Abstract
Introduction
The aim of this study was to explore the impact of augmented creatinine clearance and differing minimum inhibitory concentrations (MIC) on piperacillin pharmacokinetic/pharmacodynamic (PK/PD) target attainment (time above MIC (fT>MIC)) in critically ill patients with sepsis receiving intermittent dosing.
Methods
To be eligible for enrolment, critically ill patients with sepsis had to be receiving piperacillin-tazobactam 4.5 g intravenously (IV) by intermittent infusion every 6 hours for presumed or confirmed nosocomial infection without significant renal impairment (defined by a plasma creatinine concentration greater than 171 μmol/L or the need for renal replacement therapy). Over a single dosing interval, blood samples were drawn to determine unbound plasma piperacillin concentrations. Renal function was assessed by measuring creatinine clearance (CLCR). A population PK model was constructed, and the probability of target attainment (PTA) for 50% and 100% fT>MIC was calculated for varying MIC and CLCR values.
Results
In total, 48 patients provided data. Increasing CLCR values were associated with lower trough plasma piperacillin concentrations (P < 0.01), such that with an MIC of 16 mg/L, 100% fT>MIC would be achieved in only one-third (n = 16) of patients. Mean piperacillin clearance was approximately 1.5-fold higher than in healthy volunteers and correlated with CLCR (r = 0.58, P < 0.01). A reduced PTA for all MIC values, when targeting either 50% or 100% fT>MIC, was noted with increasing CLCR measures.
Conclusions
Standard intermittent piperacillin-tazobactam dosing is unlikely to achieve optimal piperacillin exposures in a significant proportion of critically ill patients with sepsis, owing to elevated drug clearance. These data suggest that CLCR can be employed as a useful tool to determine whether piperacillin PK/PD target attainment is likely with a range of MIC values.
Introduction
Effective antibacterial therapy is crucial for improving outcomes for patients with sepsis [1]. Current international guidelines stress the importance of early administration of broad-spectrum agents [2], with the caveat that significant dose adjustment may be required in patients who are critically ill. This is a reflection of the unique physiology often encountered in these patients [3], which may dramatically distort pharmacokinetics (PK). Changes in antibacterial volume of distribution, plasma protein binding and drug clearance are well described in the literature [4]. They lead to poorly predictable and often subtherapeutic plasma concentrations [5].
The minimum antibacterial exposure required in clinical practice to maximize bacterial killing and optimize clinical outcomes remains controversial. For β-lactams, preclinical studies support maintaining prolonged free drug concentrations above the minimum inhibitory concentration (MIC) of the likely pathogen [6]. In vivo animal data indicate that this period should be at least 40% to 70% of the dosing interval (40% to 70% fT>MIC) [7], although various retrospective clinical evaluations have recommended more aggressive targets, such as 100% fT>MIC [8] and trough concentration to MIC ratios (Cmin:MIC) >5 [9]. More recently, in a large multinational point prevalence study of antibacterial concentrations in critical illness, researchers demonstrated that clinical failure is three times more likely when β-lactam exposure is less than 50% fT>MIC [5] and that increasing exposures are associated with an increased likelihood of clinical cure.
Whereas these data demonstrate why achievement of target drug exposures is important, they do not robustly define which patients are at risk of subtherapeutic dosing. Clinicians commonly consider the likely pathogen, its susceptibility profile and the potential for drug toxicity when selecting an empirical dosing regimen. An assessment of renal function is common, with evidence of acute or chronic renal impairment often triggering dose reduction to avoid drug accumulation. For β-lactams, the converse—dose escalation in the setting of augmented renal function—has been infrequently reported in clinical practice.
Previously published data, however, suggest that patients with augmented renal clearance (ARC), which often manifests as an elevated urinary creatinine clearance (CLCR), are at particular risk of subtherapeutic β-lactam concentrations [10,11]. This, therefore, represents an attractive measure to guide dose selection, although few data are available that integrate CLCR, pathogen susceptibility and drug exposures. As such, the aim of this analysis was to explore the impact of elevated CLCR and different pathogen susceptibilities, on piperacillin pharmacokinetic/pharmacodynamic (PK/PD) target attainment in critically ill patients receiving intermittent dosing.
Material and methods
Setting
This single-centre observational study was undertaken in a tertiary level, university-affiliated intensive care unit (ICU). Ethical approval was obtained from the Royal Brisbane and Women’s Hospital Human Research Ethics Committee (HREC 2007/188), with written informed consent obtained from either the patient or the patient’s nominated substitute decision-maker.
Study population
Patients were eligible for enrolment if they were between 18 and 80 years of age and were receiving piperacillin-tazobactam for the treatment of sepsis (defined as presumed or confirmed nosocomial infection while manifesting a systemic inflammatory response syndrome [12]). Patients were excluded if they (1) did not have an intraarterial line inserted as part of routine management (to allow repeated plasma sampling without additional venipuncture), (2) had renal impairment (defined by a plasma creatinine concentration (CR) greater than 171 μmol/L or the need for renal replacement therapy) or (3) had a history of allergy to piperacillin or iodine. This, therefore, represents a convenience sample of critically ill septic patients admitted to our institution without significant renal impairment.
Study protocol
The protocol pertaining to this study has been published in detail elsewhere [13]. In brief, 4.5 g of piperacillin-tazobactam (Tazocin EF; Pfizer Australia Pty Ltd, West Ryde, NSW, Australia) diluted in 50 ml of 0.9% sodium chloride was administered over 20 minutes as part of the patient’s prescribed course of therapy. Blood samples to determine plasma piperacillin concentrations were drawn predose and at 20 minutes (end of infusion), 40 minutes, 60 minutes, 210 minutes and 360 minutes. All urine samples were collected via an indwelling urinary catheter over the dosing interval, following which urine volume and urinary CR concentration were determined by laboratory analysis. Plasma CR concentrations on the day of investigation were used to calculate CLCR. No specific CLCR value was used to define renal impairment or ARC, as our aim was to use these measures as continuous variables in analysis.
Additional data, including the requirement for mechanical ventilation, vasopressor support, modified Sequential Organ Failure Assessment (SOFA) score (excluding the neurological component) and 24-hour fluid balance, were documented on the day of drug administration. Admission Acute Physiology and Chronic Health Evaluation (APACHE) II score, body mass index (BMI) and ICU and in-hospital mortality were also recorded.
Sample handling, storage and measurement
Blood samples were immediately placed on ice and centrifuged within 60 minutes at 3,000 rpm for 10 minutes. Plasma samples were then stored at −80°C until analysis. An ultraviolet high-performance liquid chromatography (HPLC-UV) assay was used to measure piperacillin concentrations in plasma. All bioanalytical techniques were validated and conducted in accordance with the criteria of the US Food and Drug Administration’s guidance for industry on bioanalysis [14]. To isolate the unbound fraction for analysis, protein-bound piperacillin was removed from the plasma sample with centrifugal filter devices (Centrifree 30,000 NMWL; Merck Millipore, Tullagreen, Ireland). The lower limit of quantification for unbound piperacillin concentrations was 1 mg/L.
Plasma concentration data
The final (360 minutes) plasma sample was taken as the trough unbound piperacillin concentration (Cmin). These samples were then compared to an MIC of 16 mg/L to determine whether 100% fT>MIC would have been achieved. This represents the highest MIC for susceptible bacteria (for example, the Pseudomonas aeruginosa MIC is 16 mg/L for piperacillin/tazobactam) as per the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [15]. We chose to assess the adequacy of dosing in terms of this ‘worst-case scenario’, as MIC data are often not initially available to the clinician. The 100% fT>MIC target was utilized in this analysis, as more aggressive drug exposures have been demonstrated to improve both microbiological and clinical outcomes [5,8,9].
Population pharmacokinetics modelling
Concentration-time data were analysed using nonlinear mixed-effects modelling (NONMEM version 7.1; GloboMax, Ellicott City, MD, USA). A Digital Fortran compiler was used, and the runs were executed using Wings for NONMEM [16]. The first-order conditional estimation method with interaction was used throughout model building. Unbound plasma piperacillin concentrations were fitted to one-, two- or three-compartment linear models using subroutines from the NONMEM library. Between-subject variability (BSV) was evaluated using an exponential variability model. Various models for residual unexplained variability (RUV) were also tested.
Pharmacokinetics model diagnostics
Visual inspection of diagnostic scatter plots and the NONMEM objective function value (OFV) were used to evaluate goodness of fit. Statistical comparison of nested models was undertaken in the NONMEM program using log-likelihood ratios, which are assumed to be χ2-distributed. On the basis of a χ2 test of the difference in OFV, a decrease in the OFV of 3.84 units (P < 0.05) for 1 degree of freedom was considered statistically significant. Decreases in BSV of one of the parameters of at least 10% were also accepted for inclusion in a more complicated model.
Population pharmacokinetics bootstrap
A nonparametric bootstrap method (n = 1,000) was used to study the uncertainty of the pharmacokinetic parameter estimates in the final model. From the bootstrap empirical posterior distribution, we were able to obtain the 95% confidence interval (2.5% to 97.5% percentiles) for the parameters, as described previously [17].
Probability of target attainment
Monte Carlo simulations performed in NONMEM were employed to determine the probability of target attainment (PTA) with varying MIC (2 to 64 mg/L) and CLCR (10 to 300 ml/min in 10-ml/min increments) values using a standard 4.5-g piperacillin-tazobactam dose administered every 6 hours as an intermittent 20-minute infusion. The PTAs for achieving 50% fT>MIC and 100% fT>MIC with piperacillin were calculated.
Cumulative fraction of response
The cumulative fraction of response (CFR) determines the likely success of treatment by comparing the pharmacodynamic exposure (PTA) against the MIC distribution of a specific population of microorganisms [18]. The wild-type MIC distribution for Pseudomonas aeruginosa against piperacillin was obtained from the EUCAST database [15]. The CFR was calculated for both 50% fT>MIC and 100% fT>MIC by using a range of CLCR values. Dosing was a priori considered successful if the CFR was at least 80%.
Statistical analysis
Continuous data are presented as the mean (standard deviation) or median (interquartile range). Categorical data are presented as counts (%). Correlation was assessed by means of a scatter graph and Pearson correlation coefficient (r). For comparisons between groups, we used an independent Student’s t-test, Mann–Whitney U test, or one-way analysis of variance for continuous data, as well as a χ2 or Fisher’s exact test for categorical data, where analysis assumptions were met. A P-value <0.05 was considered statistically significant, and all analyses were performed using SPSS version 21 software (IBM SPSS, Chicago, IL, USA).
Results
Demographic data
Forty-eight patients were included in the analysis. All patients were receiving 4.5 g of piperacillin-tazobactam by intermittent IV infusion over 20 minutes every 6 hours for treatment of presumed or confirmed nosocomial infection. Demographic, admission, anthropometric, illness severity and outcome data are presented in Table 1. Sampling occurred after a median of 9 (4 to 13) doses and, on average, 5.2 (3.7) days postadmission to the ICU. Half of the patients were receiving piperacillin-tazobactam for treatment of nosocomial pneumonia. The cohort was relatively young and overweight with moderate to severe illness severity. The vast majority required invasive mechanical ventilation (>90%), whereas only one-fourth needed vasopressor support. As per the inclusion criteria, plasma CR concentrations were relatively low (79.5 (31.4) μmol/L). The mean CLCR over the study period was 122 (59.2) ml/min, although there was significant variability between patients: 15 patients had a CLCR <90 ml/min, 22 had a CLCR >130 ml/min and 16 had a CLCR >150 ml/min.
Table 1.
Demographic, anthropometric, illness severity and outcome data a
| Variable | N = 48 | ||
|---|---|---|---|
| Age, yr | 47.3 (17.9) | ||
| Male sex, n (%) | 27 (56.3) | ||
| Height, m | 1.70 (0.11) | ||
| Weight, kg | 88.4 (24.2) | ||
| BMI, kg/m2 | 30.7 (8.78) | ||
| BSA, m2 | 1.98 (0.26) | ||
| Admission category/system, n (%) | |||
| Medical | n = 13 | ||
| Cardiac | 1 (7.7) | ||
| Gastrointestinal | 3 (23.1) | ||
| Neurological | 2 (15.4) | ||
| Respiratory | 5 (38.5) | ||
| Primary bacteraemia | 1 (7.7) | ||
| Oncology | 1 (7.7) | ||
| Surgical | n = 13 | ||
| Gastrointestinal | 7 (53.8) | ||
| Gynaecological | 1 (7.7) | ||
| Maxillofacial | 2 (15.4) | ||
| Neurological | 2 (15.4) | ||
| Vascular | 1 (7.7) | ||
| Trauma | n = 22 | ||
| Burns | 6 (27.3) | ||
| Facial | 1 (4.5) | ||
| Abdominal | 4 (18.2) | ||
| Neurological | 5 (22.7) | ||
| Orthopaedic | 3 (13.6) | ||
| Thoracic | 3 (13.6) | ||
| APACHE II score | 19.4 (6.79) | ||
| Modified SOFA score, median (IQR) | 3.5 (2 to 6) | ||
| Mechanical ventilation, n (%) | 45 (93.8) | ||
| Use of vasopressors, n (%) | 12 (25.0) | ||
| Presumed/confirmed site of infection, n (%) | |||
| Intraabdominal | 14 (29.2) | ||
| Skin/soft tissue | 5 (10.4) | ||
| Respiratory | 24 (50.0) | ||
| Urinary | 1 (2.1) | ||
| Primary bacteraemia | 1 (2.1) | ||
| Unknown | 3 (6.3) | ||
| 24-hr fluid balance, ml | 553 (1836) | ||
| Plasma CR, μmol/L | 79.5 (31.4) | ||
| Measured CLCR, ml/min | 122 (59.2) | ||
| ICU length of stay, days | 18.2 (11.5) | ||
| ICU mortality, n (%) | 4 (8.3) | ||
| Hospital mortality, n (%) | 5 (10.4) | ||
aAPACHE, Acute Physiology and Chronic Health Evaluation; BMI, Body mass index; BSA, Body surface area; CLCR, Creatinine clearance; CR, Creatinine; ICU, Intensive care unit; IQR, Interquartile range; SD, Standard deviation; SOFA, Sequential Organ Failure Assessment. Data presented are mean (SD) unless otherwise stated.
Plasma concentration data
In 11 patients, the Cmin value was either below the lower limit of detection (n = 10) or not available (n = 1). Using an MIC of 16 mg/L, piperacillin intermittent infusion would achieve 100% fT>MIC (Cmin >16 mg/L) in only 16 (34.0%) of 47 patients. Older age (P = 0.01), higher modified SOFA score (P < 0.01), lower CLCR (P < 0.01), greater positive 24-hour fluid balance (P = 0.04) and more frequent application of vasopressor therapy (P = 0.01) were characteristics of patients likely to have higher Cmin values, and therefore achieve this PK/PD target. No statistically significant differences were noted in BMI, APACHE II scores or the need for mechanical ventilation. Unbound plasma piperacillin concentration time profiles grouped by CLCR quartile are presented in Figure 1. Higher CLCR was significantly associated with lower unbound trough plasma piperacillin concentration (P < 0.01).
Figure 1.
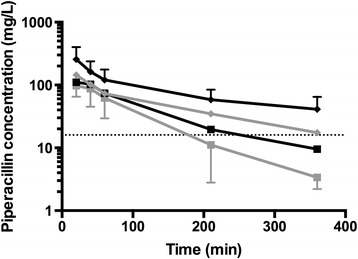
Piperacillin concentrations over time grouped according to creatinine clearance quartile. Mean piperacillin concentration (log10 scale) and time grouped according to creatinine clearance (CLCR) quartile: <68 ml/min (black diamonds), 68 to 114 ml/min (grey diamonds), 115 to 170 ml/min (black squares) and >170 ml/min (grey squares). The dotted line at 16 mg/L represents minimum inhibitory concentration.
Pharmacokinetic model building
The time course of unbound plasma piperacillin concentrations was best described by a two-compartment linear model with combined residual error and BSV on the volume of the central (Vc) and peripheral compartments (Vp), drug clearance (CL) and an infusion lag descriptor (ALAG; defined as the time taken for the residual drug to get through the IV line after completion of the 20-minute infusion, which was included as zero order input). The only covariate that improved the fit of the model was, for piperacillin CL, CLCR normalized to 100 ml/min, which decreased the OFV by 18.62 (P < 0.01). In the final model, this was described as follows:
where TVCL is the typical value of piperacillin CL. The mean parameter estimates from the final covariate model, as well as the 95% confidence intervals from all bootstrap runs, are shown in Table 2. Total volume of distribution (Vd) was calculated as the sum of Vc and Vp. The goodness-of-fit plots were acceptable with an r-value of 0.95 for the observed versus individual predicted concentrations.
Table 2.
Mean parameter estimates and bootstrap mean (95% confidence interval) estimates for the final population pharmacokinetic model a
| Parameter | Model | Bootstrap | |||
|---|---|---|---|---|---|
| Mean | Mean | 95% confidence interval | |||
| 2.5% | 97.5% | ||||
| Fixed effects | |||||
| CL (L/hr) | 16.3 | 16.2 | 14.0 | 18.9 | |
| Vc (L) | 19.9 | 15.9 | 0.7 | 27.8 | |
| Vp (L) | 18.8 | 21.3 | 11.1 | 32.3 | |
| Q (L/h) | 37.3 | 42.9 | 22.0 | 73.3 | |
| ALAG (hr) | 0.8 | 0.13 | 0.01 | 0.31 | |
| Random effects BSV (% CV) | |||||
| CL (L/hr) | 56.0 | 55.3 | 45.2 | 65.4 | |
| Vc (L) | 29.6 | 23.5 | 0.2 | 45.7 | |
| Vp (L) | 67.6 | 69.2 | 24.6 | 158.4 | |
| ALAG (hr) | 0.3 | 0.4 | 0.05 | 0.8 | |
| Random error | |||||
| RUV (% CV) | 1.0 | 1.3 | 0.3 | 3.1 | |
| RUV (SD, mg/L) | 0.3 | 0.3 | 0.2 | 0.4 | |
aALAG, Infusion lag time; BSV, Between-subject variability; CL, Clearance; CV, Coefficient of variation; Q, Intercompartmental clearance; RUV, Residual unexplained variability; SD, Standard deviation; Vc, Volume of the central compartment; Vp, Volume of the peripheral compartment.
Those patients who would fail to achieve 100% fT>MIC (16 mg/L) had higher drug CL (P < 0.01) and no statistically significant difference in piperacillin Vd (P = 0.647). As demonstrated in Figure 2, a moderate correlation was noted between CLCR and piperacillin CL overall (r = 0.58, P < 0.01). The PTAs for 50% fT>MIC and 100% fT>MIC with varying MIC and CLCR values are presented in Figure 3.
Figure 2.
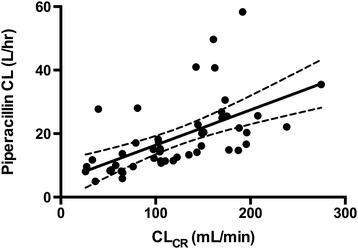
Piperacillin drug clearance compared with creatinine clearance. Scatter plot of piperacillin clearance (CL) and creatinine clearance (CLCR). A linear regression line (solid) with 95% confidence interval (dashed line) has been fitted to the data points (r = 0.58, P < 0.001).
Figure 3.
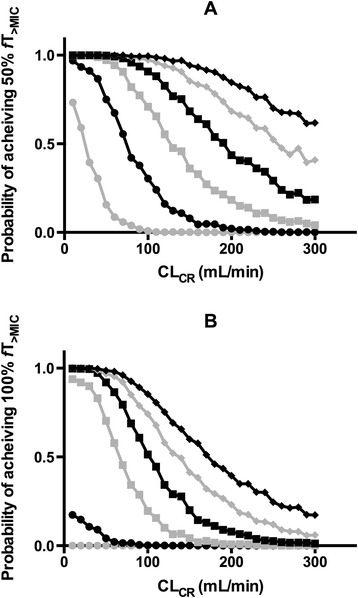
Probability of target attainment with varying creatinine clearance. Graphs depict the probability (%) of attaining 50% (A) and 100% (B) time above the minimum inhibitory concentration (fT>MIC) following a 4.5 g piperacillin-tazobactam dose administered every 6 hours as an intermittent 20-minute infusion, and CLCR values. Varying minimum inhibitory concentrations (MIC) are displayed: 2 mg/L (black diamonds), 4 mg/L (grey diamonds), 8 mg/L (black squares), 16 mg/L (grey squares), 32 mg/L (black circles), and 64 mg/L (grey circles).
The CFR for piperacillin over a range of CLCR values using the EUCAST MIC distribution for Pseudomonas aeruginosa is presented in Table 3.
Table 3.
Cumulative fraction of response for piperacillin over a range of creatinine clearance values a
| CL CR (ml/min) | CFR, 50% f T >MIC (%) | CFR, 100% fT >MIC (%) |
|---|---|---|
| 10 | 87.2 | 81.3 |
| 30 | 86.0 | 80.6 |
| 60 | 83.5 | 72.6 |
| 90 | 78.6 | 57.2 |
| 120 | 71.7 | 40.4 |
| 150 | 62.7 | 28.5 |
| 180 | 55.5 | 20.0 |
| 210 | 46.3 | 13.3 |
| 240 | 40.8 | 9.9 |
| 270 | 31.0 | 6.3 |
| 300 | 28.3 | 4.9 |
a50% fT>MIC, 50% time above the minimum inhibitory concentration; 100% fT>MIC, 100% time above the minimum inhibitory concentration; CFR, Cumulative fraction of response; CLCR, Creatinine clearance. Data represent the CFR when targeting 50% fT>MIC and 100% fT>MIC with a range of CLCR values following a 4.5 g piperacillin-tazobactam dose administered every 6 hours as an intermittent 20 minute infusion. The wild-type MIC distrubiton of Pseudomonas aeruginosa has been employed. Boldface represents those CLCR values where target exposures would be achieved in at least 80% of isolates.
Discussion
In this clinical analysis, we sought to explore the impact of elevated CLCR and different susceptibilities of bacteria on piperacillin PK/PD target attainment when administered by intermittent infusion in a large cohort of critically ill patients with sepsis. With a MIC at the upper limit of the susceptible range for piperacillin (16 mg/L), only about one-third of patients would achieve 100% fT>MIC. The primary mechanism underlying this observation appears to be significantly elevated drug CL in parallel with higher CLCR in some patients. This phenomenon of augmented renal clearance [19,20] is being described in critically ill patients with increasing frequency [21,22], which suggests that clinicians should be wary of conventional dosing in this setting. Indeed, the CFR suggests that only with lower CLCR values (<90 ml/min) will an adequate fraction of Pseudomonas aeruginosa isolates be suitably covered with this dosing regimen.
Pharmacokinetic modelling for the study cohort revealed a mean piperacillin CL of approximately 1.5-fold that reported in healthy volunteers [23]. Similar derangement in piperacillin CL has been reported in previous studies of critically ill patients [24,25], highlighting the unique PK observed in this setting [4]. Of note, piperacillin Vd was also significantly larger overall. Changes in Vd have been observed with other β-lactams in critical illness [26] and likely reflect the substantial fluid shifts frequently encountered [27]. Use of unbound piperacillin concentrations in the PK model may also have contributed to the higher Vd observed, although use of free concentrations is essential to describe the pharmacologically active fraction of piperacillin.
The specific mechanisms underlying ARC in critically ill patients remain uncertain. Researchers have recently identified a modest correlation between cardiac index and CLCR in patients with sepsis [28], suggesting that increased renal perfusion and solute delivery may be a key factor. Younger age and lower illness severity scores have previously been identified as risk factors for ARC [28,29], which is further demonstrated by our study data. Work by Shimamoto et al. highlights the role of systemic inflammation, with an increasing number of systemic inflammatory response syndrome criteria being strongly correlated with higher drug clearance and lower plasma concentrations in nonventilated patients receiving vancomycin [30]. Together, these findings imply an important interaction between systemic inflammation and available organ reserve, factors that are often neglected in antibacterial dosing regimens [31]. As such, the characteristics of patients included in this study (younger age, admission posttrauma and less organ dysfunction) are useful in defining scenarios where a measured CLCR or therapeutic drug monitoring should be employed to determine the need for higher than standard piperacillin dosing.
The prevalence of ARC varies significantly, depending upon the definition employed and institutional case mix [32-35]. In a recent multicentre study of CLCR in critically ill patients with ‘normal’ plasma CR concentrations, researchers identified an incidence of ARC (CLCR ≥130 ml/min/1.73 m2) of approximately 65% in the first 7 days in the ICU [36]. Importantly, patients who manifested ARC on day 1 were significantly more likely to do so on subsequent measures, indicating that augmented drug CL is unlikely to be short-lived. A lower incidence (about 52%) of ARC was observed in a mixed cohort of critically ill patients receiving antibacterial therapy, although an association with greater therapeutic failure was demonstrated [29].
Other investigators have highlighted the importance of renal function in informing β-lactam dosing. Patel and colleagues examined piperacillin PK data from 105 hospitalized patients to determine optimal intermittent and extended-infusion dosing in those with renal impairment [37]. Of interest, with an estimated CLCR of 100 ml/min and a traditional regimen of 4.5 g administered IV every 6 hours, the probability of target attainment (50% fT>MIC) decreased sharply (<80%) with MIC values greater than 4 mg/L [34]. With our data, we identified higher CLCR thresholds (Figure 3), although this likely reflects differences in case mix and the use of measured rather than estimated CLCR. Importantly, previous data reinforce the significant disparity between calculated and measured CLCR in patients manifesting ARC [38,39].
More recently, Carlier et al. examined meropenem and piperacillin-tazobactam target attainment in a cohort of critically ill patients receiving extended-infusion dosing. In those patients manifesting ARC (CLCR ≥130 ml/min), the probability of achieving either 50% fT>MIC or 100% fT>MIC was significantly reduced [40]. Regression analysis also identified CLCR as the only significant predictor of target attainment, a finding also demonstrated by Hites et al. in their work exploring β-lactam exposure in obese, non-critically ill patients [41]. Our data compare favourably with these studies, although the current analysis involves a wider range of MIC values, highlighting the impact of variable bacterial susceptibility. Pea and colleagues similarly examined meropenem continuous infusion dosing in critically ill patients with severe Gram-negative infection [42]. Dosing nomograms to achieve specific steady-state concentrations were developed with a linear relationship between dose and estimated CLCR [42].
Changing bacterial susceptibility represents a global challenge in medical practice. Our analyses highlight the importance of the MIC as the denominator in the PK/PD relationship in determining adequate antibacterial dosing. Less susceptible pathogens are more common in the ICU [43], and significant regional variation in resistance patterns also exists [44]. In two large antibacterial susceptibility prevalence studies, MIC90 values for piperacillin-tazobactam against Pseudomonas aeruginosa were reported at or above 64 mg/L [45,46]. Our data suggest that achieving 50% fT>MIC in such a scenario is unlikely with conventional piperacillin intermittent dosing and reinforce the use of a much lower susceptibility breakpoint (16 mg/L) with this dosing strategy. However, even in this circumstance, an elevated CLCR will significantly reduce the probability of target attainment, as demonstrated in Figure 3. In contrast, where a highly susceptible pathogen is identified and piperacillin is used as directed therapy, standard doses may still be sufficient, even with extremely high CLCR values. Importantly, this underlines the significance of such microbiological susceptibility data in accurate clinical decision-making.
Our study has several limitations. The data were drawn from a single centre and as such may not be representative of case mix at other institutions. Our inclusion criteria were designed to select a cohort of patients without significant renal impairment, as effective antibacterial dosing in this group is less likely with standard dosing. As such, ARC is likely to have been more common in our study cohort than in a broader ICU population. However, our relatively high plasma CR cutoff allowed us to explore renal function (as determined by CLCR) as a covariate in PK modelling. We did not include tazobactam PK, because it is not active against Pseudomonas aeruginosa, although, as it is renally eliminated, increased drug CL is also likely in ARC. We examined the likelihood of target attainment using varying susceptibility levels rather than clinical MIC data. This improves generalizability for empiric dosing, although it limits conclusions about the adequacy of drug exposure in any specific patient. Furthermore, given the small sample size, we are unable to make any firm conclusions about the clinical implications of these analyses.
Conclusions
Our results indicate that an empiric intermittent infusion of 4.5 g of piperacillin-tazobactam is unlikely to achieve optimal piperacillin exposure in a significant proportion of critically ill patients with sepsis, particularly when targeting less susceptible pathogens. This appears to be driven primarily by an increase in drug CL in some patients, and our data reinforce the value of CLCR in predicting whether optimal exposures are likely. As such, clinicians should be wary of the adequacy of conventional dosing in patients manifesting ARC, and research should now be focused on the use of novel administration strategies in such patients, in addition to correlating changes in antibacterial PK with clinical outcomes.
Key messages
Elevated CLCR in septic critically ill patients is associated with higher piperacillin CL, a concept referred to as augmented renal clearance, or ARC.
When employing intermittent administration, this will result in suboptimal plasma concentrations for significant periods of the dosing interval.
Increasing CLCR reduces the probability of target attainment (fT>MIC), particularly with more resistant organisms.
When considering the MIC distribution of Pseudomonas aeruginosa, 6-hourly dosing of 4.5 g piperacillin-tazobactam is unlikely to provide sufficient piperacillin exposure when the CLCR is ≥90 ml/min.
Acknowledgements
This work was supported by a grant from the National Health and Medical Research Council of Australia (NHMRC project grant 519702). JAR is supported in part by an Australian National Health and Medical Research Council Research Fellowship (NHMRC APP1048652). AAU was supported in part by a Royal Brisbane and Women’s Hospital Foundation research grant. This work was carried out in the Department of Intensive Care Medicine, Royal Brisbane and Women’s Hospital, Herston, Queensland, Australia, and in the Burns, Trauma, and Critical Care Research Centre, The University of Queensland.
Abbreviations
- ALAG
Infusion lag descriptor
- APACHE
Acute Physiology And Chronic Health Evaluation
- ARC
Augmented renal clearance
- BMI
Body mass index
- BSV
Between-subject variability
- CFR
Cumulative fraction of response
- CL
Drug clearance
- CLCR
Creatinine clearance
- Cmin
Trough concentration
- CR
Creatinine
- CV
Coefficient of variation
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- fT>MIC
Fraction of the dosing interval for which the concentration remains above the minimum inhibitory concentration
- HPLC-UV
High-performance liquid chromatography-ultraviolet
- ICU
Intensive care unit
- IDC
Indwelling urinary catheter
- IQR
Interquartile range
- LOS
Length of stay
- MIC
Minimum inhibitory concentration
- NONMEM
Nonlinear mixed-effects modelling
- OFV
Objective function value
- PD
Pharmacodynamic
- PK
Pharmacokinetic
- PTA
Probability of target attainment
- Q
Intercompartmental clearance
- RUV
Residual unexplained variability
- SD
Standard deviation
- SOFA
Sequential Organ Failure Assessment
- TVCL
Typical value of piperacillin clearance
- Vc
Volume of the central compartment
- Vd
Volume of distribution
- Vp
Volume of the peripheral compartment
Footnotes
Competing interests
JL has received an honorarium from AstraZeneca. MSR has previously consulted for Janssen-Cilag, AstraZeneca, Pfizer and Gilead; has been involved in advisory boards for Janssen-Cilag and AstraZeneca; and has received unrestricted grants from Janssen-Cilag, AstraZeneca and Novartis. AAU has received an honorarium and travel support from Pfizer. DLP has received a research grant from AstraZeneca and acted as a consultant for Merck, Acureon, Johnson & Johnson, Sanofi-Aventis and Three Rivers Pharmaceuticals. AstraZeneca and Edwards Lifesciences provide an annual unrestricted donation to the Burns, Trauma and Critical Care Research Centre (BTCCRC), The University of Queensland. All of the remaining authors declare that they have no competing interests in relation to this article.
Authors’ contributions
JL, MSR, DLP, PSK and JAR conceived of the study. AAU, PJ, JAR, PSK and JL were involved in protocol development, ethical approval and implementation. AAU, PJ and JAR collected the data. SCW completed the laboratory analysis and quality assurance. CMJK, KP, JAR and KK performed the statistical and pharmacokinetic analyses. AAU wrote the initial manuscript draft, and all of the remaining authors contributed to subsequent revisions. AAU takes responsibility for archiving the data and guarantees the integrity of the paper from inception to publication. All of the authors have read and approved the article for publication.
Contributor Information
Andrew A Udy, Email: a.udy@alfred.org.au.
Jeffrey Lipman, Email: j.lipman@uq.edu.au.
Paul Jarrett, Email: paul.jarrett@health.qld.gov.au.
Kerenaftali Klein, Email: Kerenaftali.Klein@qimrberghofer.edu.au.
Steven C Wallis, Email: s.wallis@uq.edu.au.
Kashyap Patel, Email: Kashyap.Patel@monash.edu.
Carl MJ Kirkpatrick, Email: Carl.Kirkpatrick@monash.edu.
Peter S Kruger, Email: peter.kruger@health.qld.gov.au.
David L Paterson, Email: david.antibiotics@gmail.com.
Michael S Roberts, Email: m.roberts@uq.edu.au.
Jason A Roberts, Email: j.roberts2@uq.edu.au.
References
- 1.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosein S, Udy AA, Lipman J. Physiological changes in the critically ill patient with sepsis. Curr Pharm Biotechnol. 2011;12:1991–5. doi: 10.2174/138920111798808248. [DOI] [PubMed] [Google Scholar]
- 4.Udy AA, Roberts JA, Lipman J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 2013;39:2070–82. doi: 10.1007/s00134-013-3088-4. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al. DALI: Defining Antibiotic Levels in Intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58:1072–83. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 6.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 7.Drusano GL. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug’. Nat Rev Microbiol. 2004;2:289–300. doi: 10.1038/nrmicro862. [DOI] [PubMed] [Google Scholar]
- 8.McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T > MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31:345–51. doi: 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Du X, Kuti JL, Nicolau DP. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother. 2007;51:1725–30. doi: 10.1128/AAC.00294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts JA, Lipman J. Optimal doripenem dosing simulations in critically ill nosocomial pneumonia patients with obesity, augmented renal clearance, and decreased bacterial susceptibility. Crit Care Med. 2013;41:489–95. doi: 10.1097/CCM.0b013e31826ab4c4. [DOI] [PubMed] [Google Scholar]
- 11.Udy AA, Varghese JM, Altukroni M, Briscoe S, McWhinney BC, Ungerer JP, et al. Subtherapeutic initial β-lactam concentrations in select critically ill patients: association between augmented renal clearance and low trough drug concentrations. Chest. 2012;142:30–9. doi: 10.1378/chest.11-1671. [DOI] [PubMed] [Google Scholar]
- 12.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 13.Roberts JA, Roberts MS, Semark A, Udy AA, Kirkpatrick CM, Paterson DL, et al. Antibiotic dosing in the ‘at risk’ critically ill patient: linking pathophysiology with pharmacokinetics/pharmacodynamics in sepsis and trauma patients. BMC Anesthesiol. 2011;11:3. doi: 10.1186/1471-2253-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. Guidance for industry: bioanalytical method validation. US Department of Health and Human Services. May 2001. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf. Accessed 7 Feb 2015.
- 15.European Committee on Antimicrobial Susceptibility Testing (EUCAST). http://www.eucast.org/. Accessed 19 Aug 2014.
- 16.Wings for NONMEM. http://wfn.sourceforge.net/. Accessed 7 Feb 2015.
- 17.Parke J, Holford NH, Charles BG. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Programs Biomed. 1999;59:19–29. doi: 10.1016/S0169-2607(98)00098-4. [DOI] [PubMed] [Google Scholar]
- 18.Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J Antimicrob Chemother. 2005;55:601–7. doi: 10.1093/jac/dki079. [DOI] [PubMed] [Google Scholar]
- 19.Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet. 2010;49:1–16. doi: 10.2165/11318140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Udy AA, Roberts JA, Lipman J. Implications of augmented renal clearance in critically ill patients. Nat Rev Nephrol. 2011;7:539–43. doi: 10.1038/nrneurol.2011.141. [DOI] [PubMed] [Google Scholar]
- 21.Baptista JP, Sousa E, Martins PJ, Pimentel JM. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int J Antimicrob Agents. 2012;39:420–3. doi: 10.1016/j.ijantimicag.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Grootaert V, Willems L, Debaveye Y, Meyfroidt G, Spriet I. Augmented renal clearance in the critically ill: how to assess kidney function. Ann Pharmacother. 2012;46:952–9. doi: 10.1345/aph.1Q708. [DOI] [PubMed] [Google Scholar]
- 23.Batra VK, Morrison JA, Lasseter KC, Joy VA. Piperacillin kinetics. Clin Pharmacol Ther. 1979;26:41–53. doi: 10.1002/cpt197926141. [DOI] [PubMed] [Google Scholar]
- 24.Adnan S, Li JX, Wallis SC, Rudd M, Jarrett P, Paterson DL, et al. Pharmacokinetics of meropenem and piperacillin in critically ill patients with indwelling surgical drains. Int J Antimicrob Agents. 2013;42:90–3. doi: 10.1016/j.ijantimicag.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 25.Roberts JA, Kirkpatrick CM, Roberts MS, Dalley AJ, Lipman J. First-dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int J Antimicrob Agents. 2010;35:156–63. doi: 10.1016/j.ijantimicag.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Joynt GM, Lipman J, Gomersall CD, Young RJ, Wong EL, Gin T. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J Antimicrob Chemother. 2001;47:421–9. doi: 10.1093/jac/47.4.421. [DOI] [PubMed] [Google Scholar]
- 27.Sánchez M, Jiménez-Lendínez M, Cidoncha M, Asensio MJ, Herrero E, Collado A, et al. Comparison of fluid compartments and fluid responsiveness in septic and non-septic patients. Anaesth Intensive Care. 2011;39:1022–9. doi: 10.1177/0310057X1103900607. [DOI] [PubMed] [Google Scholar]
- 28.Udy AA, Roberts JA, Shorr AF, Boots RJ, Lipman J. Augmented renal clearance in septic and traumatized patients with normal plasma creatinine concentrations: identifying at-risk patients. Crit Care. 2013;17:R35. doi: 10.1186/cc12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claus BO, Hoste EA, Colpaert K, Robays H, Decruyenaere J, De Waele JJ. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J Crit Care. 2013;28:695–700. doi: 10.1016/j.jcrc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Shimamoto Y, Fukuda T, Tanaka K, Komori K, Sadamitsu D. Systemic inflammatory response syndrome criteria and vancomycin dose requirement in patients with sepsis. Intensive Care Med. 2013;39:1247–52. doi: 10.1007/s00134-013-2909-9. [DOI] [PubMed] [Google Scholar]
- 31.Lipman J, Udy AA, Roberts JA. Do we understand the impact of altered physiology, consequent interventions and resultant clinical scenarios in the intensive care unit? The antibiotic story. Anaesth Intensive Care. 2011;39:999–1000. doi: 10.1177/0310057X1103900602. [DOI] [PubMed] [Google Scholar]
- 32.Conil JM, Georges B, Fourcade O, Seguin T, Lavit M, Samii K, et al. Assessment of renal function in clinical practice at the bedside of burn patients. Br J Clin Pharmacol. 2007;63:583–94. doi: 10.1111/j.1365-2125.2006.02807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lautrette A, Phan TN, Ouchchane L, Aithssain A, Tixier V, Heng AE, et al. High creatinine clearance in critically ill patients with community-acquired acute infectious meningitis. BMC Nephrol. 2012;13:124. doi: 10.1186/1471-2369-13-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minville V, Asehnoune K, Ruiz S, Breden A, Georges B, Seguin T, et al. Increased creatinine clearance in polytrauma patients with normal serum creatinine: a retrospective observational study. Crit Care. 2011;15:R49. doi: 10.1186/cc10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Udy A, Boots R, Senthuran S, Stuart J, Deans R, Lassig-Smith M, et al. Augmented creatinine clearance in traumatic brain injury. Anesth Analg. 2010;111:1505–10. doi: 10.1213/ANE.0b013e3181f7107d. [DOI] [PubMed] [Google Scholar]
- 36.Udy AA, Baptista JP, Lim NL, Joynt GM, Jarrett P, Wockner L, et al. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit Care Med. 2014;42:520–7. doi: 10.1097/CCM.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 37.Patel N, Scheetz MH, Drusano GL, Lodise TP. Identification of optimal renal dosage adjustments for traditional and extended-infusion piperacillin-tazobactam dosing regimens in hospitalized patients. Antimicrob Agents Chemother. 2010;54:460–5. doi: 10.1128/AAC.00296-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Udy AA, Morton FJ, Nguyen-Pham S, Jarrett P, Lassig-Smith M, Stuart J, et al. A comparison of CKD-EPI estimated glomerular filtration rate and measured creatinine clearance in recently admitted critically ill patients with normal plasma creatinine concentrations. BMC Nephrol. 2013;14:250. doi: 10.1186/1471-2369-14-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baptista JP, Udy AA, Sousa E, Pimentel J, Wang L, Roberts JA, et al. A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Crit Care. 2011;15:R139. doi: 10.1186/cc10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlier M, Carrette S, Roberts JA, Stove V, Verstraete AG, Hoste E, et al. Meropenem and piperacillin/tazobactam prescribing in critically ill patients: does augmented renal clearance affect pharmacokinetic/pharmacodynamic target attainment when extended infusions are used? Crit Care. 2013;17:R84. doi: 10.1186/cc12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hites M, Taccone FS, Wolff F, Maillart E, Beumier M, Surin R, et al. Broad-spectrum β-lactams in obese non-critically ill patients. Nutr Diabetes. 2014;4:e119. doi: 10.1038/nutd.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pea F, Viale P, Cojutti P, Furlanut M. Dosing nomograms for attaining optimum concentrations of meropenem by continuous infusion in critically ill patients with severe Gram-negative infections: a pharmacokinetics/pharmacodynamics-based approach. Antimicrob Agents Chemother. 2012;56:6343–8. doi: 10.1128/AAC.01291-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eagye KJ, Banevicius MA, Nicolau DP. Pseudomonas aeruginosa is not just in the intensive care unit any more: implications for empirical therapy. Crit Care Med. 2012;40:1329–32. doi: 10.1097/CCM.0b013e31823bc8d0. [DOI] [PubMed] [Google Scholar]
- 44.Kiratisin P, Chongthaleong A, Tan TY, Lagamayo E, Roberts S, Garcia J, et al. Comparative in vitro activity of carbapenems against major Gram-negative pathogens: results of Asia-Pacific surveillance from the COMPACT II study. Int J Antimicrob Agents. 2012;39:311–6. doi: 10.1016/j.ijantimicag.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Fujimura T, Anan N, Sugimori G, Watanabe T, Jinushi Y, Yoshida I, et al. Susceptibility of Pseudomonas aeruginosa clinical isolates in Japan to doripenem and other antipseudomonal agents. Int J Antimicrob Agents. 2009;34:523–8. doi: 10.1016/j.ijantimicag.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Zhanel GG, Adam HJ, Low DE, Blondeau J, Decorby M, Karlowsky JA, et al. Antimicrobial susceptibility of 15,644 pathogens from Canadian hospitals: results of the CANWARD 2007–2009 study. Diagn Microbiol Infect Dis. 2011;69:291–306. doi: 10.1016/j.diagmicrobio.2010.10.025. [DOI] [PubMed] [Google Scholar]


