Abstract
Cellular engulfment of particles, cells or solutes displaces large domains of plasma membrane into intracellular membranous vacuoles. This transfer of membrane is accompanied by major transitions of the phosphoinositide (PI) species that comprise the cytoplasmic face of membrane bilayers. Mapping of membrane PIs during engulfment reveals distinct patterns of protein and PI distributions associated with each stage of engulfment, which correspond with activities that regulate the actin cytoskeleton, membrane movements and vesicle secretion. Experimental manipulation of PI chemistry during engulfment indicates that PIs integrate organelle identity and orient signal transduction cascades within confined subdomains of membrane. These pathways are exploited by microbial pathogens to direct or redirect the engulfment process.
Introduction
Engulfment is the active cellular ingestion of particles, microbes or apoptotic cells by phagocytosis and of solutes by macropinocytosis. All share common component activities: distortion of plasma membrane by actin polymerization and contractility into a cup-shaped domain of plasma membrane which closes to form an intracellular vesicle or vacuole. Often, the vacuole fuses with the endolysosomal network, which includes early endosomes, late endosomes and lysosomes. Engulfment can be distinguished from other kinds of endocytic activity such as clathrin-mediated endocytosis, caveolin-mediated endocytosis and others, not only by the larger size of vesicles created by engulfment, but also by the significant involvement of the actin cytoskeleton (Flannagan et al, 2012; Kerr & Teasdale, 2009; Ravichandran, 2011). Phosphoinositides (PIs) are phospholipids which localize predominantly to the cytosolic face of cellular membranes. Through reversible binding interactions with cytosolic proteins and irreversible hydrolysis reactions, PIs coordinate activities of the actin cytoskeleton and regulate vesicle formation and fusion between membranous compartments essential to engulfment. Many microbial pathogens manipulate the PI chemistry of engulfment to direct the formation of more hospitable vacuolar environments (Ham et al, 2011), which indicates the relevance of PIs to the mechanisms of engulfment. This review examines membrane PI chemistry during the engulfment process, focusing on how plasma membrane is transformed into an intracellular vacuole.
Phosphoinositides and Membrane Identity
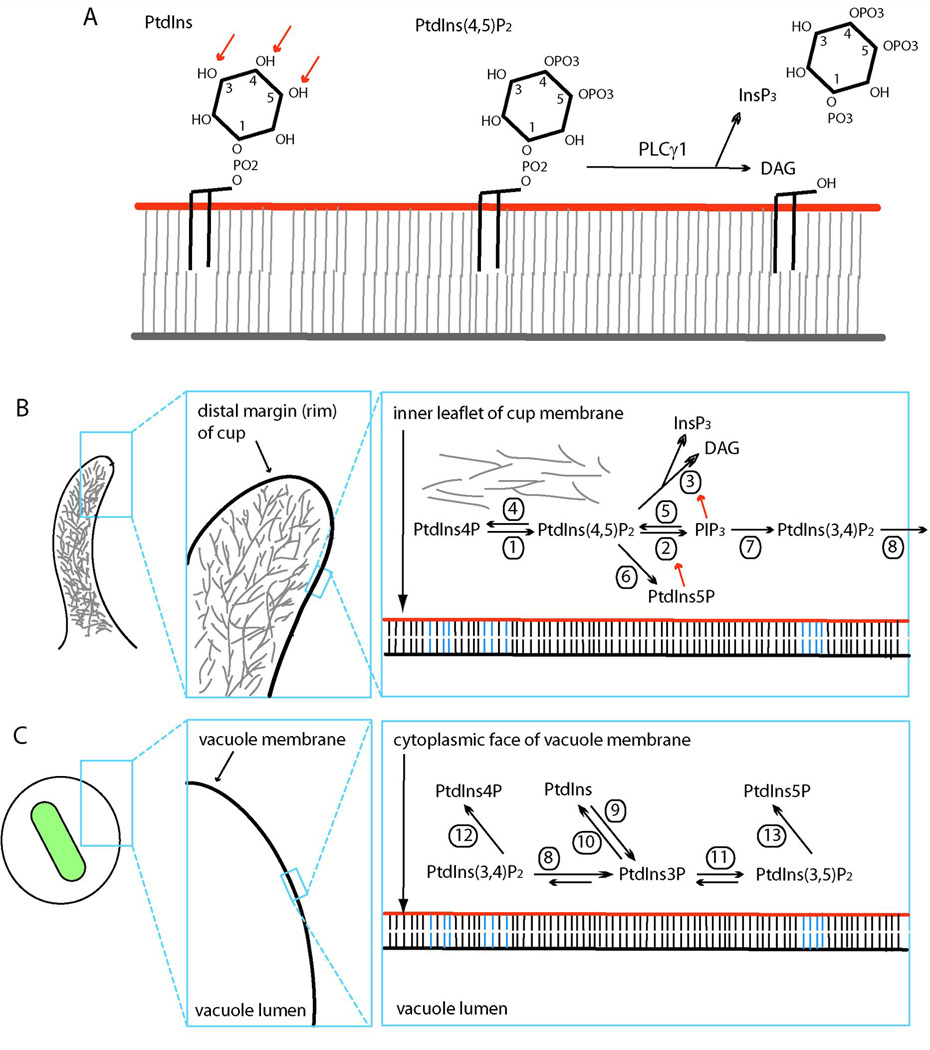
The phospholipids which form cellular membrane bilayers are mainly phosphatidyl-choline, phosphatidylserine, phosphatidylethanolamine, phosphatidic acid and the PIs (for a comprehensive review of phospholipids and endocytosis, see (Bohdanowicz & Grinstein, 2013)). The cytoplasmic leaflets of membranes involved in engulfment contain minor but significant quantities of the PI phosphatidylinositol (PtdIns), which is comprised of diacylglycerol linked to D-myo-inositol-1-phosphate by a phosphodiester linkage (Fig. 1A). The inositol hydroxyls may be reversibly altered by phosphoinositide kinases and phosphatases, generating PIs with phosphate groups in the 3, 4 or 5 positions: phosphatidylinositol 3-phosphate (PtdIns3P), PtdIns4P, PtdIns5P, phosphatidylinositol (4,5)-bisphosphate (PtdIns(4,5)P2), PtdIns(3,4)P2PtdIns(3,5)P2 and phosphatidylinositol (3,4,5)-trisphosphate (PIP3). PIs can also be hydrolyzed by phospholipases and the products of these irreversible reactions are important signal transduction intermediates. Phospholipase C (PLC) hydrolyzes PtdIns(4,5)P2 to form two important regulators of signal transduction: diacylglycerol (DAG), which activates protein kinase C (PKC), and InsP3, which releases calcium from intracellular stores (Fig. 1B). Two hydrolases which mainly hydrolyze other phospholipids are phospholipase D (PLD) and phospholipase A2 (PLA2), which generate phosphatidic acid (PA) and arachadonic acid, respectively. These hydrolases can be regulated by PIs or by enzymes which modify PIs (Bohdanowicz & Grinstein, 2013).
Figure 1. Phosphoinositide structure and dynamics on membranes.
(A) Schematic diagram of the structures of PtdIns, PtdIns(4,5)P2 and the products of PLC hydrolysis. DAG is indicated by black lines, with acyl chains extending from the cytosolic surface (orange line) into the bilayer. The hydroxyl groups at the 3, 4 and 5 positions of inositol (red arrows) can be phosphorylated by PI kinases and dephosphorylated by PI phosphatases. (B) Phosphoinositide dynamics in ruffles and cups. Surface extensions of plasma membrane are enriched in actin filaments (grey lines), whose polymerization and depolymerization are regulated by PIs. Significant transitions in PI profiles are indicated for the enlarged region of plasma membrane (right box). The red arrows indicate enzyme activation by PIs. (C) PI dynamics on vacuolar membranes (phagosomes and macropinosomes). The sequence of PIs appearing during macropinosome formation include PtdIns(4,5)P2PIP3PtdIns(3,4)P2 and PtdIns3P. The subsequent transition to PI(3,5)P2 on membranes has not been demonstrated directly. Microbial pathogens drive engulfment or modify vacuole maturation using PI-modifying enzymes. (1) PtdIns4P 5-kinase, (2) PI 3-kinase type I, (3) PLCγ1, (4) OCRL and Inpp5B, (5) PTEN, (6) PtdIns-4,5-bisphosphate 4-phosphatase, Salmonella SopB and Shigella flexneri IpgD, (7) SHIP1 and SHIP2, (8) Inpp4B, (9) PI 3-kinase type III, (10) myotubularin and Legionella SidP, (11) PYKEfyve, (12) Legionella SidF, (13) Legionella SidP.
Imaging of PIs and related protein activities during phagocytosis or macropinocytosis reveals the dynamics that maintain steady state membrane identities. Many proteins involved in signal transduction or organelle trafficking contain structural domains which bind to PIs with high specificity. For example, the pleckstrin homology (PH) domain of the kinase Akt (AktPH) binds with high affinity to PtdIns(3,4)P2 and PIP3 (Haugh et al, 2000) and the PH domain of PLC-δ binds specifically to PtdIns(4,5)P2 (Kavran et al, 1998; Rameh et al, 1997). Fluorescent protein (FP) chimeras of PI-binding domains, such as green fluorescent protein (GFP)-AktPH, can localize the target species of PIs when expressed in living cells. When combined with other fluorescent probes for labeling compartments, they can be used to map the subcellular distributions of PIs. These and other methods have revealed that plasma membrane and the membranes of various endocytic compartments have distinct and characteristic profiles of PI species. Plasma membranes are enriched for PtdIns(4,5)P2 and PtdIns4P and sometimes also the 3’phosphoinositides (3’PIs) PIP3PtdIns3P, PtdIns(3,4)P2 or PtdIns(3,5)P2 (Haugh et al, 2000; Varnai et al, 1999). The Golgi apparatus, some secretory vesicles and regions of endoplasmic reticulum are enriched for PtdIns4P and endocytic compartments contain 3’PIs in different proportions (Di Paolo & De Camilli, 2006). The PI profiles of different membrane surfaces within the cell restrict the subcompartments where soluble proteins can bind, or where transmembrane proteins become activated, and thereby define the chemical reactions that occur on those surfaces and the kinds of organelles that can interact with those surfaces (Bohdanowicz & Grinstein, 2013). Thus, the apparently static and uniform chemical identities of plasma membrane and endocytic organelles represent the steady state distributions of the substrates and products of localized PI-modifying activities and the selective fission and fusion between compartments (Munro, 2004).
PI metabolism is also essential for signal transduction at the plasma membrane. Signaling by growth factor receptors and hormones initiates changes in PI chemistry which shape and propagate signals (Balla, 2013). The changes in PIs which follow receptor signaling are also evident during engulfment. The plasma membrane increases concentrations of PtdIns(4,5)P2 in ruffles and cup-shaped extensions of the cell surface (Botelho et al, 2000). As the cup begins to close, concentrations of PtdIns(4,5)P2 in cup membranes decline and concentrations of PIP3 and DAG increase (Botelho et al, 2000)(Fig. 1B). After the cup closes into a phagosome or macropinosome, the levels of PIP3 and DAG decrease and PtdIns3P levels increase (Henry et al, 2004; Vieira et al, 2001)(Fig. 1C). Continued maturation of the vacuole or merger of the vacuole with late endosomes replaces PtdIns3P with PtdIns(3,5)P2. Thus, each morphogenetic stage of engulfment can be considered a transiently stable state, which is integrated by lateral diffusion of phospholipids over large domains of membrane and which transitions to other stable states by a limited set of vectorial reactions (Swanson, 2008; Welliver & Swanson, 2012).
Phagocytosis
Phagocytosis is usually initiated by receptors in the plasma membrane of the phagocytic cell, which bind to ligand molecules on particle surfaces and stimulate the movements of engulfment (Flannagan et al, 2012). Often this begins with the formation of actin-rich, cell surface ruffles which capture the target particle (Flannagan et al, 2010). Fcγ receptor (FcR)-mediated phagocytosis creates a tightly adherent phagocytic cup which extends over a particle surface coated (opsonized) with IgG, a ligand for FcR (Fig. 2A). As the cup advances over the surface, submembranous actin is organized into a tight cuff surrounding the particle, with actin filaments growing at the forward edge, contracting by the action of myosin molecules, and disassembling at the rear (Hoppe & Swanson, 2004; Scott et al, 2005). At the same time, membrane from intracellular vesicles is inserted into the forming cup (Bajno et al, 2000; Braun et al, 2004). These very different activities of actin filaments polymerizing, then depolymerizing, and vesicle fusion suggest that FcR signal transduction during phagocytosis is organized spatially and temporally. How does activation of a single kind of receptor control such different activities?
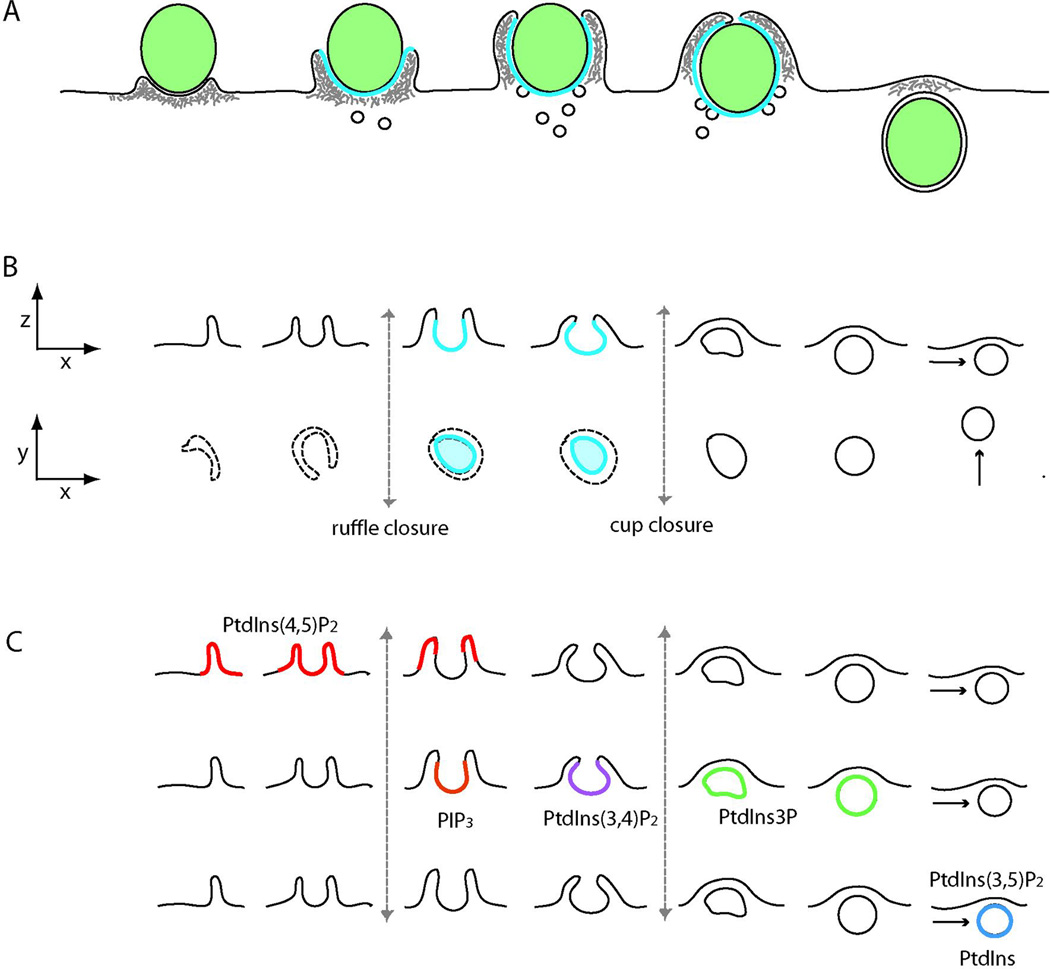
Figure 2. Cellular movements of engulfment.
(A) The cytoplasmic movements of FcR-mediated phagocytosis. The sequence left to right shows the movements of membranes during phagocytosis of a particle (green ovals). Actin filaments (grey) form a contractile cuff which advances over the particle. Membrane from intracellular organelles is inserted into the forming phagosome. Blue lines indicate plasma membrane domains with limited lateral mobility of PIs due to diffusion barriers in cup structure. (B) Stereotypical movements of macropinocytosis. The top row shows a progression, left to right, of side-view projections (x-z) of macropinocytic cup formation (ruffle closure) and membrane scission to form an intracellular macropinosome (cup closure). The second row shows the corresponding images as typically seen by light microscopy (x-y projection). Dotted lines indicate ruffles and cups in plasma membrane. Solid lines denote discrete membrane compartments. Blue lines indicate regions of plasma membrane where lateral mobility of inner leaflet molecules is constrained by diffusion barriers. (C) Dynamics of PIs during macropinosome formation. The top row shows the distributions of PtdIns(4,5)P2 (red lines) which localizes to plasma membrane and is enriched in ruffles and very early cups. The middle row shows the stages of macropinosome formation which have maximal labeling with probes for PIP3 (orange), PtdIns(3,4)P2 (violet) and PtdIns3P (green). The bottom row indicates speculated synthesis of PtdIns or PtdIns(3,5)P2, which have not been demonstrated by microscopy.
Much of this complex behavior is organized by membrane phospholipids. Binding to IgG leads to FcR clustering, which in turn activates receptor tyrosine phosphorylation by Src-family kinases (Fitzer-Attas et al, 2000). This is followed by recruitment and activation of the kinase Syk (Crowley et al, 1997), the recruitment of adapter proteins (Gu et al, 2003; Lee et al, 2007) and the activation of lipid-modifying enzymes, including phosphatidylinositol 3-kinase (PI 3-kinase) and PLCγ1 (Liao et al, 1992). The Rho-family GTPases Cdc42, Rac1 and Rac2 are activated (Hoppe & Swanson, 2004), as well as the GTPases Arf1 (Beemiller et al, 2006), Arf6 (Niedergang et al, 2003; Zhang et al, 1998) and PKC-ε (Larsen et al, 2002). Other regulatory proteins are necessary for phagocytosis, including the phospholipid-modifying enzymes PtdIns4P 5-kinase-γ (PI4P5Kγ, (Mao et al, 2009)) and PI4P5Kα (Coppolino et al, 2002), PLD1 and PLD2 (Iyer et al, 2004) and PLA2 (Lennartz et al, 1997). The mechanisms of their activation by FcR are not fully known. PLD-generated PA activates PI4P5K, which activates Arf6 (Honda et al, 1999), promoting the early movements.
PtdIns(4,5)P2 organizes the early movements of engulfment
Imaging of signaling during FcR-mediated phagocytosis revealed at least two stages of engulfment: early and late. One early event is the activation of PI4P5Kα and PI4P5Kγ, which generate PtdIns(4,5)P2 from PtdIns4P. PtdIns(4,5)P2 concentrations increase in cell surface ruffles and in early phagocytic cups (Botelho et al, 2000). The increased concentrations of PtdIns(4,5)P2 in plasma membrane stimulate actin polymerization by activating Cdc42 and Rac, which activate Wiscott-Aldrich Syndrome protein (WASP) and (WASP)-family verprolin homologous proteins 1/2 (WAVE1/2), respectively (Miki et al, 1998; Park & Cox, 2009). WASP and WAVE1/2 bind to Arp2/3 and stimulate actin polymerization (May et al, 2000). Fluorescence resonance energy transfer (FRET)-based microscopic methods for localizing GTPase activities during phagocytosis determined that Cdc42, Arf6 and Rac1 are active (ie., in their GTP-bound configurations) in ruffles and the advancing edge of the phagocytic cup, indicating that signals which generate and respond to PtdIns(4,5)P2 are active in the early stages of phagocytosis (Beemiller et al, 2006; Hoppe & Swanson, 2004).
PtdIns(4,5)P2 is depleted from the cup membranes even before the cup closes into the cell (Botelho et al, 2002). PtdIns(4,5)P2 is removed by three distinct enzyme activities which are necessary for phagocytosis: hydrolysis by PLCγ1 to DAG and IP3 (Botelho et al, 2000), phosphorylation by PI-3-kinase to form PIP3 (Araki et al, 1996) and, to a lesser extent, dephosphorylation by the PtdIns5P phosphatases, oculocerebrorenal syndrome of Lowe protein (OCRL) and inositol polyphosphate 5-phosphatase B (Inpp5B), which are activated by the GTPase Rab5a (Bohdanowicz et al, 2012)(Fig. 1B). Loss of PtdIns(4,5)P2 from the cup membrane facilitates removal of actin, which may help to maintain the advancing actin cuff (Scott et al, 2005). Consistent with this model, imaging showed that DAG, PIP3 and PI(3,4)P2 concentrations increased in cup membranes as the PtdIns(4,5)P2 was depleted (Botelho et al, 2000; Hoppe & Swanson, 2004; Vieira et al, 2001). Thus, as the cup advances over the particle, PtdIns(4,5)P2 is elevated at the front of the actin cuff and depleted at the rear.
Another pathway for the activation of Rac during phagocytosis could involve the localized synthesis of PtdIns5P, which was recently shown to increase circular ruffle formation through activation of the Rac guanine nucleotide exchange factor (GEF) Tiam1 and consequent activation of Rac (Viaud et al, 2014). PtdIns5P is synthesized via phosphorylation of PtdIns by the PI 5-kinase PYKfyve (Shisheva, 2012) or via dephosphorylation of PtdIns(4,5)P2 by PtdIns-4,5-bisphosphate 4-phosphatases (Niebuhr et al, 2002; Ungewickell et al, 2005). A direct role for PtdIns5P in phagocytosis has not been demonstrated, although the synthesis of PtdIns5P by the Shigella flexneri effector protein IpgD is required for invasion of epithelial cells (Niebuhr et al, 2002)(see below).
3’PIs organize late stages of engulfment
Concentrations of PIP3 and PtdIns(3,4)P2 increase on phagocytic cup membranes and remain elevated until after the phagosome closes into the cell (Kamen et al, 2007; Marshall et al, 2001; Vieira et al, 2001). In macrophages, PI 3-kinase inhibition does not stop ruffling or cup formation, but prevents cup closure into phagosomes, indicating that PI 3-kinase is necessary for the contractile activities that close cups into phagosomes (Araki et al, 1996). The exact functions of PI 3-kinase during FcR-mediated phagocytosis are not fully known but, overall, its activity in phagocytic cups organizes many signals associated with engulfment. PIP3 activates PLCγ1 (Falasca et al, 1998), myosin X-based contractile activities (Cox et al, 2002), the GEFs which activate Rac and the GTPase activating proteins (GAPs) which inactivate Cdc42 (Beemiller et al, 2010). FRET microscopy showed that, as cups develop, the GTPases Cdc42, Arf6 and Rac1, are activated early and remain active in the advancing edge, and Rac2 and Arf1 become active later, at the cup base and during phagosome closure (Beemiller et al, 2006; Hoppe & Swanson, 2004). When GTPase activities were imaged during phagocytosis in the presence of PI 3-kinase inhibitors, which stop phagocytosis of large particles midway through the process, the actin-rich cups which could not close around the particles contained persistent early signals associated with phagocytosis (Cdc42, Rac1 and Arf6), and the late signals (Rac2 and Arf1) were not activated. This indicated that PIP3 generated in cup membranes is necessary for the signal transition from the early GTPases which regulate actin polymerization to the late GTPases which regulate phagosome closure. This suggests that PIP3 or DAG in cup membranes activates the GAPs which turn off the early GTPases Arf6 and Cdc42 and the GEFs which activate late-stage GTPases (Arf1 and Rac2). Consistent with this model of sequential signaling, phagocytosis requires cyclical activation and deactivation of Cdc42. Cdc42 activates PI 3-kinase (Zheng et al, 1994) and the PI 3-kinase product PIP3 deactivates Cdc42 (Beemiller et al, 2010).
The requirement of PI 3-kinase for cup closure and transitions to later stages of signaling suggests that PIP3 in cup membranes must exceed some concentration threshold level for phagocytosis to proceed. Zhang et al (Zhang et al, 2010) examined the relationship between PIP3 concentrations and phagosome progression by developing quantitative microscopic methods to measure the magnitudes of signals generated in phagocytic cups as a function of ligand (IgG) density on particle surfaces. When macrophages ingested microspheres opsonized with high densities of IgG, phagosomes recruited the chimeras yellow fluorescent protein (YFP)-actin, YFP-Syk (an early signal associated with ligated Fc receptors), YFP-AktPH (indicating PIP3 and PtdIns(3,4)P2) and YFP-PKCε. Recruitment of YFP chimeras occurred in a distinct sequence of early (YFP-Syk, YFP-actin), middle (YFP-AktPH) and late (YFP-PKCε) signals. Phagocytic cups containing low IgG-density microspheres often stalled without completing phagocytosis. These stalled phagocytic cups recruited early signal reporters YFP-Syk and YFP-actin and low levels of YFP-AktPH, indicating that low concentrations of PIP3 were generated in the phagosomes. The late signal YFP-PKCε did not appear. Overall, recruitment of YFP-chimeras for early signals (Syk, actin, PIP3) was proportional to IgG density, whereas YFP-PKCε recruitment was all-or-none, indicating that suprathreshold concentrations of PIP3 in cup membranes (or of the PtdIns(3,4)P2 or DAG which appear after PIP3) were required for the appearance of late signals and for completion of phagocytosis. Consistent with this model, cells engaging high IgG-density microspheres in the presence of PI 3-kinase inhibitors made cups with YFP-Syk and YFP-actin, but without YFP-AktPH or YFP-PKCε.
Thus, the movements of FcR-mediated phagocytosis can be described as early, reversible exploratory behavior, governed by PtdIns(4,5)P2, and later commitment stages. What is distinct about commitment to late signals? Early signals are governed by largely reversible PI phosphorylation and dephosphorylation reactions, whereas late signals require irreversible hydrolase activities.
Macropinocytosis
Macropinosome formation is analogous to phagocytosis. Nearly all eukaryotic cells make macropinosomes, often in response to growth factors (Kerr & Teasdale, 2009). Immature dendritic cells and cells transformed by K-Ras or v-Src form macropinosomes constitutively (Amyere et al, 2000; Bar-Sagi & Feramisco, 1986).
The cellular movements leading to macropinosome formation are irregular in morphology and timing (Mercer & Helenius, 2009). In a macrophage stimulated by its growth factor, construction of a 3 µm diameter macropinosome takes about 3 minutes (Yoshida et al, 2009). The process begins with the formation of ruffles which extend and curve into circular profiles (a.k.a. ruffle closure; Fig. 2B). These 1–6 µm diameter, crater-like subdomains of plasma membrane called macropinocytic cups either recede inconsequentially or close at their distal margins to form a discrete intracellular vesicle, the macropinosome (a.k.a. cup closure)( Yoshida et al, 2009). The macropinosome then either fuses with other endocytic compartments, which may include early endosomes, late endosomes, lysosomes or other macropinosomes, or it recycles to the plasma membrane without further maturation. In cells transformed by v-Src, macropinosome formation requires the sequential activities of PI 3-kinase and PLC (Amyere et al, 2000). In macrophages, PI 3-kinase inhibitors do not affect cell surface ruffling or circular ruffle formation, but instead inhibit cup closure into macropinosomes (Araki et al, 1996).
The PI profile of membranes changes during macropinosome formation. PtdIns(4,5)P2 concentrations increase early (Araki et al, 2007), followed by increases in PIP3 and PtdIns3P (Araki et al, 2006). Temporal ordering of PIs relative to the stages of macropinosome formation in macrophages revealed a sequence of PI transitions in cups (Araki et al, 2007; Welliver & Swanson, 2012; Yoshida et al, 2009)(Fig. 2C). PtdIns(4,5)P2 levels peaked transiently following ruffle closure, then decreased. PIP3 and DAG levels then increased for about 30 seconds and were followed by transient increases in PtdIns(3,4)P2 and PtdIns3P, similar to the sequence of PIs seen in phagocytic cups. PI-modifying phosphatases associated with these transitions have been implicated in circular ruffle formation and macropinocytosis. SHIP2, which dephosphorylates PIP3 to PtdIns(3,4)P2, is required for circular ruffle formation (Hasegawa et al, 2011). A mutant screen using Caenorhabditis elegans cells identified the PtdIns3P phosphatase myotubularin-related protein-6 (MTMR6) and its associated protein myotubularin-related protein-9 (MTMR9) as necessary for macropinosome formation (Maekawa et al, 2014). Additionally, Maekawa, et al., showed a requirement for inositol polyphosphate 4-phosphatase type II (Inpp4B), which dephosphorylates PtdIns(3,4)P2 to PtdIns3P, supporting a model in which macropinosome formation requires a sequential progression of 3’PIs in macropinocytic cups similar to the sequence delineated by microscopy (Welliver & Swanson, 2012). These schemes may not be universal, however. In D. discoideum, although PIP3 localizes to phagocytic and macropinocytic cups (Mercanti et al, 2006), the two activities are differentially regulated by PI 3-kinase and PLC (Cardelli, 2001).
Mechanisms
Physical isolation of cup domains in plasma membrane
As mentioned above, the phospholipid composition of the cup membrane differs from plasma membrane even before the phagosome or macropinosome separates into cytoplasm (Fig. 2A, B>). The lateral segregation of membrane PIs is especially pronounced during phagocytosis of large (8 µm diameter) microspheres (Botelho et al, 2000). D. discoideum amebae have high concentrations of PIP3 in phagocytic and macropinocytic cups, with none in the contiguous plasma membrane (Mercanti et al, 2006). Sharp boundaries localized to the edges of the cups delineate the two domains. The boundaries appear to be due to structural barriers to lateral diffusion of phospholipids. Analysis of PtdIns(4,5)P2 dynamics in phagocytic cups identified barriers intrinsic to the cup wall structure that limited diffusion of PtdIns(4,5)P2 from the cups into the contiguous plasma membrane (Golebiewska et al, 2011). Similar barriers to lateral diffusion were identified in macropinocytic cups, using membrane-tethered photoactivatable GFP probes (Welliver et al, 2011). The molecular nature of these diffusion barriers remains unknown, but the fact that lateral diffusion of PIs is limited by cup structure suggests that the cup can function as a crucible for signal amplification. That is, the physical structure of a cup may provide an essential component for positive feedback amplification of PI-dependent signaling.
Size-dependent requirements for PI3K
FcR signaling can be triggered by IgG on many different kinds of surfaces, and the requirement of PI 3-kinase for cellular responses varies with the geometry of the surface. Notably, although PI 3-kinase inhibitors block phagocytosis of microspheres larger than 3 µm, ingestion of smaller microspheres is not affected. Rod-shaped particles coated with IgG are not ingested unless they contact the cell at their narrow, highly curved ends (Champion & Mitragotri, 2006). Likewise, filamentous bacteria opsonized with IgG are internalized only when the macrophage locates a free end. It then builds an actin cuff that advances along the length of the filament, pulling it into the cell and forming an elongated phagocytic cup (Prashar et al, 2013). The prolonged phagocytic response needed to ingest a long filament is not inhibited by PI 3-kinase inhibitors (Prashar et al, 2013).
What explains the particle size-dependence of PI 3-kinase inhibition? One possible mechanism is that small particles are ingested before late signals appear, rendering the late signals unnecessary. However, early and late GTPase signals, as well as PIP3, also appear on phagosomes that internalize smaller microspheres (Beemiller et al, 2010). Another possibility is that small particles can be ingested by a PI 3-kinase-independent mechanism that cannot work on larger particles. Clathrin-mediated endocytosis could be a candidate for such a mechanism. So far, however, no studies have identified a pathway which preferentially inhibits engulfment of small but not large particles. Another possibility is that PI 3-kinase is required for the actin turnover necessary for the advance an actin cuff over large particles. However, PI 3-kinase inhibitors do not block engulfment of long filaments, whose ingestion is sometimes quite prolonged (Prashar et al, 2013). Thus, a PI 3-kinase-independent phagocytosis internalizes small microspheres and elongated filaments, which suggests that 3’PIs are only required for the ingestion of wide loads.
How could cup width affect the cell’s ability to complete phagocytosis? Particle size and shape do not measurably limit actin polymerization or early signals of phagocytosis, but they may influence the ability of the cup to generate the suprathreshold concentrations of PIP3 necessary for transitions to late stages of signaling. Accordingly, a phagocytic cell may use actin-based motility to explore particle surfaces and only initiate a full phagocytic response (ie., late signals) when surface dimensions or ligand densities are right. PIP3 synthesized in cup membranes may override a brake that prevents the ingestion of large particles. The phagocytosis of large particles entails a considerable shift in organelle dimensions; cup size and 3’PI concentration thresholds may be related to mechanisms that regulate cellular dimensions. Accordingly, the concentrations of PIP3 that can be generated in cup membranes may be influenced by a cell’s size or its capacity for increasing its volume through engulfment.
Invasion
Many pathogenic microbes manipulate PI chemistry to their advantage. Some inhibit engulfment by actively inhibiting the signal transduction of phagocytosis (Ham et al, 2011). Others trigger phagocytosis as a mechanism for establishing intracellular vacuolar environments. Yersinia entercolitica invades epithelial cells by an integrin-mediated engulfment that requires Rac and Arf6-dependent activation of PI4P5K (Wong & Isberg, 2003). PtdIns(4,5)P2 accumulates in the forming phagosome and is necessary for invasion, but phagosome closure requires removal of PtdIns(4,5)P2 from the cup membranes, which is mediated by the PtdIns 5-phosphatase activities of OCRL and Inpp5b (Sarantis et al, 2012). Listeria monocytogenes invades epithelial cells by an unusual process that requires PI 3-kinase (Ireton et al, 1996), PI 4-kinase (Pizarro-Cerda et al, 2007) and clathrin (Veiga & Cossart, 2005), indicating yet another novel mechanism of engulfment. Salmonella enterica var. Typhimurium enters epithelial cells by stimulating host cell ruffling and capture by macropinosomes (Francis et al, 1993). A Salmonella type III secretion system (TTSS) delivers effector proteins into the host cell cytoplasm, including the PtdIns-4,5-bisphosphate 4-phosphatase SopB (also known as SigD) (Mallo et al, 2008; Mason et al, 2007). SopB promotes increased PIP3 and PtdIns(3,4)P2 in ruffles (Mallo et al, 2008), phagosome closure by depletion of PtdIns(4,5)P2 (Terebiznik et al, 2002) and the synthesis of PtdIns3P on Salmonella-containing vacuoles. Depletion of phagosomal PtdIns(4,5)P2 by SopB increases recruitment of Rab5, which activates the type III PI 3-kinase Vps34 to generate PtdIns3P (Mallo et al, 2008). Like Salmonella, Shigella flexneri uses a TTSS to deliver a PtdIns-4,5-bisphosphate 4-phosphatase, IpgD, into host cells (Niebuhr et al, 2002). The IpgD product PtdIns5P activates PI 3-kinase (Pendaries et al, 2006) and stimulates Tiam-1 and Rac-dependent ruffling (Viaud et al, 2014). Thus, SopB and IpgD may drive the engulfment of bacteria both through localized synthesis of PtdIns5P, activating PI 3-kinase and Rac, and localized depletion of PtdIns(4,5)P2, leading to Rab5 recruitment, activation Vps34 and increased levels of PtdIns3P necessary for vacuole maturation.
Some effector enzymes modify PIs of the cytosolic surface of the vacuole after engulfment (Hilbi, 2006). Legionella pneumophila enters macrophages by a macropinocytosis-like mechanism (Watarai et al, 2001), then uses a type four secretion system (TFSS) to deliver effector proteins which modify the intracellular vacuole (Haneburger & Hilbi, 2013). Imaging of PIs during infection of D. discoideum with L. pneumophila indicated that the Legionella-containing vacuole (LCV) forms and matures by mechanisms similar to those described for macropinocytosis or FcR-mediated phagocytosis (Weber et al, 2013): PIP3 localizes to forming phagosomes, PtdIns(4,5)P2 is stripped from phagosome and PtdIns3P appears on the closed phagosome. Then, in a manner dependent on the TFSS, PtdIns4P accumulates on the LCV over the next several hours. The Legionella TFSS effector proteins SidF and SidP are phosphoinositide 3-phosphatases which modify the PI profile of LCV membranes and potentially inhibit LCV fusion with lysosomes (Hsu et al, 2012; Toulabi et al, 2013).
Perspective
Although PIs are clearly necessary for engulfment, universal organizing principles are scant. It seems certain that the different movements that underlie engulfment require different PIs and that transitions of membrane PI chemistry organize transitions in cytoskeletal activities. Actin polymerization requires generation of PtdIns(4,5)P2 or PtdIns5P. PIP3 and PtdIns(3,4)P2 can facilitate actin dynamics and determine thresholds for commitment to late stages of engulfment, yet their requirements vary with the target particle geometry and with other conditions in the engulfing cells. Completion of engulfment requires the removal of PtdIns(4,5)P2 from cup membranes, but may also require the DAG derived from PtdIns(4,5)P2. Refined mechanistic understanding of how PIs organize the movements of engulfment may reveal an essential chemical cascade. Alternatively, we may eventually recognize that PIs organize engulfment by many different routes.
Literature Cited
- Amyere M, Payrastre B, Krause U, Van der Smissen P, Veithen A, Courtoy PJ. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation phosphoinositide 3-kinase and phospholipase C. Mol Biol Cell. 2000;11:3453–3467. doi: 10.1091/mbc.11.10.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki N, Egami Y, Watanabe Y, Hatae T. Phosphoinositide metabolism during membrane ruffling and macropinosome formation in EGF-stimulated A431 cells. Exp Cell Res. 2007;313:1496–1507. doi: 10.1016/j.yexcr.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Araki N, Hamasaki M, Egami Y, Hatae T. Effect of 3-methyladenine on the fusion process of macropinosomes in EGF-stimulated A431 cells. Cell structure and function. 2006;31:145–157. doi: 10.1247/csf.06029. [DOI] [PubMed] [Google Scholar]
- Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis in macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajno L, Peng X-R, Schreiber AD, Moore H-P, Trimble WS, Grinstein S. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J Cell Biol. 2000;149:697–705. doi: 10.1083/jcb.149.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiological reviews. 2013;93:1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sagi D, Feramisco JR. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1066. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- Beemiller P, Hoppe AD, Swanson JA. A phosphatidylinositol-3-kinase-dependent signal transition regulates ARF1 and ARF6 during Fcγ receptor-mediated phagocytosis. PLoS biology. 2006;4:e162. doi: 10.1371/journal.pbio.0040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemiller P, Zhang Y, Mohan S, Levinsohn E, Gaeta I, Hoppe AD, Swanson JA. A Cdc42 activation cycle coordinated by PI 3-kinase during Fc receptor-mediated phagocytosis. Mol Biol Cell. 2010;21:470–480. doi: 10.1091/mbc.E08-05-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohdanowicz M, Balkin DM, De Camilli P, Grinstein S. Recruitment of OCRL and Inpp5B to phagosomes by Rab5 and APPL1 depletes phosphoinositides and attenuates Akt signaling. Mol Biol Cell. 2012;23:176–187. doi: 10.1091/mbc.E11-06-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohdanowicz M, Grinstein S. Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiological reviews. 2013;93:69–106. doi: 10.1152/physrev.00002.2012. [DOI] [PubMed] [Google Scholar]
- Botelho RJ, Tapper H, Furuya W, Mojdami D, Grinstein S. FcγR-mediated phagocytosis stimulates localized pinocytosis in human neutrophils. J Immunol. 2002;169:4423–4429. doi: 10.4049/jimmunol.169.8.4423. [DOI] [PubMed] [Google Scholar]
- Botelho RJ, Teruel M, Dierckman R, Anderson R, Wells A, York JD, Meyer T, Grinstein S. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J Cell Biol. 2000;151:1353–1367. doi: 10.1083/jcb.151.7.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V, Fraisier V, Raposo G, Hurbain I, Sibarita JB, Chavrier P, Galli T, Niedergang F. TI-VAMP/VAMP7 is required for optimal phagocytosis of opsonised particles in macrophages. Embo J. 2004;23:4166–4176. doi: 10.1038/sj.emboj.7600427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardelli J. Phagocytosis and macropinocytosis in Dictyostelium: Phosphoinositide-based processes, biochemically distinct. Traffic. 2001;2:311–320. doi: 10.1034/j.1600-0854.2001.002005311.x. [DOI] [PubMed] [Google Scholar]
- Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppolino MG, Dierckman R, Loijens J, Collins RF, Pouladi M, Jongstra-Bilen J, Schreiber AD, Trimble WS, Anderson R, Grinstein S. Inhibition of phosphatidylinositol-4-phosphate 5 kinase Iα impairs localized actin remodeling and suppresses phagocytosis. J Biol Chem. 2002;277:43849–43857. doi: 10.1074/jbc.M209046200. [DOI] [PubMed] [Google Scholar]
- Cox D, Berg JS, Cammer M, Chinegwundoh JO, Dale BM, Cheney RE, Greenberg S. Myosin X is a downstream effector of PI(3)K during phagocytosis. Nature Cell Biol. 2002;4:469–477. doi: 10.1038/ncb805. [DOI] [PubMed] [Google Scholar]
- Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VLJ, DeFranco AL. A critical role for Syk in signal transduction and phagocytosis mediated by Fcγ receptors on macrophages. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J. Activation of phospholipase C-γ by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzer-Attas CJ, Lowry M, Crowley MT, Finn AJ, Meng F, DeFranco AL, Lowell CA. Fcγ receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn. J Exp Med. 2000;191:669–681. doi: 10.1084/jem.191.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannagan RS, Harrison RE, Yip CM, Jaqaman K, Grinstein S. Dynamic macrophage "probing" is required for the efficient capture of phagocytic targets. J Cell Biol. 2010;191:1205–1218. doi: 10.1083/jcb.201007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannagan RS, Jaumouille V, Grinstein S. The cell biology of phagocytosis. Annual review of pathology. 2012;7:61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- Francis CL, Ryan TA, Jones BD, Smith SJ, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- Golebiewska U, Kay JG, Masters T, Grinstein S, Im W, Pastor RW, Scarlata S, McLaughlin S. Evidence for a fence that impedes the diffusion of phosphatidylinositol 4,5-bisphosphate out of the forming phagosomes of macrophages. Mol Biol Cell. 2011;22:3498–3507. doi: 10.1091/mbc.E11-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Botelho RJ, Yu M, Grinstein S, Neel BG. Critical role for scaffolding adapter Gab2 in FcγR-mediated phagocytosis. J Cell Biol. 2003;161:1151–1161. doi: 10.1083/jcb.200212158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham H, Sreelatha A, Orth K. Manipulation of host membranes by bacterial effectors. Nature reviews Microbiology. 2011;9:635–646. doi: 10.1038/nrmicro2602. [DOI] [PubMed] [Google Scholar]
- Haneburger I, Hilbi H. Phosphoinositide lipids and the legionella pathogen vacuole. Curr Top Microbiol Immunol. 2013;376:155–173. doi: 10.1007/82_2013_341. [DOI] [PubMed] [Google Scholar]
- Hasegawa J, Tokuda E, Tenno T, Tsujita K, Sawai H, Hiroaki H, Takenawa T, Itoh T. SH3YL1 regulates dorsal ruffle formation by a novel phosphoinositide-binding domain. J Cell Biol. 2011;193:901–916. doi: 10.1083/jcb.201012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh JM, Codazzi F, Teruel M, Meyer T. Spatial sensing in fibroblasts mediated by 3' phosphoinositides. J Cell Biol. 2000;151:1269–1279. doi: 10.1083/jcb.151.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RM, Hoppe AD, N J, Swanson JA. The uniformity of phagosome maturation in macrophages. J Cell Biol. 2004;164:185–194. doi: 10.1083/jcb.200307080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbi H. Modulation of phosphoinositide metabolism by pathogenic bacteria. Cell Microbiol. 2006;8:1697–1706. doi: 10.1111/j.1462-5822.2006.00793.x. [DOI] [PubMed] [Google Scholar]
- Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris AJ, Frohman MA, Kanaho Y. Phosphatidylinositol 4-phosphate 5-kinase a is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- Hoppe AD, Swanson JA. Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol Biol Cell. 2004;15:3509–3519. doi: 10.1091/mbc.E03-11-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F, Zhu W, Brennan L, Tao L, Luo ZQ, Mao Y. Structural basis for substrate recognition by a unique Legionella phosphoinositide phosphatase. Proc Natl Acad Sci U S A. 2012;109:13567–13572. doi: 10.1073/pnas.1207903109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K, Payrastre B, Chap H, Ogawa W, Sakaue H, Kasuga M, Cossart P. A role for phosphoinositide 3-kinase in bacterial invasion. Science. 1996;274:780–782. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- Iyer SS, Barton JA, Bourgoin S, Kusner DJ. Phospholipases D1 and D2 Coordinately Regulate Macrophage Phagocytosis. J Immunol. 2004;173:2615–2623. doi: 10.4049/jimmunol.173.4.2615. [DOI] [PubMed] [Google Scholar]
- Kamen LA, Levinsohn J, Swanson JA. Differential association of phosphatidylinositol 3-kinase, SHIP-1, and PTEN with forming phagosomes. Mol Biol Cell. 2007;18:2463–2472. doi: 10.1091/mbc.E07-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavran JM, Klein DE, Lee A, Falasca M, Isakoff SJ, Skolnik EY, Lemmon MA. Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains. J Biol Chem. 1998;273:30497–30508. doi: 10.1074/jbc.273.46.30497. [DOI] [PubMed] [Google Scholar]
- Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- Larsen EC, Ueyama T, Brannock PM, Shirai Y, Saito N, Larsson C, Loegering D, Weber PB, Lennartz MR. A role for PKC-ε in FcγR-mediated phagocytosis by RAW 264.7 cells. J Cell Biol. 2002;159:939–944. doi: 10.1083/jcb.200205140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WL, Cosio G, Ireton K, Grinstein S. Role of CrkII in Fcγ receptor-mediated phagocytosis. J Biol Chem. 2007;282:11135–11143. doi: 10.1074/jbc.M700823200. [DOI] [PubMed] [Google Scholar]
- Lennartz MR, Yuen AFC, Masi SM, Russell DG, Buttle KF, Smith JJ. Phospholipase A2 inhibition results in sequestration of plasma membrane into electronlucent vesicles during IgG-mediated phagocytosis. J Cell Sci. 1997;110:2041–2052. doi: 10.1242/jcs.110.17.2041. [DOI] [PubMed] [Google Scholar]
- Liao F, Shin HS, Rhee SG. Tyrosine phosphorylation of phospholipase C-γ 1 induced by cross-linking of the high-affinity or low-affinity Fc receptor for IgG in U937 cells. Proc Natl Acad Sci U S A. 1992;89:3659–3663. doi: 10.1073/pnas.89.8.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M, Terasaka S, Mochizuki Y, Kawai K, Ikeda Y, Araki N, Skolnik EY, Taguchi T, Arai H. Sequential breakdown of 3-phosphorylated phosphoinositides is essential for the completion of macropinocytosis. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1311029111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo GV, Espina M, Smith AC, Terebiznik MR, Aleman A, Finlay BB, Rameh LE, Grinstein S, Brumell JH. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J Cell Biol. 2008;182:741–752. doi: 10.1083/jcb.200804131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao YS, Yamaga M, Zhu X, Wei Y, Sun HQ, Wang J, Yun M, Wang Y, Di Paolo G, Bennett M, Mellman I, Abrams CS, De Camilli P, Lu CY, Yin HL. Essential and unique roles of PIP5K-γ and -α in Fcγ receptor-mediated phagocytosis. J Cell Biol. 2009;184:281–296. doi: 10.1083/jcb.200806121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JG, Booth JW, Stambolic V, Mak T, Balla T, Schreiber AD, Meyer T, Grinstein S. Restricted accumulation of phosphatidylinositol 3-kinase products in a plasmalemmal subdomain during Fcγ receptor-mediated phagocytosis. J Cell Biol. 2001;153:1369–1380. doi: 10.1083/jcb.153.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D, Mallo GV, Terebiznik MR, Payrastre B, Finlay BB, Brumell JH, Rameh L, Grinstein S. Alteration of epithelial structure and function associated with PtdIns(4,5)P2 degradation by a bacterial phosphatase. The Journal of general physiology. 2007;129:267–283. doi: 10.1085/jgp.200609656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May RC, Caron E, Hall A, Machesky LM. Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR and CR3. Nature Cell Biol. 2000;2 doi: 10.1038/35008673. [DOI] [PubMed] [Google Scholar]
- Mercanti V, Charette SJ, Bennett N, Ryckewaert JJ, Letourneur F, Cosson P. Selective membrane exclusion in phagocytic and macropinocytic cups. J Cell Sci. 2006;119:4079–4087. doi: 10.1242/jcs.03190. [DOI] [PubMed] [Google Scholar]
- Mercer J, Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 1998;17:6932–6941. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. Organelle identity and the organization of membrane traffic. Nature Cell Biol. 2004;6:469–472. doi: 10.1038/ncb0604-469. [DOI] [PubMed] [Google Scholar]
- Niebuhr K, Giuriato S, Pedron T, Philpott DJ, Gaits F, Sable J, Sheetz MP, Parsot C, Sansonetti PJ, Payrastre B. Conversion of PtdIns(4,5)P2 into PtdIns(5)P by the S.flexneri effector IpgD reorganizes host cell morphology. EMBO J. 2002;21:5069–5078. doi: 10.1093/emboj/cdf522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedergang F, Colucci-Guyon E, Dubois T, Raposo G, Chavrier P. ADP ribosylation factor 6 is activated and controls membrane delivery during phagocytosis in macrophages. J Cell Biol. 2003;161:1143–1150. doi: 10.1083/jcb.200210069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Cox D. Cdc42 regulates Fcγ receptor-mediated phagocytosis through the activation and phosphorylation of Wiskott-Aldrich syndrome protein (WASP) and neural-WASP. Mol Biol Cell. 2009;20:4500–4508. doi: 10.1091/mbc.E09-03-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendaries C, Tronchere H, Arbibe L, Mounier J, Gozani O, Cantley L, Fry MJ, Gaits-Iacovoni F, Sansonetti PJ, Payrastre B. PtdIns5P activates the host cell PI3-kinase/Akt pathway during Shigella flexneri infection. EMBO J. 2006;25:1024–1034. doi: 10.1038/sj.emboj.7601001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Cerda J, Payrastre B, Wang YJ, Veiga E, Yin HL, Cossart P. Type II phosphatidylinositol 4-kinases promote Listeria monocytogenes entry into target cells. Cell Microbiol. 2007;9:2381–2390. doi: 10.1111/j.1462-5822.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- Prashar A, Bhatia S, Gigliozzi D, Martin T, Duncan C, Guyard C, Terebiznik MR. Filamentous morphology of bacteria delays the timing of phagosome morphogenesis in macrophages. J Cell Biol. 2013;203:1081–1097. doi: 10.1083/jcb.201304095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameh LE, Arvidsson A-k, Carraway KL, Couvillon AD, Rathbun G, Crompton A, VanRenterghem B, Czech MP, Ravichandran KS, Burakoff SJ, Wang D-S, Chen C-S, Cantley LC. A comparative analysis of the phosphoinositide binding specificity of pleckstrin homology domains. J Biol Chem. 1997;272:22059–22066. doi: 10.1074/jbc.272.35.22059. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–455. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantis H, Balkin DM, De Camilli P, Isberg RR, Brumell JH, Grinstein S. Yersinia entry into host cells requires Rab5-dependent dephosphorylation of PI(4,5)P2 and membrane scission. Cell host & microbe. 2012;11:117–128. doi: 10.1016/j.chom.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CC, Dobson W, Botelho RJ, Coady-Osberg N, Chavrier P, Knecht DA, Heath C, Stahl P, Grinstein S. Phosphatidylinositol-4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis. J Cell Biol. 2005;169:139–149. doi: 10.1083/jcb.200412162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shisheva A. PIKfyve and its Lipid products in health and in sickness. Curr Top Microbiol Immunol. 2012;362:127–162. doi: 10.1007/978-94-007-5025-8_7. [DOI] [PubMed] [Google Scholar]
- Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008;9:639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terebiznik MR, Vieira OV, Marcus SL, Slade A, Yip CM, Trimble WS, Meyer T, Finlay BB, Grinstein S. Elimination of host cell PtdIns(4,5)P2 by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat Cell Biol. 2002;4:766–773. doi: 10.1038/ncb854. [DOI] [PubMed] [Google Scholar]
- Toulabi L, Wu X, Cheng Y, Mao Y. Identification and structural characterization of a Legionella phosphoinositide phosphatase. J Biol Chem. 2013;288:24518–24527. doi: 10.1074/jbc.M113.474239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell A, Hugge C, Kisseleva M, Chang SC, Zou J, Feng Y, Galyov EE, Wilson M, Majerus PW. The identification and characterization of two phosphatidylinositol-4,5-bisphosphate 4-phosphatases. Proc Natl Acad Sci U S A. 2005;102:18854–18859. doi: 10.1073/pnas.0509740102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P, Rother KI, Balla T. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton's tyrosine kinase pleckstrin homology domain visualized in living cells. J Biol Chem. 1999;274:10983–10989. doi: 10.1074/jbc.274.16.10983. [DOI] [PubMed] [Google Scholar]
- Veiga E, Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat Cell Biol. 2005;7:894–900. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- Viaud J, Lagarrigue F, Ramel D, Allart S, Chicanne G, Ceccato L, Courilleau D, Xuereb JM, Pertz O, Payrastre B, Gaits-Iacovoni F. Phosphatidylinositol 5-phosphate regulates invasion through binding and activation of Tiam1. Nature communications. 2014;5:4080. doi: 10.1038/ncomms5080. [DOI] [PubMed] [Google Scholar]
- Vieira OV, Botelho RJ, Rameh L, Brachmann SM, Matsuo T, Davidson HW, Schreiber A, Backer JM, Cantley LC, Grinstein S. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J Cell Biol. 2001;155:19–25. doi: 10.1083/jcb.200107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai M, Derre I, Kirby J, Growney JD, Dietrich WF, Isberg RR. Legionella pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus. J Exp Med. 2001;194:1081–1095. doi: 10.1084/jem.194.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S, Wagner M, Hilbi H. Live-cell imaging of phosphoinositide dynamics and membrane architecture during Legionella infection. mBio. 2013;5:e00839-00813. doi: 10.1128/mBio.00839-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welliver TP, Chang SL, Linderman JJ, Swanson JA. Ruffles limit diffusion in the plasma membrane during macropinosome formation. J Cell Sci. 2011;124:4106–4114. doi: 10.1242/jcs.091538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welliver TP, Swanson JA. A growth factor signaling cascade confined to circular ruffles in macrophages. Biology open. 2012;1:754–760. doi: 10.1242/bio.20121784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KW, Isberg RR. Arf6 and phosphoinositol-4-phosphate-5-kinase activities permit bypass of the Rac1 requirement for β1 integrin-mediated bacterial uptake. J Exp Med. 2003;198:603–614. doi: 10.1084/jem.20021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Hoppe AD, Araki N, Swanson JA. Sequential signaling in plasma-membrane domains during macropinosome formation in macrophages. J Cell Sci. 2009;122:3250–3261. doi: 10.1242/jcs.053207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Cox D, Tseng C-C, Donaldson JG, Greenberg S. A requirement for ARF6 in Fcγ receptor-mediated phagocytosis in macrophages. J Biol Chem. 1998;273:19977–19981. doi: 10.1074/jbc.273.32.19977. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoppe AD, Swanson JA. Coordination of Fc receptor signaling regulates cellular commitment to phagocytosis. Proc Natl Acad Sci U S A. 2010;107:19332–19337. doi: 10.1073/pnas.1008248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Bagrodia S, Cerione RA. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J Biol Chem. 1994;269:18727–18730. [PubMed] [Google Scholar]