Abstract
RET/PTC rearrangements are one of the genetic hallmarks of papillary thyroid carcinomas. RET/PTC oncoproteins lack extracellular or transmembrane domains, and activation takes place through constitutive dimerization mediated through coiled-coil motifs in the N-terminus of the chimeric protein. Based on the observation that the EGFR kinase inhibitor PKI166 decreased RET/PTC kinase autophosphorylation and activation of downstream effectors in thyroid cells, despite lacking activity on the purified RET kinase, we proceeded to examine possible functional interactions between RET/PTC and EGFR. Conditional activation of RET/PTC oncoproteins in thyroid PCCL3 cells markedly induced expression and phosphorylation of EGFR, which was mediated in part through MAP kinase signaling. RET and EGFR were found to coimmunoprecipitate. The ability of RET to form a complex with EGFR was not dependent on recruitment of Shc, or on their respective kinase activities. Ligand induced activation of EGFR resulted in phosphorylation of a kinase-dead RET, an effect that was entirely blocked by PKI166. These effects were biologically relevant, as the EGFR kinase inhibitors PKI166, gefitinib and AEE788 inhibited cell growth induced by various constitutively active mutants of RET in thyroid cancer cells as well as NIH3T3 cells. These data indicate that EGFR contributes to RET kinase activation, signaling and growth stimulation, and may therefore be an attractive therapeutic target in RET-induced neoplasms.
Keywords: Epidermal growth factor receptor, RET/PTC, Thyroid, Tyrosine Kinase inhibitors, PKI166, AEE788, gefitinib
INTRODUCTION
The RET gene encodes the signaling subunit of a receptor complex for ligands of the glial-derived neurotrophic factor family (GFL) (1). Germline point mutations of RET confer predisposition to multiple endocrine neoplasia type 2, familial medullary thyroid carcinoma and Hirschprung's disease. RET is normally expressed at very low levels in thyroid follicular cells. Chromosomal rearrangements linking the promoter and N terminal domains of unrelated gene/s to the C-terminal fragment of RET result in the aberrant production of a chimeric form of the receptor in thyroid cells that is constitutively active (2). Several forms have been identified that differ according to the 5’ partner gene involved in the rearrangement, with RET/PTC1 and RET/PTC3 being the most common. Multiple lines of evidence point to RET/PTC as one of the key first steps in papillary thyroid cancer (PTC) pathogenesis, in particular in those arising after radiation exposure, and in pediatric PTC (reviewed in (3)). Besides RET, recombinational activation of the NTRK tyrosine kinase receptor is present in a small fraction of PTCs.
Dimerization is required for the constitutive activation of the RET/PTC oncoproteins, a property conferred by the partners of RET in the respective fusion proteins. For RET/PTC1, a leucine zipper region within the N terminus of H4 mediates dimerization (4), whereas in RET/PTC2 this is likely dependent on domains in the N terminus of RIα cAMP-dependent protein kinase A (5). In the case of RET/PTC3 this has not been formally tested, but there is a coiled-coiled motif within ELE1 (6). This results in constitutive activation of the tyrosine kinase function of RET, autophosphorylation at selected tyrosine residues, and initiation of intracellular signaling by engagement with effectors through specific tyrosine-phosphorylated domains of the receptor (2).
In addition to RET and NTRK, which are activated by genetic recombination, other tyrosine kinase receptors may play a role in the development and progression of thyroid neoplasms. The epidermal growth factor receptor (EGFR) has been reported to be overexpressed in various types of thyroid carcinomas by some (7-10) but not all (11) groups, and EGFR overexpression may correlate with poor prognosis (12, 13). Increased expression of EGF or transforming growth factor alpha has also been found in PTC and ATC. In addition, co-expression of EGF and EGFR is associated with bone metastasis of FTC (14). The mechanisms responsible for EGFR overexpression in thyroid cancer are not known, however it does not appear to be due to gene amplification (15). EGFR activity may contribute to thyroid cancer growth, since anti-EGFR antibodies (16) or small-molecule EGFR kinase inhibitors such as AG 1478, gefitinib, and AEE788 (17, 18) have shown antiproliferative effects against thyroid carcinoma cell lines in vitro and in vivo. A recent study challenged these observations, and failed to observe inhibition of growth of thyroid cancer cell lines using a range of concentrations of AEE788 within the IC50 for the EGFR kinase (11).
EGFR activity is elevated in many human solid tumors and in many cases this is associated with progression and poor prognosis (19, 20). Several mechanisms are involved in aberrant EGFR signaling in cancer (reviewed in (21)): 1) Activating mutations of EGFR, which are present in a variety of tumor types including glioma, non-small cell lung cancer (NSCLC), prostate, breast, ovary, and stomach. 2) Overexpression or activation of EGFR cognate ligands. 3) Overexpression of wild-type EGFR resulting from gene amplification, increased transcription, translation or other posttranscriptional mechanisms. 4) Heterodimerization with other Erb family members (i.e. ErbB-2, ErbB-3 or ErbB-4) leading to signal amplification. 5) Transactivation by other tyrosine kinase, cytokine and G-protein coupled receptors.
Ligand activation of the IGF-IR and PDGFR, respectively, transactivate EGFR, albeit through different mechanisms. Thus, phosphorylation of Shc and activation of the MAPK pathway by IGF-1 in Cos-7 cells is due primarily to transactivation of EGFR through metalloproteinase activation and release of HB-EGF (22). By contrast, the PDGFβR and EGFR form heterodimers basally, and PDGF-induced MAPK activation is impaired when these heterodimers are disrupted (23).
Signaling activated by RET/PTC oncoproteins is initiated by formation of homodimers through coiled-coil motifs in the N terminus of the respective fusion proteins, leading to autophosphorylation of at least 12 tyrosine residues, which then serve as docking sites for intracellular signaling molecules. Our interest in exploring possible interactions between oncogenic RET and EGFR activity was prompted by our observation that RET-induced signaling and cell growth were inhibited by EGFR kinase inhibitors. Interactions between RET and other transmembrane receptors have, to our knowledge, not been previously identified. Here we show that RET/PTC induces EGFR gene expression and kinase activity, and forms a complex with EGFR in a kinase-independent manner. EGFR in turn stimulates RET phosphorylation. Moreover, inhibition of EGFR kinase activity markedly impairs cell growth induced by a spectrum of activated RET mutants in different cellular contexts, supporting the functional relevance of these observations.
MATERIALS AND METHODS
DNA Constructs
The wild-type and kinase-dead (K721M) EGFR expression plasmids were a gift from Dr. Gibbes R. Johnson (Center for Biologics Evaluation and Research, Food and Drug Administration). The RET/PTC1 and RET/PTC3 cDNAs were gifts from Sissy Jhiang (Ohio State University) and Massimo Santoro (University of Naples), respectively. pUG10-3 RET/PTC3, and pUG10-3 RET/PTC1 have been described previously (24). RET/PTC2-PDZ cDNA, in which nucleotides encoding the 22 C-terminal amino acids (including Y1062) were replaced with the Enigma PDZ domain (provided by Susan Taylor, University of California San Diego (25)), was subcloned into pUG10-3. pEGFRpr-Luc, which contains nucleotides −1,180 to +29 of the mouse EGFR promoter, was cloned upstream of the firefly luciferase gene in the pGL2 basic plasmid, and was a gift from Elaine Fuchs (26). To generate epitope tagged RET/PTC3 the RET/PTC3 Sma I/Xba I fragment was excised from pUG10-3 and ligated in frame into Eco RV/Xba I-digested pcDNA4HisMax vector C (Invitrogen, Carlsbad, CA). To generate the kinase defective tagged RET/PTC3S765P, wild-type RET/PTC3 in pUG10-3 was digested with Blp I and a double stranded oligo (Table 1) containing the S765P substitution was annealed and ligated into the Blp I site. The RET/PTC3S765P Sma I/Xba I fragment was excised from pUG10-3 and ligated into pcDNA4HisMax vector C. All constructs were confirmed by DNA sequencing.
Table 1.
Primer pairs used.
| Gene | Sequence | Product Size (bp) |
|---|---|---|
| RET/PTC3S765P |
5’TGCTGAAAGAGAACGCCCCCCCGAGTGAGCTT3’ 5’CACTCGGGGGGGTTCTCTTTCAGCATCTTC3’ |
N/A |
| EGFR |
5’AACTCATGCCCTATGGTTGC3’ 5’GTTCATGCCCTTTGCAATCT3’ |
106 |
| cMET |
5’ACGTACGGTGTCTCCAGCAT3’ 5’GGCCCATGAATAAATCCACA3’ |
70 |
| ErbB2 |
5’CTCAACTGGTGTGTTCAGATTGC3’ 5’TTCCGGGCAGCCAGGTC3’ |
82 |
| ErbB3 |
5’CTGTGCTTCCTTCTCAGCCT3’ 5’GGTCAGCATTGTGTCCTGTG3’ |
192 |
| β-actin |
5’CTGAACCCTAAGGCCAACCGTG3’ 5’GGCATACAGGGACAGCACAGCC3’ |
105 |
Cell lines
All PCCL3 cell lines were maintained in H4 complete medium consisting of Coon's medium/F12 high zinc supplemented with 5% FBS, 0.3 mg/ml L-glutamine, 1 mIU/ml TSH, 10 μg/ml insulin, 5 μg/ml apo-transferrin, 10 nM hydrocortisone, and penicillin/streptomycin. H3 complete media was identical to H4 but without the addition of TSH. Dox-inducible cell lines expressing RET/PTC3, RET/PTC1, and RET/PTC2-PDZ (24), BRAFV600E (27), rTTA, and HRASV12 (28) have been described previously. The TT cell line (a gift from Robert Gagel, University of Texas M.D. Anderson Cancer Center), which was derived from a human medullary thyroid carcinoma expressing RETC634W (MEN2A), was grown in Ham's F12 medium with 10% FBS. NIH3T3 cells expressing RETC634W were a gift from Dr. Massimo Santoro. NIH3T3 expressing RET/PTC3 or RETM918T (MEN2B) were generated by stable transfection of pcDNA3.1-RET/PTC3 or pBABE puro RETM918T, respectively, and G418-resistant clones isolated and expression of the respective proteins determined by Western blotting. All NIH3T3- derived cell lines were grown in DMEM containing 10% calf serum and 1.0 μg/ml puromycin (Sigma, St. Louis, MO) unless indicated. COS-7 cells were grown in DMEM supplemented with 10% FBS and penicillin/streptomycin.
Antibodies and reagents
The goat anti-EGFR, goat anti-RET, anti-PKCα, anti-PLCγ, anti-phospho Y783 PLCγ, anti-MET, and the species-specific horseradish peroxidase conjugated IgGs were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The anti-phospho tyrosine and rabbit anti-EGFR were obtained from Upstate Biotechnology (Lake Placid, NY). Anti-phospho Y783 PLCγ, anti- phospho Y905 RET were purchased from Cell Signaling Technology (Beverly, MA). Anti phospho Y1173 EGFR was from BIOMOL Research Laboratories (Plymouth Meeting, PA). Mouse anti-EGFR was from Sigma (St. Louis, MO). The mouse anti-RET was a kind gift from Dr Yuri Nikiforov (University of Cincinnati). The pan matrix metalloprotease inhibitor GM6001 was from Chemicon International (Temecula, CA), indomethacin and U0126 from Calbiochem (San Diego, CA).
Transient transfection and immunoprecipitation
COS-7 cells were transfected using Lipofectamine 2000 as directed by the manufacturer (Invitrogen, Carlsbad, CA). Ten hours after transfection the medium was replaced with serum free medium and cells incubated for 10-16 hours. Cells were then washed with ice-cold PBS containing 0.2 mM sodium ortho-vanadate, and lysed by incubating with ice-cold RIPA buffer (20 mM Tris-HCl [pH7.4], 150 mM NaCl, 1.0 % Nonidet P-40, 1.0 % Tween 20, 20 mM sodium fluoride, 1 mM sodium ortho-vanadate, 1 mM EGTA, 5 mM EGTA, 0.2 mM PMSF, Sigma (St Louis, MO) for 20 minutes and passage through a 26-gauge needle. Lysates were centrifuged for 30 minutes at 4°C, the supernatant collected, and the protein concentration determined using Coomassie Plus (Pierce, Rockford, IL) as directed by manufacturer. Equal amounts of protein from lysates were incubated with anti-EGFR (Upstate) for 2 hours at 4°C, and then with pre-washed protein A/G agarose (Santa Cruz) for 1 hour at 4° C. The immunoprecipitate was washed 3 times with washing buffer (50 mM HEPES [pH 7.2], 20 mM MnCl2, 5 mM MgCl2), incubated with SDS-PAGE loading buffer for 10 minutes at 95°C and subjected to SDS-PAGE. Proteins were transferred to nitrocellulose or PVDF membranes and Western blotted with the indicated antibody.
Western blotting
Cell lysates were collected by centrifugation at 4°C for 20 min. Protein concentrations were determined using Coomassie Plus and 100 μg of total protein was subjected to SDS-PAGE, transferred to nitrocellulose or PVDF, and membranes probed with the indicated antibody. Bands were detected by incubating with species-specific horseradish peroxidase conjugated IgG's and then with enhanced chemiluminescence reagent (Amersham Biosciences Corp, Piscataway, NJ). Images were captured using the Kodak Image Station 440CF. Band densities were quantitated using Kodak 1D image analysis software.
Quantitative RT-PCR
The indicated cell lines were grown until confluent and incubated in H3 medium for 5 d prior to being incubated with H3 or H4 medium with or without doxycycline (dox) for 48 hours. RNA was then isolated using TRI reagent as directed by the manufacturer (Molecular Research Center, Inc., Cincinnati, OH). Total RNA (2 μg) was reverse transcribed with 200 U of Superscript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA) in the presence of 2.5 μM of random 9-mer primers, 20 μM dNTP for 60 min at 50°C. Quantitative PCR amplifications were performed using the QuantiTect Sybr Green PCR Kit as directed by the manufacturer (Qiagen, Inc. Valencia, CA). The amplification conditions were optimized for the LightCycler instrument (Cepheid, Sunnyvale, CA, USA) and demonstrated to result in a single PCR product by melting curve and electrophoretic analysis. Primer pairs for each gene (Table 1) were designed with the Primer3 Software (Whitehead Institute for Biomedical Research). PCR primer pairs were designed to span a large intron whose location was determined by BLAST analysis of the cDNA sequence of EGFR, ErbB2, ErbB3, cMET or β-actin against the NCBI rat genome database. This was further verified by the lack of a signal when reactions were performed in the absence of reverse transcriptase. The CT value, which was determined using the second derivative, was used to calculate the β-actin normalized expression of the different mRNAs using the Q-Gene program (29). Reactions were performed in triplicate.
EGFR promoter assay
PC-PTC3 cells were grown until confluent and incubated in H3 medium for 3 d. Cells were transfected with CMV-Renilla and pEGFRpr-Luc or pGL3-Basic using Fugene6 (Roche, Indianapolis, IN), and then incubated with H3 or H4 medium with or without doxycycline (dox) for 48 hours. Luceriferase activity was determined using the Dual-Luciferase Reporter Assay System directed by the manufacturer (Promega, Madison, WI). Fold-induction between cells incubated with or without dox was calculated after normalizing to CMV-Renilla and subtracting luceriferase activity in pGL3-Basic transfected cells.
Preparation of GST-RET and RET Kinase Assays
The activity profile of PKI166 has been reported previously (30). To explore its effects on RET kinase, a GST-fused RET kinase domain was expressed in baculovirus and purified over glutathione-Sepharose. Kinase activity was tested by measuring the phosphorylation of a synthetic substrate (poly[Glu, Tyr]), by purified GST-fusion kinase domains of the respective protein kinase in the presence of radiolabeled ATP; ATP-concentrations used were optimized within the Km range for the individual kinases. Briefly, each kinase was incubated under optimized buffer conditions in 20 mM Tris-HCl (pH 7.5), 1–3 mM MnCl2, 3–10 mM MgCl2, 10 μM Na3VO4, 1 mM DTT, 0.2 μCi [γ-33P]ATP, 1–8 μM ATP, 3–8 μg/ml poly(Glu:Tyr, 4:1), and 1% DMSO in a total volume of 30 μl in the presence or absence of NVP-AST487 for 10 min at ambient temperature. Reactions were terminated by adding 10 μl of 250 mM EDTA, and the reaction mixture was transferred onto an Immobilon-polyvinylidene difluoride membrane (Millipore, Bedford, MA). Filters were washed (0.5% H3PO4), soaked in ethanol, dried and counted in a liquid scintillation counter. IC50s for PKI166 was calculated by linear regression analysis of the percentage inhibition.
Growth Curves
The various cell lines were seeded in multiple 6 well plates and 24 hours later the number of attached cells in a representative plate was determined by counting the trypsinized cells with a Z1 Coulter counter (Beckman Coulter, Fullerton, CA). The remaining plates were incubated in the indicated experimental condition and the cells counted as described above. All experiments were performed in triplicate and medium was replaced every 48 hours.
Statistical evaluation
A single-sample t test was used to compare experimental samples where control values were normalized to 1. In all other cases a standard two-tailed t test was used.
RESULTS
Induction of EFGR by RET/PTC1 and RET/PTC3 in PCCL3 cells
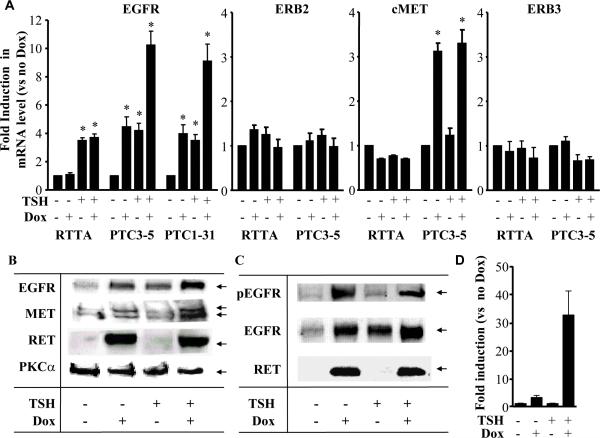
We first examined factors controlling EGFR gene expression in thyroid cells. TSH induced EGFR mRNA by >3-fold in PCCL3 cells expressing the reverse tetracycline transactivator (rTTA) as the sole heterologous gene. These served as controls for the other cell lines modified to conditionally express the indicated oncoproteins. Similar effects of TSH were seen in all other PCCL3-derived cell lines when grown without dox (Fig 1A). Accordingly, TSH also increased EGFR abundance as determined by Western blotting (Fig 1B). Dox-inducible expression of RET/PTC1 or 3 increased EGFR mRNA levels by ~3- to 4-fold in TSH-deprived cells. The combined effects of TSH and RET/PTC1 or 3 on EGFR mRNA (Fig 1A) and protein (Fig 1B) were at least additive. In addition, RET/PTC induced EGFR phosphorylation as determined by Western blotting with an anti EGFR pY1173 antibody, consistent with activation of the receptor. The pEGFR/total EGFR ratio was not significantly increased by RET/PTC3 (Fig 1C), indicating that RET-induced EGFR phosphorylation could primarily be due to overexpression of the receptor. To determine whether RET/PTC increases EGFR transcription, PC-PTC3 cells were transiently transfected with a reporter construct consisting of – 1210 bp (−1,180 to 29) of the mouse EGFR promoter. RET/PTC activation was associated with 3.2 and 32.6-fold induction in EGFR promoter activity in the absence and presence of TSH, respectively (Fig. 1D). To explore whether other factors may also have contributed to RET-induced EGFR activation, we first explored whether RET-induced release of heparin-bound EGF (HB-EGF) may have participated in this process. For this, PC-PTC3 cells were grown with or without TSH for 3 days and then incubated with doxycycline in the presence or absence of 20 μm GM6001, a pan MMP inhibitor that prevents release of HBEGF. Treatment with GM6001 did not alter EGFR phosphorylation in either basal conditions or after stimulation with TSH or dox-induced RET/PTC (not shown). Similarly, RET-induced prostaglandin biosynthesis was not involved, since EGFR phosphorylation was not prevented by a 24h treatment with 50 μm indomethacin (not shown).
Figure 1. Effects of TSH and/or RET/PTC on EGFR, MET, ErbB2 and ErbB3 mRNA abundance in PCCL3 cells.
A) PCCL3 cell lines expressing only the tetracycline transactivator protein (rtTA), or dox-inducible RET-PTC1 (PTC1) or RET-PTC3 (PTC3) were grown to confluence, placed in H3 medium (no TSH) for 4 to 6 days prior to switching to the indicated conditions for 48 hours. Quantitative RT-PCR was performed in triplicate using β-actin as the internal control. Data are expressed relative to cells grown in the absence of TSH and dox, and expressed as mean ± SD from at least two different experiments. * p < 0.05 vs control. B) Conditions were as described in A. Cell lysates containing 100 μg protein were separated on a 4 – 20% gradient polyacrylamide denaturating gel. The membrane was immunoblotted sequentially with antibodies to EGFR, MET and RET. Protein loading was determined by immunoblotting with PKC α. C) A second set of cell lysates containing 100 μg protein were separated on a 4 – 20% gradient polyacrylamide denaturating gel. The membrane was immunoblotted sequentially with antibodies to EGFR (pY1173), EGFR, and RET. D) PC-PTC3 cells were transfected with pEGFRpr-Luc and incubated with or without dox for 48 hours. Bars represent the mean ± SEM fold-increase in lucerifase activity versus –dox . *p<0.02.
By contrast to the effects on EGFR, neither TSH nor oncogenic RET altered expression of other members of the ERB family, namely ERB2 or ERB3 (Fig 1A). RET/PTC oncoproteins, but not TSH, did induce expression of the tyrosine kinase receptor MET, which is often overexpressed in thyroid cancers (31).
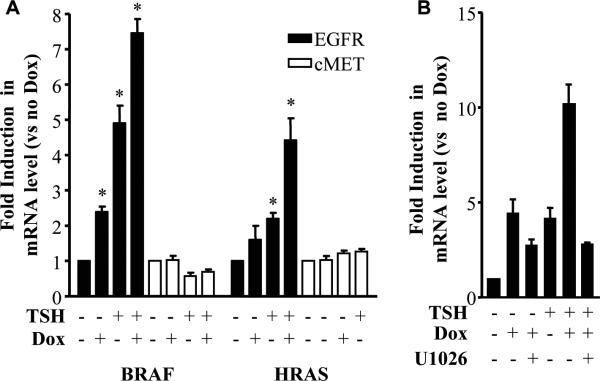
RET/PTC signaling via RAS-RAF-MEK-ERK has been implicated in thyroid cell transformation towards a papillary lineage. We explored the contribution of this pathway to RET/PTC-induced EGFR overexpression. Dox-inducible expression of oncogenic mutants of HRAS and BRAF (HRASV12 or BRAFV600E, respectively) also increased EGFR mRNA abundance (Fig 2A), although to a lesser extent than activated RET. Neither HRASV12 nor BRAFV600E significantly affected MET mRNA levels. The RET/PTC-induced increase in EGFR mRNA showed a partial requirement for MEK/ERK, as treatment of cells with the MEK inhibitor U0126 at the time dox was added markedly blunted RET-induction of EGFR (Fig 2B).
Figure 2. Effects of oncogenic HRAS or BRAF on EGFR and MET mRNA abundance in PCCL3 cells.
A) PCCL3 cell lines with dox-inducible expression of HRASG12V (HRAS) or BRAFV600E (BRAF) were grown as described in 1A. RNA was isolated and quantitative RT-PCR for MET or EGFR was performed in triplicate using β-actin as the internal control. B) PCCL3 cells with dox-inducible expression of RET-PTC3 were treated with or without 10 μM U0126 added simultaneously with dox. RNA was prepared as described in the Methods and qRT-PCR for EFGR was performed. Data are expressed relative to cells grown in the absence of TSH and dox, and expressed as mean ± SD from at least two different experiments. *p < 0.05.
Co-immunoprecipitation of EGFR and RET/PTC3
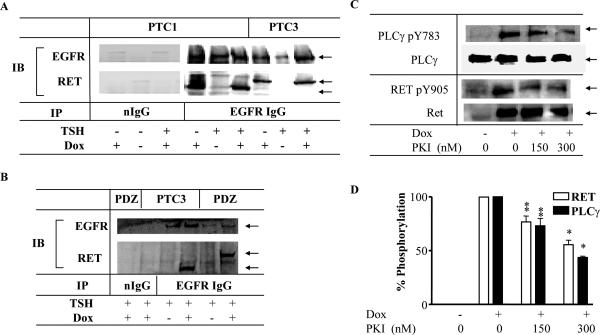
As will be shown in greater detail further below, we began to entertain the hypothesis that RET and EGFR may reciprocally transactivate each other because PKI166, a potent EGFR kinase antagonist, inhibited RET/PTC phosphorylation in PCCL3 cell extracts despite lacking inhibitory activity on a purified GSTRET kinase. To determine if RET/PTC was present in a complex with EGFR, extracts prepared from PC-PTC1 or PC-PTC3 cells treated with or without dox were immunoprecipitated with rabbit anti-EGFR, and blotted with mouse anti-RET. Both RET/PTC1 and 3 coimmunoprecipitated with EGFR (Fig 3A). The RET/PTC oncoproteins were not detected in extracts prepared from cells not treated with dox or when extracts were immunoprecipitated with a normal rabbit IgG (Fig 3A). None of the commercially available RET antisera immunoprecipitated RET in our hands, so were unable to confirm the association using a reciprocal approach. The EGFR-RET association does not require the extracellular domain of RET (which is absent in RET/PTC chimeric proteins) or RET-Y1062, as the PDZ-RET/PTC fusion protein, which lacks the C-terminal 22 amino acids including Y1062, also co-IP with EGFR (Fig 3B). As this fusion protein lacks Y1062, it cannot recruit Shc, thus indicating that signaling via Shc-Grb2-RAS is not required for the interaction between these two proteins. Moreover, coimmunoprecipitation with EGFR is not dependent on the specific N-terminal domains of the respective RET/PTC oncoproteins, since RET/PTC1, RET/PTC2 and 3 associate with EGFR.
Figure 3. EGFR co-immunoprecipitates with RET/PTC1 and RET/PTC3 in PCCL3 cells.
A) PC-PTC1 or PC-PTC3 cells were grown to near-confluence, and then placed in H3 medium (no TSH) for 5 to 6 day prior to switching to the indicated conditions for 48 hours. Cell lysates containing 600 μg total protein were immunoprecipitated with non-immune IgG or anti-EGFR antibodies. The washed immuno-complexes were then separated on a gradient gel (4 – 20%) and subjected to Western blot analysis. The membranes were cut and immunoblotted with either anti-RET or EGFR IgGs. B) Cells with dox-inducible expression of RET/PTC3 (PTC3) or RET/PTC2-PDZ (PDZ) were grown in H4 medium (with TSH) until almost confluent, and then treated with or without dox for 48 hours. Cell lysates were prepared, and immunoprecipitated with non-immune IgG or EGFR antibodies. Representative blot of four experiments is shown. The arrows mark EGFR, RET/PTC2-PDZ and RET/PTC3. C) Representative Western blot of PC-PTC3 cell extracts treated with or without 1 μM dox in the presence of TSH for 24 h to activate RET/PTC3, in the presence or absence of the indicated concentration of PKI166. Blot was sequentially probed with anti phospho-Y905 RET, anti phospho-Y783 PLCγ, anti RET, and anti PLCγ. D) Dose-dependent inhibition of RET pY905 and PLCγ pY783 by PKI166. Bars represent the average percent decrease compared to untreated cells from 3 independent experiments. *p<0.001 and ** p<0.01 vs no PKI166.
Role for EFGR in activation of RET/PTC
To investigate the contribution of EGFR activity to RET/PTC-mediated signaling, cells were treated with PKI166 (30), a potent EGFR kinase inhibitor with no in vitro activity on GST-RET kinase (see Methods). PKI166 inhibited RET/PTC-induced RET-Y905 and PLC γ phosphorylation in PCCL3 cells (Fig 3C&D). These data could be explained if PKI166 effects on RET kinase were indirect and mediated via inhibition of EGFR. As shown in Fig 4A, association of EGFR with RET/PTC was likely not dependent on their respective kinase activities, as KD-EGFR and KD-RET/PTC3S765P, also coimmunoprecipitated. This is further supported by the observation that KD-RET/PTCS765P and EGFR co-immunoprecipitate in the presence of 500 nM PKI166, which completely blocks tyrosine phosphorylation of both proteins (Fig 4C). The RET/PTC association with EGFR did not result in EGF-independent EGFR phosphorylation. The trace amount of EGFR immunoreactivity observed with the pY antibody in cells transfected with KD-EGFR is likely the result of endogenous EGFR activation in the host cells.
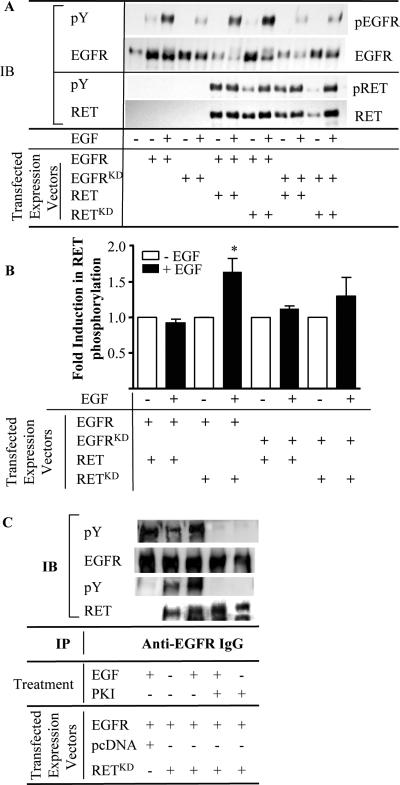
Figure 4. EGFR-dependent RET phosphorylation in Cos-7 cells.
A) Representative Western blot of anti-EGFR immunoprecipitates of Cos-7 cells transiently transfected with the following constructs: empty vector, EGFR, EGFRKD, EGFR + RET, EGFR + RETKD, EGFRKD + RET, or EGFRKD + RETKD. Prior to harvesting, cells were washed and placed in complete medium for 10 h prior to switching to serum free media for 16 h. Where indicated, 60 ng/ml EGF was added for 5 min prior to harvesting. Lysates were prepared and 400 μg of total protein was immunoprecipitated with anti-EGFR IgG. The washed immuno-complexes were then separated, and sequentially immunoblotted with antibodies to anti-pY, total EGFR, and RET. B) Fold-induction of RET phosphorylation by EGF is shown as the mean ± SEM from three different experiments. * p = 0.037. C) Representative Western blot of anti-EGFR immunoprecipitates of Cos-7 cells transiently transfected with the indicated constructs. Cells were treated as in A except that where indicated 500 nM PKI166 was added 12 hours prior to harvest.
By contrast, EGF-mediated activation of EGFR was associated with greater phosphorylation of kinase defective RET/PTC (RET-KD) (Fig 4B). There was no effect of EGF on wild-type RET/PTC3 phosphorylation, possibly because the baseline phosphorylation state of the overexpressed oncoprotein was very high, making additional increments hard to detect.
To confirm phosphorylation of RET/PTC by EGFR, RET-KD and EFGR were co-transfected into COS-7 cells and cells were treated with EGF. The addition of EGF produced a marked increase in phosphorylation of EGFR and RET-KD. The EGF-induced increase in phosphorylation of both EGFR and RET-KD was completely blocked by the addition of PKI166 (Fig 4C), consistent with a requirement of EGFR kinase activity for phosphorylation of the kinase-defective RET mutant.
Role EGFR in RET-mediated growth
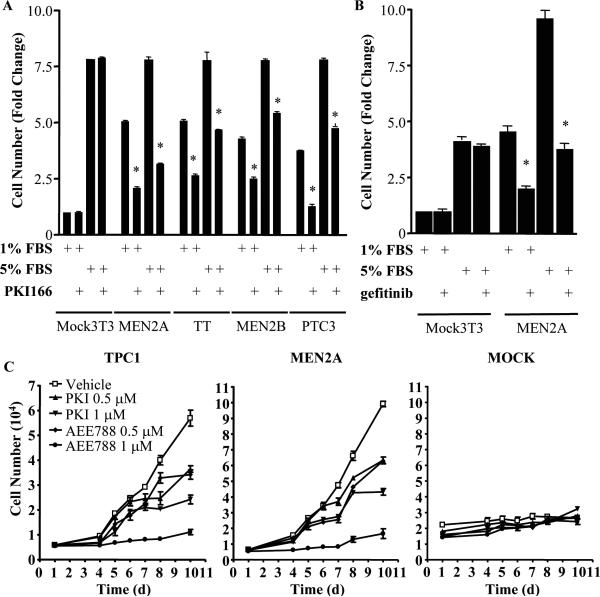
Growth of NIH3T3 cells stably expressing constitutively active mutants of RET: RET-C634W (RET-MEN2A), RET-M918T (RET-MEN2B) or RET/PTC3 was markedly inhibited by 300 nM PKI166, whereas growth of vector-transfected NIH3T3 cells was not (Fig 5A,C). Similar inhibitory effects were seen on the medullary carcinoma cell line TT. These effects were reproduced with the EGFR kinase inhibitors gefitinib (Fig 5B) and AEE788 (Fig 5C), which inhibited RET-mediated growth of NIH3T3 cells and of the human papillary thyroid cancer cell line TPC-1, which harbors a naturally occurring RET/PTC1 rearrangement.
Figure 5.
EFGR kinase inhibitors impair growth of cell lines expressing constitutively active RET-mutant proteins. A) The following cell lines were grown in 1 or 5% FBS in the presence or absence of 300 nM PKI166: human TT and NIH3T3 cells stably transfected with empty vector, RET-MEN2A, RET-MEN2B, or RET/PTC3. Cells were plated in 6-well dishes and harvested after 10 days, with media changes every two days. Data represent fold-change compared to vector-transfected NIH3T3 in 1% serum. * p < 0.001, PKI166 vs vehicle. Data are mean ± SD of two independent experiments, performed in triplicate. B) Gefitinib inhibits RET-MEN2A-induced growth of NIH3T3 cells. NIH3T3 cells stably transfected with empty vector or an expression vector for RET-MEN2A were grown in 1 or 5% FBS in the presence or absence of 1 μM gefitinib for ten days. Data represent the mean ± SD from two different experiments performed in triplicate. * p < 0.001, gefitinib vs. vehicle. C) NIH3T3 cells stably expressing RET-MEN2A, human papillary cancer TPC1 cells and vector-transfected NIH3T3 cells were incubated with the indicated concentration of inhibitors and counted at the indicated times.
DISCUSSION
EGFR is frequently overexpressed in cancer cells. As EGFR transduces growth and survival signals in many cell types, pharmacological approaches to inhibit its enzymatic activity have been the subject of considerable interest for cancer therapy. EGFR signaling can be increased in cancer cells through several mechanisms: higher expression, activating mutations, heterodimerization with other members of the erbB family, greater ligand concentrations, and alterations in signaling molecules downstream of EGFR that enhance signal output (32).
Most studies report increased EGFR expression in thyroid cancer, but the mechanisms responsible for this are not well understood. To explore factors regulating EGFR gene expression in thyroid cells we examined the effects of TSH, RET/PTC3 and BRAF in PCCL3 cells. TSH induced a robust increase in EGFR mRNA and protein. This is consistent with the observations of Westermark who reported that ligand activation of the TSH receptor, likely acting via cAMP, increased the number of EGFR in porcine thyroid membranes (33). In UMR 106-01 cells, PTH and cAMP also induce EGFR gene transcription (34). Indeed, there is a cAMP responsive element in the EGFR gene promoter, likely mediating these effects (35). RET/PTC1 and RET/PTC3 also upregulated EGFR expression, with a magnitude of induction similar to that seen with TSH. RET/PTC induction of EGFR expression showed a partial requirement for MAPK signaling, as it was decreased by pharmacological inhibition of MEK, and recapitulated by expression of activated RAS or BRAF. The effects of TSH and RET/PTC were at least additive, suggesting that independent pathways are involved in its regulation. Taken together, these data indicate that two of the critical signaling pathways implicated in thyroid oncogenesis, TSHR-adenyl cyclase-cAMP-PKA and RET-RAS-RAF-MEK cooperate to evoke a major increase in EGFR abundance in thyroid cells.
Besides increasing EGFR expression, activation of RET/PTC resulted in EGFR phosphorylation, but this was proportional to the increase in receptor mass. We examined whether other mechanisms of EGFR transactivation may also be contributing to this effect. Tyrosine kinase receptors and G-protein coupled receptors have been reported to stimulate metalloproteinases which cleave and release EGF-like precursors in the extracellular surface of the plasma membrane (36). Activation of the EP1 receptor by PGE2, which is commonly elevated in a variety of cancers as a result of overexpression of the cyclooxygenase COX-2 (37), increases phosphorylation of EGFR in cholangiocarcinoma (38) and hepatocellular carcinoma (39) cell lines. Neither of these mechanisms appeared to be involved in RET-induced EGFR phosphorylation, as the process was not prevented by incubation with MMP or COX inhibitors.
We were prompted to explore additional interactions between RET and EGFR because of the observation that PKI166, a potent EGFR kinase inhibitor, decreased RET autophosphorylation and signaling in cell extracts despite lacking effect on kinase activity of a purified GST-RET fusion protein. As autophosphorylation of RET/PTC is ligand independent, and there is no other known intracellular mediator of RET phosphorylation, this pointed to the possibility that EGFR itself may mediate RET activation. RET/PTC was found to co-immunoprecipitate with EGFR, indicating that they were part of a common complex. RET/PTC oncoproteins differ in their N-terminal domains, which are encoded by the upstream gene recombination partner in the rearrangement. These domains of H4, PRKAR1A and ELE1, which are the corresponding partners for RET/PTC1, RET/PTC2 and RET/PTC3, respectively, have either been shown (5) or are believed to encode for coiled-coil motifs which mediate oligomerization of the fusion proteins. We considered the possibility that one of these domains could also mediate the association with EGFR. Our data suggest that this is unlikely because all three RET oncoproteins co-immunoprecipitated with EGFR. Alternatively, the RET/PTC-EGFR interaction could be mediated by adaptors or other signaling effectors recruited following activation and autophosphorylation of each of these receptors. To test this proposition we examined whether a mutant of RET/PTC modified to prevent association with Shc retained the association with EGFR, and this was indeed the case. Moreover, a kinase defective mutant of RET (RET/PTC3S765P) co-immunoprecipitated with EGFR. It is unlikely that the RET-EGFR complex is formed through recruitment of proteins to phosphotyrosine residues of these receptors, as RET/PTC3S765P associated with KD-EGFR, and with wild-type EGFR in the presence of PKI166, which completely blocks tyrosine phosphorylation of both RET/PTC3S765P or EGFR. Src has been implicated in transactivation of EGFR following agonist induced G protein coupled receptor signaling (40, 41). As Src kinase activity is regulated by RET, and may mediate its mitogenic effects, it is conceivable that this tyrosine kinase may play a role in the RET-EGFR interaction. The precise nature of the association of Src and EGFR is not fully understood but it is likely dependent on receptor autophosphorylation, as is the case with PDGFR (42). Indeed, there is evidence that Src may bind to pY891 of EGFR through its SH2 domain (42). As KDEGFR lacks any detectable autophosphorylation yet retains its ability to associate with RET, Src is an unlikely mediator of this phenomenon. At this point we have not further attempted to identify the components of this complex, or to explore the contribution of individual proteins to the EGFR-RET association.
We did not find any evidence that the immunoprecipitated complex of RET/PTC3 with KDEGFR was associated with increased EGFR phosphorylation. This is again consistent with our conclusion that RET-induced EGFR activation is largely due to increased expression of EGFR. By contrast, ligand-stimulated EGFR does transactivate RET, as EGF-induced EGFR activation increases phosphorylation of KD-RET/PTC, which is completely blocked by treatment with the EGFR kinase inhibitor PKI166. As the KD-RET/PTC lacks an extracellular domain, this must be mediated by EGFR or an EGFR-regulated intracellular kinase, the identity of which is unknown.
The potential significance of this observation was demonstrated in a human thyroid cancer cell line harboring an endogenous activating RET rearrangement (PTC1, derived from a PTC), as well as NIH3T3 cells stably expressing the most common activating mutants of RET. Three multikinase inhibitors with potent effects on EGFR kinase activity were tested, PKI166, gefitinib, and AEE788, all of which exerted consistent growth inhibitory effects on the thyroid cancer line and on RET-transfected cell lines at sub-micromolar concentrations. PKI166 and AEE788 are pyrrolo-pyrimidines (30), whereas gefitinib is a quinazoline (43). Gefitinib and PKI166 lack activity against RET, whereas AEE788 is moderately active on this kinase (IC50 0.74 μM), so we cannot exclude that some of the biological effects of the latter compound may have been exerted via RET, perhaps explaining its more potent effects. However, AEE788 inhibited RET-induced growth at concentrations below its IC50 for this kinase, indicating that other targets were involved. Based on our data, it is reasonable to postulate that the growth inhibitory properties of these compounds were mediated via EGFR, although we cannot exclude interference with other distal effectors of RET.
This has implications for preclinical development. There is now considerable interest in developing compounds that hit more than one target, either to overcome resistance or to interfere with multiple pathways of pathogenetic significance (44). ZD6474, a lead compound showing considerable effectiveness in phase 2 trials for medullary thyroid carcinomas that harbor RET mutations is also a potent EGFR inhibitor.
Acknowledgments
This work was supported in part by CA50706, CA72597 and a grant from Novartis Oncology.
Abbreviations
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein/extracellular signal-regulated kinase kinase
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- RET
rearranged by transfection
- ERK
extracellular signal-regulated kinase
- TSH
thyrotropin
- dox
doxycycline’
- PLCγ
phospholipase C gamma
Reference List
- 1.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–94. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 2.Santoro M, Melillo RM, Carlomagno F, Fusco A, Vecchio G. Molecular mechanisms of RET activation in human cancer. Ann N Y Acad Sci. 2002;963:116–21. 116–21. doi: 10.1111/j.1749-6632.2002.tb04102.x. [DOI] [PubMed] [Google Scholar]
- 3.Fagin JA. How thyroid tumors start and why it matters: kinase mutants as targets for solid cancer pharmacotherapy. J Endocrinol. 2004;183:249–56. doi: 10.1677/joe.1.05895. [DOI] [PubMed] [Google Scholar]
- 4.Tong Q, Xing S, Jhiang SM. Leucine zipper-mediated dimerization is essential for the PTC1 oncogenic activity. Journal of Biological Chemistry. 1997;272:9043–7. doi: 10.1074/jbc.272.14.9043. [DOI] [PubMed] [Google Scholar]
- 5.Durick K, Yao VJ, Borrello MG, Bongarzone I, Pierotti MA, Taylor SS. Tyrosines outside the kinase core and dimerization are required for the mitogenic activity of RET/ptc2. J Biol Chem. 1995;270:24642–5. doi: 10.1074/jbc.270.42.24642. [DOI] [PubMed] [Google Scholar]
- 6.Santoro M, Dathan NA, Berlingieri MT, et al. Molecular characterization of RET/PTC3; a novel rearranged version of the RETproto-oncogene in a human thyroid papillary carcinoma. Oncogene. 1994;9:509–16. [PubMed] [Google Scholar]
- 7.Lemoine NR, Hughes CM, Gullick WJ, Brown CL, Wynford-Thomas D. Abnormalities of the EGF receptor system in human thyroid neoplasia. Int J Cancer. 1991;49:558–61. doi: 10.1002/ijc.2910490414. [DOI] [PubMed] [Google Scholar]
- 8.Aasland R, Akslen LA, Varhaug JE, Lillehaug JR. Co-expression of the genes encoding transforming growth factor-alpha and its receptor in papillary carcinomas of the thyroid. Int J Cancer. 1990;46:382–7. doi: 10.1002/ijc.2910460308. [DOI] [PubMed] [Google Scholar]
- 9.Schiff BA, McMurphy AB, Jasser SA, et al. Epidermal growth factor receptor (EGFR) is overexpressed in anaplastic thyroid cancer, and the EGFR inhibitor gefitinib inhibits the growth of anaplastic thyroid cancer. Clin Cancer Res. 2004;10:8594–602. doi: 10.1158/1078-0432.CCR-04-0690. [DOI] [PubMed] [Google Scholar]
- 10.Duh QY, Gum ET, Gerend PL, Raper SE, Clark OH. Epidermal growth factor receptors in normal and neoplastic thyroid tissue. Surgery. 1985;98:1000–7. [PubMed] [Google Scholar]
- 11.Mitsiades CS, Kotoula V, Poulaki V, et al. Epidermal growth factor receptor as a therapeutic target in human thyroid carcinoma: mutational and functional analysis. J Clin Endocrinol Metab. 2006;91:3662–6. doi: 10.1210/jc.2006-0055. [DOI] [PubMed] [Google Scholar]
- 12.Akslen LA, Varhaug JE. Oncoproteins and tumor progression in papillary thyroid carcinoma: presence of epidermal growth factor receptor, c-erbB-2 protein, estrogen receptor related protein, p21-ras protein, and proliferation indicators in relation to tumor recurrences and patient survival. Cancer. 1995;76:1643–54. doi: 10.1002/1097-0142(19951101)76:9<1643::aid-cncr2820760922>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Chen BK, Ohtsuki Y, Furihata M, et al. Co-overexpression of p53 protein and epidermal growth factor receptor in human papillary thyroid carcinomas correlated with lymph node metastasis, tumor size and clinicopathologic stage. Int J Oncol. 1999;15:893–8. doi: 10.3892/ijo.15.5.893. [DOI] [PubMed] [Google Scholar]
- 14.Gorgoulis V, Aninos D, Priftis C, et al. Expression of epidermal growth factor, transforming growth factor-alpha and epidermal growth factor receptor in thyroid tumors. In Vivo. 1992;6:291–6. [PubMed] [Google Scholar]
- 15.Namba H, Gutman RA, Matsuo K, Alvarez A, Fagin JA. H-ras protooncogene mutations in human thyroid neoplasms. J Clin Endocrinol Metab. 1990;71:223–9. doi: 10.1210/jcem-71-1-223. [DOI] [PubMed] [Google Scholar]
- 16.Gabler B, Aicher T, Heiss P, Senekowitsch-Schmidtke R. Growth inhibition of human papillary thyroid carcinoma cells and multicellular spheroids by anti-EGF-receptor antibody. Anticancer Res. 1997;17:3157–9. [PubMed] [Google Scholar]
- 17.Bergstrom JD, Westermark B, Heldin NE. Epidermal growth factor receptor signaling activates met in human anaplastic thyroid carcinoma cells. Exp Cell Res. 2000;259:293–9. doi: 10.1006/excr.2000.4967. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, Schiff BA, Yigitbasi OG, et al. Targeted molecular therapy of anaplastic thyroid carcinoma with AEE788. Mol Cancer Ther. 2005;4:632–40. doi: 10.1158/1535-7163.MCT-04-0293. [DOI] [PubMed] [Google Scholar]
- 19.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 20.Brabender J, Danenberg KD, Metzger R, et al. Epidermal growth factor receptor and HER2- neu mRNA expression in non-small cell lung cancer Is correlated with survival. Clin Cancer Res. 2001;7:1850–5. [PubMed] [Google Scholar]
- 21.Normanno N, De LA, Bianco C, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Roudabush FL, Pierce KL, Maudsley S, Khan KD, Luttrell LM. Transactivation of the EGF receptor mediates IGF-1-stimulated shc phosphorylation and ERK1/2 activation in COS-7 cells. J Biol Chem. 2000;275:22583–9. doi: 10.1074/jbc.M002915200. [DOI] [PubMed] [Google Scholar]
- 23.Saito Y, Haendeler J, Hojo Y, Yamamoto K, Berk BC. Receptor heterodimerization: essential mechanism for platelet-derived growth factor-induced epidermal growth factor receptor transactivation. Mol Cell Biol. 2001;21:6387–94. doi: 10.1128/MCB.21.19.6387-6394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knauf JA, Kuroda H, Basu S, Fagin JA. RET/PTC-induced dedifferentiation of thyroid cells is mediated through Y1062 signaling through SHC-RAS-MAP kinase. Oncogene. 2003;22:4406–12. doi: 10.1038/sj.onc.1206602. [DOI] [PubMed] [Google Scholar]
- 25.Durick K, Gill GN, Taylor SS. Shc and Enigma are both required for mitogenic signaling by Ret/ptc2. Mol Cell Biol. 1998;18:2298–308. doi: 10.1128/mcb.18.4.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Bolotin D, Chu DH, Polak L, Williams T, Fuchs E. AP-2alpha: a regulator of EGF receptor signaling and proliferation in skin epidermis. J Cell Biol. 2006;172:409–21. doi: 10.1083/jcb.200510002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitsutake N, Knauf JA, Mitsutake S, Mesa C, Jr., Zhang L, Fagin JA. Conditional BRAFV600E expression induces DNA synthesis, apoptosis, dedifferentiation, and chromosomal instability in thyroid PCCL3 cells. Cancer Res. 2005;65:2465–73. doi: 10.1158/0008-5472.CAN-04-3314. [DOI] [PubMed] [Google Scholar]
- 28.Shirokawa JM, Elisei R, Knauf JA, et al. Conditional apoptosis induced by oncogenic ras in thyroid cells. Mol Endocrinol. 2000;14:1725–38. doi: 10.1210/mend.14.11.0559. [DOI] [PubMed] [Google Scholar]
- 29.Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 2002;32:1372–9. [PubMed] [Google Scholar]
- 30.Bruns CJ, Solorzano CC, Harbison MT, et al. Blockade of the Epidermal Growth Factor Receptor Signaling by a Novel Tyrosine Kinase Inhibitor Leads to Apoptosis of Endothelial Cells and Therapy of Human Pancreatic Carcinoma. Cancer Res. 2000;60:2926–35. [PubMed] [Google Scholar]
- 31.Wasenius VM, Hemmer S, Kettunen E, Knuutila S, Franssila K, Joensuu H. Hepatocyte growth factor receptor, matrix metalloproteinase-11, tissue inhibitor of metalloproteinase-1, and fibronectin are up-regulated in papillary thyroid carcinoma: a cDNA and tissue microarray study. Clin Cancer Res. 2003;9:68–75. [PubMed] [Google Scholar]
- 32.Arteaga C. Targeting HER1/EGFR: a molecular approach to cancer therapy. Semin Oncol. 2003;30:3–14. [PubMed] [Google Scholar]
- 33.Westermark K, Karlsson FA, Westermark B. Thyrotropin modulates EGF receptor function in porcine thyroid follicle cells. Molecular and Cellular Endocrinology. 1985;40:17–23. doi: 10.1016/0303-7207(85)90153-4. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez EA, Disthabanchong S, Kowalewski R, Martin KJ. Mechanisms of the regulation of EGF receptor gene expression by calcitriol and parathyroid hormone in UMR 106-01 cells. Kidney Int. 2002;61:1627–34. doi: 10.1046/j.1523-1755.2002.00327.x. [DOI] [PubMed] [Google Scholar]
- 35.Hudson LG, Thompson KL, Xu J, Gill GN. Identification and Characterization of a Regulated Promoter Element in the Epidermal Growth Factor Receptor Gene. PNAS. 1990;87:7536–40. doi: 10.1073/pnas.87.19.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prenzel N, Zwick E, Daub H, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–8. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 37.Puxeddu E, Mitsutake N, Knauf JA, et al. Microsomal prostaglandin E2 synthase-1 is induced by conditional expression of RET/PTC in thyroid PCCL3 cells through the activation of the MEK-ERK pathway. J Biol Chem. 2003;278:52131–8. doi: 10.1074/jbc.M306003200. [DOI] [PubMed] [Google Scholar]
- 38.Han C, Wu T. Cyclooxygenase-2-derived prostaglandin E2 promotes human cholangiocarcinoma cell growth and invasion through EP1 receptor-mediated activation of the epidermal growth factor receptor and Akt. J Biol Chem. 2005;280:24053–63. doi: 10.1074/jbc.M500562200. [DOI] [PubMed] [Google Scholar]
- 39.Han C, Michalopoulos GK, Wu T. Prostaglandin E2 receptor EP1 transactivates EGFR/MET receptor tyrosine kinases and enhances invasiveness in human hepatocellular carcinoma cells. J Cell Physiol. 2006;207:261–70. doi: 10.1002/jcp.20560. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Liao Z, Camden J, et al. Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem. 2004;279:8212–8. doi: 10.1074/jbc.M312230200. [DOI] [PubMed] [Google Scholar]
- 41.Luttrell LM, la Rocca GJ, van Biesen T, Luttrell DK, Lefkowitz RJ. Gbeta gamma Subunits Mediate Src-dependent Phosphorylation of the Epidermal Growth Factor Receptor. A SCAFFOLD FOR G PROTEIN-COUPLED RECEPTOR-MEDIATED Ras ACTIVATION. Journal of Biological Chemistry. 1997;272:4637–44. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- 42.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. 513-609. [DOI] [PubMed] [Google Scholar]
- 43.Anderson NG, Ahmad T, Chan K, Dobson R, Bundred NJ. ZD1839 (Iressa), a novel epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, potently inhibits the growth of EGFR-positive cancer cell lines with or without erbB2 overexpression. Int J Cancer. 2001;94:774–82. doi: 10.1002/ijc.1557. [DOI] [PubMed] [Google Scholar]
- 44.McNeil C. Two Targets, One Drug for New EGFR Inhibitors. J Natl Cancer Inst. 2006;98:1102–3. doi: 10.1093/jnci/djj350. [DOI] [PubMed] [Google Scholar]