Abstract
The purpose of this study was to investigate the therapeutic effect of functional electrical stimulation for improving gait and tibialis anterior (TA) muscle activity in individuals with stroke or brain injury who were enrolled in an inpatient rehabilitation program. Twenty-six individuals, 2-33 days post injury, were randomly assigned to an experimental group or control group. No significant differences were observed between groups at the conclusion of the study as both groups achieved similar improvements in gait speed, TA muscle activity, and FIM™ locomotion scores. This single site study found a low dose of gait training sessions with single channel FES did not augment gait nor EMG activity beyond gait training with sham stimulation.
Keywords: stroke, foot drop, functional electrical stimulation
Stroke is the leading cause of disability in the United States with approximately 13 million people having suffered a stroke.(Lloyd-Jones et al., 2009) Fifty percent of ischemic stroke survivors over 65 years of age exhibit hemiparesis, and thirty percent are unable to walk without assistance.(Lloyd-Jones, et al., 2009) In addition, brain injury is a significant contributor to disability in the United States.(Faul, Xu, Wald, & Coronado, 2010) Foot drop is a common impairment that leads to disability and limits ambulation in survivors of neurological injury with resulting hemiparesis.(DeQuervain, Simon, Leurgans, Pease, & McCallister, 1996; Fish & Kosta, 1999; Lehmann, 1993; Lehmann, Condon, Price, & deLateur, 1987) Functional electrical stimulation (FES) to the peroneal nerve and the tibialis anterior (TA) muscle is an intervention for treating foot drop in patients with hemiparesis. The use of FES as a neuroprosthesis has been shown to increase gait velocity, decrease the physiological demand of walking, improve gait symmetry, increase balance during gait, and improve social integration for individuals with chronic stroke when a FES device is in use.(Burridge, Taylor, Hagan, Wood, & Swain, 1997; Hausdorff & Ring, 2008; Kesar et al., 2010; Kesar et al., 2011; Kim, Chung, Kim, & Hwang, 2012; Kottink et al., 2004; Laufer, Hausdorff, & Ring, 2009; Laufer, Ring, Sprecher, & Hausdorff, 2009; Ring, Treger, Gruendlinger, & Hausdorff, 2009; Robbins, Houghton, Woodbury, & Brown, 2006; Stein et al., 2010; Taylor et al., 1999; van Swigchem, van Duijnhoven, den Boer, Geurts, & Weerdesteyn, 2012; van Swigchem et al., 2011)
A therapeutic effect has also been demonstrated after use of FES for durations of 3 months to one year in patients with chronic stroke.(Laufer, Ring, et al., 2009; Stein, et al., 2010) FES has been shown to facilitate improved gait velocity in individuals with chronic stroke after the device is no longer in use.(Daly et al., 2006; Kottink, et al., 2004; Laufer, Ring, et al., 2009; Robbins, et al., 2006) Long term use of FES has been shown to increase activation of motor cortical areas as demonstrated by increased motor-evoked potentials from transcranial magnetic stimulation for individuals with chronic stroke.(Everaert, Thompson, Chong, & Stein, 2012) Regular use of FES may improve control over voluntary neural motor pathways for the TA muscle and provide reciprocal inhibition of abnormal reflexes in the soleus muscle.(Sabut, Sikdar, Kumar, & Mahadevappa, 2011a; Thompson, Estabrooks, Chong, & Stein, 2009)
However, long term use of FES is not always feasible as cost and lack of insurance coverage can be prohibitive. Studies investigating the short term use of peroneal FES (3-5 days/week for 6-12 weeks) for individuals with chronic stroke have demonstrated improvements in gait; however, these studies have lacked a control group for comparison,(Hakansson, Kesar, Reisman, Binder-Macleod, & Higginson, 2011; Israel, Kotowski, Talbott, Fisher, & Dunning, 2011; Sabut, Kumar, Lenka, & Mahadevappa, 2010; Sabut, Lenka, Kumar, & Mahadevappa, 2010; Sabut, Sikdar, Kumar, & Mahadevappa, 2011b) Randomized controlled trials that have investigated short term use of peroneal FES for individuals with chronic stroke have not been able to demonstrate that FES assisted gait training is superior to a control group receiving gait training without peroneal FES.(Everaert et al., 2013; Kluding et al., 2013; Pereira, Mehta, McIntyre, Lobo, & Teasell, 2012; Prado-Medeiros et al., 2011; Sabut, Sikdar, Mondal, Kumar, & Mahadevappa, 2010; Sheffler et al., 2013) Sabut et al have suggested starting FES during the sub-acute phase of stroke recovery may result in better outcomes as compared to performing FES during the chronic phase of stroke recovery.(Sabut, et al., 2011b) Salisbury et al(Salisbury, Shiels, Todd, & Dennis, 2013) and Dunning et al(Dunning, Black, Harrison, McBride, & Israel, 2009) have demonstrated that FES can be applied during inpatient rehabilitation, and Morone et al(Morone, Fusco, Di Capua, Coiro, & Pratesi, 2012) suggest the use of FES during inpatient rehabilitation may improve gait outcomes. Additionally, it has been suggested that FES during early gait training may promote motor learning.(Dimitrijevic, 2008; Weingarden & Ring, 2006) However, little is known regarding the use of FES for individuals enrolled in an inpatient rehabilitation program, and multiple authors have suggested a need for randomized controlled trials to determine the efficacy and optimal dose of peroneal FES.(Dunning, et al., 2009; Morone, et al., 2012; Salisbury, et al., 2013) Compounding this problem is the actuality that time allotted for inpatient rehabilitation has significantly decreased (Ottenbacher et al., 2004) and a large dose (>3 months of daily use) of FES in this setting is not plausible.
The purpose of the current research is to determine if there is a therapeutic effect on gait speed, FIM™ locomotion scores, or TA muscle activity after applying peroneal FES during an inpatient rehabilitation program for individuals with hemiplegia and resulting foot drop. We hypothesize that individuals receiving FES and the control group will both demonstrate improvements in gait and muscle activity; however we expect greater improvements for the group receiving FES.
Method
A 2-group, randomized, parallel-comparison groups design with treatment blinding was implemented. The study was approved by a local institutional review board and followed the ethical standards set forth in the declaration of Helsinki.
Participants
Thirty-two individuals were recruited for the study. Participants were 2 to 33 days post first stroke or brain injury with resulting foot drop and were participating in an inpatient rehabilitation program. Only individuals with non-progressive forms of brain injury such as traumatic brain injury (n=3), surgical removal of an aneurysm (n=1), or stroke (n=28) were included in the study. Participants were included if they were ≥18 years old, were able to walk 10 meters with moderate or less assistance as determined by the participants treating physical therapist using FIM™ guidelines, and had ankle dorsiflexion passive range of motion to 0° or greater. Participants were excluded if they were receiving other forms of electrical stimulation to the lower extremity, had contra-indications to electrical stimulation, or any prior condition that limited the ability to walk. Subject characteristics are reported in Table 1.
Table 1. Participant characteristics.
| Group | Experimental group (FES) | Control group (Sensory) |
|---|---|---|
| Side of hemiplegia (right: left) | 6:7 | 7:6 |
| Days since injury; mean (SD) | 15.5 (8.2) | 12.9 (5.9) |
| Days since injury; range | 2 - 33 | 6 - 29 |
| Type of injury (CVA: BI) | 11:2 | 11:2 |
| Sex (male: female) | 10:3 | 6:7 |
| Age (years); mean (SD) | 54.8 (13.4) | 47.8 (18.6) |
| Age (years); range | 20 - 71 | 22 - 71 |
| Number of sessions; mean (SD) | 3.9 (1.0) | 4.5 (1.2) |
| Days enrolled in study; mean (SD) | 10.4 (3.4) | 11.4 (3.9) |
Abbreviations: BI, brain injury; CVA, cerebral vascular accident; FES, functional electrical stimulation group; SD, standard deviation
Measures
Data were collected prior to FES or sham sensory stimulation interventions and again at the conclusion of the study. The investigator collecting GAITRite and electromyography (EMG) data was blinded to treatment groups. Data collection sessions consisted of participants walking 10 meters at a self-selected speed for 3 repetitions. During data collection sessions participants walked without the FES device and without any type of ankle orthosis. Participants were allowed to use a one handed assistive device during data collection, but were required to use the same device for both pre and post testing. In order to minimize the effect of acceleration and deceleration, the GAITRite walkway was placed in the middle of the 10 meters walked and data were collected in the middle 3.5 meters of the distance walked. EMG and spatiotemporal gait parameters were collected simultaneously during the walking task.
The primary outcome was the change in gait speed from baseline to post-testing. Secondary analyses were performed on EMG of the TA muscle and FIM™ locomotion scores. All post-testing occurred within a window of 12 - 48 hours after the last administration of FES.
Gait speed data and phases of gait were collected using a GAITRite system5 as participants walked at a comfortable self-selected speed. The GAITRite walkway contains six arrays of force sensitive sensors imbedded in a walking surface with an active area of 3.66 m long × 0.61 m wide. As subjects ambulated across the GAITRite walkway, the system continuously scanned the sensors to detect footfalls and computed temporal and spatial parameters including gait speed and the phases of gait.
EMG data during gait were collected from the hemiparetic lower extremity TA muscle at a rate of 2,000 Hz using a Myopac6 16-channel unit. Self-adhesive 2×3 cm silver – silver/chloride passive electrodes7 were used to record surface EMG and serve as the reference electrode. Standardized procedures for EMG electrode placement and data collection were followed based on Surface EMG for Non-Invasive Assessment of Muscles (SENIAM) recommendations.(H. Hermens et al., 1999; H. J. Hermens, Freriks, Disselhorst-Klug, & Rau, 2000) The reference electrode was placed over the C-7 spinous process. A single researcher performed all electrode placements.
EMG data were synchronized with the GAITRite system so that muscle activity could be examined during the swing and loading phases of gait. The GAITRite system served as the master system for synchronization, outputting a 5 volt signal when data collection was started. This 5 volt signal was detected by the Myopac system triggering simultaneous initiation of EMG data collection resulting in the data being time synchronized. The data being time synchronized allowed for isolation of EMG data during the swing and loading phases of gait as detected by the GAITRite system.
Data processing was performed using a custom analysis program written on MatLab 6.1. EMG data were filtered with a 10-500 Hz band pass filter and enveloped via root mean square (RMS) with 50 ms intervals. EMG activity was quantified by integration of the RMS signal (RMS area) during the swing and loading phases of gait. Mean values for each phase of gait were then calculated from the three strides for each subject and normalized to the mean EMG RMS data of the entire gait cycle of the same test as normalizing to the mean of the gait cycle has been found to decrease variability for the TA muscle.(Winter & Yack, 1987; Yang & Winter, 1984) The change in EMG activity of the tibialis anterior muscle during the swing and loading phases of gait were compared across groups.
FIM™ locomotion scores were assigned by the subjects' physical therapists at admission to the study and discharge from the hospital and were derived from the medical records. All physical therapists assigning FIM™ locomotion scores were trained in administering the FIM™ and had passed an annual exam administered by the Uniform Data System for Medical Rehabilitation to enhance reliability of scoring. Finally, the amount of change in FIM™ locomotion scores was compared across groups.
Procedures
Stratified random sampling was used to assign participants to groups and counterbalance the number of participants with stroke and brain injury in each group. Allocation to groups was concealed, performed after singing an informed consent, and prior to baseline testing. Participants were informed the study would investigate the effects of a new device on walking but were blinded to group assignment.
A Bioness L300™8 unit was used to deliver FES (experimental group) or sham sensory stimulation (control group) during gait training. The Bioness™ L300 is a neuroprosthesis that delivers electrical pulses over the peroneal nerve and the TA muscle causing the ankle to dorsiflex during the swing phase of gait. The unit was fitted and stimulation parameters set by a single, trained researcher. For the experimental group stimulation was delivered according to the manufacturer's specifications with adequate amplitude to provide ankle dorsiflexion during the swing phase of gait.(Bioness, 2006) The intensity of the stimulation varied from 15 -76 milliamps (mA) and was set at the lowest amplitude that produced a muscle contraction that provided foot clearance during the swing phase of gait. As sensory stimulation does not have an effect on the recovery of gait for individuals with sub-acute stroke enrolled in a stroke rehabilitation program (Yavuzer, Oken, Atay, & Stam, 2007) we employed sensory stimulation as a control. For the control group the intensity of stimulation varied from 3-12 mA and was set at the lowest amplitude that produced a mild sensory stimulus without producing a palpable muscle contraction. Also, to assure no motor response in the control group, the Bioness stimulating electrodes were placed over the tibia. For both groups, electrical stimulation was delivered using a continuous, biphasic symmetric waveform with a pulse width of 200 microseconds with a pulse rate of 30 Hz.
All participants were enrolled in an inpatient rehabilitation program and received 1.5 hours of physical therapy 5 days per week. During physical therapy sessions, the experimental group received FES and the control group received sham sensory stimulation during gait training 3 times a week for a forty-five minute duration. The physical therapists were trained to administer gait training (with rest as needed) and no other type of training during the 45 minute sessions. For all participants, gait training was based upon Neuro-Developmental Treatment (NDT) principles. Four physical therapists administered gait training and had a mean of 10 years of experience. During the gait training sessions, physical therapists provided tactile cues and manual assistance to facilitate a normalized gait pattern. Orthoses or other assistive devices to assist with preventing foot drop were not used during gait training. Instead, Moleskin was applied to the anterior plantar surface of participants' shoes to allow the hemiparetic foot to slide forward during gait training.
All participants wore the device during gait training receiving either sham sensory stimulation or FES and participants were kept separate during treatment sessions. At the end of data collection no subjects were able to identify if they were in the experimental or control group. Physical therapists performing gait training were not provided group information. However, it is unlikely therapists were truly blinded as they have prior knowledge that FES should elicit a muscle contraction, and the experimental group had a discernible motor response to the FES and the control group did not. Physical therapists were randomly assigned to treat participants in both the experimental and control groups to help minimize any effect of bias.
Analysis
The change in gait speed, EMG activity of the Tibialis anterior muscle, and FIM™ locomotion scores were compared between groups. Predictive Analytic Software (PASW) version 17.0 was used for data analysis. Histograms revealed a normal distribution of the data for changes in gait speed and EMG data. This data was analyzed for between group differences using independent sample T-tests. Changes in FIM™ locomotion scores, which are ordinal data, were analyzed using a Mann-Whitney test. The overall alpha level for the experiment was set at 0.05. Because multiple comparisons were made, a Bonferroni correction was performed with a resulting alpha level of 0.0167 for each variable.
Results
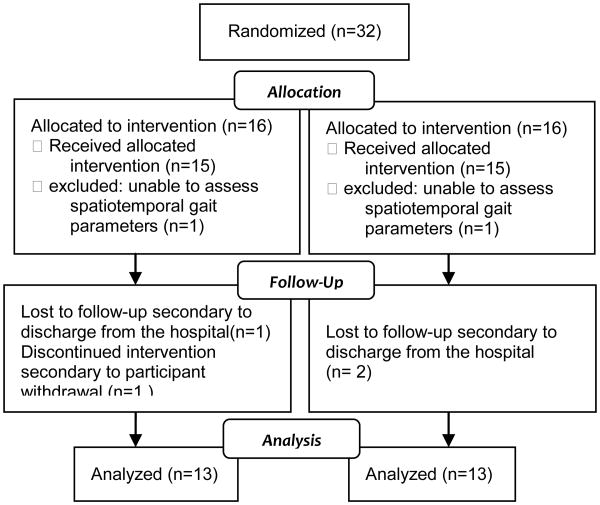
Of the 32 individuals enrolled, 13 participants comprised the experimental group and 13 the control group (flow of participants is shown in Figure 1). Three experimental group participants and 3 control group participants did not complete the study, and their data were excluded. One subject withdrew from the study to receive electrical stimulation during cycling. During initial data collection, two subjects' stance and swing phases of gait were unable to be determined using the GAITRite system and both were excluded from the study. Three subjects failed to complete the study because they were discharged early from the rehabilitation facility.
Figure 1. Flow of Participants.
Outcomes are presented in table 2. When comparing the change in gait speed between the experimental and control groups there was no significant difference. Change in FIM™ locomotion scores were also not statistically different when comparing the experimental and control groups. Additionally, a significant difference was not observed when comparing the change in EMG activity of the TA muscle during the swing phase of gait, nor in the loading phase of gait.
Table 2.
Mean (SD) of groups, mean (SD) difference within groups, and mean (95% CI) difference between groups.
| Outcome | Groups | Difference within groups | Difference between groups | ||||
|---|---|---|---|---|---|---|---|
| Pre Test | Post Test | Post Test minus Pre test | |||||
| Exp (n = 13) | Con (n = 13) | Exp (n = 13) | Con (n = 13) | Exp | Con | Exp minus Con (95% CI) | |
| Walking speed (m/s) | 0.15 (0.09) | 0.20 (0.14) | 0.28* (0.21) | 0.31* (0.21) | 0.13 (0.13) | 0.11 (0.11) | 0.02 (-0.11 to 0.09) |
| EMG TA activity in swing (normalized to mean) | 57.3 (31.7) | 53.1 (20.1) | 77.5* (23) | 70.3* (33.5) | 20.2 (24.9) | 17.2 (31.2) | 3 (-25.9 to 19.8) |
| EMG TA activity in loading (normalized to mean) | 31.6 (24.6) | 35.9 (19) | 28.7 (8.9) | 37.2 (21.6) | -2.8 (25.5) | 1.3 (29.5) | -4.1 (-18.2 to 26.4) |
| FIM locomotion (1 to 7) | 1.5 (0.9) | 1.9 (1.3) | 3.7* (0.6) | 4.0* (1.5) | 2.2 (0.9) | 2.1 (1.2) | 0.1 |
Exp = experimental group, Con = control group,
Indicates significant change for within group comparisons from pre-testing to post-testing (p<0.0167)
As expected, both groups exhibited improvements from pre-test to post-test (Table 2). Within-group comparisons demonstrated increased gait velocity, increased FIM™ locomotion scores, and increased EMG activity during the swing phase of gait for both groups.
Discussion
This study examined the therapeutic effect of single channel FES used in conjunction with gait training for individuals with stroke or brain injury with hemiparesis and subsequent foot drop who were participating in an inpatient rehabilitation program. While all individuals enrolled in the study demonstrated improvements from pre-test to post-test, the results indicate individuals receiving FES did not have significantly more improvements in gait nor TA muscle activity when compared to the control group who received sham sensory stimulation during gait training.
In contrast to the current study, research that has investigated the therapeutic effect of multichannel FES has demonstrated improvements in gait.(Bogataj, Gros, Kljajić, Aćimović, & Malezic, 1995; Daly et al., 2011; Robbins, et al., 2006; Solopova, Tihonova, Grishin, & Ivanenko, 2011; Yan, Hui-Chan, & Li, 2005) Therefore, increasing the number of muscles stimulated with FES may have resulted in different outcomes. Robbins et al suggest that performing multichannel FES may be more effective than administering single channel FES for individuals with sub-acute stroke.(Robbins, et al., 2006) Bogataj et al(Bogataj, et al., 1995), Yan et al(Yan, et al., 2005), and Solopova et al(Solopova, et al., 2011) have all demonstrated improvements for patients with a sub-acute stroke receiving multichannel FES. Yan et al performed 30 minute sessions of multichannel FES 5 times per week for three weeks in individuals with sub-acute stroke and found an increase in TA muscle EMG when compared to a control group.(Yan, et al., 2005) Bogataj et al found that performing multichannel FES 5 times per week for a duration of 30-60 minutes for 3 weeks in individuals with sub-acute stroke resulted in improved gait parameters.(Bogataj, et al., 1995) Solopota et al found that performing multichannel FES combined with assisted leg movement and progressive limb loading resulted in greater improvements in lower extremity limb function, muscle strength, and muscle activity when compared to a control group.(Solopova, et al., 2011) Therefore, future studies investigating the use of FES in individuals with sub-acute stroke should focus on multichannel FES systems as compared to single channel systems.
Use of FES during inpatient rehabilitation may be beneficial even though this study did not find a difference between the experimental and control groups. Other studies suggest that starting FES early with sufficient intensity may have a long term benefit for skill acquisition and long term recovery. Biernaskie et al found that a 5 week rehabilitation program (without use of FES) started 5 days after infarct produces superior results when compared to starting a rehabilitation program 30 days after an infarct.(Biernaskie, Chernenko, & Corbett, 2004) Additionally, employing a crossover design study Bogotaja et al found that starting FES early was superior to starting the FES protocol later.(Bogataj, et al., 1995) The current study has demonstrated that starting FES early in the recovery process is possible. However, it is likely that more frequent use of FES for a longer duration or stimulation of multiple muscles is required to achieve improvements in gait and muscle activity.
Additionally, studies that have investigated the therapeutic effect of long term use of single channel FES (3 months to one year) in subjects with chronic stroke have demonstrated improvements in gait.(Everaert, Thompson, Chong, & Stein; Everaert, et al., 2012; Laufer, Ring, et al., 2009; Stein, et al., 2010) Laufer et al found gait speed and single limb stance is significantly improved after one year of use of single channel FES in patients with chronic stroke.(Laufer, Ring, et al., 2009) Stein et al found a therapeutic effect on gait speed and physiological cost index after using single channel FES for 3 months and continued improvements after 11 months of use for individuals with chronic non-progressive neurological disorders.(Stein, et al., 2010) Additionally, Everaert et al found that use of single channel FES on a daily basis for 3 to 12 months in individuals with non-progressive neurological disorders resulted in increased motor cortex activity and improved gait speed.(Everaert, et al., 2012) Augmentation of gait with single channel FES may therefore depend upon the dosage of treatment. Compared with the studies by Everaert et al(Everaert, et al., 2012), Laufer et al(Laufer, Ring, et al., 2009), and Stein et al(Stein, et al., 2010) the quantity of FES during the current study was relatively small (mean of 3.85 sessions of FES for 45 minute sessions). The low dosage may not have resulted in achievement of full potential outcomes, as a large number of repetitions of movement are required to re-learn a skill and promote neuro-plasticy. Kleim and Jones suggest it is likely that several days of training at a high intensity are required to produce lasting changes in the neural structure.(Kleim & Jones, 2008) It is also important to indicate none of the aforementioned studies had a control group. Therefore, future research investigating single channel FES should have a control group for comparison and the FES intervention should be more robust than the intervention in the current study.
As we designed this study as a stage 2 trial(Dobkin, 2009), the results should not be generalized beyond the setting and dosage, and the results should be confirmed by more rigorous randomized controlled trials. As the intent of the study was to examine the effects of FES for gait training in an inpatient rehabilitation environment the dosage was small. We provided the largest feasible dose that could be administered at our facility during the patient's length of stay. This small dosage was likely insufficient to produce an effect on the subjects' gait and muscle activity, and these results should not be interpreted as FES in general has no therapeutic effect. Additionally, few patients who were screened at admission to the rehabilitation facility met the inclusion criteria of being able to walk 10′ with moderate assistance or less. This resulted in a small subset of the population being included in the study. Therefore, one cannot generalize the findings to patients who are non-ambulatory.
This study indicates that small amounts of FES provided during inpatient rehabilitation (4 sessions for 45 minutes) for individuals with stroke or brain injury may not augment gait nor EMG activity of the TA muscle beyond gait training with sham sensory stimulation. It is likely the low dosage of single channel FES administered during inpatient rehabilitation was not sufficient to induce a therapeutic effect observed in other studies. Further research could build on the results by investigating larger doses of FES, investigating the timing for initiation of FES, and investigating the optimal number of muscles to stimulate during FES.
Footnotes
CIR Systems, Inc., Havertown, PA
RUN technologies, Mission Viejo, CA
Vermed, Bellows Falls, VT
Bioness Inc., Valencia, CA
Contributor Information
Chad I. Lairamore, University of Central Arkansas.
Mark K. Garrison, University of Central Arkansas.
Laetitia Bourgeon, University of Central Arkansas.
Mark Mennemeier, University Of Arkansas For Medical Sciences.
References
- Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J Neurosci. 2004;24(5):1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bioness I. Unpublished manuscript. Santa Clarita, CA: 2006. Ness L300 Clinician's Guide. [Google Scholar]
- Bogataj U, Gros N, Kljajić M, Aćimović R, Malezic M. The rehabilitation of gait in patients with hemiplegia: a comparison between conventional therapy and multichannel functional electrical stimulation therapy. Phys Ther. 1995;75(6):490–502. doi: 10.1093/ptj/75.6.490. [DOI] [PubMed] [Google Scholar]
- Burridge JH, Taylor PN, Hagan SA, Wood DE, Swain ID. The effects of common peroneal stimulation on the effort and speed of walking: a randomized controlled trial with chronic hemiplegic patients. Clin Rehabil. 1997;11(3):201–210. doi: 10.1177/026921559701100303. [DOI] [PubMed] [Google Scholar]
- Daly JJ, Roenigk K, Holcomb J, Rogers JM, Butler K, Gansen J, et al. A randomized controlled trial of functional neuromuscular stimulation in chronic stroke subjects. Stroke. 2006;37(1):172–178. doi: 10.1161/01.STR.0000195129.95220.77. [DOI] [PubMed] [Google Scholar]
- Daly JJ, Zimbelman J, Roenigk KL, McCabe JP, Rogers JM, Butler K, et al. Recovery of coordinated gait: randomized controlled stroke trial of functional electrical stimulation (FES) versus no FES, with weight-supported treadmill and over-ground training. Neurorehabil Neural Repair. 2011;25(7):588–596. doi: 10.1177/1545968311400092. [DOI] [PubMed] [Google Scholar]
- DeQuervain I, Simon S, Leurgans S, Pease W, McCallister D. Gait pattern in the early recovery period after stroke. J Bone Joint Surg Am. 1996;78:1506–1514. doi: 10.2106/00004623-199610000-00008. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR. Clinical practice of functional electrical stimulation: from “Yesterday” to “Today”. Artif Organs. 2008;32(8):577–580. doi: 10.1111/j.1525-1594.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- Dobkin BH. Progressive Staging of Pilot Studies to Improve Phase III Trials for MotorInterventions. Neurorehabil Neural Repair. 2009;23(3):197–206. doi: 10.1177/1545968309331863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning K, Black K, Harrison A, McBride K, Israel S. Neuroprosthesis peroneal functional electrical stimulation in the acute inpatient rehabilitation setting: a case series. Phys Ther. 2009;89(5):499–506. doi: 10.2522/ptj.20080241. [DOI] [PubMed] [Google Scholar]
- Everaert DG, Stein RB, Abrams GM, Dromerick AW, Francisco GE, Hafner BJ, et al. Effect of a foot-drop stimulator and ankle-foot orthosis on walking performance after stroke: a multicenter randomized controlled trial. Neurorehabil Neural Repair. 2013;27(7):579–591. doi: 10.1177/1545968313481278. [DOI] [PubMed] [Google Scholar]
- Everaert DG, Thompson AK, Chong SL, Stein RB. Does functional electrical stimulation for foot drop strengthen corticospinal connections? Neurorehabil Neural Repair. 2012;24(2):168–177. doi: 10.1177/1545968309349939. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald M, Coronado V. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006 2010 [Google Scholar]
- Fish D, Kosta CS. Walking impediments and gait inefficiencies in the CVA patient. JPO. 1999;11:33–37. [Google Scholar]
- Hakansson NA, Kesar T, Reisman D, Binder-Macleod S, Higginson JS. Effects of fast functional electrical stimulation gait training on mechanical recovery in poststroke gait. Artif Organs. 2011;35(3):217–220. doi: 10.1111/j.1525-1594.2011.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Ring H. Effects of a new radio frequency-controlled neuroprosthesis on gait symmetry and rhythmicity in patients with chronic hemiparesis. Am J Phys Med Rehabil. 2008;87(1):4–13. doi: 10.1097/PHM.0b013e31815e6680. [DOI] [PubMed] [Google Scholar]
- Hermens H, Freriks B, Merletti R, Hagg G, Stegeman D, Blok J. SENIAM 8: European recommendations for surface electromyography. Roessingh Research and Development; 1999. [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(5):361–374. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Israel S, Kotowski S, Talbott N, Fisher K, Dunning K. The therapeutic effect of outpatient use of a peroneal nerve functional electrical stimulation neuroprosthesis in people with stroke: a case series. Top Stroke Rehabil. 2011;18(6):738–745. doi: 10.1310/tsr1806-738. [DOI] [PubMed] [Google Scholar]
- Kesar TM, Perumal R, Jancosko A, Reisman DS, Rudolph KS, Higginson JS, et al. Novel patterns of functional electrical stimulation have an immediate effect on dorsiflexor muscle function during gait for people poststroke. Phys Ther. 2010;90(1):55–66. doi: 10.2522/ptj.20090140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesar TM, Reisman DS, Perumal R, Jancosko AM, Higginson JS, Rudolph KS, et al. Combined effects of fast treadmill walking and functional electrical stimulation on post-stroke gait. Gait Posture. 2011;33(2):309–313. doi: 10.1016/j.gaitpost.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Chung Y, Kim Y, Hwang S. Functional electrical stimulation applied to gluteus medius and tibialis anterior corresponding gait cycle for stroke. Gait Posture. 2012;36(1):65–67. doi: 10.1016/j.gaitpost.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):S225–239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- Kluding PM, Dunning K, O'Dell MW, Wu SS, Ginosian J, Feld J, et al. Foot drop stimulation versus ankle foot orthosis after stroke: 30-week outcomes. Stroke. 2013;44(6):1660–1669. doi: 10.1161/STROKEAHA.111.000334. [DOI] [PubMed] [Google Scholar]
- Kottink AI, Oostendorp LJ, Buurke JH, Nene AV, Hermens HJ, MJ IJ. The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: a systematic review. Artif Organs. 2004;28(6):577–586. doi: 10.1111/j.1525-1594.2004.07310.x. [DOI] [PubMed] [Google Scholar]
- Laufer Y, Hausdorff JM, Ring H. Effects of a foot drop neuroprosthesis on functional abilities, social participation, and gait velocity. Am J Phys Med Rehabil. 2009;88(1):14–20. doi: 10.1097/PHM.0b013e3181911246. [DOI] [PubMed] [Google Scholar]
- Laufer Y, Ring H, Sprecher E, Hausdorff JM. Gait in individuals with chronic hemiparesis: one-year follow-up of the effects of a neuroprosthesis that ameliorates foot drop. J Neurol Phys Ther. 2009;33(2):104–110. doi: 10.1097/NPT.0b013e3181a33624. [DOI] [PubMed] [Google Scholar]
- Lehmann JF. Push-off and propulsion of the body in normal and abnormal gait. Correction by ankle-foot orthoses. Clin Orthop Relat Res. 1993;(288):97–108. [PubMed] [Google Scholar]
- Lehmann JF, Condon SM, Price R, deLateur BJ. Gait abnormalities in hemiplegia: their correction by ankle-foot orthoses. Arch Phys Med Rehabil. 1987;68(11):763–771. [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson T, Flegal K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- Morone G, Fusco A, Di Capua P, Coiro P, Pratesi L. Walking training with foot drop stimulator controlled by a tilt sensor to improve walking outcomes: a randomized controlled pilot study in patients with stroke in subacute phase. Stroke Res Treat. 2012;2012:523564. doi: 10.1155/2012/523564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenbacher KJ, Smith PM, Illig SB, Linn RT, Ostir GV, Granger CV. Trends in length of stay, living setting, functional outcome, and mortality following medical rehabilitation. JAMA. 2004;292(14):1687–1695. doi: 10.1001/jama.292.14.1687. [DOI] [PubMed] [Google Scholar]
- Pereira S, Mehta S, McIntyre A, Lobo L, Teasell RW. Functional electrical stimulation for improving gait in persons with chronic stroke. Top Stroke Rehabil. 2012;19(6):491–498. doi: 10.1310/tsr1906-491. [DOI] [PubMed] [Google Scholar]
- Prado-Medeiros CL, Sousa CO, Souza AS, Soares MR, Barela AM, Salvini TF. Effects of the addition of functional electrical stimulation to ground level gait training with body weight support after chronic stroke. Rev Bras Fisioter. 2011;15(6):436–444. doi: 10.1590/s1413-35552011005000030. [DOI] [PubMed] [Google Scholar]
- Ring H, Treger I, Gruendlinger L, Hausdorff JM. Neuroprosthesis for footdrop compared with an ankle-foot orthosis: effects on postural control during walking. J Stroke Cerebrovasc Dis. 2009;18(1):41–47. doi: 10.1016/j.jstrokecerebrovasdis.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Robbins SM, Houghton PE, Woodbury MG, Brown JL. The therapeutic effect of functional and transcutaneous electric stimulation on improving gait speed in stroke patients: a meta-analysis. Arch Phys Med Rehabil. 2006;87(6):853–859. doi: 10.1016/j.apmr.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Sabut SK, Kumar R, Lenka PK, Mahadevappa M. Surface EMG analysis of tibialis anterior muscle in walking with FES in stroke subjects. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:5839–5842. doi: 10.1109/IEMBS.2010.5627503. [DOI] [PubMed] [Google Scholar]
- Sabut SK, Lenka PK, Kumar R, Mahadevappa M. Effect of functional electrical stimulation on the effort and walking speed, surface electromyography activity, and metabolic responses in stroke subjects. J Electromyogr Kinesiol. 2010;20(6):1170–1177. doi: 10.1016/j.jelekin.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Sabut SK, Sikdar C, Kumar R, Mahadevappa M. Functional electrical stimulation of dorsiflexor muscle: effects on dorsiflexor strength, plantarflexor spasticity, and motor recovery in stroke patients. NeuroRehabilitation. 2011a;29(4):393–400. doi: 10.3233/NRE-2011-0717. [DOI] [PubMed] [Google Scholar]
- Sabut SK, Sikdar C, Kumar R, Mahadevappa M. Improvement of gait & muscle strength with functional electrical stimulation in sub-acute & chronic stroke patients. Conf Proc IEEE Eng Med Biol Soc. 2011b;2011:2085–2088. doi: 10.1109/IEMBS.2011.6090387. [DOI] [PubMed] [Google Scholar]
- Sabut SK, Sikdar C, Mondal R, Kumar R, Mahadevappa M. Restoration of gait and motor recovery by functional electrical stimulation therapy in persons with stroke. Disabil Rehabil. 2010;32(19):1594–1603. doi: 10.3109/09638281003599596. [DOI] [PubMed] [Google Scholar]
- Salisbury L, Shiels J, Todd I, Dennis M. A feasibility study to investigate the clinical application of functional electrical stimulation (FES), for dropped foot, during the sub-acute phase of stroke - A randomized controlled trial. Physiother Theory Pract. 2013;29(1):31–40. doi: 10.3109/09593985.2012.674087. [DOI] [PubMed] [Google Scholar]
- Sheffler LR, Taylor PN, Gunzler DD, Buurke JH, Ijzerman MJ, Chae J. Randomized Controlled Trial of Surface Peroneal Nerve Stimulation for Motor Relearning in Lower Limb Hemiparesis. Arch Phys Med Rehabil. 2013 doi: 10.1016/j.apmr.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solopova IA, Tihonova DY, Grishin AA, Ivanenko YP. Assisted leg displacements and progressive loading by a tilt table combined with FES promote gait recovery in acute stroke. NeuroRehabilitation. 2011;29(1):67–77. doi: 10.3233/NRE-2011-0679. [DOI] [PubMed] [Google Scholar]
- Stein RB, Everaert DG, Thompson AK, Chong SL, Whittaker M, Robertson J, et al. Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil Neural Repair. 2010;24(2):152–167. doi: 10.1177/1545968309347681. [DOI] [PubMed] [Google Scholar]
- Taylor PN, Burridge JH, Dunkerley AL, Wood DE, Norton JA, Singleton C, et al. Clinical use of the Odstock dropped foot stimulator: its effect on the speed and effort of walking. Arch Phys Med Rehabil. 1999;80(12):1577–1583. doi: 10.1016/s0003-9993(99)90333-7. [DOI] [PubMed] [Google Scholar]
- Thompson AK, Estabrooks KL, Chong S, Stein RB. Spinal reflexes in ankle flexor and extensor muscles after chronic central nervous system lesions and functional electrical stimulation. Neurorehabil Neural Repair. 2009;23(2):133–142. doi: 10.1177/1545968308321067. [DOI] [PubMed] [Google Scholar]
- van Swigchem R, van Duijnhoven HJ, den Boer J, Geurts AC, Weerdesteyn V. Effect of peroneal electrical stimulation versus an ankle-foot orthosis on obstacle avoidance ability in people with stroke-related foot drop. Phys Ther. 2012;92(3):398–406. doi: 10.2522/ptj.20100405. [DOI] [PubMed] [Google Scholar]
- van Swigchem R, Weerdesteyn V, van Duijnhoven HJ, den Boer J, Beems T, Geurts AC. Near-normal gait pattern with peroneal electrical stimulation as a neuroprosthesis in the chronic phase of stroke: a case report. Arch Phys Med Rehabil. 2011;92(2):320–324. doi: 10.1016/j.apmr.2010.10.038. [DOI] [PubMed] [Google Scholar]
- Weingarden H, Ring H. Functional electrical stimulation-induced neural changes and recovery after stroke. Eura Medicophys. 2006;42(2):87–90. [PubMed] [Google Scholar]
- Winter DA, Yack HJ. EMG profiles during normal human walking: stride-to-stride and inter-subject variability. Electroencephalogr Clin Neurophysiol. 1987;67(5):402–411. doi: 10.1016/0013-4694(87)90003-4. [DOI] [PubMed] [Google Scholar]
- Yan T, Hui-Chan CW, Li LS. Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke: a randomized placebo-controlled trial. Stroke. 2005;36(1):80–85. doi: 10.1161/01.STR.0000149623.24906.63. [DOI] [PubMed] [Google Scholar]
- Yang JF, Winter DA. Electromyographic amplitude normalization methods: improving their sensitivity as diagnostic tools in gait analysis. Arch Phys Med Rehabil. 1984;65(9):517–521. [PubMed] [Google Scholar]
- Yavuzer G, Oken O, Atay MB, Stam HJ. Effect of sensory-amplitude electric stimulation on motor recovery and gait kinematics after stroke: a randomized controlled study. Arch Phys Med Rehabil. 2007;88(6):710–714. doi: 10.1016/j.apmr.2007.02.030. [DOI] [PubMed] [Google Scholar]