Abstract
Importance
Posttraumatic stress disorder (PTSD) appears to increase obesity risk, but the pathways by which PTSD leads to weight gain are not known. Identification of the links between PTSD and obesogenic eating behaviors is necessary to clarify this pathway and inform development of obesity prevention strategies in PTSD-affected populations.
Study objective
To determine whether women with PTSD symptoms are more likely to report food addiction, a measure of perceived dependence on food, than women without PTSD symptoms. To determine whether age at PTSD symptom onset and type of trauma influence the PTSD food addiction association.
Design, setting, and participants
Cross-sectional analysis of 49,408 participants in the Nurses’ Health Study II (NHSII), a cohort comprised of female nurses who were aged 25–42 at 1989 recruitment from 14 US states.
Exposure and outcome measures
The NHSII ascertained lifetime trauma exposure and PTSD symptoms in 2008 and current food addiction in 2009. Food addiction was defined as ≥3 clinically significant symptoms on a modified version of the Yale Food Addiction Scale. Confounder-adjusted prevalence ratios (PRs) and 95% confidence intervals (CIs) were estimated using modified Poisson regression.
Results
Roughly 80% of the study sample reported some type of trauma exposure, with 66% of the trauma-exposed reporting at least 1 lifetime PTSD symptom. Eight percent of the cohort met the criteria for food addiction. The prevalence of food addiction increased with number of lifetime PTSD symptoms, and women with the greatest number of PTSD symptoms (6–7 symptoms) had more than twice the prevalence of food addiction as women with neither PTSD symptoms nor trauma histories (PR=2.68; 95% CI: 2.41, 2.97). PTSD symptoms were more strongly related to food addiction when symptom onset occurred at an earlier age. The PTSD food addiction association did not differ substantially by trauma type.
Conclusions and relevance
PTSD symptoms were associated with increased food addiction prevalence in this cohort of women. Strategies to reduce obesity associated with PTSD may require psychological and behavioral interventions that address dependence on food and/or use of food to cope with distress.
Introduction
Posttraumatic stress disorder (PTSD) is a potentially severe psychiatric condition affecting both military and civilian populations1–3. PTSD is characterized by a set of three symptom clusters—reexperiencing, arousal and avoidance/numbing4 — that arise in response to a potentially traumatic event. In the United States civilian population, lifetime PTSD occurrence is 6.8%, with a higher rate (roughly 12%) among women2,5. In addition to causing acute psychological suffering, PTSD may have long-term effects on physical health and well-being. In particular, a growing body of evidence suggests that PTSD is an important risk factor for obesity and obesity-related diseases, including type 2 diabetes and cardiovascular disease6–12. PTSD may be linked to cardiometabolic illness in part through direct biological mechanisms such as inflammatory pathways13,14, but it is likely that behavioral factors also play an important role, and these remain to be clarified.
A recent cross-sectional study by Hirth et al. found PTSD symptoms to be associated with increased consumption of fast food and soda in young women 15. As noted by Brewerton in a commentary 16, these findings are suggestive of the use of food to self-medicate, and are consistent with animal models indicating that stress may provoke increased consumption of highly palatable (high-fat/high-sugar) food, leading to weight gain 17–19. Neurological studies indicate that ingestion of high-fat and high-sugar foods may blunt feelings of distress by triggering dopaminergic reward responses 20,21, and animal studies suggest that stress-related consumption of palatable food can ultimately become addiction-like in nature 22–24. This growing body of animal and clinical literature has led to public health interest in the potential role of “food addiction” in the obesity epidemic 25. In previous work, we found that childhood abuse is strongly associated with adult reports of food addiction, and that women reporting food addiction were an average of 6 units of body mass index heavier than women without food addiction 26. Food addiction is not currently established as a psychiatric diagnosis, but the concept may nonetheless be helpful for identifying the reliance on food to cope with psychological distress, one plausible pathway from PTSD to obesity.
Understanding the links between PTSD and obesogenic eating behaviors is crucial for development of effective strategies to prevent and treat obesity in PTSD-affected populations. In this analysis, we examined the association between post-traumatic stress disorder symptoms and food addiction among 49,408 participants in the Nurses’ Health Study II.
Methods
Data sources
The Nurses’ Health Study II (NHSII) follows 116,430 female registered nurses recruited at ages 25–42 in 1989. Biennial questionnaires are used to gather sociodemographic, behavioral, and medical data. Participants indicate their consent by returning completed questionnaires. In 2009, the biennial questionnaire included a modified version of the Yale Food Addiction Scale (mYFAS) 27, which ascertains the extent to which participants’ eating behavior can be characterized as a dependency. In 2008, a supplemental PTSD Questionnaire assessing trauma exposure and PTSD symptoms was sent to 60,894 NHSII participants who had responded to an earlier supplemental questionnaire on exposure to violence. The 2008 PTSD Questionnaire was returned by 54,700 women (a response rate of 90%). Of these, 53,616 responded to the 2009 biennial questionnaire, with 49,408 having complete food addiction and PTSD information. This study was approved by the Institutional Review Board at Brigham and Women’s Hospital, Boston.
Variables and variable definitions
Exposures
The 2008 PTSD Questionnaire used a modified version of the Brief Trauma Questionnaire28,29 to ascertain women’s experiences of sixteen traumatic events, including violent death of a loved one, childhood physical abuse, or “a serious traumatic event not already covered.” Participants were further asked which event they considered to be the worst30 and their age when it first occurred. Suggestive PTSD symptoms (hereafter referred to as “PTSD symptoms”) were queried with respect to the worst traumatic event using the 7-item Brief Screening Scale for DSM-IV PTSD 31. Participants were asked the age at which they first started experiencing the PTSD symptoms. More than half of individuals with PTSD do not seek treatment 32, and we therefore focus on PTSD symptoms rather than diagnosis to avoid missing the majority of cases.
Breslau et al. recommend using a cutoff of 4+ symptoms on this screening scale for identifying PTSD cases, based on trade-offs between sensitivity and specificity31. Using a cutoff of 4+ symptoms yields a positive predictive value of 71% compared to diagnostic interview, while a cutoff of 6+ symptoms yields a positive predictive value of 87%31. To avoid overemphasizing a single cut-point, we classified participants into 5 exposure categories: 1) no trauma exposure, 2) exposed to a traumatic event but reported no PTSD symptoms, 3) trauma with 1–3 PTSD symptoms, 4) trauma with 4–5 PTSD symptoms, and 5) trauma with 6–7 PTSD symptoms. PTSD symptoms are queried with respect to a specific traumatic event; by definition, women without trauma were classified as having no PTSD symptoms.
Outcome
Food addiction was assessed in 2009 using a modified version of the Yale Food Addiction Scale (YFAS) 27, which is modeled after measures of drug and alcohol addiction. The mYFAS defines food addiction as 3 or more of the following symptoms plus clinically significant impairment or distress: (a) eating when no longer hungry four or more times per week, (b) worrying about cutting down on certain foods four or more times per week, (c) feeling sluggish or fatigued from overeating two or more times per week, (c) experiencing negative feelings from overeating that interfere with other activities two or more times per week, (c) having physical withdrawal symptoms when cutting down on certain foods two or more times per week, (d) continuing to consume the same amount of food despite significant emotional or physical problems due to overeating at any frequency, and (e) feeling the need to eat an increasing amount of food to reduce distress at any frequency. The mYFAS defines clinically significant impairment or distress as either (a) experiencing significant distress related to eating behavior two or more times per week or (b) experiencing a decrease in ability to function due to issues related to food two or more times per week. For further details on the adaptation of the original YFAS see eMethods in the Supplement.
Covariates
We included the following potential confounders in adjusted models, because they are plausible predictors of both PTSD and food addiction 33: age at NHSII baseline (continuous with a squared term), race (indicators for African American, Asian, Hispanic, and other, with non-Hispanic white as the referent), mother’s and father’s educational attainment when participant was an infant (indicators for <9, 9–11, 12, and 13–15 years, with 16+ as the referent), indicators for mother in professional occupation and father in professional occupation, indicator for parental home ownership when participant was an infant, recalled body size at age 5 (continuous; the participant could choose one of nine female figures ranging from very lean, a score of 1, to very obese, a score of 9, that best represented her body type at age five years 34), and parental lifetime history of depression (indicator if either parent had depression history) assessed in 2005.
Data analysis
We used modified Poisson regression (Poisson regression with robust standard errors 35) to estimate prevalence ratios for food addiction as a function of trauma exposure and number of PTSD symptoms. Analyses included women who responded to both the 2008 Stress Questionnaire, on which abuse was ascertained, and the 2009 biennial questionnaire, on which food addiction was assessed (n=53,616). We excluded women who left 3 or more food addiction symptom questions blank (n=2,270); who were missing information on clinical significance of symptoms (n=291); or whose trauma-PTSD status could not be ascertained, due to large amounts of missing data (n=1,607) or inconsistency in their reports (n=40), leaving 49,408 women (92% of all respondents to the PTSD and 2009 biennial questionnaires).
We ran three main analyses. First, we examined the cross-sectional association between lifetime trauma and PTSD symptoms, reported in 2008, and current food addiction, assessed in 2009. We ran an age-adjusted model and a model adjusted for additional potential confounders. Because the 2009 questionnaire did not ask about timing of food addiction symptom onset, the temporal order of PTSD and food addiction is unknown, and the results of these analyses are therefore presented as prevalence rather than risk ratios.
Second, in order to gain some insight into the temporal order of PTSD and food addiction, we examined both the number of PTSD symptoms and the timing of their onset. We were particularly interested in whether onset of PTSD in childhood would be associated with food addiction, because eating disorders typically manifest in adolescence or later 36, and thus an association between pre-adolescent PTSD and food addiction would support a temporal sequence from PTSD to food addiction. To conduct this analysis, we categorized women into 19 cross-classifications based on symptom level (none, 1–3 symptoms, 4–5 symptoms, 6–7 symptoms) and age at onset in 6 intervals (<10, 10–19, 20–29, 30–39, 40–49, and 50+ years). We report food addiction prevalence ratios for each category of symptom level and age at onset, with no symptoms at any time as the referent. Among women with at least one PTSD symptom, we conducted a test for trend of age of symptom onset. We also tested the interaction between categorical PTSD symptoms and age at onset.
We ran two sensitivity analyses related to timing of PTSD onset. Because women were asked to report symptoms in reference to their worst trauma, timing of PTSD symptoms could plausibly be defined using age at worst trauma; we therefore re-ran our analyses using reported age at worst trauma to time the onset of PTSD symptoms. In addition, because we were concerned about the plausibility of recall from a very young age, we ran a sensitivity analysis excluding the 136 women reporting symptom onset prior to age 4.
For our third analysis, we explored PTSD food addiction associations by type of trauma. We categorized women based on cross-classifications of the trauma they identified as their worst and the number of PTSD symptoms experienced in response, with no trauma as the reference category. We previously reported strong relationships of childhood and adolescent physical and sexual abuse victimization with food addiction 26, and thus we were particularly interested in examining the influence of these traumas. While childhood physical abuse (physical abuse before the age of 18) was included in the list of traumas on the PTSD questionnaire, childhood sexual abuse was not—rather, women were asked about unwanted sexual contact at any age. For this analysis, because we were interested in childhood sexual abuse, we defined a participant has having PTSD symptoms in response to childhood sexual abuse if she indicated unwanted sexual contact as her worst trauma and reported that her worst trauma occurred prior to the age of 18 years. We modeled food addiction as a function of the cross-classification of 16 trauma types and 3 PTSD symptom levels using modified Poisson regression and adjusting for potential confounders. Approximately 6.5% of trauma-exposed women did not specify which trauma was their worst and were excluded from this analysis.
Results
Of 49,408 women meeting inclusion criteria for our main analyses, 81% reported experiencing at least one traumatic event (Table 1). Of these, 34% reported no PTSD symptoms, 39% reported 1–3 symptoms, 17% reported 4–5 symptoms, and 10% reported 6–7 symptoms. On average, women with PTSD symptoms reported that their first symptom occurred at age 30.5 years (SD=14.7 years). The food addiction prevalence was 8%, varying from 6% among women with no lifetime PTSD symptoms, to almost 18% among women with 6–7 PTSD symptoms. Family depression history, but not socioeconomic and demographic variables, was associated with PTSD symptoms (Table 1).
Table 1.
Age-adjusted characteristics of the study sample, by reported trauma exposure and number of lifetime PTSD symptoms.
| Variable | Trauma and number of lifetime post-traumatic stress disorder symptomsc
|
||||
|---|---|---|---|---|---|
| No trauma (n=9445) | Trauma but no symptoms (n=13,445) | Trauma and l–3 symptoms (n=15,757) | Trauma and 4–5 symptoms (n=6,859) | Trauma and 6–7 symptoms (n=3,902) | |
| Age in years at first PTSD symptoma (mean, SD) | -- | -- | 30.9(14.3) | 30.5(14.9) | 28.4(15.4) |
|
| |||||
| Food addiction cases, n (prevalence in exposure category) | 575 (6.1) | 785 (5.9) | 1210 (7.7) | 774 (11.3) | 698 (17.8) |
|
| |||||
|
Covariates
| |||||
| Continuous | Mean(SD) | Mean(SD) | Mean(SD) | Mean(SD) | Mean(SD) |
| Age at NHSII baseline (years)a | 35.1(4.7) | 35.3(4.7) | 35.3(4.6) | 35.3(4.5) | 35.3(4.4) |
| Mother’s educational attainment (years) | 12.4(1.9) | 12.3(2.0) | 12.4(2.0) | 12.4(2.0) | 12.4(2.0) |
| Father’s educational attainment (years) | 12.5(2.4) | 12.4(2.4) | 12.5(2.5) | 12.5(2.5) | 12.5(2.5) |
| Somatogram at age 5b | 2.5(1.2) | 2.5(1.2) | 2.5(1.2) | 2.6(1.3) | 2.6(1.3) |
| Categorical | Column% | Column% | Column% | Column% | Column% |
| Race/ethnicity | |||||
| Black | 0.8 | 1.1 | 1.1 | 0.7 | 0.6 |
| Latina | 0.9 | 1.2 | 1.4 | 1.2 | 1.1 |
| Asian | 1.7 | 1.3 | 1.1 | 0.9 | 0.9 |
| White | 93.4 | 93.4 | 94.0 | 94.7 | 94.5 |
| Other | 1.8 | 1.6 | 1.3 | 1.4 | 1.7 |
| Mother in professional occupation | 11.5 | 11.7 | 11.3 | 11.1 | 11.5 |
| Father in professional occupation | 28.0 | 27.3 | 28.4 | 29.0 | 27.7 |
| Parents owned home | 53.0 | 52.6 | 50.6 | 50.5 | 49.9 |
| Parental history of depression | 10.1 | 11.2 | 15.4 | 20.4 | 25.6 |
Value is not age adjusted
Participants chose the image of a female figure that best approximated their body type at age 5, ranging from 1 (very lean) to 9 (obese)
Traumatic symptoms are queried with regard to trauma exposure, and thus by definition women without trauma could not have traumatic symptoms
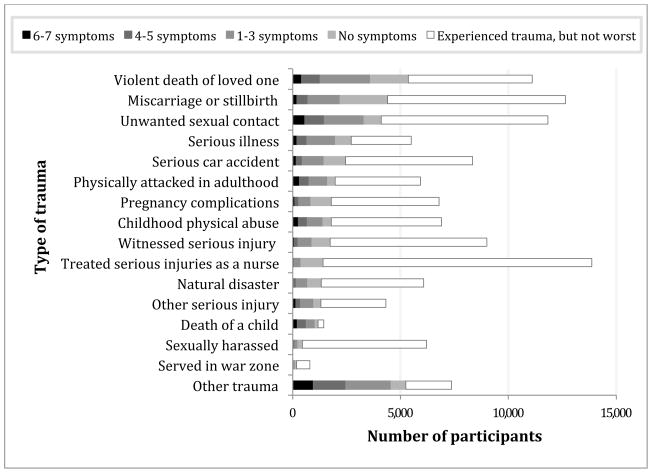
Violent death of a family member or friend was most frequently cited as the respondent’s worst trauma, followed by miscarriage or stillbirth (Figure 1). Child death was most strongly associated with a high level of PTSD symptomatology. The most commonly reported trauma experience in this nurse population was treating civilians with traumatic injuries (Figure 1), though this was rarely cited as the respondent’s worst trauma.
Figure 1.
Number of participants reporting each trauma type as their worst, by the level of PTSD symptoms they reported experiencing in response. Traumas are ordered by descending frequency with which they were reported as worst. Note: Participants could choose only one worst trauma, and thus participants in one worst trauma category will not be in any other worst trauma category; in contrast, participants may be represented in the ‘experienced trauma, but not worst’ category for multiple trauma types.
In age-adjusted analyses, PTSD symptoms were associated with the prevalence of food addiction in a dose-response manner (Table 2), with prevalence ratios (PRs), relative to no trauma, of 0.96 (95% CI: 0.87–1.07), 1.26 (95% CI 1.14–1.39), 1.85 (95% CI: 1.67–2.05), and 2.93 (95% CI: 2.64–3.25) for trauma but no PTSD symptoms, and 1–3, 4–5, and 6–7 PTSD symptoms, respectively. Covariate adjustment attenuated these modestly, yielding PRs of 0.95 (95% CI: 0.86–1.05), 1.22 (95% CI 1.11–1.34), 1.73 (95% CI: 1.56–1.92), and 2.68 (95% CI: 2.41–2.97) for trauma but no symptoms, and 1–3, 4–5, and 6–7 symptoms, respectively. Inclusion of family history of depression resulted in the greatest attenuation on the estimates, though its impact was still small (resulting in a roughly 3% change in the estimate).
Table 2.
Prevalence ratios for 2009 food addiction, by reported lifetime number of posttraumatic stress disorder symptoms
| Trauma and number of PTSD symptoms | n (cases) | Age-adjusted | Covariate-adjusteda | ||
|---|---|---|---|---|---|
|
| |||||
| PR | 95% CI | PR | 95% CI | ||
| No trauma | 9445 (575) | 1.00 | -- | 1.00 | -- |
| Trauma, no symptoms | 13,445 (785) | 0.96 | (0.87, 1.07) | 0.95 | (0.86, 1.06) |
| Trauma and 1–3 symptoms | 15,757 (1210) | 1.26 | (1.14, 1.39) | 1.22 | (1.11, 1.34) |
| Trauma and 4–5 symptoms | 6,859 (774) | 1.85 | (1.67, 2.05) | 1.73 | (1.56, 1.92) |
| Trauma and 6–7 symptoms | 3,902 (698) | 2.93 | (2.64, 3.25) | 2.68 | (2.41, 2.97) |
Adjusted for age in 1989, race, mother’s educational attainment, father’s educational attainment, mother in professional occupation, father in professional occupation, parental home ownership, parental history of depression. Additional adjustment for parental history of diabetes mellitus, early myocardial infarction, and stroke did not have an important impact on the results.
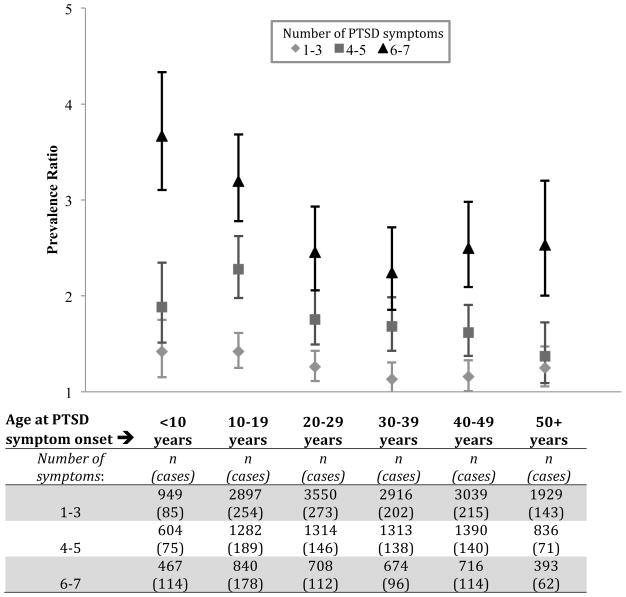
When PTSD symptom onset occurred at <10 years of age, the adjusted PR for food addiction associated with 6–7 PTSD symptoms, relative to no PTSD symptoms, was 3.67 (95% CI: 3.10, 4.33) (Figure 2); this declined to 2.24 (95% CI: 1.85, 2.71) when symptom onset occurred between 30 and 39 years of age. This pattern of diminishing effect sizes with increasing age was less apparent for lower levels of PTSD symptomatology. Findings were robust to using reported age at worst trauma to define the age increments and excluding women reporting trauma symptoms prior to age 4. Among women with at least one PTSD symptom, the age at symptom onset was statistically significantly associated with food addiction (p for trend<0.001), but the interaction between age at symptom onset and number of PTSD symptoms was not (p=0.33). This suggests that, among women with at least one PTSD symptom, earlier onset predicts a higher baseline prevalence of food addiction, but does not influence the dose-response association between additional PTSD symptoms and prevalence of food addiction.
Figure 2.
Prevalence ratio for food addiction as a function of number of reported PTSD symptoms, by age at onset of first symptom. Reference group reported no PTSD symptoms (n=22,890; 1360 cases of food addiction).
The type of trauma reported as worst did not have a clear influence on the magnitude of the PTSD food addiction association (Figure 2 and eTable 1 in the Supplement). Traumatic symptoms in response to physical abuse in childhood showed the strongest associations with food addiction overall (PR ranged from 1.6 (95% CI: 1.1,2.1) for physical abuse with no PTSD symptoms to 3.7 (95% CI: 3.0, 4.7) for physical abuse with 6–7 symptoms). However, confidence intervals were overlapping across various trauma types, providing little statistical evidence for differences in PTSD food addiction associations, and the overall pattern of associations were fairly similar.
Discussion
In this analysis, we found a dose-response association between number of lifetime PTSD symptoms and prevalence of food addiction in middle adulthood. Food addiction prevalence appeared to be further elevated when PTSD symptoms occurred earlier in life. To our knowledge, this is the first study to look at the association between PTSD symptoms and food addiction. Our findings are relevant to ongoing questions regarding the mechanisms behind observed associations between PTSD and obesity 12, and provide support for hypotheses suggesting that associations between PTSD and obesity might partly originate in maladaptive coping and use of food to blunt trauma-associated distress. If replicated longitudinally, these results may have implications for both the etiology of obesity and for treatment of individuals suffering from PTSD.
Our findings are consistent with a small body of literature on the comorbidity of PTSD and disordered eating 37, including two cross-sectional studies documenting an association between PTSD and Binge Eating Disorder (BED) 10,38, a related but distinct overeating phenotype39. The value of the food addiction measure is that it explicitly captures feelings of dependence on food and distress as a trigger for eating, and is thus more directly in line with hypothesized mechanisms involving eating as a coping strategy.
While we were limited to cross-sectional analyses by lack of data on timing of food addiction onset, we were able to examine the relationship between age at PTSD onset and food addiction. We found PTSD symptoms to be associated with food addiction even when they are experienced prior to adolescence, the typical age of eating disorder onset, consistent with a pathway from PTSD to food addiction. Early-onset PTSD food addiction associations are also consistent with the idea that childhood is a critical window of exposure during which health insults may have especially profound effects 40–42. However, it is also possible that these results arise from recall bias: women may have difficulty recalling early-life trauma unless it was severe, and self-reports of childhood PTSD may therefore over-represent the most severe PTSD exposure.
While there was some variation in the PTSD food addiction association across trauma types, evidence for an influence of trauma type on the association was limited. We found that childhood physical abuse and unwanted sexual contact in adulthood were unique in being associated with food addiction even in the absence of PTSD symptoms, but these findings may be due to chance, and overall patterns of association between PTSD symptoms and food addiction were otherwise similar.
Several limitations of our study warrant comment. First, we assessed PTSD retrospectively with a screening questionnaire (rather than a diagnostic interview), which likely reduced the accuracy of PTSD classification, but enabled the collection of PTSD data from a large cohort. Second, it should be noted that women who reported more than one PTSD symptom may not have experienced all of their symptoms in the same age range that they reported symptom onset; the classification of women by age of PTSD onset is therefore a crude measure of the timing of PTSD and does not completely capture the nuances of PTSD symptoms over the lifecourse. Finally, food addiction is controversial, and the clinical applicability of our findings will depend in part upon whether mental health providers determine that food addiction is a legitimate psychiatric diagnosis and identify effective prevention and treatment strategies for it. However, we believe that the value of the food addiction construct goes beyond its identification of psychiatric illness by capturing a potentially important maladaptive coping behavior that may provide insight into mechanisms linking trauma and PTSD to obesity.
CONCLUSION
This study provides the first evidence of an association between PTSD symptoms and food addiction, two disorders of emerging concern for obesity risk. Our findings are consistent with the hypothesis that observed links between PTSD and obesity12 might be partly explained by a tendency to use food to self-medicate traumatic stress symptoms16. If replicated longitudinally, these findings suggest that interrupting the pathway from PTSD to obesity may require psychological and behavioral interventions that address dependence on eating to cope with distress. Our study provides additional support for the recent recommendations by Kubzansky et al. for integration of health behavior modification into PTSD treatment 12, and highlights one eating behavior phenotype that might be modified to reduce long-term obesity risk among populations with PTSD.
Supplementary Material
Figure 3.
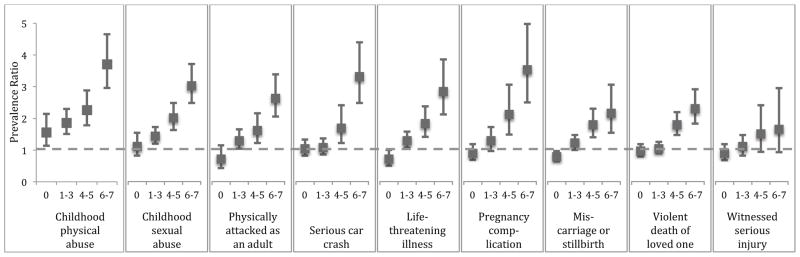
Prevalence ratios for food addiction as a function of type of worst trauma and number of PTSD symptoms experienced in response. Figure presents the nine queried traumas most commonly reported as worst.
Acknowledgments
Dr. Mason conducted all data analyses, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This project was supported by grants RO1HL081557 and RO1HL064108 from the National Institutes of Health. The funder had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Conflicts of interest: The authors report no conflicts of interest
References
- 1.Thomas JL, Wilk JE, Riviere LA, McGurk D, Castro CA, Hoge CW. Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Arch Gen Psychiatry. 2010;67(6):614–23. doi: 10.1001/archgenpsychiatry.2010.54. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. 2000. p. 943. Text Revision. [Google Scholar]
- 5.Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61:984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- 6.Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol. 2011;108:29–33. doi: 10.1016/j.amjcard.2011.02.340. [DOI] [PubMed] [Google Scholar]
- 7.Boyko EJ, Jacobson IG, Smith B, et al. Risk of diabetes in U.S. military service members in relation to combat deployment and mental health. Diabetes Care. 2010;33:1771–1777. doi: 10.2337/dc10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dedert EA, Becker ME, Fuemmeler BF, Braxton LE, Calhoun PS, Beckham JC. Childhood traumatic stress and obesity in women: the intervening effects of PTSD and MDD. J Trauma Stress. 2010;23:785–763. doi: 10.1002/jts.20584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dedert EA, Calhoun PS, Watkins LL, Sherwood A, Beckham JC. Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann Behav Med. 2010;39(1):61–78. doi: 10.1007/s12160-010-9165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagoto SL, Schneider KL, Bodenlos JS, et al. Association of post-traumatic stress disorder and obesity in a nationally representative sample. Obesity (Silver Spring) 2012;20:200–5. doi: 10.1038/oby.2011.318. [DOI] [PubMed] [Google Scholar]
- 11.Roberts AL, Agnew-Blais J, Spiegelman D, et al. Posttraumatic stress disorder and type 2 diabetes incidence in women: An 18-year longitudinal study. Under Rev. 2013 doi: 10.1001/jamapsychiatry.2014.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubzansky LD, Bordelois P, Jun HJ, et al. The Weight of Traumatic Stress: A Prospective Study of Posttraumatic Stress Disorder Symptoms and Weight Status in Women. JAMA Psychiatry. 2013 doi: 10.1001/jamapsychiatry.2013.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieck A, Grassi-Oliveira R, Hartmann do Prado C, Teixeira A, Bauer M. Neuroimmunoendocrine interactions in post-traumatic stress disorder: focus on long-term implications of childhood maltreatment. Neuroimmunomodulation. 2014;21(2–3):145–51. doi: 10.1159/000356552. [DOI] [PubMed] [Google Scholar]
- 14.Rasmusson AM, Schnurr PP, Zukowska Z, Scioli E, Forman DE. Adaptation to extreme stress: post-traumatic stress disorder, neuropeptide Y and metabolic syndrome. Exp Biol Med (Maywood) 2010;235:1150–1162. doi: 10.1258/ebm.2010.009334. [DOI] [PubMed] [Google Scholar]
- 15.Hirth JM, Rahman M, Berenson AB. The association of posttraumatic stress disorder with fast food and soda consumption and unhealthy weight loss behaviors among young women. J Womens Health (Larchmt) 2011;20:1141–1149. doi: 10.1089/jwh.2010.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brewerton TD. Posttraumatic stress disorder and disordered eating: food addiction as self-medication. J Womens Health (Larchmt) 2011;20:1133–1134. doi: 10.1089/jwh.2011.3050. [DOI] [PubMed] [Google Scholar]
- 17.Corwin RL, Avena NM, Boggiano MM. Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav. 2011;104:87–97. doi: 10.1016/j.physbeh.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowland NE, Antelman SM. Stress-induced hyperphagia and obesity in rats: a possible model for understanding human obesity. Science. 1976;191:310–312. doi: 10.1126/science.1246617. [DOI] [PubMed] [Google Scholar]
- 19.Dallman MF, Pecoraro N, Akana SF, et al. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cota D, Tschöp MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res Rev. 2006;51:85–107. doi: 10.1016/j.brainresrev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Ulrich-Lai YM, Christiansen AM, Ostrander MM, et al. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci U S A. 2010;107:20529–20534. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- 24.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gearhardt AN, Grilo CM, DiLeone RJ, Brownell KD, Potenza MN. Can food be addictive? Public health and policy implications. Addiction. 2011;106:1208–1212. doi: 10.1111/j.1360-0443.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason SM, Flint AJ, Field AE, Austin SB, Rich-Edwards JW. Abuse victimization in childhood or adolescence and risk of food addiction in adult women. Obesity (Silver Spring) 2013;200:1–33. doi: 10.1002/oby.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009;52:430–436. doi: 10.1016/j.appet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Schnurr P, Lunney C, Sengupta A, Spiro A. A longitudinal study of retirement in older male veterans. J Consult Clin Psychol. 2005;73(3):561–566. doi: 10.1037/0022-006X.73.3.561. [DOI] [PubMed] [Google Scholar]
- 29.Schnurr P, Spiro A, Velhauer M, Findler M, Hamblen J. Trauma in the lives of older men: findings from the Normative Aging Study. J Clin Geropsychol. 2002;8(3):175–187. [Google Scholar]
- 30.Breslau N, Peterson EL, Poisson LM, Schultz LR, Lucia VC. Estimating post-traumatic stress disorder in the community: lifetime perspective and the impact of typical traumatic events. Psychol Med. 2004;34:889–898. doi: 10.1017/s0033291703001612. [DOI] [PubMed] [Google Scholar]
- 31.Breslau N, Peterson EL, Kessler RC, Schultz LR. Short screening scale for DSM-IV posttraumatic stress disorder. Am J Psychiatry. 1999;156:908–911. doi: 10.1176/ajp.156.6.908. [DOI] [PubMed] [Google Scholar]
- 32.Roberts A, Gilman S, Breslau J, Breslau N, Koenen K. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychol Med. 2011;41(1):71–83. doi: 10.1017/S0033291710000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koenen KC, Moffitt TE, Poulton R, Martin J, Caspi A. Early childhood factors associated with the development of post-traumatic stress disorder: results from a longitudinal birth cohort. Psychol Med. 2007;37:181–192. doi: 10.1017/S0033291706009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stunkard A, Sorenson T, Schulsinger F. The Genetics of Neurological and Psychiatric Disorders. New York: Raven Press; 1983. [Google Scholar]
- 35.Zou G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 36.Kessler RC, Berglund Pa, Chiu WT, et al. The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol Psychiatry. 2013;73:904–14. doi: 10.1016/j.biopsych.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell KS, Mazzeo SE, Schlesinger MR, Brewerton TD, Smith BN. Comorbidity of partial and subthreshold ptsd among men and women with eating disorders in the national comorbidity survey-replication study. Int J Eat Disord. 2012;45:307–15. doi: 10.1002/eat.20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dansky BS, Brewerton TD, Kilpatrick DG, O’Neil PM. The National Women’s Study: relationship of victimization and posttraumatic stress disorder to bulimia nervosa. Int J Eat Disord. 1997;21:213–228. doi: 10.1002/(sici)1098-108x(199704)21:3<213::aid-eat2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 39.Gearhardt AN, White Ma, Masheb RM, Morgan PT, Crosby RD, Grilo CM. An examination of the food addiction construct in obese patients with binge eating disorder. Int J Eat Disord. 2012;45:657–63. doi: 10.1002/eat.20957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann N Y Acad Sci. 2006;1071:313–323. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- 41.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 42.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.