Abstract
The OPTAMA Program is intended to examine typical antimicrobial regimens used in the treatment of common nosocomial pathogens and the likelihood of these regimens attaining appropriate pharmacodynamic exposure in different parts of the world. A 5,000-subject Monte Carlo simulation was used to estimate pharmacodynamic target attainment for meropenem, imipenem, ceftazidime, cefepime, piperacillin-tazobactam, and ciprofloxacin against Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa. Standard dosing regimens from North America were used. Pharmacokinetic parameter variability was derived from existing healthy volunteer data, and MIC data came from the 2002 MYSTIC Program. Ciprofloxacin displayed the lowest target attainment against all bacterial species (41 to 46% for A. baumannii, 53 to 59% for P. aeruginosa, and 80 to 85% for the Enterobacteriaceae). Increasing the dose to 400 mg every 8 h did not significantly increase target attainment against nonfermenters. Piperacillin-tazobactam target attainments were similar to that of ceftazidime against all pathogens. Higher doses of both compounds were needed to achieve better target attainments against P. aeruginosa. Overall, meropenem, imipenem, and cefepime attained the highest probabilities of attainment against the Enterobacteriaceae (99 to 100%). The carbapenems appear to be the most useful agents against A. baumannii (88 to 92%), and these agents, along with higher doses of any of the β-lactams, would be the most appropriate choices for empirical therapy for P. aeruginosa infection. Given the lack of agreement between percent susceptibility and probability of target attainment for certain antimicrobial regimens, a methodology employing stochastic pharmacodynamic analyses may be a more useful tool for differentiating the most-optimal compounds and dosing regimens in the clinical setting of initial empirical therapy.
Antimicrobial resistance among common nosocomial pathogens is a significant contributor to patient morbidity and mortality and therefore must carefully be considered when choosing empirical treatment regimens (17, 21). In numerous studies, resistance was the predominant reason leading to the initiation of inappropriate antimicrobial therapy (17, 18, 31). Accordingly, a shift in the thinking regarding the selection of the initial empirical antimicrobial regimen has been suggested by which more broad-spectrum agents are selected up front, usually in combination with a compound from another class to provide adequate coverage for the anticipated pathogens in severe infections, such as ventilator-associated pneumonia. After identification and susceptibility testing have been performed, therapy is streamlined to agents directed at the causative pathogen. This approach would lead to initiation of appropriate antimicrobial therapy for the majority of patients and is based on the local resistance patterns of specific pathogens to commonly used antibiotics.
Large surveillance studies are designed to identify key areas of resistance and report these as changes in the MIC (4, 9-11). Unfortunately, although commonly reported and utilized as such, the MIC alone is not an ideal marker by which to choose an antibiotic or dose, since it does not consider the pharmacokinetics of the antibiotic or the pharmacodynamic exposure necessary for successful clinical outcomes (9).
The goal of the OPTAMA (Optimizing Pharmacodynamic Target Attainment Using the MYSTIC Antibiogram) Program is to impart greater understanding about the appropriate antibiotic options for empirical therapy of common nosocomial pathogens. This is accomplished by incorporating the variability in pharmacokinetic parameter estimates, dosage regimens, and MIC distributions from various regions of the world to calculate the probability of attaining critical pharmacodynamic targets. In this report, the probabilities of attaining targeted pharmacodynamic exposure for six commonly used intravenous antimicrobials against populations of Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa collected from North America are described.
(This work was presented in part at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., September 2003.)
MATERIALS AND METHODS
Pharmacodynamic model.
Pharmacodynamic exposures, as measured by percent time above the MIC (% T>MIC) for free (i.e., unbound) drug, were modeled for intravenous (i.v.) bolus regimens of meropenem, 1 g every 8 h (1 g q8h) (all pathogens); imipenem, 1 g q8h; ceftazidime, 1 g q8h; ceftazidime, 2 g q8h (A. baumannii and P. aeruginosa only); cefepime, 1 g every 12 h (q12h); cefepime, 2 g q12h (A. baumannii and P. aeruginosa only); and piperacillin-tazobactam, 3.375 g every 6 h (q6h) and every 4 h (q4h) (A. baumannii and P. aeruginosa only). Pharmacodynamic exposures for ciprofloxacin (400 mg q12h [all pathogens] and 400 mg q8h [A. baumannii and P. aeruginosa only]) were measured by calculation of the total drug 24-h area-under-the-concentration-curve (AUC) over the MIC, i.e., the AUC/MIC ratio. Dosage regimens were chosen based on the most common regimens used in North America. A one-compartment i.v.-bolus equation was used to calculate % T>MIC for the β-lactams:
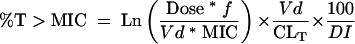 |
where Ln is the natural logarithm, f is the fraction of unbound drug, Vd is the volume of distribution in liters at steady state, CLT is the total body clearance in liters per hour, and DI is the dosing interval for the regimen.
Total-drug AUCs for ciprofloxacin regimens were calculated by dividing the dose by the CLT value. The total-drug AUC/MIC ratio was used instead of that for free drug because the original studies evaluating the pharmacodynamic breakpoint for ciprofloxacin did not account for free drug in those patients (7). The AUC/MIC ratio was then calculated by dividing the total-drug AUC for the ciprofloxacin regimen by the MIC.
Microbiology.
Microbiology data used during the pharmacodynamic analyses were derived from the Meropenem Yearly Susceptibility Test Information Collection (MYSTIC) database. The MYSTIC Program contains a large set of data for nosocomial isolates from around the world and associated information on the MICs for these isolates. It is a global, multicenter surveillance study that compares the activity of meropenem in high-prescribing centers along with that of imipenem, ceftazidime, cefepime, piperacillin-tazobactam, and ciprofloxacin against gram-positive and gram-negative nosocomial isolates (27).
The data aggregated in the present study were generated from isolates collected consecutively from patients hospitalized in North America during the 2002 edition of the MYSTIC Program. North American participants in the 2002 MYSTIC included 1 site in Canada, 15 sites distributed geographically across the United States, and 4 sites in Mexico. Four hundred thirty-three E. coli isolates, 288 K. pneumoniae isolates, 109 A. baumannii isolates, and 427 P. aeruginosa isolates were included. Multiple isolates of the same species from a single origin (same patient) were excluded. Each participant laboratory performed identification at the species level by colony morphology or simple biochemical tests (spot indole, bile solubility, oxidase, etc.) or Vitek ID cards (bioMerieux, Hazelwood, Mo.) when required.
The MICs of meropenem, imipenem, ceftazidime, cefepime, piperacillin-tazobactam, and ciprofloxacin were determined at a central laboratory by either broth microdilution or agar dilution according to National Committee for Clinical Laboratory Standards methodology (24). The detailed methodology for MIC determination has been published elsewhere (27). MICs ranged from ≤0.008 to ≥256 μg/ml in doubling dilutions for all antibiotics. MICs of less than 0.008 μg/ml or greater than 256 μg/ml were classified as 0.008 or 256 μg/ml, respectively. The percentages of isolates for which each MIC applies are listed in Table 1. Discrete MIC distributions were built for each population of bacteria based on the MIC frequencies in the MYSTIC study using Crystal Ball 2000 (Desisioneering, Inc., Denver, Colo.), whereby the percentage of bacteria for which each MIC applies is treated as a frequency and values in between the MIC do not exist.
TABLE 1.
MIC distributions for various antimicrobials tested during the 2002 MYSTIC Program in North America
| Species (no.) or antimicrobial agent | % of isolates susceptible at MIC (μg/ml) of:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.008 | 0.016 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | |
| E. coli (433) | ||||||||||||||||
| Meropenem | 61.7 | 7.2 | 20.8 | 2.8 | 2.1 | 2.3 | 1.9 | 1.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Imipenem | 0 | 0 | 0 | 13.2 | 64.4 | 12.2 | 7.6 | 2.3 | 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ceftazidime | 0 | 0 | 0 | 48.5 | 6.5 | 25.2 | 7.6 | 5.3 | 1.2 | 0.5 | 0.9 | 0.5 | 3.5 | 0.2 | 0.2 | 0 |
| Cefepime | 0 | 0 | 0 | 92.6 | 0.3 | 4.8 | 1.2 | 0.9 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Piperacillin-tazobactam | 0 | 0 | 0 | 0 | 0.7 | 2.1 | 7.2 | 39.5 | 32.8 | 10.2 | 3.7 | 1.4 | 0.2 | 0.5 | 0.5 | 1.4 |
| Ciprofloxacin | 2.5 | 5.1 | 2.5 | 1.4 | 71.8 | 4.2 | 0.9 | 4.2 | 0 | 5.3 | 0.2 | 0.5 | 0.7 | 0.5 | 0.2 | 0 |
| K. pneumoniae (288) | ||||||||||||||||
| Meropenem | 5.2 | 1.0 | 75.3 | 7.3 | 2.4 | 6.6 | 1.0 | 0.4 | 0.4 | 0 | 0 | 0.4 | 0 | 0 | 0 | 0 |
| Imipenem | 0 | 0 | 0 | 4.2 | 56.6 | 27.4 | 10.8 | 0 | 0.4 | 0.4 | 0 | 0 | 0 | 0.4 | 0 | 0 |
| Ceftazidime | 0 | 0 | 0 | 49.3 | 5.2 | 18.1 | 8.7 | 7.6 | 1.0 | 0 | 0.4 | 0.4 | 8.7 | 0.4 | 0 | 0.4 |
| Cefepime | 0 | 0 | 1.8 | 90.8 | 0.5 | 3.2 | 0.5 | 0.5 | 0.5 | 0.9 | 1.4 | 0 | 0 | 0 | 0 | 0 |
| Piperacillin-tazobactam | 0 | 0 | 0 | 0 | 0.7 | 2.4 | 3.1 | 16.0 | 37.2 | 23.6 | 6.9 | 4.9 | 1.0 | 0.7 | 0.7 | 2.8 |
| Ciprofloxacin | 0 | 0.4 | 7.3 | 2.4 | 68.1 | 10.4 | 8.0 | 1.4 | 0.7 | 1.0 | 0 | 0 | 0 | 0 | 0 | 0.4 |
| A. baumannii (109) | ||||||||||||||||
| Meropenem | 0 | 0 | 0 | 3.7 | 6.4 | 23.0 | 22.0 | 16.5 | 10.1 | 6.4 | 2.8 | 1.8 | 5.5 | 0.9 | 0 | 0.9 |
| Imipenem | 0 | 0 | 0.9 | 0 | 13.8 | 28.4 | 22.9 | 16.5 | 4.6 | 3.8 | 0.9 | 6.4 | 0.9 | 0 | 0 | 0.9 |
| Ceftazidime | 0 | 0 | 0 | 0 | 0 | 0 | 0.9 | 11.0 | 11.0 | 20.2 | 17.4 | 7.3 | 31.2 | 0 | 0 | 0.9 |
| Cefepime | 0 | 0 | 0 | 0 | 0 | 1.3 | 2.7 | 11.5 | 12.8 | 12.8 | 14.1 | 19.2 | 24.4 | 1.3 | 0 | 0 |
| Piperacillin-tazobactam | 0 | 0.9 | 0 | 0 | 19.3 | 5.5 | 5.5 | 3.7 | 6.4 | 4.6 | 11.9 | 4.6 | 8.3 | 5.5 | 9.2 | 14.7 |
| Ciprofloxacin | 0 | 0 | 0 | 4.6 | 35.8 | 6.4 | 6.4 | 15.6 | 1.8 | 26.6 | 0 | 0 | 0 | 2.8 | 0 | 0 |
| P. aeruginosa (427) | ||||||||||||||||
| Meropenem | 0.2 | 0.2 | 1.6 | 5.4 | 14.1 | 17.1 | 22.7 | 17.8 | 5.9 | 6.6 | 2.8 | 2.1 | 1.2 | 1.2 | 1.2 | 0 |
| Imipenem | 0 | 0 | 0 | 0.2 | 1.2 | 4.2 | 12.7 | 44.5 | 18.7 | 5.6 | 3.5 | 5.2 | 2.1 | 0.9 | 0 | 1.2 |
| Ceftazidime | 0 | 0 | 0 | 0 | 0.2 | 0.7 | 2.8 | 15.0 | 37.7 | 19.2 | 8.7 | 4.9 | 8.0 | 0 | 0 | 2.8 |
| Cefepime | 0 | 0 | 0 | 0 | 0 | 0.9 | 2.4 | 17.4 | 28.6 | 21.2 | 17.7 | 6.8 | 5.0 | 0 | 0 | 0 |
| Piperacillin-tazobactam | 0 | 0 | 0 | 0 | 0.7 | 0.9 | 2.1 | 3.5 | 13.8 | 36.8 | 13.8 | 11.7 | 6.1 | 3.5 | 3.0 | 4.0 |
| Ciprofloxacin | 0 | 0 | 0.5 | 2.3 | 49.2 | 7.5 | 9.4 | 4.7 | 4.2 | 18.5 | 0.2 | 0.2 | 0.2 | 0.9 | 1.2 | 0.9 |
Pharmacokinetics.
Pharmacokinetic data were obtained from previously published studies with healthy volunteers (5, 16, 23, 25, 26). For studies to be considered, they had to be conducted with at least 10 healthy volunteers, describe the assay used to determine drug concentrations, use clinically relevant dosing regimens, perform an adequate pharmacokinetic analysis as determined by the OPTAMA investigators (J.L.K. and D.P.N.), and present means and standard deviations for CLT and Vd. Because a published report on at least 10 healthy volunteers had not been found for cefepime or ceftazidime, studies which determined the pharmacokinetics of these agents in 6 and 8 healthy volunteers, respectively, and that met all other criteria were used (23, 25). Values for these parameters are listed in Table 2. Log-gaussian probability distributions for CLT and Vd values were developed using Crystal Ball.
TABLE 2.
Summary of pharmacokinetic parameters and variability for antimicrobials used in the Monte Carlo simulationc
| Antibiotic | Pharmacokinetic parameter (mean ± SD)
|
||
|---|---|---|---|
| CLT (liters/h)c | Vd (liters)c | Fraction unbound (%)a | |
| Meropenem | 14.4 ± 1.8 | 18.6 ± 3.0 | 85-98 |
| Imipenem | 10.5 ± 1.4 | 15.3 ± 3.3 | 80-95 |
| Ceftazidime | 9.3 ± 3.3 | 14.0 ± 2.9 | 80-90 |
| Cefepime | 5.3 ± 0.6 | 13.6 ± 2.4 | 80-90 |
| Piperacillin-tazobactam | 10.9 ± 1.2 | 11.9 ± 1.7 | 65-75 |
| Ciprofloxacinb | 31.2 ± 6.3 | 60-80 | |
Fraction unbound, presented as a range.
Fraction unbound for ciprofloxacin is listed in the table but was not used in analysis.
Results are expressed as means ± standard deviations.
Estimates of the fraction unbound for meropenem, imipenem, ceftazidime, cefepime, and piperacillin-tazobactam were derived from the package insert for each antibiotic among the other studies previously described. The unbound fractions for these agents were treated as ranges and are also listed in Table 2. Uniform distributions for the unbound fraction were developed using Crystal Ball 2000.
Monte Carlo simulation.
A 5,000-patient Monte Carlo simulation (Crystal Ball 2000) was conducted to calculate estimates of % T>MIC or AUC/MIC ratio for each antibiotic regimen-bacterial population combination. During each iteration, different values for CLT, Vd, f, and MIC were substituted in the appropriate equations based on the probability distributions for each, thereby resulting in 5,000 different estimates of pharmacodynamic exposure for each antibiotic regimen against each bacterial population. Values for % T>MIC and AUC/MIC were plotted on frequency curves for further analysis. The probabilities of obtaining a % T>MIC of 20, 30, 40, 50, 60, 70, 80, 90, and 100% were calculated for the β-lactams. The probabilities of achieving an AUC/MIC ratio greater than or equal to 100, 125, 150, 175, 200, 225, and 250 were calculated for ciprofloxacin (3, 7, 30, 33). For comparative purposes, bactericidal pharmacodynamic breakpoints were considered 40% for meropenem and imipenem, 50% for ceftazidime, cefepime, and piperacillin-tazobactam, and 125 for ciprofloxacin (3, 6, 7, 32, 33).
Statistics.
The agreement between the probability of bactericidal target attainment and percent susceptibility was assessed by the methods of Bland and Altman (2) and is reported as the mean difference and 95% confidence interval (95% CI) of the difference. The two methods will be considered in agreement when the lower and upper bounds of the 95% CI of the difference are within −5 and 5, respectively.
RESULTS
Pharmacokinetic and microbiology parameters.
Simulated distributions for all pharmacokinetic parameters and MICs were consistent with log-gaussian (CLT and Vd), uniform (f), and discrete distributions (MICs) in accordance with the data inputted into the models (Tables 1 and 2).
Pharmacodynamic target attainment.
Table 3 demonstrates the probabilities of bactericidal pharmacodynamic target attainment for the antimicrobial regimens tested against. Both meropenem and imipenem demonstrated the highest target attainments overall, with 99 to 100% probabilities against E. coli and K. pneumoniae and excellent target attainment percentages against A. baumannii and P. aeruginosa (88 and 91% for meropenem and 92 and 89% for imipenem, respectively). The ceftazidime and cefepime simulated regimens also had excellent activity against the Enterobacteriaceae (90 to 100%). Against A. baumannii and P. aeruginosa, target attainments were reduced; however, using higher doses of these agents (1 g q8h for ceftazidime and 2 g q12h for cefepime) resulted in acceptable probabilities against P. aeruginosa. For a piperacillin-tazobactam regimen of 3.375 g q6h, target attainments for E. coli and K. pneumoniae isolates were 95 and 89%, respectively. Against P. aeruginosa, the q6h simulated regimen only achieved T>MIC for 70% of the isolates, but this was increased to 85% with the q4h regimen. Both piperacillin-tazobactam simulated dosing regimens faired less well against A. baumannii (56 and 65%). Ciprofloxacin achieved the lowest target attainment against all bacteria. Increasing the dose to 400 mg q8h did not significantly improve target attainment against A. baumannii or P. aeruginosa.
TABLE 3.
Probability of bactericidal pharmacodynamic target attainment for various antimicrobial regimens
| Regimen | Target attainment (%)d
|
|||
|---|---|---|---|---|
| EC | KP | AB | PSA | |
| Meropenem, 1 g q8ha | 100 | 100 | 88 | 91 |
| Imipenem, 1 g q8ha | 100 | 99 | 92 | 89 |
| Ceftazidime, 1 g q8hb | 96 | 90 | 59 | 84 |
| Ceftazidime, 2 g q8hb | — | — | 69 | 89 |
| Cefepime, 1 g q12hb | 100 | 99 | 50 | 82 |
| Cefepime, 2 g q12hb | — | — | 67 | 93 |
| Piperacillin-tazobactam, 3.375 g q6hb | 95 | 89 | 56 | 70 |
| Piperacillin-tazobactam, 3.375 g q4hb | — | — | 65 | 85 |
| Ciprofloxacin, 400 mg q12hc | 85 | 80 | 41 | 53 |
| Ciprofloxacin, 400 mg q8hc | — | — | 46 | 59 |
Bactericidal target assessed as free drug T>MIC of more than/equal ≥40%.
Bactericidal target assessed as free drug T>MIC of ≥50%.
Bactericidal target assessed as total drug AUC/MIC of ≥125.
— indicates not tested. EC, E. coli; KP, K. pneumoniae; AB, A. baumannii; PSA, P. aeruginosa.
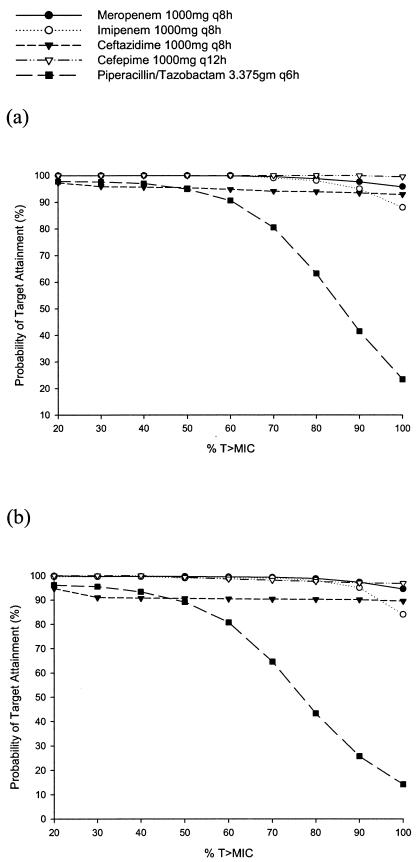
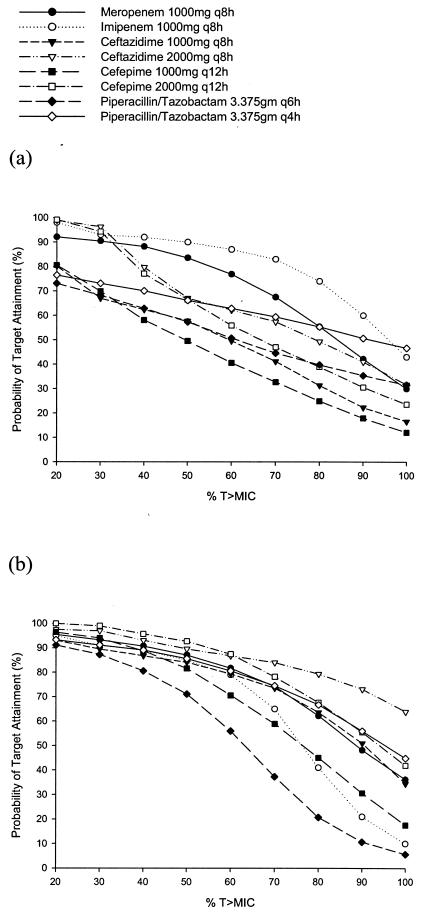
The probabilities of target attainment for the β-lactams at various % T>MIC exposures are displayed in Fig. 1 and 2. Against the Enterobacteriaceae, the probability for all of the β-lactam agents of maintaining free concentrations above the MIC for up to 100% of their respective dosing intervals was >90% with one exception (Fig. 1a and 1b). The probability fell precipitously for piperacillin-tazobactam once concentrations above 50% of the dosing interval were targeted. For A. baumannii, in general imipenem had the best probability for maintaining concentrations above the MIC throughout the dosing interval, and the cefepime 1 g g12h regimen had the worst (Fig. 2a). Against P. aeruginosa, the ceftazidime 2 g q8h regimen had the best probability of maintaining T>MIC for 100% of the dosing interval, and piperacillin-tazobactam at 3.375g q6h had the least probability (Fig. 2b).
FIG. 1.
Probabilities of target attainment at different free drug % T>MIC targets for β-lactams against E. coli (a) and K. pneumoniae (b).
FIG. 2.
Probabilities of target attainment at different free drug % T>MIC targets for β-lactams against A. baumannii (a) and P. aeruginosa (b).
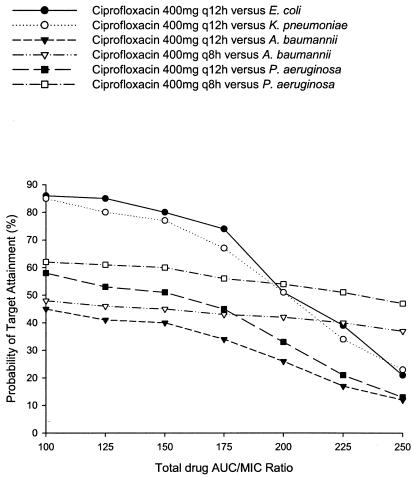
Probabilities of target attainment for ciprofloxacin at various AUC/MIC ratio exposures against all pathogens are presented in Fig. 3. For all pathogens and doses, a lower AUC/MIC target of 100 resulted in no better target attainment than exposure probabilities at 125. In contrast, as the exposure target was increased, the probabilities fell precipitously, especially once the target was greater than or equal to 200 for the q12h dosing regimens. Every 8-h regimen against the nonfermenters appeared to maintain similar, albeit poor, target attainments up to an AUC/MIC of 250.
FIG. 3.
Probabilities of target attainment at different total drug AUC/MIC ratio targets for ciprofloxacin against E. coli, K. pneumoniae, A. baumannii, and P. aeruginosa.
There was excellent agreement between the probability of target attainment and percent susceptibility for the carbapenems and ceftazidime at 1 g q8h. Pharmacodynamic exposure of the cefepime 1 g q12h regimen resulted in −3.0% (95% CI, −8.76 to 2.76) difference compared with the percent susceptibility. Increasing the doses of ceftazidime and cefepime to 2 g against A. baumannii and P. aeruginosa resulted in a positive difference of 6.5% (95% CI, 2.34 to 10.66) and 8.5% (95% CI, −1.2 to 18.2), respectively. In contrast, the probabilities of attaining bactericidal exposure for piperacillin-tazobactam and ciprofloxacin were consistently lower than reported by the percent susceptibility. Agreement for piperacillin-tazobactam 3.375 g q6h was −9.25% (95% CI, −27.60 to 9.10), and that for ciprofloxacin at 400 mg q12h was −18.8% (95% CI, −35.02 to −2.48). Increasing the doses to 3.375g q4h and 400 mg q8h against the nonfermenters did not improve the agreement in probability of target attainment and percent susceptibility.
DISCUSSION
Surveillance programs and trials to monitor antimicrobial resistance are an important part in the war against pathogenic bacteria (10). MYSTIC is one example of a program designed to monitor susceptibilities of important nosocomial pathogens. The decision to use specific antimicrobials is often made by referring to the agent's MIC for a bacteria population (i.e., the concentration at which 50% or 90% of the organisms are inhibited) or the percent susceptibility. However, antibacterial potency, as typically measured by MICs, has little applicability in patient care unless further parameters can be added to the equation. By incorporating these susceptibility data with an antimicrobial agent's pharmacokinetic properties and, most importantly, knowledge of how concentrations best correlate with bactericidal effect relative to its MIC (i.e., pharmacodynamics), more clinically relevant information can be attained. For β-lactam and related antimicrobials, the amount or percentage of time above the MIC is the more important parameter, whereas for fluoroquinolones, the ratio of the AUC to MIC becomes a more crucial factor in eradication of bacteria. Ideal pharmacodynamic goals for each antimicrobial class have been studied and discussed in previous works (3, 6, 7, 30, 32, 33).
In this analysis, we intended to examine typical antimicrobial regimens used in eradicating certain nosocomial pathogens and which ones are likely to be the most suitable as empirical choices in different parts of the world. This report represents just one portion of the MYSTIC MIC data used in conjunction with pharmacokinetic data to assess the probability of attaining pharmacodynamic targets for commonly prescribed i.v. antimicrobials used to empirically treat nosocomial infections. Other regions currently included in the OPTAMA Program are South America, Northern Europe, Eastern Europe, and Southern Europe.
Of all antimicrobials and dosage regimens tested, ciprofloxacin had the lowest probabilities of pharmacodynamic target attainment. This was the case against all pathogens, including the Enterobacteriaceae. Although target attainments of 80 to 85% were achieved, these values are low relative to the high target attainment for some of the other agents tested. Currently, no consensus has been made regarding what is considered an appropriate target attainment; ideally, agents and dosages should be chosen that achieve probabilities as close to 100% as possible, without causing untoward toxicity. Increasing MICs and resistance to fluoroquinolones in E. coli and K. pneumoniae have become a greater concern in some parts of the world, including North America, and the lower target attainments found in this analysis reflect changes in MICs often not seen when only percent susceptibility is reported (8, 12, 28, 29). Moreover, because of a higher frequency of A. baumannii and P. aeruginosa isolates for which MICs are elevated, increasing the dose to 400 mg q8h did not significantly improve target attainment, although this dosing regimen was able to maintain target attainments up to AUC/MIC ratios of 250 (Fig. 3). Of note, we modeled total-drug AUCs for ciprofloxacin because the original studies deriving the pharmacodynamic target for this compound (i.e., AUC/MIC ratio of ≥125) did not consider protein binding (7). Protein binding for ciprofloxacin is reported as 20 to 40%; thus, probabilities of target attainment for free drug AUC/MIC ratios would be that much lower than we reported.
The percent susceptibility of all bacteria to ciprofloxacin in this data set was not in agreement with the target attainment. Ciprofloxacin target attainment exposure was consistently lower than what was reported as susceptible (−18.8%), even with the higher dose used against A. baumannii and P. aeruginosa (−19.0%). The potential to treat pathogens reported as susceptible and yet not achieve the desired pharmacodynamic exposure is a matter for concern. Therefore, against these organisms, ciprofloxacin and the other currently available fluoroquinolones should most likely be reevaluated for susceptibility breakpoints in relation to their normally achievable plasma concentrations and resulting AUCs with standard intravenous dosing (22).
Against the Enterobacteriaceae, all of the β-lactams achieved high target attainment, but only cefepime, meropenem, and imipenem obtained their targets at approximately 100%. Of the β-lactams, target attainment was consistent across various exposures (20 to 100% T>MIC) for all agents except piperacillin-tazobactam, which began to decline after a target of 60% T>MIC for E. coli and 50% for K. pneumoniae. Only the two carbapenems achieved high target attainment against A. baumannii at the bactericidal target (i.e., 40% T>MIC), with imipenem maintaining consistent attainment over a range of exposures up to 60% T>MIC. In contrast, all of the β-lactams achieved relatively high levels of exposure against P. aeruginosa, but larger doses of ceftazidime, cefepime, and piperacillin-tazobactam were needed. Of note is the increase in target attainment for cefepime and piperacillin-tazobactam with the larger daily doses compared with the standard lower doses against this pathogen. Target attainments for all β-lactams declined as the target exposure was increased for this pathogen. Importantly, no extended-spectrum β-lactamases were isolated among these isolates for North America in 2002, and target attainment for the noncarbapenem β-lactams might be significantly lower in the presence of pathogens harboring these enzymes, as was demonstrated in a previous Monte Carlo simulation comparing piperacillin-tazobactam and cefepime (1).
In general, agreement between the probability of bactericidal target attainment and the percent susceptibility for the β-lactams at standard doses and dosing intervals was excellent. However, the one exception to this observation was piperacillin-tazobactam. Like ciprofloxacin, noticeable discrepancies between target attainment and percent susceptibility of bacteria for piperacillin-tazobactam were discovered in this analysis, specifically for P. aeruginosa. The percent susceptibility to piperacillin-tazobactam for P. aeruginosa was 93%, whereas target attainment for even the higher dose regimen was 85%. Mean agreement for achieving optimal exposure against all pathogens was 9.25% lower than reported by the percent susceptibility. From a clinical perspective, these piperacillin-tazobactam regimens may seem appropriate for most patients with nosocomial gram-negative bacilli infections due to concomitant antimicrobial therapy and immune response, but the inability to achieve a higher % T>MIC value among these bacteria might lead to further environmental pressures and greater antimicrobial resistance (6, 22). Cefepime exposure at a dose of 1 g q12h also just fell outside of what was considered adequate for agreement with percent susceptibility. This slight disagreement is due to the very low MICs for the Enterobacteriaceae populations and higher ones for the Acinetobacter population. As MICs increase for these bacterial populations, the percent susceptibility will consistently overestimate the probability of achieving optimal exposure at the dose of 1 g q12h. Although not tested in this analysis, a cefepime dose of 1 g q8h would likely achieve target attainment that agrees better with percent susceptibility. Meanwhile, higher doses of both ceftazidime and cefepime actually resulted in target attainment above what would be reported as susceptible.
Compared with data from other regions of the world (i.e., South America and Northern, Southern, and Eastern Europe), the target attainment for the antimicrobial regimens simulated in OPTAMA North America is markedly better overall (14; R. Masterton, J. L. Kuti, P. Turner, and D. P. Nicolau, submitted for publication). For example, target attainment percentages for the agents reported in the current evaluation were 20 to 30% lower against South American P. aeruginosa isolates that those observed in North America (14). This is due to substantial differences in MIC distributions among these regions of the world.
It is important to understand certain assumptions that we made in our model when applying these results to daily clinical practice. First of all, as mentioned above, isolates were derived from a total of 20 hospitals geographically distributed throughout the United States, Mexico, and Canada during the MYSTIC Surveillance Study. The MYSTIC study is one of many large surveillance databases that track resistance to numerous antimicrobials throughout the world, and while susceptibility results tend to be similar to those found in other programs (13), the MIC distributions for such pathogens in a specific institution may be different than that reported here. Ideally, the use of hospital- or unit-specific MIC distributions in these Monte Carlo simulations would provide the most reliable data for designing empirical dosing regimens. This approach has been done at some centers to justify the use of novel dosing regimens, such as continuous infusion (20). Additionally, we chose to employ a one-compartment i.v. bolus model to determine % T>MIC for these β-lactams, whereas in clinical practice they are commonly administered as 30 min infusions. Other investigators have used the same one-compartment model to calculate % T>MIC for many of the β-lactams (1, 19); furthermore, a previous pharmacodynamic analysis with piperacillin-tazobactam revealed that the additional time above the MIC allotted by the 30-min infusion was insignificant with respect to total exposure (15). Last, for comparative purposes, we defined the bactericidal targets for these antibiotic classes. While the bactericidal targets for the carbapenems, penicillins, and fluoroquinolones against gram-negatives are well referenced at 40% T>MIC, 50% T>MIC, and an AUC/MIC ratio of 125, the absolute pharmacodynamic target for the various antimicrobial classes has been disputed. For example, the bactericidal exposure for cephalosporins seems to vary depending on the specific agent and bacterium studied, and higher targets, such as 70%, have been reported (32). Likewise, AUC/MIC exposures greater than or equal to 250 have also demonstrated outcomes equivalent to if not better than those found at 125 (7, 33). For this reason, we modeled target attainment over a range of % T>MIC and AUC/MIC ratio exposures, allowing readers to compare probabilities at their preferred targets.
Conclusion
In line with the goal of the OPTAMA Program, opportunities to employ optimal empirical antimicrobial therapy based on MIC, pharmacokinetic, and pharmacodynamic data are achievable. In certain cases, making empirical antimicrobial decisions based on such data may prove to correlate better with clinical outcomes than simply choosing agents based on susceptibility reports. Ciprofloxacin monotherapy appears to be a poor empirical choice for the treatment of infections presumed to be caused by these pathogens relative to the target attainment of other antimicrobials. Since the carbapenems were the only antimicrobials to achieve high target attainment against A. baumannii, these agents should be first line when this pathogen is suspected. When A. baumannii is not suspected, the carbapenems, as well as larger or more frequent doses of the cephalosporins and piperacillin-tazobactam, should be appropriate first-line therapy for the treatment of the Enterobacteriaceae and P. aeruginosa. Proper streamlining of empirical antimicrobial therapy is encouraged after pathogen identification and susceptibility results are available. Given the lack of agreement between percent susceptibility and probability of target attainment for certain antimicrobial regimens, a methodology employing stochastic pharmacodynamic analyses may be a more useful tool for differentiating the most optimal compounds and dosing regimens in the clinical setting of initial empirical therapy.
Acknowledgments
The OPTAMA Program is made possible through a research grant provided by AstraZeneca LP, Wilmington, Del.
REFERENCES
- 1.Ambrose, P. G., S. M. Bhavnani, and R. N. Jones. 2003. Pharmacokinetics-pharmacodynamics of cefepime and piperacillin-tazobactam against Escherichia coli and Klebsiella pneumoniae strains producing extended-spectrum β-lactamases: report from the ARREST program. Antimicrob. Agents Chemother. 47:1643-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bland, J. M., and D. G. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307-310. [PubMed] [Google Scholar]
- 3.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 4.Diekema, D. J., M. A. Pfaller, R. N. Jones, G. V. Doern, K. C. Kugler, and M. L. Bench. 2000. Trends in antimicrobial susceptibility of bacterial pathogens isolated from patients with bloodstream infection in the USA, Canada and Latin America. SENTRY Participants Group. Int. J. Antimicrob. Agents. 18:257-271. [DOI] [PubMed] [Google Scholar]
- 5.Dreetz, M., J. Hamacher, J. Eller, K. Borner, P. Koepe, T. Schberg, and H. Lode. 1996. Serum bactericidal activities and comparative pharmacokinetics of meropenem and imipenem-cilastatin. Antimicrob. Agents Chemother. 40:105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drusano G. L. 2003. Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin. Infect. Dis. 36(Suppl. 1):S42-S50. [DOI] [PubMed] [Google Scholar]
- 7.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez, L., J. Garau, C. Estrada, M. Marquez, D. Dalmau, M. Xercavins, J. M. Marti, and C. Estany. 2003. Ciprofloxacin prophylaxis in patients with acute leukemia and granulocytopenia in an area with high prevalence of ciprofloxacin-resistant Escherichia coli. Cancer 97:419-424. [DOI] [PubMed] [Google Scholar]
- 9.Jones, R. N. 1996. The emergent needs for basic research, education, and surveillance of antimicrobial resistance. Problems facing the report from the American Society for Microbiology Task Force on Antibiotic Resistance. Diagn. Microbiol. Infect. Dis. 25:153-161. [DOI] [PubMed] [Google Scholar]
- 10.Jones, R. N., and R. G. Masterton. 2001. Establishing the value of antimicrobial surveillance programs. Diagn. Microbiol. Infect. Dis. 41:171-175. [DOI] [PubMed] [Google Scholar]
- 11.Jones, R. N., and M. A. Pfaller. 1998. Bacterial resistance: a worldwide problem. Diagn. Microbiol. Infect. Dis. 31:379-388. [DOI] [PubMed] [Google Scholar]
- 12.Karlowsky, J. A., C. Thornsberry, M. E. Jones, and D. F. Sahm. 2003. Susceptibility of antimicrobial-resistant urinary Escherichia coli isolates to fluoroquinolones and nitrofurantoin. Clin. Infect. Dis. 3:183-187. [DOI] [PubMed] [Google Scholar]
- 13.Karlowsky, J. A., D. C. Draghi, M. E. Jones, C. Thornsberry, I. R. Friedland, and D. F. Sahm. 2003. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob. Agents Chemother. 47:1681-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiffer, C. R. V., C. Mendes, J. L. Kuti, and D. P. Nicolau. Pharmacodynamic comparisons of antimicrobials against nosocomial isolates of E. coli, K. pneumoniae, A. baumannii and P. aeruginosa from the MYSTIC Surveillance Program: the OPTAMA Program, South America 2002. Diagn. Microbiol. Infect. Dis., in press. [DOI] [PubMed]
- 15.Kim, M. K., B. Capitano, H. M. Mattoes, D. Xuan, R. Quintiliani, C. H. Nightingale, and D. P. Nicolau. 2002. Pharmacokinetic and pharmacodynamic evaluation of two dosing regimens for piperacillin-tazobactam. Pharmacotherapy 22:569-577. [DOI] [PubMed] [Google Scholar]
- 16.Klepser, M. E., K. B. Patel, D. P. Nicolau, R. Quintiliani, and C. H. Nightingale. 1995. Comparison of the bactericidal activities of ofloxacin and ciprofloxacin alone and in combination with ceftazidime and piperacillin against clinical strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 39:2503-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kollef, M. H., and S. Ward. 1998. The influence of mini-BAL cultures on patient outcomes: implications for the antibiotic management of ventilator-associated pneumonia. Chest 113:412-420. [DOI] [PubMed] [Google Scholar]
- 18.Kollef, M. H., G. Sherman, S. Ward, and V. J. Fraser. 1999. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462-474. [DOI] [PubMed] [Google Scholar]
- 19.Kuti, J. L., N. F. Florea, C. H. Nightingale, and D. P. Nicolau. 2004. Pharmacodynamics of meropenem and imipenem against Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa. Pharmacotherapy 24:8-15. [DOI] [PubMed] [Google Scholar]
- 20.Kuti, J. L., C. H. Nightingale, R. Quintiliani, and D. P. Nicolau. 2002. Pharmacodynamic profiling of continuously infused piperacillin/tazobactam against Pseudomonas aeruginosa using Monte Carlo analysis. Diagn. Microbiol. Infect. Dis. 44:51-57. [DOI] [PubMed] [Google Scholar]
- 21.Mendes, C., and P. J. Turner. 2001. Unit differences in pathogen occurrence among European MYSTIC Program (1997-2000). Diagn. Microbiol. Infect. Dis. 41:191-196. [DOI] [PubMed] [Google Scholar]
- 22.Mouton, J. W. 2002. Breakpoints: current and future perspectives. Int. J. Antimicrob. Agents 19:323-331. [DOI] [PubMed] [Google Scholar]
- 23.Mouton, J. W., A. M. Horrevorts, P. G. Mulder, E. P. Prens, and M. E. Michel. 1990. Pharmacokinetics of ceftazidime in serum and suction blister fluid during continuous and intermittent infusion in healthy volunteers. Antimicrob. Agents Chemother. 34:2307-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NCCLS. 2002. Performance standards for antimicrobial susceptibility testing: eleventh informational supplement M100-S11. NCCLS, Wayne, Pa.
- 25.Nye, K. J., Y. G. Shi, J. M. Andrew, and R. Wise. 1989. Pharmacokinetics and tissue penetration of cefepime. J. Antimicrob. Chemother. 24:23-28. [DOI] [PubMed] [Google Scholar]
- 26.Occhipinti, D. J., S. L. Pendland, L. L. Schoonover, E. B. Rypins, L. H. Danzinger, and K. A. Rodvold. 1997. Pharmacokinetics and pharmacodynamics of two multiple-dose piperacillin-tazobactam regimens. Antimicrob. Agents Chemother. 41:2511-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhomberg, P. R., R. N. Jones, and The MYSTIC Program (USA) Study Group. 2003. Antimicrobial spectrum of activity for meropenem and nine broad-spectrum antimicrobials: report from the MYSTIC Program (2002) in North America. Diagn. Microbiol. Infect. Dis. 47:365-372. [DOI] [PubMed] [Google Scholar]
- 28.Sahm, D. F., L. A. Critchley, L. J. Kelly, J. A. Karlowsky, D. C. Mayfield, C. Thornsberry, Y. Mauriz, and J. Kahn. 2001. Evaluation of current activities of fluoroquinolones against gram-negative bacilli using centralized in vitro testing and electronic surveillance. Antimicrob. Agents Chemother. 45:267-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheld, W. M. 2003. Maintaining fluoroquinolone class efficacy: review of influencing factors. Emerg. Infect. Dis. 9:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas, J. K., A. Forrest, S. M. Bhavnani, J. M. Hyatt, A. Cheng, C. H. Ballow, and J. J. Schentag. 1998. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob. Agents Chemother. 42:521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trouillet, J.-L., J. Chastre, A. Vuagnat, M.-L. Joly-Guillou, D. Combaux, M.-C. Dombret, and C. Gibert. 1998. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am. J. Respir. Crit. Care Med. 157:531-539. [DOI] [PubMed] [Google Scholar]
- 32.Turnidge, J. D. 1998. The pharmacodynamics of β-lactams. Clin. Infect. Dis. 27:10-22. [DOI] [PubMed] [Google Scholar]
- 33.Wright, D. H., G. H. Brown, M. L. Peterson, and J. C. Rotschafer. 2000. Application of fluoroquinolone pharmacodynamics. J. Antimicrob. Chemother. 46:669-683. [DOI] [PubMed] [Google Scholar]