Abstract
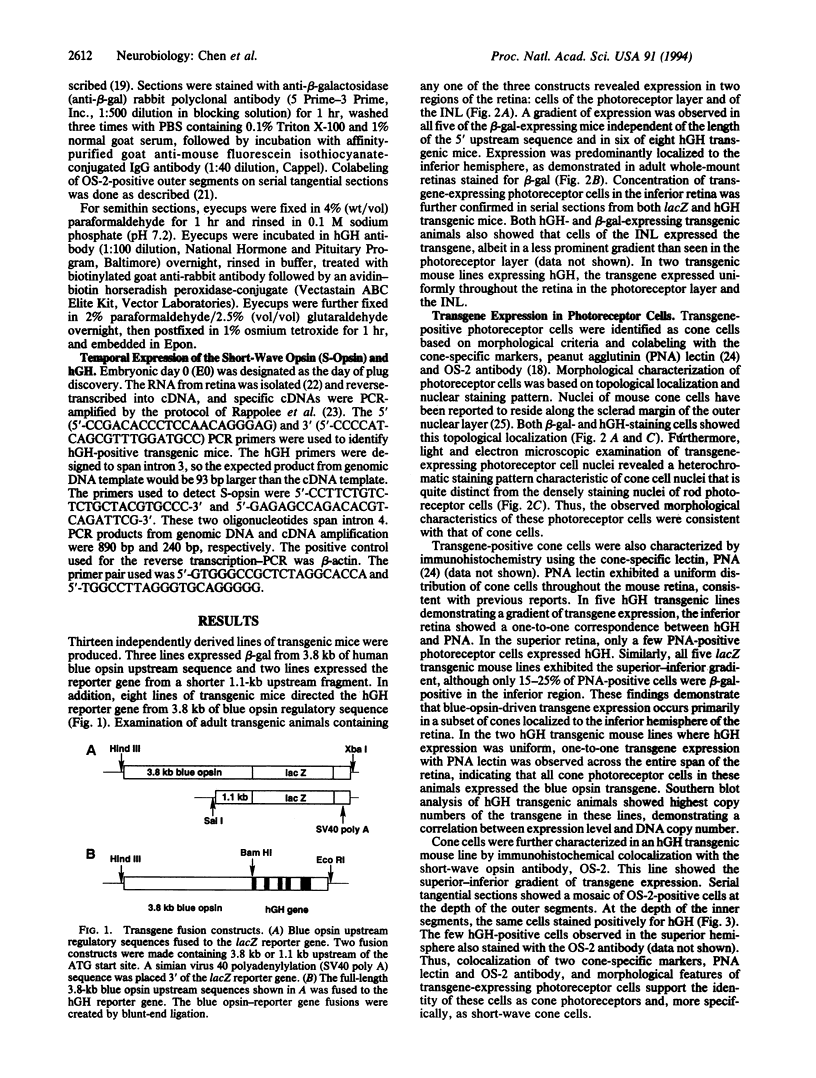
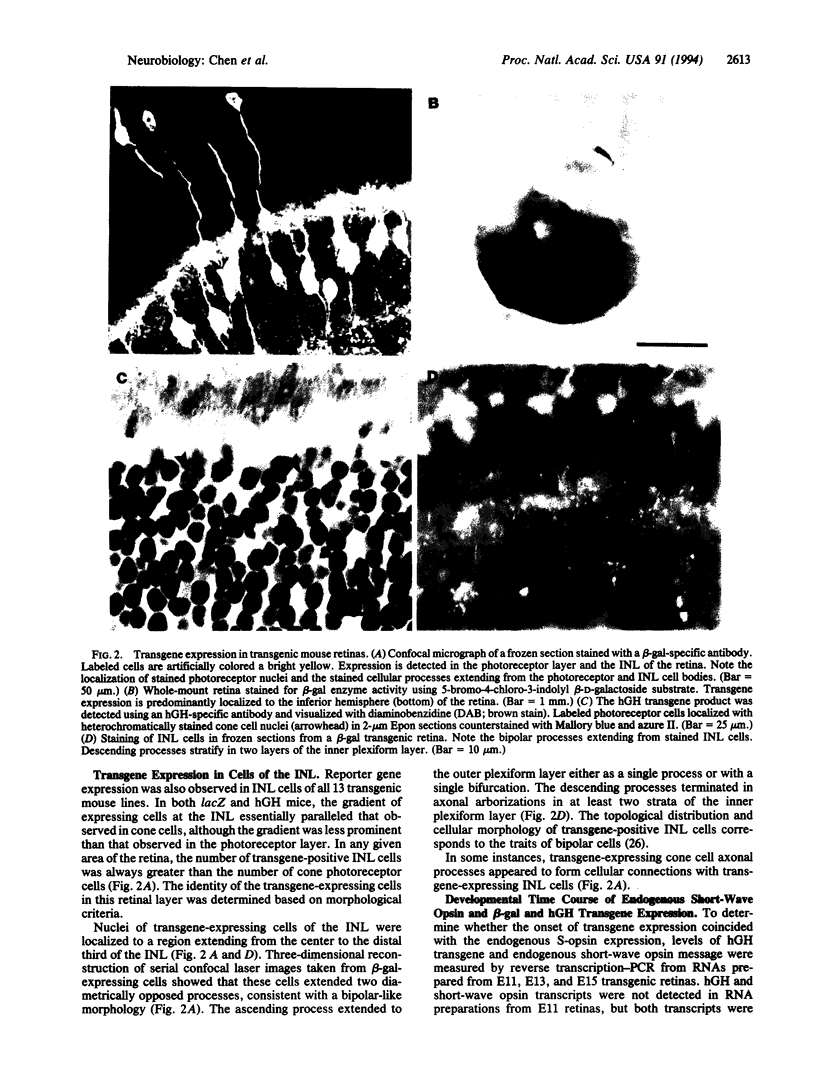
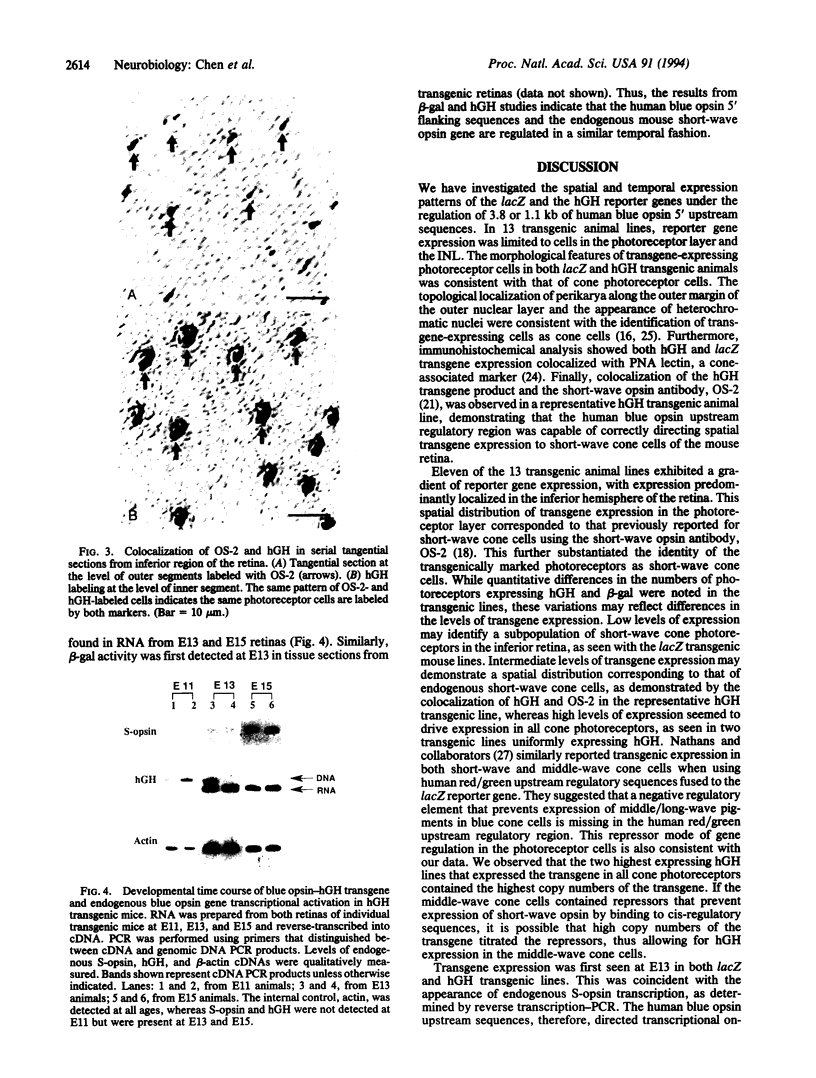
Transgenic mouse lines were generated using either 3.8 or 1.1 kb of 5' upstream flanking sequence from the human blue opsin gene fused to the lacZ or human growth hormone reporter gene. Mice were analyzed for appropriate cell-specific and developmental expression patterns. In 13 independently derived lines of animals, transgene expression was limited to photoreceptor and inner nuclear layer cells. Photoreceptors were identified as cone cells based on morphological criteria and colocalization of transgene expression with the cone-associated marker, peanut agglutinin lectin. More specifically, transgene-positive photoreceptors were identified as short-wave cone cells (S-cones) by using the short-wave color opsin-specific antibody, OS-2. Reporter-gene-positive cells of the inner nuclear layer were identified as bipolar cells based on morphological criteria. Transgenes and the endogenous mouse short-wave opsin gene were transcriptionally coactivated at embryonic day 13. These results show that 3.8 or 1.1 kb of human blue opsin upstream flanking sequences are capable of directing expression in short-wave cone cells in a spatially and temporally appropriate fashion and that the human blue opsin gene is the homologue of the short-wave-sensitive pigment, S-opsin, in the short-wave cones of the mouse retina. Expression in the bipolar cells may reflect regulatory mechanisms that are common to these cells and to the cone photoreceptors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Falk J. D., Bugra K., Triantafyllos J. T., McGinnis J. F. Isolation and analysis of the mouse opsin gene. FEBS Lett. 1988 Oct 10;238(2):253–256. doi: 10.1016/0014-5793(88)80490-3. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J. Immunological studies of the diversity and development of the mammalian visual system. Immunol Rev. 1987 Dec;100:47–78. doi: 10.1111/j.1600-065x.1987.tb00527.x. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson L. D., LaVail M. M. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979 Nov 15;188(2):245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dizhoor A. M., Ray S., Kumar S., Niemi G., Spencer M., Brolley D., Walsh K. A., Philipov P. P., Hurley J. B., Stryer L. Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science. 1991 Feb 22;251(4996):915–918. doi: 10.1126/science.1672047. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gillespie P. G., Beavo J. A. Characterization of a bovine cone photoreceptor phosphodiesterase purified by cyclic GMP-sepharose chromatography. J Biol Chem. 1988 Jun 15;263(17):8133–8141. [PubMed] [Google Scholar]
- Hageman G. S., Johnson L. V. Biochemical characterization of the major peanut-agglutinin-binding glycoproteins in vertebrate retinae. J Comp Neurol. 1986 Jul 22;249(4):499-510, 482-3. doi: 10.1002/cne.902490406. [DOI] [PubMed] [Google Scholar]
- Hurwitz R. L., Bunt-Milam A. H., Chang M. L., Beavo J. A. cGMP phosphodiesterase in rod and cone outer segments of the retina. J Biol Chem. 1985 Jan 10;260(1):568–573. [PubMed] [Google Scholar]
- Ibbotson R. E., Hunt D. M., Bowmaker J. K., Mollon J. D. Sequence divergence and copy number of the middle- and long-wave photopigment genes in Old World monkeys. Proc Biol Sci. 1992 Feb 22;247(1319):145–154. doi: 10.1098/rspb.1992.0021. [DOI] [PubMed] [Google Scholar]
- Jacobs G. H., Neitz J., Deegan J. F., 2nd Retinal receptors in rodents maximally sensitive to ultraviolet light. Nature. 1991 Oct 17;353(6345):655–656. doi: 10.1038/353655a0. [DOI] [PubMed] [Google Scholar]
- Kouyama N., Marshak D. W. Bipolar cells specific for blue cones in the macaque retina. J Neurosci. 1992 Apr;12(4):1233–1252. doi: 10.1523/JNEUROSCI.12-04-01233.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. H., Lieberman B. S., Yamane H. K., Bok D., Fung B. K. A third form of the G protein beta subunit. 1. Immunochemical identification and localization to cone photoreceptors. J Biol Chem. 1992 Dec 5;267(34):24776–24781. [PubMed] [Google Scholar]
- Lem J., Applebury M. L., Falk J. D., Flannery J. G., Simon M. I. Tissue-specific and developmental regulation of rod opsin chimeric genes in transgenic mice. Neuron. 1991 Feb;6(2):201–210. doi: 10.1016/0896-6273(91)90356-5. [DOI] [PubMed] [Google Scholar]
- Lerea C. L., Bunt-Milam A. H., Hurley J. B. Alpha transducin is present in blue-, green-, and red-sensitive cone photoreceptors in the human retina. Neuron. 1989 Sep;3(3):367–376. doi: 10.1016/0896-6273(89)90261-4. [DOI] [PubMed] [Google Scholar]
- Lerea C. L., Somers D. E., Hurley J. B., Klock I. B., Bunt-Milam A. H. Identification of specific transducin alpha subunits in retinal rod and cone photoreceptors. Science. 1986 Oct 3;234(4772):77–80. doi: 10.1126/science.3529395. [DOI] [PubMed] [Google Scholar]
- Li T. S., Volpp K., Applebury M. L. Bovine cone photoreceptor cGMP phosphodiesterase structure deduced from a cDNA clone. Proc Natl Acad Sci U S A. 1990 Jan;87(1):293–297. doi: 10.1073/pnas.87.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam A. H., Dacey D. M., Dizhoor A. M. Recoverin immunoreactivity in mammalian cone bipolar cells. Vis Neurosci. 1993 Jan-Feb;10(1):1–12. doi: 10.1017/s0952523800003175. [DOI] [PubMed] [Google Scholar]
- Nathans J., Piantanida T. P., Eddy R. L., Shows T. B., Hogness D. S. Molecular genetics of inherited variation in human color vision. Science. 1986 Apr 11;232(4747):203–210. doi: 10.1126/science.3485310. [DOI] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Neitz J., Jacobs G. H. Reexamination of spectral mechanisms in the rat (Rattus norvegicus). J Comp Psychol. 1986 Mar;100(1):21–29. [PubMed] [Google Scholar]
- Peng Y. W., Robishaw J. D., Levine M. A., Yau K. W. Retinal rods and cones have distinct G protein beta and gamma subunits. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10882–10886. doi: 10.1073/pnas.89.22.10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee D. A., Wang A., Mark D., Werb Z. Novel method for studying mRNA phenotypes in single or small numbers of cells. J Cell Biochem. 1989 Jan;39(1):1–11. doi: 10.1002/jcb.240390102. [DOI] [PubMed] [Google Scholar]
- Szél A., Diamantstein T., Röhlich P. Identification of the blue-sensitive cones in the mammalian retina by anti-visual pigment antibody. J Comp Neurol. 1988 Jul 22;273(4):593–602. doi: 10.1002/cne.902730413. [DOI] [PubMed] [Google Scholar]
- Szél A., Röhlich P., Caffé A. R., Juliusson B., Aguirre G., Van Veen T. Unique topographic separation of two spectral classes of cones in the mouse retina. J Comp Neurol. 1992 Nov 15;325(3):327–342. doi: 10.1002/cne.903250302. [DOI] [PubMed] [Google Scholar]
- Szél A., Röhlich P., Mieziewska K., Aguirre G., van Veen T. Spatial and temporal differences between the expression of short- and middle-wave sensitive cone pigments in the mouse retina: a developmental study. J Comp Neurol. 1993 May 22;331(4):564–577. doi: 10.1002/cne.903310411. [DOI] [PubMed] [Google Scholar]
- Turner D. L., Snyder E. Y., Cepko C. L. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990 Jun;4(6):833–845. doi: 10.1016/0896-6273(90)90136-4. [DOI] [PubMed] [Google Scholar]
- Vollrath D., Nathans J., Davis R. W. Tandem array of human visual pigment genes at Xq28. Science. 1988 Jun 17;240(4859):1669–1672. doi: 10.1126/science.2837827. [DOI] [PubMed] [Google Scholar]
- Wang Y., Macke J. P., Merbs S. L., Zack D. J., Klaunberg B., Bennett J., Gearhart J., Nathans J. A locus control region adjacent to the human red and green visual pigment genes. Neuron. 1992 Sep;9(3):429–440. doi: 10.1016/0896-6273(92)90181-c. [DOI] [PubMed] [Google Scholar]
- Wikler K. C., Rakic P. Relation of an array of early-differentiating cones to the photoreceptor mosaic in the primate retina. Nature. 1991 May 30;351(6325):397–400. doi: 10.1038/351397a0. [DOI] [PubMed] [Google Scholar]