Abstract
Dried leaves of Artemisia annua show promise as an inexpensive and sustainable antimalarial therapeutic, especially for use in developing countries. Along with the potent terpene, artemisinin, many other small molecules produced by the plant seem to aid in the therapeutic response. However, little is known about the ontogenic and phenological production of artemisinin in the plant, and its plethora of other important secondary metabolites. From a consistently high artemisinin-producing A. annua clone (SAM) we extracted and analyzed by GC/MS 22 different metabolites including terpenes, flavonoids, a coumarin, and two phenolic acids as they varied during leaf development and growth of the plant from the vegetative stage through the reproductive, full flower stage. As leaves developed, the maximum amount of most metabolites was in the shoot apical meristem. Artemisinin, on the other hand, maximized once leaves matured. Leaf and apical tissues (e.g. buds, flowers) varied in their metabolite content with growth stage with maximum artemisinin and other important secondary metabolites determined to be at floral bud emergence. These results indicated that plants at the floral bud stage have the highest level of artemisinin and other therapeutic compounds for the treatment of malaria.
Keywords: malaria, artemisinin, flavonoids, monoterpenes, phenolic acids, scopoletin
1.0 Introduction
Worldwide more than three billion people are at risk of getting malaria with nearly a million deaths in 2010, most of which were African children (WHO 2012). The current preferred antimalarial therapeutic is artemisinin (Fig. 1), a sesquiterpene lactone produced by the plant Artemisia annua L. (Asteraceae).
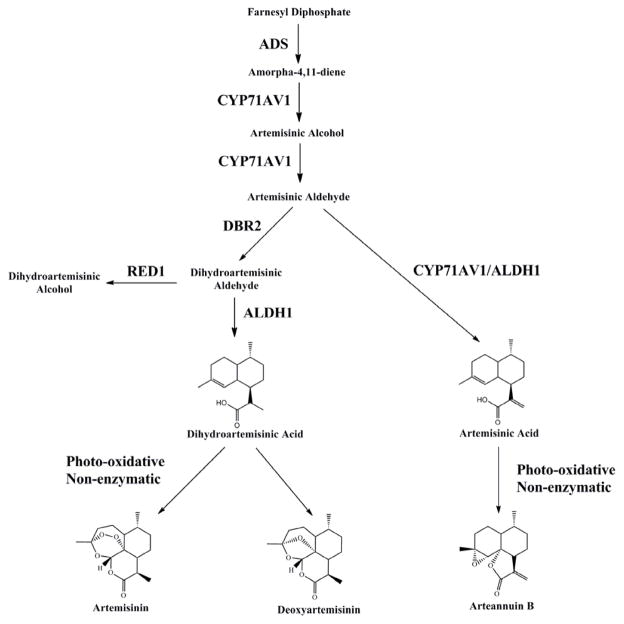
Figure 1.
Simplified artemisinic biosynthetic pathway. ADS, amorphadiene synthase; ALDH1, aldehyde dehydrogenase; CYP71AV1, cytochrome P450 monooxygenase; DBR2, double-bond reductase 2; RED1, dihydroartemisinic aldehyde reductase.
Recently there has been interest in the use of more plant-based delivery of artemisinin consumed either as a tea infusion (Mueller et al. 2004; Räth et al. 2004; Suberu et al. 2013) or via ingestion of dried leaves of the plant (Elfawal et al. 2012, 2014; ICIPE 2005; Onimus et al. 2013; Weathers et al. 2011). Evidence is building to suggest these approaches to malaria therapy are not only highly cost effective, but more importantly, efficacious as a plant-based combination therapy (pACT; see recent reviews by van der Kooy and Sullivan 2013 and Weathers et al. 2014a,b). Indeed, within A. annua a plethora of compounds (Bhakuni et al. 2001; Brown 2010; Ferreira et al. 2010) may themselves act as weak antimalarials (Elford et al. 1987; Lehane and Saliba 2008; Liu et al. 1992; Suberu et al. 2013; van Zyl et al. 2006), and may improve artemisinin bioavailability (Weathers et al. 2014c) or its activity (Liu et al. 1992; Suberu et al. 2013; also see reviews by Weathers et al. 2014a, b). These compounds include flavonoids, monoterpenes, and other phenolics. Many of these compounds change with drying and other post-harvest processing of the plant material (Weathers and Towler 2014). Although much is known about where and when during the development of the plant artemisinin is produced, less is known about these other compounds. If dried leaf A. annua is to be used therapeutically, then it is important to know how these compounds change relative to artemisinin production and when and where they are produced in order to determine a preferred harvest time.
Some prior studies showed how some of the metabolites in A. annua vary during development. For example, Graham et al. (2010) measured artemisinin in leaves as they matured down the stem and found that at about node 10–11, the concentration in the leaves maximized and thereafter remained constant. Developmentally, maximum artemisinin production is usually observed at floral bud formation, but before full flowering (Ferreira 2008; Ferreira and Janick 1996; Ferreira et al. 1995; Ma et al. 2008; Nair et al. 2013; Singh et al. 1988; Woerdenbag et al. 1994). Even senesced leaves contain substantial amounts of artemisinin (Lommen et al. 2006; 2007).
A. annua can produce at least 40 different flavonoids, but not necessarily the same ones in every cultivar or line (Ferreira et al. 2010). Baraldi et al. (2008) showed that although total flavonoid production paralleled that of artemisinin at three growth stages, a few specifically tracked flavonoids did not. Namely, artemisinin was highest in leaves and flowers during full bloom; the eupatin flavonoid was greatest in flowers at full flower and post-flower, but in leaves it peaked post flowering. Recently, we showed that flavonoids in shoot apical meristematic tissues were about fourfold that in mature leaves (Weathers and Towler 2014).
A. annua is also rich in essential oil, including a variety of mono- and sesquiterpenes (Bhakuni et al. 2001), but content varies significantly with cultivar (Reale et al. 2011) and also with developmental stage (Rana et al. 2013; Yang et al. 2012). These compounds seem to be associated with the glandular trichomes (GLTs) because the glandless mutant of A. annua produces barely detectable amounts of oils and artemisinin (Tellez et al. 1999). Artemisinin is produced and stored in the GLTs, which are located mainly on the leaves and floral buds of the plant. Flavonoid production also seems to be associated with the GLTs. Both Maes et al. (2010) and Wang et al. (2009) showed that key genes from the artemisinin, terpenoid, and flavonoid pathways were expressed in isolated GLTs and upregulated after jasmonic acid elicitation.
Use of a high artemisinin-producing clonal cultivar of A. annua would be particularly useful in analyzing changes in this wide variety of compounds in the plant throughout its development. Such information will inform decisions regarding harvest for maximum therapeutic efficacy. Here we used a consistent high artemisinin-producing (~1.4% w/w) A. annua clone (SAM; Weathers and Towler 2012) that has also been used in our recent animal studies that included orally delivered dried leaves as antiparasitic proof-of-concept (Elfawal et al. 2012), resilience to emergence of artemisinin drug resistance (Elfawal et al. 2014), and pharmacokinetics (Weathers et al. 2014a). This study will further aid in correlating animal-pathogen responses with the phytochemistry of the plant. Five key artemisinic metabolites in the artemisinin biosynthetic pathway were studied as they varied during leaf development and growth of the plant from the vegetative stage through the reproductive, full flower stage. Also compared were changes in five other terpenes (including four monoterpenes and a nonartemisinic sesquiterpene), nine flavonoids, a coumarin, and two phenolic acids. Most of the measured compounds are reported to have weak antimalarial activity; see summary Table 1 in Weathers and Towler (2014).
2.0 Materials and Methods
2.1 Plant material and cultivation conditions
An isolated clonal line of Artemisia annua L. (SAM; Weathers and Towler 2012) propagated by cuttings was used in the study. SAM (voucher MASS 00317314) originated from a nonclonal Chinese variety stemming from the seeds of an F2 generation of line PEG01 received from and identified by Chunzhao Liu (Chinese Academy of Science, Beijing, China). Plants were rooted and grown in Metro-Mix® 360 and fertilized weekly with Miracle-Gro all-purpose plant food (NPK: 24-8-16). All plants were kept in a Percival incubator at 25°C and a 16-hr (vegetative) or 8-hr (flowering) photo period under Philips F32T8/TL735 ALTO II fluorescent bulbs; light intensity ranged from 100–300 μmol m−2sec−1 depending on height of plants and proximity to lights. Budding occurred approximately two weeks after photo period shift, and full flowering two weeks after that. For this work, the shoot apical meristem area (ShAM) was defined as those leaves surrounding the terminal bud that were not fully expanded and flattened (Figure 2). Flower buds or flowers, if present, were removed from terminal shoot and axillary shoots in the upper 15 cm of each plant. Mature leaves in flowering plants were taken as the first 4 leaves located 15 cm below the apex. See Figure 2 for photos of this A. annua line at various developmental stages. There were 5 to 8 replicates for each condition. Immediately after excision and weighing, plant tissues were extracted as subsequently described.
Figure 2.
Photos of main developmental stages of A. annua (SAM clonal line) and timeline definition of various sampling stages. Top: close-up view of shoot apical meristem region (ShAM, oval) and leaves 1–3 of A. annua as defined for this study.
2.2 Harvest and extraction of metabolites
Metabolites were extracted from the selected leaves, buds, or flowers by adding MeCl2 to the tissue in glass test tubes and sonicating in a water bath for 30 min. Biomass:solvent ratios ranged from 2.5 to 150 mg fresh weight (FW) per mL depending on tissue weight, which is below the 100 mg dry weight (DW) per mL ratio (equivalent to 400 g FW per mL) proposed by Malwade et al. (2013). Solvent was decanted and dried under N2, then stored at −20°C until analysis. Aliquots were later transferred to GC/MS sample vials and dried. For chlorogenic and rosmarinic acids, tissue was extracted as above but with MeOH. Stems were extracted in a similar manner, but after being dried at room temperature and pulverized in a commercial coffee grinder.
2.3 Metabolite analysis
Artemisinin (AN), deoxyartemisinin (deoxyAN), arteannuin B (AB), artemisinic acid (AA), dihydroartemisinic acid (DHAA), artemisia ketone, nerolidol, scopoletin, α-pinene, eucalyptol, camphor, artemetin, casticin, chrysoplenetin, chrysoplenol-D, eupatorin, kaempferol, luteolin, myrcetin, quercetin, chlorogenic and rosmarinic acids were all measured using GC/MS and extraction and analytical methods are detailed in Weathers and Towler (2014). Briefly the system included: GC, Agilent 7890A; MS, Agilent 5975C; column, Agilent HP-5MS (30 m × 0.25 mm × 0.25 μm) and He carrier gas at 1 mL/min. All compounds were identified and quantified using validated standards and/or NIST library. Authentic standards of artemisinin, camphor, chlorogenic acid, eucalyptol, luteolin, trans-nerolidol, α-pinene, rosmarinic acid, and scopoletin were obtained from Sigma-Aldrich (St. Louis, MO, USA); deoxyartemisinin was sourced from Toronto Research Chemicals Inc. (Toronto, Canada); artemetin, casticin, eupatorin, quercetin were from ChromaDex (Irvine, CA, USA); chrysoplenol-D and chrysoplenetin were from ChemFaces (Wuhan, PRC); artemisinic acid and arteannuin B were gifts of Dr. Nancy Acton from Walter Reed Army Institute of Research; artemisia ketone was identified via NIST library. dihydroartemisinic acid was quantified as artemisinic acid equivalents and measured according to Mannan et al. (2010).
Total flavonoids were quantified using the AlCl3 method of Arvouet-Grand et al. (1994) and based on quercetin as the standard; results are expressed as quercetin equivalents.
2.4 Statistical analysis
All samples were analyzed at least in triplicate and statistically compared for significance using Student’s t-test.
3.0 Results
In this study we tracked 22 metabolites in real time in a clonal cultivar of A. annua that produces 1.4% DW artemisinin (Weathers and Towler 2014). Except for stems (Section 3.3) all results are expressed on a FW basis; DW comparisons can be calculated using the DW/FW ratio of 0.25.
3.1 Changes with leaf maturity
3.1.1 Variations in artemisinin and other artemisininic metabolites
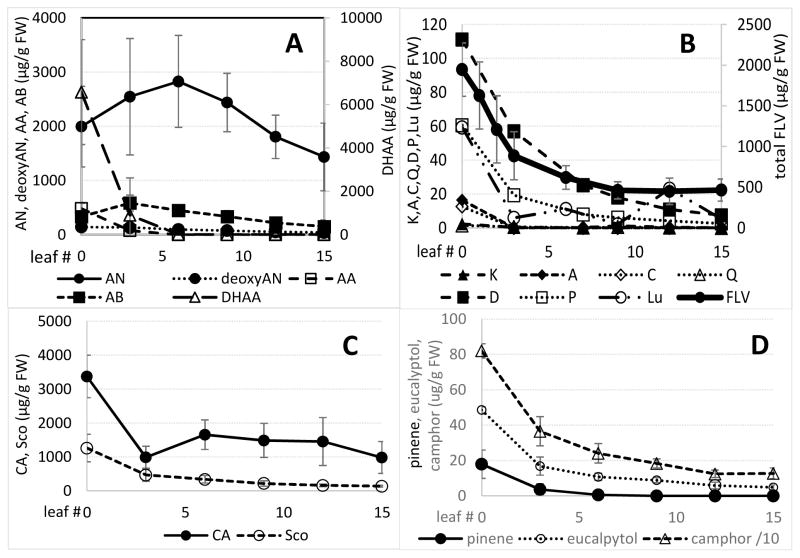
Although not statistically different from either younger (ShAM) or older more mature leaves, artemisinin reached its peak level at about leaf six in this cultivar declining somewhat as leaves matured to leaf 15 (Fig. 3). For the other key artemisinic metabolites, to our knowledge there are no reports on changes in either artemisinic metabolites or other compounds in A. annua leaves during their maturation. Of the artemisinic metabolites measured, dihydroartemisinic acid, the precursor to artemisinin (Fig. 1), was highest in the ShAM by more than threefold that of artemisinin, declining by >90% by leaf five, correlating slightly with the observed rise in artemisinin (Fig. 3). In the ShAM, artemisinic acid, arteannuin B, and deoxyartemisinin were present at <25% that of artemisinin and also declined with leaf maturity.
Figure 3.
Changes in targeted metabolites as leaves develop from the shoot apical meristem leaf #0) through leaf 15. Error bars ± SD. A - artemisinic compounds: AN, artemisinin, AA, artemisinic acid, deoxyAN, deoxyartemisinin, DHAA, dihydrodartemisinic acid, AB, arteannuin B. B -flavonoids: K, kaempferol, A, artemetin, C, casticin, Q, quercetin, D, chrysoplenol-D, P, chrysoplenetin, Lu, luteolin, FLV, total flavonoids (error bars on individual flavonoids omitted for clarity). C - CA, chlorogenic acid, Sco, scopoletin. D - monoterpenes.
3.1.2 Variations in nonartemisinic metabolites
All other non-artemisinic metabolites measured in this study in developing vegetative leaves were highest in the ShAM, but declined as leaves matured (Fig. 3). Although present in reproductive buds and leaves of only a few plants, rosmarinic acid was undetectable in vegetative leaves.
3.2 Changes with shift from vegetative to reproductive growth
3.2.1 Variations in artemisinin and other artemisininic metabolites
As plants shifted into reproductive growth, leaves always had the highest level of artemisinin versus ShAM-related tissues, e.g. bolting ShAM, floral buds, and flowers, with maximum amounts in leaves during budding (BL) stage (Table 1). Conversely, the ShAM-related tissues all had dihydroartemisinic acid levels that exceeded that in the mature leaves, which often had undetectable amounts (Table 1). dihydroartemisinic acid levels declined substantially as plants entered the reproduction stage, artemisinic acid was usually found at low levels, but nearly always higher in apical vs. mature leaf tissues (Table 1). Deoxyartemisinin, an undesirable byproduct post-dihydroartemisinic acid (Fig. 1), remained low throughout all of the measured growth stages in SAM (Table 1).
Table 1.
Artemisinic compounds in Artemisia annua at each developmental stage. (μg g−1 FW)
| Compound | Vegetative stage | Reproductive stages | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bolting | Budding | Full flowering | Post flowering | |||||||
| ShAM | VL | ShAM | BL | Buds | BuL | Flowers | FL | Flowers | PFL | |
| Artemisinin | 2047.2 a | 2845.1 w | 1198.7 b | 2338.0 xyz | 206.1 c | 3031.2 w | 99.8 d | 2307.8 y | 184.3 ad | 2338.7 wz |
| Dihydroartemisinic acid | 10052.6 a | nd | 1655.2 b | nd | 121.5 c | nd | 69.9 d | 28.8 x | 31.9 e | 2.1 x |
| Deoxyartemisinin | 246.1 a | 215.1 x | 167.5 b | 216.2 xz | 29.4 c | 120.0 y | 12.82 d | 149.1 yz | 24.4 ce | 128.1 yz |
| Artemisinic acid | 552.2 a | nd | 178.9 b | nd | 15.1 c | nd | 8.8 cd | 3.9 x | 2.6 e | 3.8 x |
| Arteannuin B | nd | 24.2 w | 132.8 a | 386.8 xy | nd | 303.4 x | 6.3 b | 208.9 xz | 22.2 c | 209.9 xz |
ShAM, shoot apical meristem region; VL, mature vegetative leaves (1st four leaves located 15 cm below apex in all cases); BL, mature leaves during bolting stage; Buds, unopened floral buds from top 15 cm of plant; BuL, mature leaves during floral budding stage; Flowers, flowers from top 15 cm of plant; FL, mature leaves during full flower; PFL, mature leaves 2 weeks post flowering; nd, not detectable; letters a,b,c,d,e indicate statistical significance among shoot apical meristems and reproductive structures; letters w,x,y,z indicate statistical significance among mature leaves.
3.2.2 Variations in flavonoids
Total flavonoid content of tissues was maximum in vegetative ShAMs (Table 2). In mature leaves, the maximum amount was during the bolting, budding, and full-flowering stages. Of the specific flavonoids measured in this study, only chrysoplenetin and chrysoplenol-D were regularly detected; artemetin, casticin, eupatorin, kaempferol, luteolin, myrcetin, and quercetin were at best barely detected in the analyzed tissues (Table 2). The sum of individually detected flavonoids represented at most 30% of total flavonoids measured using the AlCl3 method (Table 2). Only those flavonoids reported to have antimalarial activity with commercially available standards were analyzed in this study, so others as yet unidentified in this cultivar likely comprise the remainder. As plants shifted into reproduction (bolting), flavonoid levels in apical tissues (bolting ShAM, buds, flowers) declined and then stabilized. Once budding was reached, amounts in leaves were greater (Table 2).
Table 2.
Flavonoids and coumarins in Artemisia annua at each developmental stage. (μg g−1 FW)
| Compound | Vegetative stage | Reproductive stage | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bolting | Budding | Full flowering | Post flowering | |||||||
| ShAM | VL | ShAM | BL | Buds | BuL | Flowers | FL | Flowers | PFL | |
| Total flavonoids (AlCl3 assay) | 1514.7 a | 388.6 w | 978.3 c | 537.0 xz | 351.8 b | 567.7 xy | 290.4 b | 627.6 x | 394.9 b | 429.9 wyz |
| Artemetin | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Casticin | 2.7a | nd | nd | nd | 1.0a | 2.9x | nd | 5.1x | nd | nd |
| Chrysoplenetin | 77.5a | 20.9x | 6.1b | 19.6x | 12.4c | 22.3x | 11.9c | 41.7xy | 9.2c | 43.9y |
| Chrysoplenol-D | 175.9a | 52.8x | 15.2bd | 39.1xy | 31.3c | 50.3x | 35.7c | 79.2xz | 24.1cd | 82.4xz |
| Eupatorin | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Kaempferol | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Luteolin | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Myrcetin | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Quercetin | nd | nd | 6.9a | nd | 0.1a | 1.2x | nd | nd | nd | 0.6x |
| Sum as % of total flavonoids | 17 | 19 | 3 | 11 | 13 | 13 | 16 | 20 | 8 | 30 |
ShAM, shoot apical meristem region; VL, mature vegetative leaves (1st four leaves located 15 cm below apex in all cases); BL, mature leaves during bolting stage; Buds, unopened floral buds from top 15 cm of plant; BuL, mature leaves during floral budding stage; Flowers, flowers from top 15 cm of plant; FL, mature leaves during full flower; PFL, mature leaves 2 weeks post flowering; nd, not detectable; letters a,b,c,d,e indicate statistical significance among shoot apical meristems and reproductive structures; letters x,y,z indicate statistical significance among mature leaves.
3.2.3 Variations in monoterpenes
Artemisinin is the main therapeutic compound in the plant, so when the initial measurements of artemisinin in the study showed maximum levels in vegetative (VL) and floral budding leaves (BuL), additional plants were grown such that monoterpenes, phenolic acids, and scopoletin were measured only in those two stages. The sum of the measured monoterpenes remained relatively constant between vegetative and budding stages, but was significantly greater in apical tissues (ShAM and buds) than in leaves (Table 3). Camphor represented the majority of the three measured compounds; α-pinene was only detected in leaves and apical tissues after plants shifted into reproductive phase (Table 3). Eucalyptol (1,8-cineole) increased in buds versus the vegetative ShAM but decreased as those young leaves matured. Neither nerolidol nor artemisia ketone were detectable in our cultivar.
Table 3.
Other terpenes in Artemisia annua at vegetative and budding stages. (μg g−1 FW)
| Compound | Vegetative stage | Reproductive stage Budding | ||
|---|---|---|---|---|
| ShAM | VL | Buds | BuL | |
| α-pinene | nd | nd | 204.5 | 97.5 |
| 1,8-cineole | 275.4a | 75.4x | 989.5b | 28.7y |
| Camphor | 2530.3a | 892.5x | 1712.5b | 901.1x |
| Artemisia ketone | nd | nd | nd | nd |
| Nerolidol | nd | nd | nd | nd |
ShAM, shoot apical meristem region; VL, mature vegetative leaves (1st four leaves located 15 cm below apex); Buds, unopened floral buds; BuL, mature leaves during floral budding stage; nd, not detectable; letters a,b indicate statistical significance between shoot apical meristems and reproductive structures; letters x,y indicate statistical significance between two stages of mature leaves.
3.2.4 Variations in the coumarin, scopoletin
When the coumarin scopoletin was measured, it was highest in vegetative ShAMs, but declined significantly in floral buds (Table 4). Although low in vegetative leaves, scopoletin increased in mature leaves during the floral budding phase, correlating with the apical tissue decrease.
Table 4.
Scopoletin and chlorogenic and rosmarinic acids in Artemisia annua at vegetative and budding stages. (μg g−1 FW)
| Compound | Vegetative stage | Reproductive stage Budding | ||
|---|---|---|---|---|
| ShAM | VL | Buds | BuL | |
| Scopoletin | 1379.0a | 329.9x | 33.4b | 625.7y |
| Chlorogenic acid | 2709.9a | 1618.3x | 239.8b | 516.0y |
| Rosmarinic acid | nd | nd | 491.9# | 486.5# |
ShAM, shoot apical meristem region; VL, mature vegetative leaves (1st four leaves located 15 cm below apex); Buds, unopened floral buds; BuL, mature leaves during floral budding stage; nd, not detectable; #, 2 of 6 replicates had rosmarinic acid; letters a,b indicate statistical significance among shoot apical meristems and reproductive structures; letters x,y indicate statistical significance among mature leaves.
3.2.5 Variations in phenolic acids
Similar to observations for flavonoids and camphor, chlorogenic acid concentration was maximum in vegetative ShAMs, then declined >10-fold with floral bud formation (Table 4). Vegetative leaves also contained high levels that dropped significantly when plants entered floral budding. As was observed for α-pinene, no rosmarinic acid was detected in vegetative leaves, but it appeared when plants entered the reproductive phase. Levels were comparable in leaves and buds.
3.3 Stem metabolite content
Usually stem tissue is discarded, but since it can comprise such a large amount of the harvested biomass of A. annua, its metabolite content was also measured. Stem tissue of the SAM cv contained only five of the 22 different compounds measured in this study. Five metabolites were present in dried powdered main stems from vegetative plants in the following amounts (μg g−1 DW, with μg g−1 FW in parentheses estimated from a stem DW/FW ratio of 0.28): artemisinin, 995.51 ± 280.59 (278.74 ± 78.57); deoxyartemisinin, 33.87 ± 11.67 (9.48 ± 3.27); camphor, 1,882.98 ± 300.91 (527.23 ± 84.25); chlorogenic acid, 103.63 ± 16.40 (29.02 ± 4.59); scopoletin, 30.66 ± 6.60 μg g−1 DW (8.58 ± 1.84). Total flavonoids were measured at 406.85 ± 68.12 μg g−1 DW (113.92 ± 19.07 μg g−1 FW). Because the amounts were considerably less than that found in leaves, stems were not measured at all growth stages or at different developmental stages of shoots.
4.0 Discussion
Yadav et al. (2013) reported artemisinin results similar to ours for two cultivars, with artemisinin significantly greater by about 10% in the leaves of the upper third of the plant than those in the lower two thirds. In contrast, Graham et al. (2010) showed that artemisinin content in leaves increased by more than fourfold from node 4 to node 16 and was constant from node 11–23. It seems these inconsistencies in artemisinin content with leaf maturation may be cultivar specific. Indeed, A. annua has two main chemotypes with one producing mainly artemisinin and the other producing mainly artemisinic acid, so such discrepancies are reasonable (Wallaart et al. 2000; Wu et al. 2011).
A response in artemisinin production similar to our results was observed by Ma et al. (2008) in the 001 wild type of A. annua and its F4 transgenic strain that overexpressed farnesyl diphosphate synthase. Dihydroartemisinic acid levels declined substantially as our plants entered the reproduction stage as they also did for both the F4 transgenic line and strain 001. Arteannuin B, always low overall, was also higher in leaf versus apical tissues, but in the F4 strain it peaked during floral budding while the 001 strain remained consistently low (Ma et al. 2008). Similar to our SAM clonal line, Ma et al. (2008) found artemisinic acid levels in both the 001 and F4 strains declined as plants transitioned through the stages of flowering.
In a prior study, Baraldi et al. (2008) measured the flavonoids, eupatin, artemetin, and casticin/chrysoplenetin; the latter two were inseparable in their detection system. Those flavonoids along with artemisinin were measured in leaves and inflorescences of a nonclonal, unidentified Italian cultivar at three floral stages. Total flavonoids peaked during full flowering, in parallel with artemisinin. With a few exceptions, however, leaf and inflorescence content was similar at most stages. In contrast, our results showed fourfold greater total flavonoids in ShAMs than in mature leaves during vegetative growth. High levels of these reportedly synergistic compounds (Liu et al. 1992; Suberu et al. 2013) could be exploited in pruning-harvest scenarios. If tips were harvested mid-season, plants should grow bushier yielding even more flavonoid-rich tips. Furthermore, the ratio of many of the nonartemisinic compounds, e.g. flavonoids, monoterpenes and chlorogenic acid, to artemisinin increase two-fourfold in tips vs. leaves, so adding extra tips could potentially improve the therapeutic impact of that material. Comparative studies in animals and in vitro vs. P. falciparum would be needed to validate this hypothesis.
Similar to a trend also seen by Bagchi et al. (2003), 1,8-cineole increased in buds versus the vegetative ShAM but decreased as those young leaves matured. In contrast to those results, Rana et al. (2013) observed increases in 1,8-cineole when plants shifted from vegetative to reproductive growth. Although vegetative tissues were not specifically measured, as plants progressed through full flowering to post bloom, Yang et al. (2012) observed declines in these two monoterpenes in the reproductive tissues of cultivar Wuling-3938. In our study as plants developed from the vegetative to the reproductive stage, camphor remained constant in leaves, but decreased in the ShAMs. On the other hand, while camphor overall remained rather constant (Rana et al. 2013; Yang et al. 2012), Bagchi et al. (2003) saw an increase in leaf camphor content at the budding stage. Together these studies suggested cultivar differences.
Scopoletin is produced in leaves of Spilanthes acmella and other plants after elicitation (Singh and Chaturvedi 2010) and in leaves of tobacco after cytokinin treatment (Großkinsky et al. 2011). More recently Xia et al. (2014) observed that scopoletin increased in tobacco leaves as plants matured, results similar to this study. However, to our knowledge there are no comparative reports of developmental or phenological variations in scopoletin in A. annua.
Chlorogenic and rosmarinic acids are phenolic acids that also have some weak antiplasmodial activity (Suberu et al. 2013) and also inhibit CYP3A4, an artemisinin degrading enzyme in the liver (Svensson and Ashton 1999). These phenolic acids are present in A. annua tea infusions (de Magalhães et al. 2012; Suberu et al. 2013), but to our knowledge, there are no comparative reports of phenolic acid variations in A. annua. Appearance of both rosmarinic acid and α-pinene during flowering suggested their possible importance in defense or attraction.
Depending on the age of harvested plants and cultivar type, stem tissue in A. annua can comprise up to ~90% of the shoot dry weight in late flowering plants (Gupta et al. 2002). During the vegetative stage, however, stem dry mass is considerably less. For example, stem tissue in the SAM cv used in this study was about 35% of total shoot mass, and for cv Jeevanraksha was about 68% (Gupta et al. 2002).
Nevertheless, others have reported similar low levels of artemisinin in the main stems of A. annua with fine branch stems containing about twice the artemisinin of the main stem (Ferreira et al. 1995; Gupta et al. 2002; Yadav et al. 2013). Li et al. (2011) also measured a large variety of essential oils in the main stems with caryophyllene oxide, methyl cinnamate, and β-guaiene the top 3 compounds; they also found 1,8-cineole, nerolidol, and camphor, three compounds also tracked in this study.
5.0 Conclusions
To develop a consistent and effective A. annua dried leaf therapeutic, biomass has to be harvested when there is a reasonably consistent amount of active phytochemicals. There are significant changes in artemisinic and other metabolites as A. annua leaves develop during vegetative growth and the reproductive stages of the plant. The peak of artemisinin production is during floral budding when the other therapeutically active phytochemicals are also produced in reasonable amounts. Flavonoids, already reported to synergize with artemisinin are especially high in the growing shoot tips so periodic pruning of tips that are dried and added to may prove useful in enhancing the overall flavonoid content of this potential dried leaf therapeutic. While leaf tissue is overwhelmingly preferred for therapeutic use, there is about 0.1% artemisinin in the stem, so stems offer some therapeutic value. These results suggest that for production of a plant-based combination therapy using this cultivar, A. annua should be harvested during floral budding to provide maximum artemisinin with reasonable amounts of other important secondary metabolites for use in treating malaria and other artemisinin or A. annua-susceptible diseases.
Acknowledgments
The authors thank Andrew Butler of WPI and Darwin Reed, Canadian Research Council, for advice on GC/MS. We are also grateful to Worcester Polytechnic Institute and University of Massachusetts Center for Clinical and Translational Science (CCTS) for partially funding this project. We are also grateful for Award Number NIH-R15AT008277-01 from the National Center for Complementary and Alternative Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary and Alternative Medicine or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arvouet-Grand A, Vennat B, Pourrat A, Legret P. Standardization of a propolis extract and identification of the main constituents. J de Pharma de Belg. 1994;49:462–468. [PubMed] [Google Scholar]
- Bagchi GD, Haider F, Dwivedi PD, Singh A, Naqvi AA. Essential oil constituents of Artemisia annua during different growth periods at monsoon conditions of subtropical north indian plains. J Essent Oil Res. 2003;15:248–250. doi: 10.1080/10412905.2003.9712131. [DOI] [Google Scholar]
- Baraldi R, Isacchi B, Predieri S, Marconi G, Vincieri FF, Bilia AR. Distribution of artemisinin and bioactive flavonoids from Artemisia annua L. during plant growth. Biochem Syst Ecol. 2008;36:340–348. doi: 10.1016/j.bse.2007.11.002. [DOI] [Google Scholar]
- Bhakuni RS, Jain DC, Sharma RP, Kumar S. Secondary metabolites of Artemisia annua and their biological activity. Curr Sci. 2001;80:35–48. [Google Scholar]
- Brown GD. The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao) Molecules. 2010;15:7603–7698. doi: 10.3390/molecules15117603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhães PM, Dupont I, Hendrickx A, Joly A, Raas T, Dessy S, Sergent T, Schneider Y-J. Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal Caco-2 cells. Food Chem. 2012;134:864–871. doi: 10.1016/j.foodchem.2012.02.195. [DOI] [PubMed] [Google Scholar]
- Elfawal MA, Towler MJ, Reich NG, Golenbock D, Weathers PJ, Rich SM. Dried whole plant Artemisia annua as an antimalarial therapy. PLOS ONE. 2012;7:e52746. doi: 10.1371/journal.pone.0052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfawal MA, Towler MJ, Reich NG, Weathers PJ, Rich SM. Dried whole plant Artemisia annua may slow evolution of malaria drug resistance and overcome resistance to artemisinin. Proc Natl Acad Sci USA. 2014 doi: 10.1073/pnas.1413127112. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elford BC, Roberts MF, Phillipson D, Wilson RJM. Potentiation of the antimalarial activity of qinghaosu by methoxylated flavones. Trans Roy Soc Trop Med Hyg. 1987;81:434–436. doi: 10.1016/0035-9203(87)90161-1. [DOI] [PubMed] [Google Scholar]
- Ferreira FS, Simon JE, Janick J. Relationship of artemisinin content of tissue cultured, greenhouse and field grown plants of Artemisia anuua. Planta Med. 1995;61:351–355. doi: 10.1055/s-2006-958098. [DOI] [PubMed] [Google Scholar]
- Ferreira FS, Janick J. Distribution of artemisinin in Artemisia annua. In: Janick J, editor. Progress in new crops. ASHA Press; Arlington VA: 1996. pp. 579–584. [Google Scholar]
- Ferreira JFS. Seasonal and post-harvest accumulation of artemisinin, artemisinic acid, and dihydroartemisinic acid in three accessions of Artemisia annua cultivated in West Virginia, USA. Planta Med. 2008;74:310–311. [Google Scholar]
- Ferreira JFS, Luthria DL, Sasaki D, Heyerick A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 2010;15:3135–3170. doi: 10.3390/molecules15053135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham IA, Besser K, Blumer S, Branigan CA, Czechowski T, Elias L, Guterman I, Harvey D, Isaac PG, Khan AM, Larson TR, Li Y, Pawson T, Penfield T, Rae AM, Tathbone DA, Reid S, Ross J, Smallwood MF, Segura V, Townsend T, Vyas D, Winzer T, Bowles D. The genetic map of Artemisia annua L. identifies loci affecting yield of the antimalarial drug artemisinin. Science. 2010;327:328–331. doi: 10.1126/science.1182612. [DOI] [PubMed] [Google Scholar]
- Großkinsky DK, Naseem M, Abdelmohsen UR, Plickert N, Engelke T, Griebel T, Zeier J, Novák O, Strnad M, Pfeifhofer H, van der Graaff E, Simon U, Roitsch T. Cytokinins mediate resistance against Pseudomonas syringae in tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling. Plant Physiol. 2011;157:815–830. doi: 10.1104/pp.111.182931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Singh P, Bajpai P, Ram G, Singh D, Gupta MM, Jain DC, Khanuja SP, Kumar S. Morphogenetic variation for artemisinin and volatile oil in Artemisia annua. Indus Crops Prod. 2002;16:217–224. doi: 10.1016/S0926-6690(02)00049-3. [DOI] [Google Scholar]
- ICIPE. Whole-leaf Artemisia annua-based antimalarial drug: report on proof-of-concepts studies, unpublished report. 2005 Retrieved on December 9, 2014 from : http://bit.ly/1vCHQkH.
- Lehane AM, Saliba KJ. Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Res Notes. 2008;1:26. doi: 10.1186/1756-0500-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hu H, Zheng X, Zhu J, Liu L. Composition and antimicrobial activity of essential oil from the aerial part of Artemisia annua. J Medicin Plants Res. 2011;5:3629–3633. [Google Scholar]
- Liu KCS, Yang SL, Roberts ME, Elford BC, Phillipson JD. Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant Cell Rep. 1992;11:637–640. doi: 10.1007/BF00236389. [DOI] [PubMed] [Google Scholar]
- Lommen WJM, Schenk E, Bouwmeester HJ, Verstappen FWA. Trichome dynamics and artemisinin accumulation during development and senescence of leaves of Artemisia annua leaves. Planta Med. 2006;72:336–345. doi: 10.1055/s-2005-916202. [DOI] [PubMed] [Google Scholar]
- Lommen WJM, Elzing S, Verstappen FWA, Bouwmeester HJ. Artemisinin and sesquiterpene precursors in dead and green leaves of Artemisia annua L. crops. Planta Med. 2007;73:1133–1139. doi: 10.1055/s-2007-981567. [DOI] [PubMed] [Google Scholar]
- Ma C, Wang H, Lu X, Xu C, Liu B. Metabolic fingerprinting investigation of Artemisia annua L. in different stages of development by gas chromatography and gas chromatography-mass spectrometry. J Chromatog A. 2008;1186:412–419. doi: 10.1016/j.chroma.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Maes L, Van Nieuwerburgh FCW, Zhang Y, Reed DW, Pollier J, Vande Casteele SRF, Covello PS, Deforce DLD, Goossens A. A dissection of the phytohormonal regulation of trichome formation and biosynthesis of the antimalarial compound artemisinin in Artemisia annua plants. New Phytol. 2010;189:176–189. doi: 10.1111/j.1469-8137.2010.03466.x. [DOI] [PubMed] [Google Scholar]
- Malwade CR, Qu H, Rong BG, Christensen LP. Conceptual process synthesis for recovery of natural products from plants: a case study of artemisinin from Artemisia annua. Ind Eng Chem Res. 2013;52:7157–7169. doi: 10.1021/ie302495w. [DOI] [Google Scholar]
- Mannan A, Liu CZ, Arsenault PR, Towler MJ, Vail DR, Lorence A, Weathers PJ. DMSO triggers the generation of ROS leading to an increase in artemisinin and dihydroartemisinic acid in Artemisia annua shoot cultures. Plant Cell Rep. 2010;29:143–152. doi: 10.1007/s00299-009-0807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MS, Runyambo N, Wagner I, Borrmann S, Dietz K, Heide L. Randomized controlled trial of a traditional preparation of Artemisia annua L. (Annual Wormwood) in the treatment of malaria. Trans R Soc Trop Med Hyg. 2004;98:318–321. doi: 10.1016/j.trstmh.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Nair P, Misra A, Singh A, Shukla AK, Gupta MM, Gupta AK, Gupta V, Khanuja SPS, Shasany AK. Differentially expressed genes during contrasting growth stages of Artemisia annua for artemisinin content. PLOS ONE. 2013;8:e60375. doi: 10.1371/journal.pone.0060375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimus M, Carteron S, Lutgen P. The surprising efficiency of Artemisia annua powder capsules. Medicinal Aromatic Plants. 2013;2:3. doi: 10.4172/2167-0412.1000125. [DOI] [Google Scholar]
- Rana VS, Abirami K, Blázquez MA, Maiti S. Essential oil composition of Artemisia annua L at different growth stages. J Spices Aromatic Crop. 2013;22:181–187. [Google Scholar]
- Räth K, Taxis K, Walz G, Gleiter CH, Li SM, Heide L. Pharmacokinetic study of artemisinin after oral intake of a traditional preparation of Artemisia annua L. (annual wormwood) Am J Trop Med Hyg. 2004;70:128–132. [PubMed] [Google Scholar]
- Reale S, Fasciani P, Pace L, De Angelis F, Marcozzi G. Volatile fingerprints of artemisinin-rich Artemisia annua cultivars by headspace solid-phase microextraction gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2011;25:2511–2516. doi: 10.1002/rcm.5155. [DOI] [PubMed] [Google Scholar]
- Singh M, Chaturvedi R. Improved clonal propagation of Spilanthes acmella Murr. for production of scopoletin. Plant Cell Tiss Organ Cult. 2010;103:243–253. doi: 10.1007/s11240-010-9774-9. [DOI] [Google Scholar]
- Singh A, Vishwakarma RA, Husain A. Evaluation of Artemisia annua strains for higher artemisinin production. Planta Med. 1988;64:475–476. doi: 10.1055/s-2006-962515. [DOI] [PubMed] [Google Scholar]
- Suberu JO, Gorka AP, Jacobs L, Roepe PD, Sullivan N, Barker GC. Antiplasmodial polyvalent interactions in Artemisia annua L. aqueous extract – possible synergistic and resistance mechanisms. PLOS ONE. 2013;8:e80790. doi: 10.1371/journal.pone.0080790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson USH, Ashton M. Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Br J Clin Pharmacol. 1999;48:528–535. doi: 10.1046/j.1365-2125.1999.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez MR, Canel C, Rimando AM, Duke SO. Differential accumulation of isoprenoids in glanded and glandless Artemisia annua L. Phytochem. 1999;52:1035–1040. doi: 10.1016/S0031-9422(99)00308-8. [DOI] [Google Scholar]
- van der Kooy F, Sullivan SE. The complexity of medicinal plants: the traditional Artemisia annua formulation, current status and future perspectives. J Ethnopharmacol. 2013;150:1–13. doi: 10.1016/j.jep.2013.08.021. [DOI] [PubMed] [Google Scholar]
- van Zyl RL, Seatlholo ST, van Vuuren SF, Viljoen AM. The biological activities of 20 nature identical essential oil constituents. J Essent Oil Res. 2006;18:129–133. [Google Scholar]
- Wallaart TE, Pras N, Beekman AC, Quax WJ. Seasonal variation of artemisinin and its biosynthetic precursors in plants of Artemisia annua of different geographical origin: proof for the existence of chemotypes. Planta Med. 2000;66:57–62. doi: 10.1055/s-2000-11115. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang Y, Zhang Q, Qi Y, Guo D. Global characterization of Artemisia annua glandular trichome transcriptome using 454 pyrosequencing. BMC Genomics. 2009;10:465. doi: 10.1186/1471-2164-10-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Arsenault PR, Covello PS, McMickle A, Teoh KH, Reed DW. Artemisinin production in Artemisia annua: Studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochem Rev. 2011;10:173–183. doi: 10.1007/s11101-010-9166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Towler MJ. The flavonoids casticin and artemetin are poorly extracted and are unstable in Artemisia annua tea infusion. Planta Med. 2012;78:1024–1026. doi: 10.1055/s-0032-1314949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Reed K, Hassanali A, Lutgen P, Engeu PO. Whole plant approaches to therapeutic use of Artemisia annua, L. (Asteraceae)Artemisia annua. In: Aftab T, Feirrera JFS, Khan MMA, Naeem M, editors. Pharmacology and Biotechnology. Chapter 4. Springer; Heidelberg, GDR: 2014a. pp. 51–74. [Google Scholar]
- Weathers PJ, Towler M, Hassanali A, Lutgen P, Engeu PO. Dried-leaf Artemisia annua: A practical malaria therapeutic for developing countries? World J Pharmacol. 2014b;3:39–55. doi: 10.5497/wjp.v3.i4.39. http://dx.doi.org/10.5497/wjp.v3.i4.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Elfawal MA, Towler MJ, Acquaah-Mensah G, Rich SM. Pharmacokinetics of artemisinin delivered by oral consumption of Artemisia annua dried leaves in healthy vs. Plasmodium chabaudi-infected mice. J Ethnopharmacol. 2014c;153:732–736. doi: 10.1016/j.jep.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Towler MJ. Changes in key constituents of clonally propagated Artemisia annua L. during preparation of compressed leaf tablets for possible therapeutic use. Ind Crop Prod. 2014;62:173–178. doi: 10.1016/j.indcrop.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 10 Facts on Malaria. 2012 http://www.who.int/features/factfiles/malaria/en/index.html.
- Woerdenbag HJ, Pras N, Chan NG, Bang BT, Bos R, Uden WV, Pham VY, Nguyen VB, Boi NV, Batterman S, Lugt CB. Artemisinin related sesquiterpenes, and essential oil in Artemisia annua during a vegetation period in Vietnam. Planta Med. 1994;60:272–275. doi: 10.1055/s-2006-959474. [DOI] [PubMed] [Google Scholar]
- Wu W, Yuan M, Zhang Q, Zhu Y, Yong L, Wang W, Qi Y, Guo D. Chemotype-dependent metabolic response to methyl jasmonate elicitation in Artemisia annua. Planta Med. 2011;77:1048–53. doi: 10.1055/s-0030-1250744. [DOI] [PubMed] [Google Scholar]
- Xie B, Feng M, Xu G, Xu J, Li S, Chen X, Ding L, Zhou Y. Investigation of the chemical compositions in tobacco of different origins and maturities at harvest by GC-MS and HPLC-PDA-QTOF-MS. J Agric Food Chem. 2014;62:4979–4987. doi: 10.1021/jf5009204. [DOI] [PubMed] [Google Scholar]
- Yadav RK, Sangwan RS, Srivastava AK, Maurya S, Sangwan NS. Comparative profiling and dynamics of artemisinin related metabolites using efficient protocol and expression of biosynthetic pathway genes during developmental span of two elite varieties of Artemisia annua L. J Plant Biochem Biotechnol. 2013 doi: 10.1007/s13562-013-0249-z. [DOI] [Google Scholar]
- Yang Z, Zhu S, Yu Z. Comparison of terpene components from flowers of Artemisia annua. Bangla J Pharmacol. 2012;7:114–119. [Google Scholar]