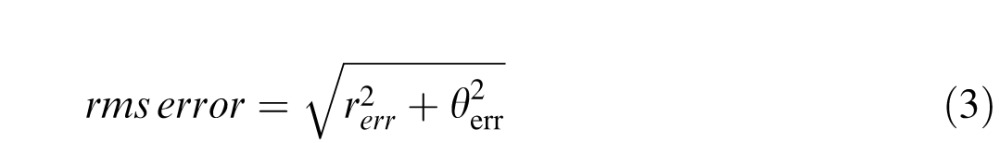 |
For each observer, we calculated the standard deviation of rms error, rerr, and θerr at each eccentricity, pooled across polar angle (i.e., the standard deviation of error at 56 points at each eccentricity). These measures estimate the overall error, and radial and tangential error for each observer at each eccentricity. For each observer, errors that exceeded 2.5 standard deviations of the mean estimate at each eccentricity, and responses that were placed in the opposite hemifield to the correct response were excluded as outliers. This criterion resulted in the exclusion of 2.4% of all responses.
Cortical magnification derived from positional error across the visual field
From Schwartz's (1980) log-conformal model of visual cortex, the topography on the cortex of points in two-dimensional space is given by the complex logarithm of their retinal polar coordinates. The magnitude and argument of the complex value, z, correspond to the radius, r, and angle, θ, of a point in polar coordinates:
 |
In cortex, z is transformed as:
 |
where k is a scaling factor in millimeters, and a quantifies the amount of cortex devoted to the central visual field. The parameter a is independent of the absolute size of visual cortex, and may be used to compare changes in visual topography across individuals and species (Grüsser, 1995; Polimeni, Balasubramanian, & Schwartz, 2006), and across groups of individuals, as we do below. The cortical magnification factor, M, is the magnitude of the complex-valued derivative of Equation 5:
 |
The above two-dimensional formulation of M is sometimes formulated in one dimension as:
 |
where E is eccentricity along a single meridian, and M is the real-valued derivative (e.g., Klein & Levi, 1987; Levi & Klein, 1990).
We define rms error in cortical units at a given location for a given response as:
 |
where w is the cortical position of the correct response, and w* the cortical position of the actual response. We denote the standard deviation of werr at each location, j, as σj.
If positional error on the task follows an M-scaling rule (i.e., is limited by cortical magnification), it should be uniform across the visual field when expressed in cortical units. In other words, σj should be equal at the 32 locations, or the standard deviation of σj across the 32 locations should approach zero. Using Equations 5 and 8, and fixing k to 1, we estimated for each observer that value of a that minimized the standard deviation of σj across the 32 locations tested, normalized by its mean across 32 locations.
Specifically, we minimized:
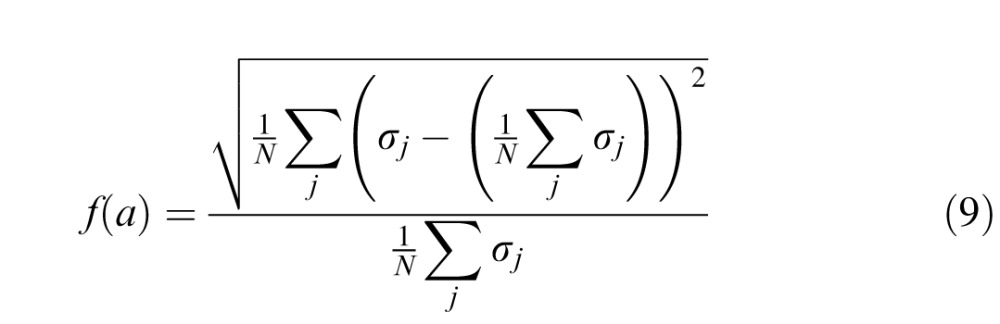 |
using a Nelder-Mead simplex algorithm (fminsearch in MATLAB 7.1). Estimates of a provide a comparison of cortical magnification between normal and amblyopic observers.
Results
Disproportionately large positional error in amblyopic central vision
Performance on the task can be visualized as a map of spatial precision at all probed locations. Figure 2 shows representative performance of a normally sighted observer, and two amblyopic observers (strabismic and anisometropic). Positional accuracy of normally sighted observers was high, and responses were closely clustered around the probed location. Positional accuracy of amblyopic observers was lower than normal, with increased variability at all probed locations. Strabismic observers' responses were shifted in the direction corresponding to their ocular deviation, resulting in a global shift of the entire configuration of responses by an amount consistent with the subjective angle of squint (see Figure 2). A subset of strabismic observers did not show a matching global shift, consistent with a compensatory mechanism that counteracts diplopia in certain cases of squint (i.e., anomalous retinal correspondence; von Noorden & Campos, 2002). Our focus is on the cortical mechanisms producing the variability in positional responses rather than the displacement of the retinal image in the strabismic eye, per se. Thus, for the analyses that follow, rms error was calculated after the average x- and y-offset across all trials and positions was subtracted from each response's x- and y-position, to realign the positional maps to the center of the visual field. This removed the ocular-motor contribution to the positional maps, revealing the purely perceptual errors. The strabismic and anisometropic amblyopes were treated as a single group (amblyopic).
Figure 2.
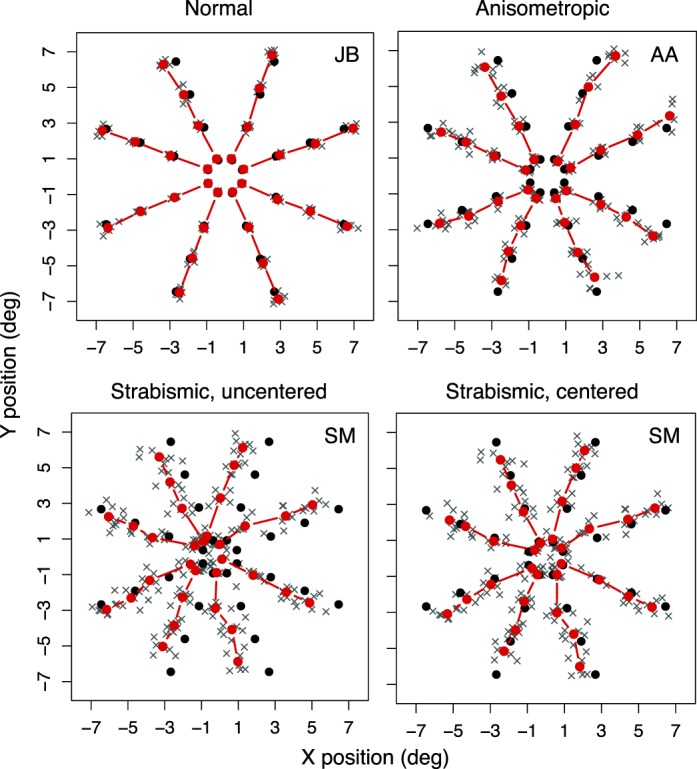
Representative performance of a normally sighted, anisometropic, and strabismic observer on the dichoptic localization task. Solid black symbols show the probed location (correct response), gray crosses show responses on individual trials, and solid red symbols show the mean of individual trials at a given probed location. The strabismic data are shown before and after centering (see Results), and removal of outliers (5% of trials for this observer).
Figure 3A shows radial error, tangential error and rms error against eccentricity for the normal and the amblyopic groups. Error increased with eccentricity for both groups, and the amblyopic group had larger-than-normal error at all eccentricities. F-tests (repeated-measures ANOVAs) were used to evaluate the effect of Group, Eccentricity, and Direction of judgment (radial vs. tangential) on error. Rms error was significantly larger for the amblyopic group than for the normal group, F(1, 58) = 15.67, p = 0.00021, and error increased significantly with eccentricity for both groups, F(3, 174) = 38.805, p < 0.0001. The interaction between Group and Eccentricity was not significant, F(3, 174) = 1.352, p = 0.259. Radial and tangential error were also significantly larger in the amblyopic group than in the normal group, F(1, 58) = 15.58, p = 0.000217, and both types of error increased significantly with eccentricity for the two groups, F(3, 174) = 81.17, p < 0.0001. Radial error was significantly larger than tangential error for both groups, F(1, 58) = 7.358, p = 0.009. No interactions of Group, Eccentricity, or Direction of judgment were significant (p > 0.20).
Figure 3.
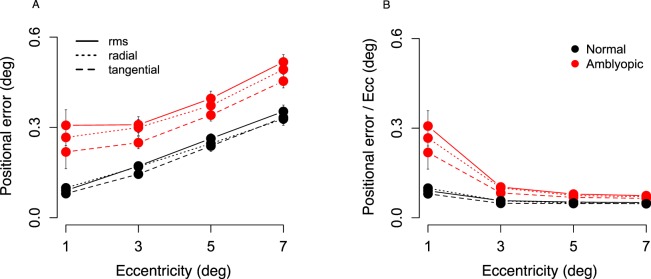
Positional error in degrees of visual angle across eccentricity for normally sighted (N = 20; black traces) and amblyopic (N = 40; red traces) observers. (A) Standard deviation of rms error, radial error, and tangential error at 56 points per eccentricity per observer, averaged for each group. (B) Standard deviation of rms error, radial error, and tangential error divided by eccentricity at the given location. Calculation of the three types of error in text. Error bars show standard error of the mean, smaller than symbol where not shown.
Figure 3B shows rms error, and radial and tangential error divided by the eccentricity at the tested location. Error plotted as a proportion of eccentricity was disproportionately large at 1° and 3° for the amblyopic group. There was a significant interaction between Group and Eccentricity for rms error, F(3, 174) = 23.584, p = 0.0002. The interaction remained significant when rms error at 1° was compared separately with each other eccentricity, and when error at 3° was compared with error at 7° (p < 0.05 for all comparisons). The interaction of Group with Eccentricity was not significant for 3° versus 5°, or 5° versus 7° comparisons. This pattern of results suggests that the amblyopic group performed substantially poorer than normal at 1° and 3° eccentricity than at the other eccentricities, and worst of all, at 1°. In other words, the gradient of error across the visual field differed between the amblyopic and normal group. Exactly the same pattern of results was obtained for radial and tangential error.
Estimates of a
For normal observers, whose positional error rose across the visual field in agreement with the log-conformal model, the average estimate of a was 0.71 (SE = 0.14). For a subset of amblyopes (10 out of 40), positional error did not increase uniformly across the eccentricities tested (e.g., error at 1° was larger than at 3°), and was therefore not consistent with the log-conformal model. For these observers, the fitting procedure produced extremely large estimates of a (range: 9 to >10,000), which would have greatly exaggerated the mean estimate of a and the variance of this group. Hence, these observers were excluded from the remaining analyses. For the remaining 30 observers, the average estimate of a was 2.02 (SE = 0.25). The difference between the estimate of a for the normally sighted group and amblyopic group was significant, t(43.06) = 4.59, p = 3.831e–05.
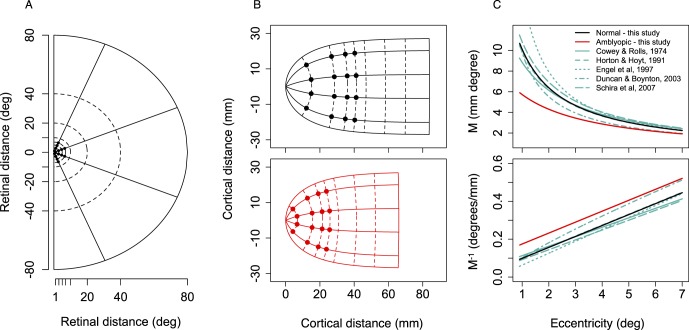
Figure 4 shows the effect of changes in a on the shape and extent of the retinotopic map of visual space. Retinal coordinates in one half of the visual field (Figure 4A) are represented in one hemisphere of cortex (Figure 4B) using Equation 5 (assuming k = 17.3 mm; Horton & Hoyt, 1991). Isoeccentric points are arranged approximately vertically, and isopolar points are arranged horizontally. The map is compressed for the amblyopic group particularly at the inner eccentricities (up to 4.3 mm at 1°), and the overall extent of visual cortex is shorter. The implications of this finding are discussed later.
Figure 4.
Effect of changes in a on the retinotopic map. (A) Retinal map of right visual field extending to 80° of visual angle. Positions probed in task are shown as small black dots within 10° eccentricity. (B) Log-conformal cortical representation of the right visual field in the left hemisphere, given by imaginary versus real part of the complex logarithm of retinal polar coordinates (Equation 5), scaled by k = 17.3 mm (Horton & Hoyt, 1991), and centered on the fovea, that is, k ∗ log(z + a) − k ∗ log(a). Top: Normally sighted group (a = 0.71); positions probed in task shown as black symbols. Bottom: Amblyopic group (a = 2.02); positions probed in task shown as red symbols. Larger values of a shrink the retinotopic map, particularly at the inner eccentricities. (C) Top: Magnification factor, M, given by Equation 7 (M = k/E + a), plotted against eccentricity for the two groups alongside M reported in five other studies. Cowey and Rolls (1974): M = 0.063 + 0.05E; Horton and Hoyt (1991): M = 17.3/(E + 0.75); Engel et al. (1997): M = 1/(E + 0.063); Duncan and Boynton (2003): M = 9.81*E–0.83; Schira et al. (2007): M = 19.2/(E + 0.77). Bottom: M−1 for groups in the present study and the five studies shown in top panel.
Figure 4C shows M and M−1 derived from a (using Equation 7, the one-dimensional formulation, and assuming k = 17.3 mm) against eccentricity for the two groups alongside magnification data from five other studies (Figure 4C). Cowey and Rolls's measurements were based on the distribution of phosphenes evoked in a single subject by stimulation of visual cortex (Cowey & Rolls, 1974). Horton and Hoyt's measurements were derived from visual field deficits in humans with lesions to the occipital lobe (Horton & Hoyt, 1991). Engel, Glover, and Wandell (1997), Duncan and Boynton (2003), and Schira, Wade, and Tyler (2007) used functional magnetic resonance imaging (fMRI) to map the primary visual cortex in humans using standard retinotopic stimuli. The normal magnification function estimated in the present study is superimposed on the estimates of the five previous studies. The estimate of the magnification function for the amblyopic group is outside this normal range.
Dichoptic versus monocular measurement
The above estimates were based on positional judgements made under dichoptic viewing conditions. The advantage of dichoptic viewing is that it enables perception of stimuli through the amblyopic eye that would otherwise be suppressed by the fellow eye, while the eyes remain in their habitual viewing position. The disadvantage of this method is that it introduces an additional (constant) error into the positional judgements of strabismic subjects because their responses are shifted in the direction of their ocular deviation. This shift is absent during monocular viewing because the deviating eye takes up central fixation when the fellow eye is occluded.
To assess whether comparable estimates of a are obtained under dichoptic and monocular viewing conditions, we repeated the same measurements under monocular viewing conditions for nine amblyopic observers (seven old subjects and two new subjects). The display conditions for the monocular task were exactly the same as during dichoptic viewing, except that both dots were set to pass through one filter. The observers did the task in three viewing conditions: dichoptically, monocularly with the amblyopic eye, and monocularly with the fellow eye (with the other eye occluded with an eye patch). Table 2 gives estimates of the parameter a for all observers in the three conditions. The mean estimate of a for these nine observers was 3.68 (SE = 1.06), 3.29 (SE = 0.73), and 0.86 (SE = 0.12), in the dichoptic, monocular amblyopic, and monocular fellow eye conditions, respectively. A repeated-measured analysis of variance confirmed a significant main effect of viewing condition, F(2, 16) = 4.631, p = 0.026. Post hoc t tests showed that a did not differ between the dichoptic and monocular amblyopic eye conditions, t(14.26) = 0.30, p = 0.76, but that there was a significant difference between the dichoptic and monocular-fellow eye conditions, t(8.218) = 2.63, p = 0.03, and the monocular-fellow and monocular-amblyopic eye conditions, t(8.45) = 3.24 p = 0.011. Hence, similar estimates of magnification were obtained for these subjects when the task was performed monocularly with the amblyopic eye, and when it was performed dichoptically.
Table 2.
Dichoptic versus monocular estimates of a. Notes: *Observers excluded from the dichoptic and monocular analyses; see text for details.
| ID |
Dichoptic |
Monocular amblyopic |
Monocular fellow |
| AB | 1.67 | 2.47 | 0.30 |
| BS | 8.76 | 2.86 | 0.84 |
| CB | 1.67 | 3.01 | 1.29 |
| CM2 | 1.11 | 0.92 | 0.67 |
| DT | 2.26 | 3.81 | 1.19 |
| JO | 9.65 | 4.89 | 0.51 |
| JP | 2.70 | 1.31 | 0.56 |
| RB | 2.51 | 2.05 | 1.34 |
| SM | 2.75 | 8.22 | 0.98 |
| LG* | 66.49 | 2.50 | 0.42 |
| CM1* | >10,000 | 2.37 | 0.64 |
| KA* | >10,000 | 2.07 | 0.11 |
For three of the 10 observers who were excluded from the dichoptic analyses due to unusually large estimates of a (also excluded from the monocular analyses reported above), the fitting procedure produced reasonable estimates of a when the task was performed monocularly with the amblyopic eye and the fellow eye (see Table 2). Hence, for certain subjects, dichoptic viewing introduced nonuniform error into the positional judgments that could not be corrected by removing the constant error due to the ocular deviation (i.e., by mean centering the data). This difference between the dichoptic and monocular conditions did not depend on the angle of squint or the visual acuity of the amblyopic eye (see Table 1 for clinical details of these observers).
Overall, the dichoptic estimates of a were consistent with the estimates obtained when viewing monocularly with the amblyopic eye. In all cases, the estimates of a were larger when measured monocularly with the amblyopic eye than when measured monocularly with the fellow eye. In a subset of subjects, measurements using the monocular method produced more reasonable estimates of a than when the task was performed dichoptically.
Discussion
We used the complex logarithmic mapping between retinal and cortical space to estimate cortical magnification in normally sighted and amblyopic individuals, using error in positional localization at 32 locations in the visual field. Our approach was based on the premise that error on this positional localization task is limited by cortical sampling for both groups, and that the retinotopic map in amblyopia is log conformal as in normal observers (see Conner, Odom, Schwartz, & Mendola, 2007, for evidence that basic retinotopic layout is intact in amblyopic subjects). For the normally sighted group, the results suggest that this was a reasonable approach: estimates of magnification were similar to those reported using physiological (Horton & Hoyt, 1991; Slotnick, Klein, Carney, & Sutter, 2001; Duncan & Boynton, 2003; Schira et al., 2007) and other behavioral methods (Levi et al., 1985; Klein & Levi, 1987; Grüsser, 1995). The estimate of magnification for the amblyopic group was significantly lower than the normal group. For the groups tested in this study, our calculations predict a difference in cortical magnification of 4.40 mm deg−1 at 1° eccentricity. By 3° eccentricity, this difference between normally sighted and amblyopic observers declines to 1.21 mm deg−1. The estimates of magnification also differed between the amblyopic eye and the fellow eye of amblyopic subjects, consistent with an earlier prediction based on orientation discrimination performance in the fovea and periphery of amblyopic subjects (Vandenbussche et al., 1986). We first discuss the implications of this result if cortical magnification is indeed altered in amblyopia. We then consider other neural changes that may have affected the estimate of cortical magnification for the amblyopic group.
Size changes of visual cortex
A difference in foveal magnification between normally sighted and amblyopic observers implies concomitant changes in eccentric cortex. If a constant scaling factor k is assumed (as we do in Figure 4), one consequence of reduced foveal magnification is that the size of the cortex would differ across groups, being smaller than normal in amblyopic individuals (linear extent of 66 mm for amblyopes vs. 84 mm for normal observers, for visual field extending to 90° of visual angle, at the present estimates of a). Alternatively, the overall size of V1 could be preserved across groups through an increase in the scaling factor, k, for the amblyopic observers. The increase in k needed to equate the horizontal extent of V1 (from 0° to 90° eccentricity) across groups, given the estimates of a for each group, can be calculated from the ratio:
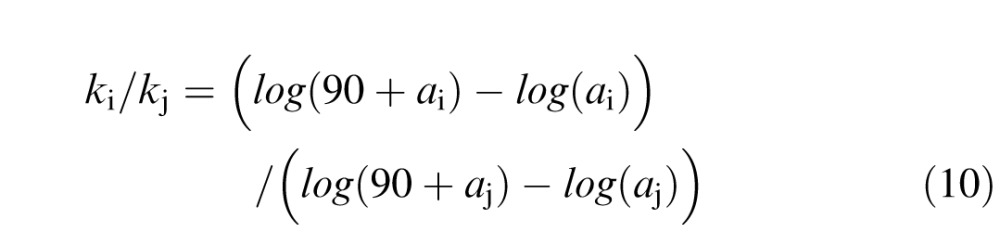 |
giving a ratio of 1.27 for normal to amblyopic k (i.e., 22 mm for the amblyopic group compared to the assumed k of 17.3 mm for normal observers). Such rescaling would equate V1 size between groups but still produce smaller-than-normal magnification at the fovea for the amblyopic observers.
Hence, alterations in magnification would be compatible with two alternatives: either both cortical magnification and V1 size could differ between normally sighted and amblyopic individuals, or changes in cortical magnification could be accompanied by changes in the scaling factor k, to preserve the size of V1 between groups. Note that reductions in cortical magnification of the central field, or reductions in the size of cortex do not entail a change in the organization of cortical topography (see Figure 4b). The intrinsic organization of visual cortex (i.e., the shape and retinotopic layout) varies little across individuals and species (Hinds et al., 2008), despite large individual differences in size (Stensaas, Eddington, & Dobelle, 1974; Andrews, Halpern, & Purves, 1997; Schira et al., 2007). Indeed, the size of V1 can vary by more than a factor of two across individuals, and has been shown to correlate positively with cortical magnification (Tootell, Switkes, Silverman, & Hamilton, 1988; Schira et al., 2007) and with vernier acuity (Duncan & Boynton, 2003; Harvey & Dumoulin, 2011). With this in mind, it is plausible that systematic differences in V1 magnification factor and/or size may exist between groups of individuals differentiated on the integrity of visual experience during development.
At least two studies have reported reductions in gray (and white) matter volume in children and adults with amblyopia (Chan et al., 2004; Li et al., 2013). Using voxel-based morphometry, both Chan et al. (2004) and Li et al. (2013) found reductions in gray matter volume in striate and extrastriate visual areas of amblyopic adults compared to normal controls, accompanied by increases in volume of oculomotor and frontal areas. These authors interpreted this pattern of gray matter changes as plasticity in intact systems compensating for the central visual deficit. Another imaging study has suggested that the boundary between areas V1 and V2 differs during activation of the amblyopic eye compared to the fellow eye, such that the overall extent of V1 is reduced during activation of the amblyopic eye (Li, Dumoulin, Mansouri, & Hess, 2007). Taken together, morphometric changes in V1 are not implausible, but more direct investigations are needed.
Relation to E2
Our estimates of cortical magnification (M) were derived from the parameter a in the two-dimensional mapping between retinal and cortical space. The parameter a along a single horizontal direction is sometimes termed E2, and is calculated as the horizontal intercept of a regression line of threshold against eccentricity (Levi et al., 1985; Klein & Levi, 1987). Values of E2 vary across tasks, but within the regime used here (i.e., beyond 0.5° eccentricity, at large stimulus separations) E2 is approximately 0.7–0.9 (Klein & Levi, 1987; Beard, Levi, & Klein, 1997; Slotnick et al., 2001). We computed E2 from our data by calculating the horizontal intercept of the linear regression line of σj against eccentricity, averaged across polar angle, for each observer (for those 30 observers to whom the log-conformal model was fit). Table 3 gives mean values of a and E2 for both groups. E2 for normally sighted observers (mean = 1.09; SE = 0.23) was in the previously reported range; E2 for amblyopic observers was significantly higher, mean = 2.28; t(47.96) = 3.23, p = 0.002. Estimates of E2 and a were highly correlated within observers, r (48) = 0.82, p = 0.0001. Therefore, although the two methods of deriving cortical magnification differ in whether they consider the one- or two-dimensional magnification function, both predict reduced foveal cortical magnification for the amblyopic group.
Table 3.
a and E2 for the three groups. Notes: Mean and standard error of the mean (SEM) shown.
| Estimate |
Normal |
Amblyopic |
| a | 0.71 (0.14) | 2.02 (0.25) |
| E2 | 1.09 (0.23) | 2.28 (0.28) |
Aetiology of amblyopia
Strabismic (misalignment of visual axes) and anisometropic (chronic unilateral blur) amblyopia are differentiated on tasks such as positional acuity and suprathreshold contrast discrimination, suggesting aetiologically distinct functional losses (Hess & Bradley, 1980; Levi & Klein, 1982; Hess & Pointer, 1985). Whereas central vision in strabismic amblyopia is modeled on the properties of normal peripheral vision, in anisometropic amblyopia it is better accounted for by blur (Levi, Whitaker, & Provost, 2009). On the other hand, suprathreshold spatial distortions have been reported for both types of amblyopia (Barrett, Pacey, Bradley, Thibos, & Morrill, 2003). We found no significant difference in a between the strabismic and anisometropic amblyopes tested here, t(8.133) = 1.35, p = 0.21, suggesting that certain neural changes—to cortical topography, for instance—may be independent of the aetiology of visual deprivation.
Other properties of cortical architecture
Receptive fields
Could other properties of the neural architecture besides cortical magnification contribute to the positional error measured here? Changes in receptive field size, number, and regularity are thought to underlie amblyopic visual deficits measured at threshold. The current task used uncrowded, high contrast, spatially broadband dots, well above the resolution limit and at large stimulus separations. These stimulus conditions are outside the threshold range limited by properties of spatial filtering mechanisms, particularly receptive field size. Therefore, it is unlikely that explanations based on receptive field size alone could account for the present data. Furthermore, where evidence for enlarged receptive fields in the amblyopic fovea has been found, the enlargement underestimates the actual behavioral loss (Swindale & Mitchell, 1994; Kiorpes et al., 1998). Finally, foveal visual acuity is not well correlated with positional accuracy across the visual field. LogMAR acuity of the amblyopic observers was not significantly correlated with the parameter a, r = 0.15, t(28) = 0.79, p = 0.43, or with average rms error across the visual field, r = 0.15, p = 0.37, consistent with a previous study that measured positional distortions in amblyopes (Mansouri, Hansen, & Hess, 2009).
Amblyopia has also been modeled without changes in receptive field size: as reductions in receptive field number (i.e., undersampling; Levi et al., 1987), irregularity in receptive field spacing and wiring (i.e., miscalibration; Hess & Field, 1994; Mansouri et al., 2009), a combination of the two (Wilson, 1991), and as changes in feedforward and feedback connectivity in thalamo-striate and striate-extrastriate networks (Li et al., 2011). An alteration in cortical magnification, arising from asynchronous binocular input is not incompatible with these models, and would arise if, for instance, the central visual field was more undersampled (or anomalously wired) than the peripheral visual field. Note also that the changes in cortical magnification may not be confined to the primary visual cortex. Topologic organization of the visual field is present in the lateral geniculate nucleus and in extrastriate areas, both of which are implicated in the amblyopic deficit (Barnes, Hess, Dumoulin, Achtman, & Pike, 2001; Anderson & Swettenham, 2006; Hess, Thompson, Gole, & Mullen, 2010).
Ocular dominance columns
Experimental amblyopia (i.e., amblyopia induced in animals through eyelid suture during the critical period of development) alters the balance of eye-specific information in cortex, through changes in the relative widths of ocular dominance columns representing monocular visual input (Löwel, 1994). Column shrinkage is the primary model for deprivation amblyopia induced early in life. Could columnar alterations account for the exaggerated positional deficits of amblyopes in this study? The evidence on this issue is unclear. On one hand, studies suggest that column shrinkage does not occur in human amblyopia, and more generally, in late-onset amblyopia. In humans, the onset of amblyopia frequently occurs after two years of age, providing a window of experience that may preserve the columnar structure already present at birth. Human postmortem studies have found no shrinkage of columns in an anisometropic and a strabismic amblyope (Horton & Stryker, 1993; Horton & Hocking, 1996), and only slight alterations in column width were found in animals induced with deprivation relatively late after birth (Sengpiel et al., 1998). Furthermore, the substantial variation in column structure is not well correlated with visual function. Periodicity and width of columns can vary across a twofold range, both within and across species (Adams et al., 2007), without apparent functional significance. In squirrel monkeys for instance, columns are sometimes present only in parts of striate cortex, or are entirely absent, with no consequences for stereoacuity. Indeed, squirrel monkeys achieve stereo thresholds comparable to human thresholds, suggesting that columnar organization is not critical (Horton, 2006). Similarly, in humans, overlapping inputs from each eye confer no functional advantage or disadvantage for positional tasks. Positional acuity in the region of the visual field corresponding to the monocular crescent (where columnar structure is absent) is not different than acuity from adjacent regions comprising interlaced input from the two eyes (Westheimer, 1982).
On the other hand, properties of global retinotopic organization (e.g., vertical–horizontal anisotropy) have been linked to the layout of local feature representations such as orientation and ocular dominance (Blasdel & Campbell, 2001; Yu et al., 2005), suggesting that a change in magnification ought to covary with changes in column width. One index of the contribution of column changes to positional error in amblyopes might be the difference between radial error and tangential error across the visual field. In humans, bisection thresholds are elevated in the radial (isopolar) direction compared to the tangential (isoeccentric) direction, and this elevation is thought to arise from columnar organization (Yap, Levi, & Klein, 1987). Columns run along isoeccentric contours parallel to the vertical meridian, with boundary crossings in the isopolar direction, and it has been proposed that thresholds are elevated when stimuli are offset across, rather than within column boundaries (Yap et al., 1987). With the above in mind, if column width (or radial error in the present task) were to account for the amblyopic data, radial error should be disproportionately larger than tangential error for the amblyopes compared to normal subjects, particularly at 1°. However, radial and tangential errors in the present task were associated with different positional judgements (bisection and alignment); therefore the data are difficult to interpret within this framework. Although changes in ocular dominance structure cannot be ruled out, overall, such changes to explain the results would have to be larger in the inner visual field than in the periphery of amblyopic subjects.
Caveats
Eye movements, fixational stability, attentional shifts and age
Cortical magnification was calculated from error on a positional localization task that required central fixation while stimuli were localized parafoveally. Subjects were told to maintain fixation at all times, but eye movements were not measured. In fact, it was clear that strabismic subjects fixated away from the true location of the fixation cross with their amblyopic eye, in the direction consistent with their angle of squint (e.g., Figure 2). Furthermore, fixation of the amblyopic group was almost certainly more unstable than that of the normal group (Schor & Levi, 1980; González, Wong, Niechwiej-Szwedo, Tarita-Nistor, & Steinbach, 2012). We suggest that the absence of eye movement measures and the differences in fixational stability between groups are not problematic for the main findings of this study. First, positional error increased from the center to the periphery for all subjects, as would be expected if subjects were accurately fixating. Second, any constant error introduced by eccentric fixation was removed from the data prior to the analyses. Third, normal variation in fixational stability as well as pronounced nystagmus (e.g., in albinism and in rod-monochromats), does not affect the accuracy of retinotopic mapping with techniques that require steady central fixation (e.g., fMRI; Baseler et al., 2002; Hoffmann, Tolhurst, Moore, & Morland, 2003; Crossland, Morland, Feely, von dem Hagen, & Rubin, 2008). For differences in fixational stability or attention to have produced the results shown here, such instability or shifts in attention would have to be disproportionately larger for centrally presented probes than for peripherally presented probes, which is inconsistent with the properties of the task: In this task, fixational or attentional shifts, if any, would be greater at large (and not small) eccentricities, when judgments were made across a wider spatial extent, producing more error in the periphery and not the center. Even if subjects' fixation alternated between probes, the positional judgment would ultimately be made with reference to a peripheral point. For the above reasons, we suggest that eye movements, and attentional or fixational differences between groups, do not explain the results. The amblyopic group was on average, 12 years older than the normal group, introducing age as another possible confound (although both groups can be considered relatively young: mean 38 years vs. 26 years). Older subjects are reported to show less cortical activation than younger subjects (Crossland et al., 2008), but age is not known to affect the cortical magnification function (see Conner, Sharma, Lemieux, & Mendola, 2004, for children vs. adults), or to produce a selective central visual field impairment (except in macular degeneration, which the observers in this study did not have). To examine any such effects of age, we conducted an analysis of covariance (ANCOVA), with a as the dependent measure, Age as a covariate and Group as a between-subjects factor. The analysis showed a significant main effect of Age, F(1, 46) = 5.80, p = 0.02, and a significant main effect of Group, F(1, 46) = 11.03, p = 0.001, confirming that age was correlated with the dependent measure, but that the group difference remained significant after controlling for the effect of age. The homogeneity of slopes assumption was confirmed by the absence of a significant interaction between Group and Age, F(1, 46) = 2.80, p = 0.15. Hence, age does not account for the difference in the estimate of cortical magnification between the normally sighted and amblyopic subjects.
Summary
We have shown how cortical magnification can be derived from positional acuity at multiple points in the visual field, and that the estimates for normally sighted individuals using this method are consistent with estimates obtained from physiological techniques. Our results from the amblyopic group suggest that in addition to changes in local cortical properties that have been reported in other studies, there may be changes in the global retinotopic map. Alterations in global retinotopy in amblyopia are perhaps less surprising given the interdependence between properties of cortical maps at local and global scales (Yu et al., 2005). The results are consistent with work showing that the refinement of retinotopic maps depends on the quality and timing of correlated binocular visual input during the critical period (Smith & Trachtenberg, 2007; Zhang, Ackman, Xu, & Crair, 2012). In monocularly deprived mice, retinotopy is disrupted both in ipsilateral and contralateral cortex, suggesting that normal mapping cannot be preserved by input through the fellow eye alone (Adams et al., 2007). In amblyopic humans, fMRI retinotopic mapping studies have shown differences in the overall amount of activation in cortical areas corresponding to the preferred and amblyopic eye (Barnes et al., 2001; Algaze, Roberts, Leguire, Schmalbrock, & Rogers, 2002; Anderson & Swettenham, 2006; Lerner et al., 2006; Conner et al., 2007), but we are not aware of studies that have examined systematic changes in cortical magnification or V1 size. For such changes to be detected, future neuroimaging studies will need to compare large numbers of normal and amblyopic individuals, given the existing variability in cortical size (and magnification) in the normal population.
Other investigators have used similar methods to map spatial distortions in the visual field of amblyopes (Fronius & Sireteanu, 1989; Sireteanu & Fronius, 1989; Mansouri et al., 2009; see also Pugh, 1958; Hess, Campbell, & Greenhalgh, 1978; Bedell & Flom, 1981, 1983; Barrett et al., 2003, for monocular spatial distortions measured using different methods). These studies single out effects of visual deprivation that are not confined to the fovea, and which differ qualitatively from the resolution and contrast deficits that are more often studied in this group. We have quantified this extrafoveal, suprathreshold deficit and interpreted it for the first time within a simple, established model of cortical topography to suggest a role for early visual input in the development of retinotopic maps in cortex. More elaborate models of cortical topography that do not assume isotropy and provide a more anatomically accurate representation of the foveal confluence and extreme periphery may help to refine the effects of different types of early visual experience on cortical maps (Polimeni et al., 2006; Schira et al., 2007).
Acknowledgments
This work was supported by The Leverhulme Trust (grant number RPG-2013-216). Ben Webb was funded by a Wellcome Trust Research Career Development Fellowship. We thank Andrew Astle for refracting amblyopic participants, Denis Schluppeck for discussion on cortical architecture and V1 size, and Eric Schwartz for feedback on the manuscript.
Commercial relationships: none.
Corresponding author: Zahra Hussain.
Email: zahra.hussain@nottingham.ac.uk.
Address: School of Psychology, University of Nottingham, Nottingham, United Kingdom.
Contributor Information
Zahra Hussain, Email: zahra.hussain@nottingham.ac.uk.
Carl-Magnus Svensson, Email: cmgsvensson@gmail.com.
Julien Besle, Email: julien.besle@gmail.com.
Ben S. Webb, Email: ben.webb@nottingham.ac.uk.
Brendan T. Barrett, Email: B.T.Barrett@Bradford.ac.uk.
Paul V. McGraw, Email: paul.mcgraw@nottingham.ac.uk.
References
- Adams D. L., Sincich L. C., Horton J. C. (2007). Complete pattern of ocular dominance columns in human primary visual cortex. Journal of Neuroscience, 27 (39), 10391–10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algaze A., Roberts C., Leguire L., Schmalbrock P., Rogers G. (2002). Functional magnetic resonance imaging as a tool for investigating amblyopia in the human visual cortex: A pilot study. Journal of the American Association for Pediatric Ophthalmology and Strabismus , 6 (5), 300–308. [DOI] [PubMed] [Google Scholar]
- Anderson S. J., Swettenham J. B. (2006). Neuroimaging in human amblyopia. Strabismus , 14 (1), 21–35. [DOI] [PubMed] [Google Scholar]
- Andrews T. J., Halpern S. D., Purves D. (1997). Correlated size variations in human visual cortex, lateral geniculate nucleus, and optic tract. Journal of Neuroscience , 17 (8), 2859–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes G. R., Hess R. F., Dumoulin S. O., Achtman R. L., Pike G. B. (2001). The cortical deficit in humans with strabismic amblyopia. Journal of Physiology , 533 (1), 281–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett B. T., Pacey I. E., Bradley A., Thibos L. N., Morrill P. (2003). Nonveridical visual perception in human amblyopia. Investigative Ophthalmology & Visual Science , 44 (4), 1555–1567, http://www.journalofvision.org/content/15/1/29. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Baseler H. A., Brewer A. A., Sharpe L. T., Morland A. B., Jägle H., Wandell B. A. (2002). Reorganization of human cortical maps caused by inherited photoreceptor abnormalities. Nature Neuroscience , 5 (4), 364–370. [DOI] [PubMed] [Google Scholar]
- Beard B. L., Levi D. M., Klein S. A. (1997). Vernier acuity with non-simultaneous targets: The cortical magnification factor estimated by psychophysics. Vision Research , 37 (3), 325–346. [DOI] [PubMed] [Google Scholar]
- Bedell H. D., Flom M. C. (1981). Monocular spatial distortion in strabismic amblyopia. Investigative Ophthalmology & Visual Science , 20 (2), 263–268, http://www.iovs.org/content/20/2/263. [PubMed] [Article] [PubMed] [Google Scholar]
- Bedell H. E., Flom M. C. (1983). Normal and abnormal space perception. American Journal of Optometry and Physiological Optics , 60 (6), 426–435. [DOI] [PubMed] [Google Scholar]
- Blasdel G., Campbell D. (2001). Functional retinotopy of monkey visual cortex. Journal of Neuroscience , 21 (20), 8286–8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S.-T., Tang K.-W., Lam K.-C., Chan L.-K., Mendola J. D., Kwong K. K. (2004). Neuroanatomy of adult strabismus: A voxel-based morphometric analysis of magnetic resonance structural scans. Neuroimage , 22 (2), 986–994. [DOI] [PubMed] [Google Scholar]
- Conner I. P., Odom J. V., Schwartz T. L., Mendola J. D. (2007). Retinotopic maps and foveal suppression in the visual cortex of amblyopic adults. Journal of Physiology , 583 (1), 159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner I. P., Sharma S., Lemieux S. K., Mendola J. D. (2004). Retinotopic organization in children measured with fMRI. Journal of Vision , 4 (6): 25 509–523, http://www.journalofvision.org/content/4/6/10, doi:10.1167/4.6.10. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Cowey A., Rolls E. T. (1974). Human cortical magnification factor and its relation to visual acuity. Experimental Brain Research , 21 (5), 447–454. [DOI] [PubMed] [Google Scholar]
- Crossland M. D., Morland A. B., Feely M. P., von dem Hagen E., Rubin G. S. (2008). The effect of age and fixation instability on retinotopic mapping of primary visual cortex. Investigative Ophthalmology & Visual Science , 49 (8), 3734–3739, http://www.iovs.org/content/49/8/3734. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Whitteridge D. (1961). The representation of the visual field on the cerebral cortex in monkeys. Journal of Physiology , 159, 203–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasdo N. (1977). The neural representation of visual space. Nature , 266 (5602), 554–556. [DOI] [PubMed] [Google Scholar]
- Duncan R. O., Boynton G. M. (2003). Cortical magnification within human primary visual cortex correlates with acuity thresholds. Neuron , 38 (4), 659–671. [DOI] [PubMed] [Google Scholar]
- Engel S. A., Glover G. H., Wandell B. A. (1997). Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cerebral Cortex , 7 (2), 181–192. [DOI] [PubMed] [Google Scholar]
- Fronius M., Sireteanu R. (1989). Monocular geometry is selectively distorted in the central visual field of strabismic amblyopes. Investigative Ophthalmology & Visual Science , 30 (9), 2034–2044, http://www.iovs.org/content/30/9/2034. [PubMed] [Article] [PubMed] [Google Scholar]
- González E. G., Wong A. M. F., Niechwiej-Szwedo E., Tarita-Nistor L., Steinbach M. J. (2012). Eye position stability in amblyopia and in normal binocular vision. Investigative Ophthalmology & Visual Science , 53 (9), 5386–5394, http://www.iovs.org/content/53/9/5386. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Grüsser O. J. (1995). Migraine phosphenes and the retino-cortical magnification factor. Vision Research , 35 (8), 1125–1134. [DOI] [PubMed] [Google Scholar]
- Harvey B. M., Dumoulin S. O. (2011). The relationship between cortical magnification factor and population receptive field size in human visual cortex: Constancies in cortical architecture. Journal of Neuroscience , 31 (38), 13604–13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R. F., Bradley A. (1980). Contrast perception above threshold is only minimally impaired in human amblyopia. Nature , 287 (5781), 463–464. [DOI] [PubMed] [Google Scholar]
- Hess R. F., Campbell F. W., Greenhalgh T. (1978). On the nature of the neural abnormality in human amblyopia: Neural aberrations and neural sensitivity loss. Pflügers Archiv , 377 (3), 201–207. [DOI] [PubMed] [Google Scholar]
- Hess R. F., Field D. J. (1994). Is the spatial deficit in strabismic amblyopia due to loss of cells or an uncalibrated disarray of cells? Vision Research , 34 (24), 3397–3406. [DOI] [PubMed] [Google Scholar]
- Hess R. F., Pointer J. S. (1985). Differences in the neural basis of human amblyopia: The distribution of the anomaly across the visual field. Vision Research , 25 (11), 1577–1594. [DOI] [PubMed] [Google Scholar]
- Hess R. F., Thompson B., Gole G. A., Mullen K. T. (2010). The amblyopic deficit and its relationship to geniculo-cortical processing streams. Journal of Neurophysiology , 104 (1), 475–483. [DOI] [PubMed] [Google Scholar]
- Hinds O., Polimeni J. R., Rajendran N., Balasubramanian M., Wald L. L., Augustinack J. C., Schwartz E. L. (2008). The intrinsic shape of human and macaque primary visual cortex. Cerebral Cortex , 18 (11), 2586–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M. B., Tolhurst D. J., Moore A. T., Morland A. B. (2003). Organization of the visual cortex in human albinism. Journal of Neuroscience , 23 (26), 8921–8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. C. (2006). Ocular integration in the human visual cortex. Canadian Journal of Ophthalmology , 41 (5), 584–593. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Hocking D. R. (1996). Pattern of ocular dominance columns in human striate cortex in strabismic amblyopia. Visual Neuroscience , 13 (4), 787–795. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Hoyt W. F. (1991). The representation of the visual field in human striate cortex: A revision of the classic Holmes map. Archives of Ophthalmology , 109 (6), 816–824. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Stryker M. P. (1993). Amblyopia induced by anisometropia without shrinkage of ocular dominance columns in human striate cortex. Proceedings of the National Academy of Sciences, USA , 90 (12), 5494–5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D., Wiesel T. N. (1962). Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. Journal of Physiology , 160, 106–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L., Kiper D. C., O'Keefe L. P., Cavanaugh J. R., Movshon J. A. (1998). Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. Journal of Neuroscience , 18 (16), 6411–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S. A., Levi D. M. (1987). Position sense of the peripheral retina. Journal of the Optical Society of America A , 4 (8), 1543–1553. [DOI] [PubMed] [Google Scholar]
- Lerner Y., Hendler T., Malach R., Harel M., Leiba H., Stolovitch C., Pianka P. (2006). Selective fovea-related deprived activation in retinotopic and high-order visual cortex of human amblyopes. Neuroimage , 33 (1), 169–179. [DOI] [PubMed] [Google Scholar]
- Levi D. M., Klein S. (1982). Hyperacuity and amblyopia. Nature , 298 (5871), 268–270. [DOI] [PubMed] [Google Scholar]
- Levi D. M., Klein S. A. (1990). The role of separation and eccentricity in encoding position. Vision Research , 30 (4), 557–585. [DOI] [PubMed] [Google Scholar]
- Levi D. M., Klein S. A., Aitsebaomo A. P. (1985). Vernier acuity, crowding and cortical magnification. Vision Research , 25 (7), 963–977. [DOI] [PubMed] [Google Scholar]
- Levi D. M., Klein S. A., Yap Y. L. (1987). Positional uncertainty in peripheral and amblyopic vision. Vision Research , 27 (4), 581–597. [DOI] [PubMed] [Google Scholar]
- Levi D. M., Whitaker D., Provost A. (2009). Amblyopia masks the scale invariance of normal central vision. Journal of Vision , 9 (1): 25 1–11, http://www.journalofvision.org/content/9/1/22, doi:10.1167/9.1.22. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Li Q., Jiang Q., Guo M., Li Q., Cai C., Yin X. (2013). Grey and white matter changes in children with monocular amblyopia: Voxel-based morphometry and diffusion tensor imaging study. British Journal of Ophthalmology , 97 (4), 524–529. [DOI] [PubMed] [Google Scholar]
- Li X., Dumoulin S. O., Mansouri B., Hess R. F. (2007). The fidelity of the cortical retinotopic map in human amblyopia. European Journal of Neuroscience , 25 (5), 1265–1277. [DOI] [PubMed] [Google Scholar]
- Li X., Mullen K. T., Thompson B., Hess R. F. (2011). Effective connectivity anomalies in human amblyopia. Neuroimage , 54 (1), 505–516. [DOI] [PubMed] [Google Scholar]
- Li Y., Fitzpatrick D., White L. E. (2006). The development of direction selectivity in ferret visual cortex requires early visual experience. Nature Neuroscience , 9 (5), 676–681. [DOI] [PubMed] [Google Scholar]
- Löwel S. (1994). Ocular dominance column development: strabismus changes the spacing of adjacent columns in cat visual cortex. Journal of Neuroscience , 14 (12), 7451–7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W., Constantine-Paton M. (2004). Eye opening rapidly induces synaptic potentiation and refinement. Neuron , 43 (2), 237–249. [DOI] [PubMed] [Google Scholar]
- Mansouri B., Hansen B. C., Hess R. F. (2009). Disrupted retinotopic maps in amblyopia. Investigative Ophthalmology & Visual Science , 50 (7), 3218–3225, http://www.iovs.org/content/50/7/3218. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Peirce J. W. (2007). PsychoPy—Psychophysics software in Python. Journal of Neuroscience Methods , 162 (1–2), 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimeni J. R., Balasubramanian M., Schwartz E. L. (2006). Multi-area visuotopic map complexes in macaque striate and extra-striate cortex. Vision Research , 46 (20), 3336–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh M. (1958). Visual distortion in amblyopia. British Journal of Ophthalmology , 42 (8), 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schira M. M., Wade A. R., Tyler C. W. (2007). Two-dimensional mapping of the central and parafoveal visual field to human visual cortex. Journal of Neurophysiology , 97 (6), 4284–4295. [DOI] [PubMed] [Google Scholar]
- Schor C. M., Levi D. M. (1980). Disturbances of small-field horizontal and vertical optokinetic nystagmus in amblyopia. Investigative Ophthalomology & Visual Science , 19 (6), 668–683, http://www.iovs.org/content/19/6/668. [PubMed] [Article] [PubMed] [Google Scholar]
- Schwartz E. L. (1980). Computational anatomy and functional architecture of striate cortex: A spatial mapping approach to perceptual coding. Vision Research , 20 (8), 645–669. [DOI] [PubMed] [Google Scholar]
- Sengpiel F., Giödecke I., Stawinski P., Huibener M., Liöwel S., Bonhoeffer T. (1998). Intrinsic and environmental factors in the development of functional maps in cat visual cortex. Neuropharmacology , 37 (4–5), 607–621. [DOI] [PubMed] [Google Scholar]
- Sireteanu R., Fronius M. (1989). Different patterns of retinal correspondence in the central and peripheral visual field of strabismics. Investigative Ophthalmology & Visual Science , 30 (9), 2023–2033, http://www.iovs.org/content/30/9/2023. [PubMed] [Article] [PubMed] [Google Scholar]
- Slotnick S. D., Klein S. A., Carney T., Sutter E. E. (2001). Electrophysiological estimate of human cortical magnification. Clinical Neurophysiology , 112 (7), 1349–1356. [DOI] [PubMed] [Google Scholar]
- Smith S. L., Trachtenberg J. T. (2007). Experience-dependent binocular competition in the visual cortex begins at eye opening. Nature Neuroscience , 10 (3), 370–375. [DOI] [PubMed] [Google Scholar]
- Stensaas S. S., Eddington D. K., Dobelle W. H. (1974). The topography and variability of the primary visual cortex in man. Journal of Neurosurgery , 40 (6), 747–755. [DOI] [PubMed] [Google Scholar]
- Swindale N. V., Mitchell D. E. (1994). Comparison of receptive field properties of neurons in area 17 of normal and bilaterally amblyopic cats. Experimental Brain Research , 99 (3), 399–410. [DOI] [PubMed] [Google Scholar]
- Tootell R. B., Switkes E., Silverman M. S., Hamilton S. L. (1988). Functional anatomy of macaque striate cortex. II. Retinotopic organization. Journal of Neuroscience , 8 (5), 1531–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche E., Vogels R., Orban G. A. (1986). Human orientation discrimination: Changes with eccentricity in normal and amblyopic vision. Investigative Ophthalmology & Visual Science , 27 (2), 237–245, http://www.iovs.org/content/27/2/237. [PubMed] [Article] [PubMed] [Google Scholar]
- von Noorden G. K., Campos E. C. (2002). Binocular vision and ocular motility - theory and management of strabismus, 6th edition. St. Louis, MO: Mosby. [Google Scholar]
- Westheimer G. (1982). Do ocular-dominance columns set spatial limits for hyperacuity processing? Vision Research , 22 (11), 1349–1352. [DOI] [PubMed] [Google Scholar]
- Wilson H. R. (1991). Model of peripheral and amblyopic hyperacuity. Vision Research , 31 (6), 967–982. [DOI] [PubMed] [Google Scholar]
- Yap Y. L., Levi D. M., Klein S. A. (1987). Peripheral hyperacuity: Isoeccentric bisection is better than radial bisection. Journal of the Optical Society of America A , 4 (8), 1562–1567. [DOI] [PubMed] [Google Scholar]
- Yu H., Farley B. J., Jin D. Z., Sur M. (2005). The coordinated mapping of visual space and response features in visual cortex. Neuron , 47 (2), 267–280. [DOI] [PubMed] [Google Scholar]
- Zhang J., Ackman J. B., Xu H.-P., Crair M. C. (2012). Visual map development depends on the temporal pattern of binocular activity in mice. Nature Neuroscience , 15 (2), 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]