Summary
Objective
C–C chemokine receptor type 5 (CCR5) has been implicated in rheumatoid arthritis and several inflammatory diseases, where its blockade resulted in reduced joint destruction. However, its role in modulating cartilage and bone changes in post-traumatic osteoarthritis (OA) has not yet been investigated. In this study, we investigated changes in articular cartilage, synovium and bone in a posttraumatic OA model using CCR5-deficient (CCR5−/−) mice.
Method
Destabilization of the medial meniscus (DMM) was performed on the right knee of 10-week old CCR5−/− and C57BL/6J wild-type (WT) mice to induce post-traumatic OA. The contralateral left knee served as sham-operated control. Knee joints were analyzed at 4-, 8- and 12-weeks after surgery to evaluate cartilage degeneration and synovitis by histology, and bone changes via micro-CT.
Results
Our findings showed that CCR5−/− mice exhibited significantly less cartilage degeneration than WT mice at 8- and 12-weeks post-surgery. CCR5−/− mice showed some altered bone parameters 18- and 22-weeks of age, but body size and weight were not affected. The effect of CCR5-ablation was insignificant at all time points post-surgery for synovitis and for bone parameters such as bone volume/total volume, connectivity density index (CDI), structure model index (SMI), subchondral bone plate thickness, and trabecular bone number, thickness and spacing.
Conclusion
These findings suggest that CCR5−/− mice developed less cartilage degeneration, which may indicate a potential protective role of CCR5-ablation in cartilage homeostasis. There were no differences in bone or synovial response to surgery suggesting that CCR5 functions primarily in cartilage during the development of post-traumatic OA.
Keywords: CCR5, Post-traumatic, Osteoarthritis, Cartilage, Bone, Synovium
Introduction
Osteoarthritis (OA), the most common degenerative joint disease of the U.S. population, affects many joint tissue processes including articular cartilage, meniscus, and ligament degeneration, subchondral bone remodeling, osteophyte formation, and inflammation of the synovium (synovitis)1–5. Despite its widespread occurrence and consequences to society, the etiopathogenesis of OA remains largely elusive. It is thought that OA is dependent on multiple factors including degradative enzymes5, inflammatory mediators6, cytokines3,7 and chemokines8,9. Chemokines are a family of small structurally-related proteins8 that are involved in a wide-array of inflammatory and infectious diseases including OA8–10. Chemokines exert their biological functions through binding to specific cell membrane receptors9,11.
We are interested in C–C chemokine receptor type 5 (CCR5) for the following specific reasons: (1) CCR5 has been identified to serve as a functional receptor for several inflammatory C–C-chemokines, including macrophage inflammatory protein 1α (MIP-1α, also called C–C motif ligand 3 or CCL3), MIP-1β (CCL4), and RANTES (regulated on activation, normal T cell expressed and secreted, also called CCL5)9,11,12, (2) in vitro studies in our laboratory have shown that levels of many chemokines including CCL3, C–C motif ligand 3 like 1 (CCL3L1) and CCL4 are elevated in human articular chondrocytes in response to the pro-inflammatory cytokine interleukin (IL)-1β and the adipokine resistin13–15, (3) CCR5 and its ligands maintain the inflammatory process in rat adjuvant-induced arthritis whereas blocking of CCR5 has resulted in reduced joint destruction16–18, (4) CCR5 has been reported to be expressed in normal and OA chondrocytes14,19,20 and its expression is elevated in OA chondrocytes14,21 as well as after RANTES stimulation19, (5) CCR5 has been found in synovial fluid and synovial tissues of patients with rheumatoid arthritis22–24 as well as accumulation of CCR5+ T cells in the inflamed joint25,26 and finally, (6) CCR5 plays an important role in the clearance of pro-inflammatory chemokines to resolve inflammation27. Despite the important role of chemokines and chemokine receptors in rheumatoid arthritis, direct evidence for the role of CCR5 in post-traumatic OA is not available.
In this study, we evaluated the progression of post-traumatic OA in CCR5-deficient (CCR5−/−) and C57BL/6J wild-type (WT) mice. We used the destabilization of the medial meniscus (DMM) model to induce OA since this model provides practical ease and reproducibility and resembles slow-progressing human OA28,29. We hypothesized that removal of CCR5 in vivo would protect mice from developing post-traumatic OA by protecting from cartilage degeneration, synovial inflammation and OA-like bone phenotype.
Method
Mice
All procedures were approved by the Washington University Animal Studies Committee. CCR5−/− (in a C57BL/6J background) and C57BL/6JWT mice were procured from The Jackson Laboratory (Bar Harbor, ME) as homozygous pairs. All mice in a genotype were bred by brother-sister mating and raised at our mouse facility operating at constant temperature of 21°C and on a 12-h light/dark cycle at high standards of sanitation. Offspring were housed with their mothers until weaning at 3-weeks of age, and then separated into sex-specific cages of 4–5 mice/cage with each cage individually ventilated. The genotypes of offspring were confirmed by using a genotyping kit (KAPA Biosystems, Boston, MA). All mice were fed on irradiated rodent chow (Purina 5053, Purina Mills St. Louis, MO) with food and water provided ad libitum. Table I depicts the number of mice and time points used in this study.
Table I.
Numbers of mice in each experimental group for each strain and time point
| Genotype | Time point | N |
|---|---|---|
| WT | 4 weeks | 16 |
| 8 weeks | 14 | |
| 12 weeks | 6 | |
| CCR5−/− | 4 weeks | 14 |
| 8 weeks | 16 | |
| 12 weeks | 6 | |
| Total | 72 |
Induction of post-traumatic OA
OA was induced through DMM surgery in which the medial meniscotibial ligament (MMTL) was transected in 10-week old mice as described elsewhere28,29. Briefly, mice were anesthetized using an intra-peritoneal injection of rodent cocktail (100 mg/kg ketamine, 20 mg/kg xylazine and 10 mg/kg acepromazine) before their right knee MMTL was resected to displace the medial meniscus. The contralateral left knee served as a sham, receiving the exact same surgery as DMM but without severing the MMTL. Mice were sacrificed by CO2 asphyxiation at indicated time points. Knees were harvested and subjected to histological and micro-CT analyses.
Histological analysis of cartilage
The harvested knees were fixed in 10% neutral-buffered-formalin, decalcified with 14% ethylenediaminetetraacetic acid for 6-days with constant shaking before embedding in paraffin. Coronal sections (5-µm) were taken through the joint at eight levels with each level separated by 80-µm intervals. From each level, three sections were stained with toluidine blue for histological assessment of cartilage and synovium. The changes in cartilage were semi-quantitatively scored29,30 by two observers blinded to mouse identity and surgical procedure. Histological cartilage scores were assigned to four quadrants (medial tibial plateau, medial femoral condyle, lateral tibial plateau, and lateral femoral condyle) of each knee joint at all sectioned levels. For each individual, summed OA scores (representing whole joint changes) and maximum OA scores (representing the highest score within all sectioned levels of a given knee) were calculated from all four quadrants of each section29. The mean of the final scores from each time-point (4-, 8-, 12-weeks), genotype (CCR5−/−, WT) and procedure (sham, DMM) were used for analysis.
Histological analysis of synovium
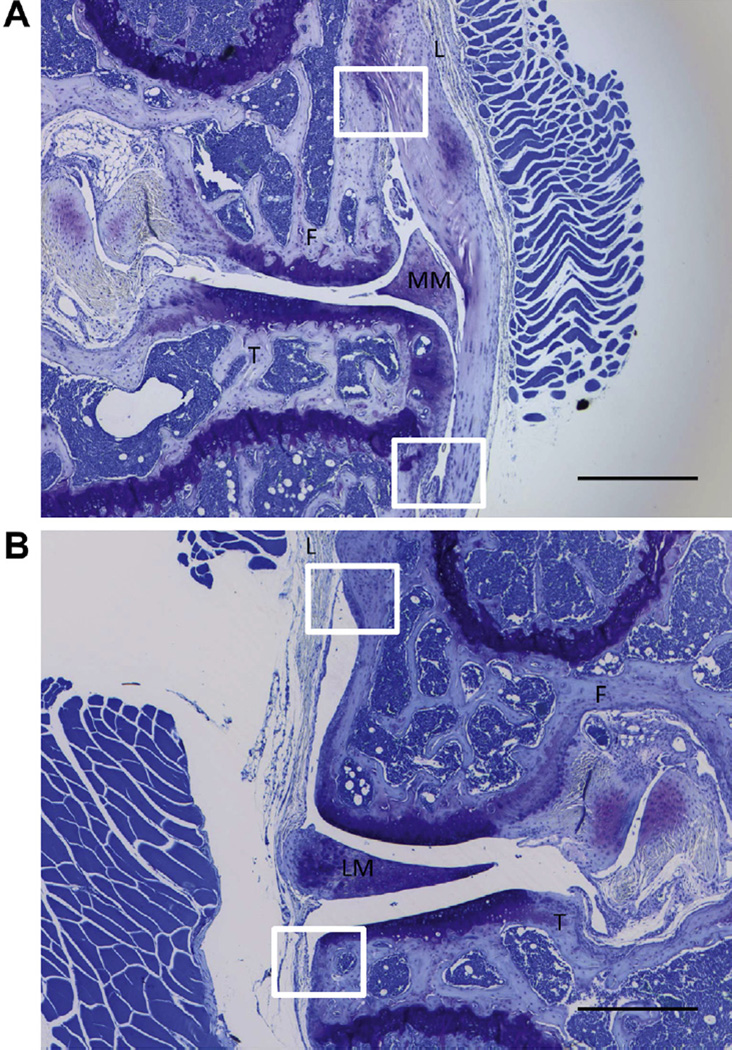
The synovial pathology (i.e., synovitis) was analyzed on all the toluidine blue stained sections from which summed and maximum OA scores were obtained. Degree of synovitis was scored using a published synovitis scoring system31 that measured the enlargement of the synovial lining cell layer on a scale of 0–3 (0 = 1–2 cells, 1 = 2–4 cells, 2 = 4–9 cells and 3 = 10 or more cells) and cellular density in the synovial stroma on a scale of 0–3 (0 = normal cellularity, 1 = slightly increased cellularity, 2 = moderately increased cellularity and 3 = greatly increased cellularity). Synovitis scores obtained from all four quadrants (medial tibia, medial femur, lateral tibia, and lateral femur) [Fig. 1(A–B)] for both of the above parameters were averaged separately and then the sum of averages from both parameters was used for analysis (on a scale of 0–6).
Fig. 1. Analysis of synovitis in CCR5−/− and WT mice.
Enlargement of synovial lining cell layer and density of the cells were obtained from all four quadrants (medial tibia, medial femur, lateral tibia, and lateral femur). The evaluation areas were white four-sided figures which were between the bone (femur or tibia) and ligament. (A) medial side of the knee (B) lateral side of the knee. Magnification 40× (Scale bar = 500 µm). F = femur; T = tibia; L = ligament; LM = lateral meniscus; MM = medical meniscus.
Micro-CT analysis of bone
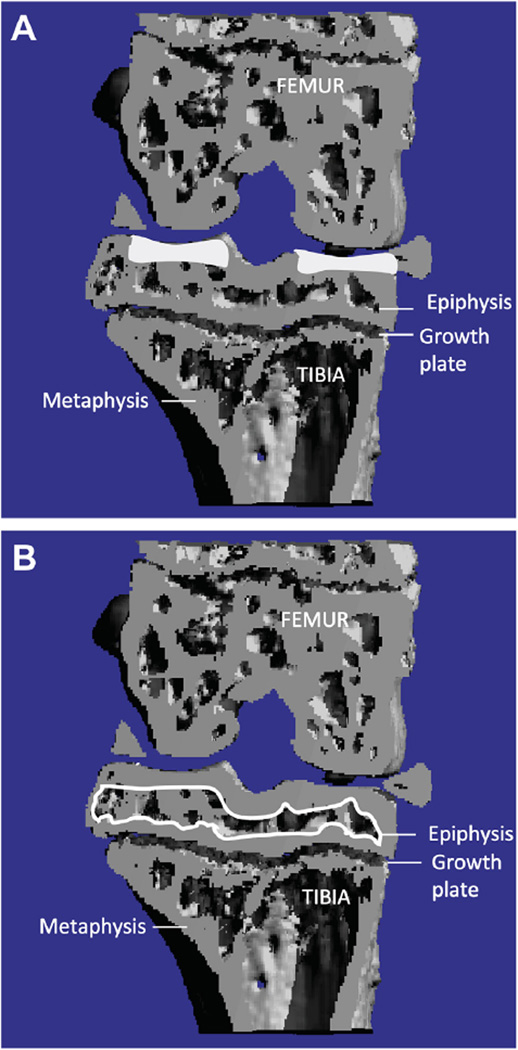
Prior to decalcification, knee joints were scanned using a viva CT-40 micro-CT scanner (Scanco-Medical, Bassersdorf, Switzerland) for analysis of 3-dimensional structure of bone for several parameters as described previously29,32 with the following setting: voxel size = 21 µm, energy = 45 kV, intensity = 177 µA and integration time = 300 ms. In order to analyze bone changes, the epiphysis of the proximal tibia was chosen as the region of interest. The region of interest was identified between the cartilage and the growth plate [Fig. 2(A)]. The outline of the epiphysis was carefully selected without inclusion of outgrowing osteophyte(s). The following morphometric parameters33 of the tibial cancellous bone were calculated for trabecular compartments: trabecular bone volume fraction (BV/TV), i.e., the ratio of trabecular bone volume to endocortical total volume, trabecular thickness (Tb.Th.), trabecular separation (Tb.Sp.), trabecular number (Tb.N.) and structure model index (SMI). SMI, an indicator of structure of trabecular bone, is designed so that a 0 = parallel plate-like trabecular bone, 3 = cylindrical rod-like structure and 4 = perfect spheres33. The bone parameters documented here are not only standard osteoporosis measurements but have also been used to study bone changes in OA29,32,34.
Fig. 2. Micro-CT analysis of bone changes.
(A) The subchondral bone plate thickness was outlined (white shaded areas) separately from trabecular bone of the tibia using the contouring methods on the cortical regions and measured the same algorithm as used for determining Tb. Th. (B) The epiphysis of proximal tibia was selected as region of interest (outlined by white line).We were only interested in bone changes occurring in the epiphysis, i.e., area between the articular cartilage and the growth plate.
The subchondral bone thickness was outlined separately from trabecular bone at the tibia using the contouring methods on the cortical regions and measured the same algorithm as used for determining Tb. Th [Fig. 2(B)].
Statistical analysis
For each phenotype, we performed analysis of variance using the factors genotype (CCR5−/−, WT) and procedure (sham, DMM) and the genotype-by-procedure interaction.
The interaction term controls for between genotype tissue differences present prior to surgery. F is the fundamental test statistic used in analysis of variance, and is calculated as the ratio of the mean squares of the factors (between group variation) and residual error (within group variation). We set the probability of significance of a factor at 0.05. When significant, the genotype-by-procedure interaction indicates differences between CCR5−/− and WT in surgical response and thus the effect of CCR5-ablation on the OA phenotype. In these cases, we used Tukey's post-hoc adjustments of standard errors in pair-wise comparisons of sham- and DMM-operated knees of WT and CCR5−/− mice, and report 95% confidence intervals for significant contrasts.
Results
Gross appearance and body weight
CCR5-ablation did not result in any overt phenotype or developmental abnormalities since both CCR5−/− and WT mice grossly appeared alike as has also been reported by The Jackson Laboratory datasheet (http://jaxmice.jax.org/strain/005427.html). The average bodyweight of CCR5−/− (22.53 ± 0.51 g) and WT (22.57 ± 0.39 g) mice at 10-weeks of age was equal.
Cartilage analysis in CCR5−/− and WT mice
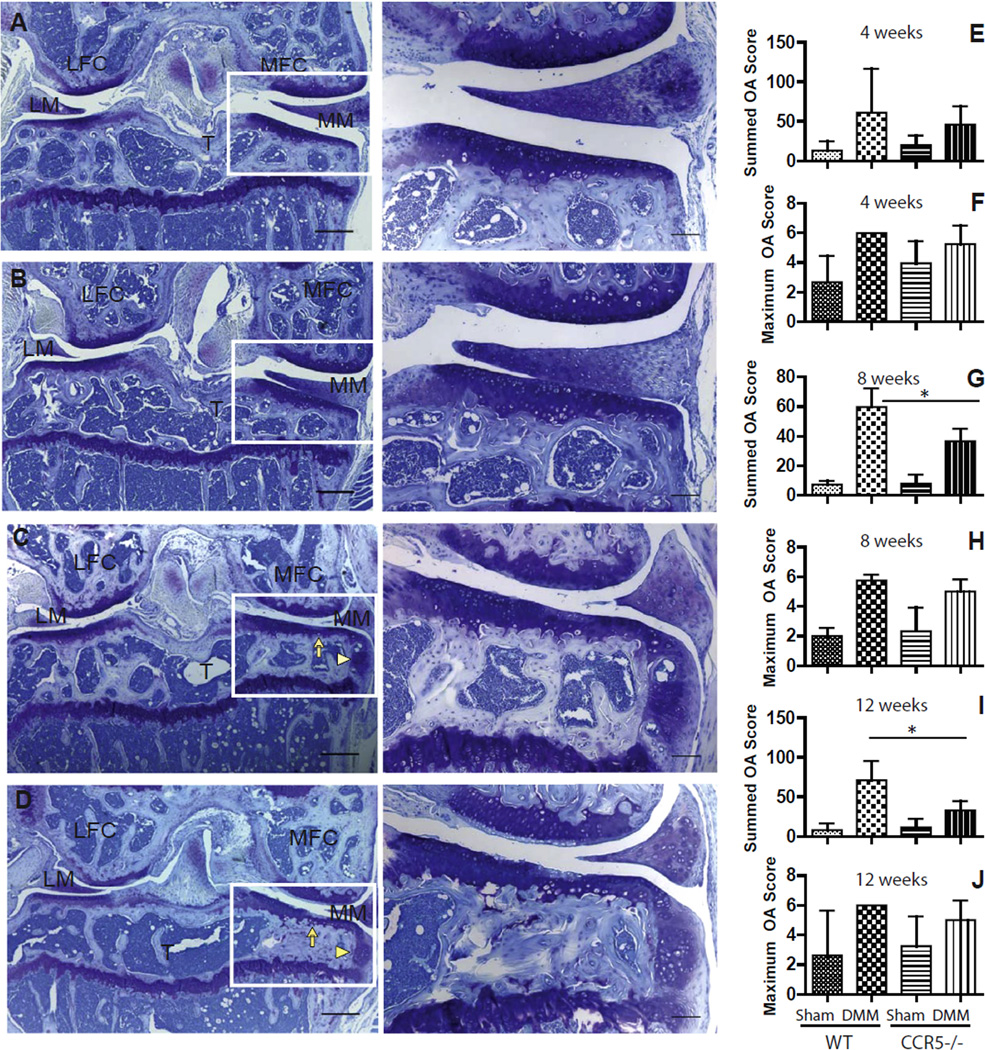
Representative sections from CCR5−/− and WT sham joints at 8-weeks post-surgery are shown in Figs. 3(A) and 1(B). There was decreased cartilage degeneration in CCR5−/− compared to WT mice at 8- [Fig. 3(C and D)] and 12-weeks after induction of DMM. Whereas WT mice showed a loss of proteoglycan staining along with cartilage fibrillation in the superficial zone [Fig.1(D)], CCR5−/− mice demonstrated a focal loss of proteoglycan staining without severe loss in cartilage post-surgery [Fig. 3(C)]. Osteophyte formation was observed on the medial side in both CCR5−/− and WT mice.
Fig. 3. Histological evaluation for cartilage degeneration in CCR5−/− and WT mice.
The sham-operated side of both CCR5−/− (A) and WT (B) mice after DMM showed normal cartilage with no osteophyte formation at 8-weeks after DMM. Following DMM at 8-weeks, decreased articular cartilage degeneration (complete arrow) in CCR5−/− (C) compared to WT mice (D) was observed. Osteophyte formation was also observed on the medial side in both CCR5−/− and WT mice (arrowhead). The sum OA score from all four quadrants of five sections from each level with eight levels from each knee was based on cartilage damage and shows that CCR5−/− mice develop less OA compared to WT mice after surgery only at 8-weeks (N = 14 for WT, N = 16 for CCR5−/−) (G) and 12-weeks (N = 6 each genotype) (I) but not at 4-weeks post-surgery (N = 16 for WT, N = 14 for CCR5−/−) (E). Pair-wise comparison showed that the summed OA score was found to be significantly lower in DMM knees of CCR5−/− compared to WT mice at 8- (P < 0.01; 95% CI = 5.52–20.02 in CCR5−/−) and 12-weeks (P < 0.01; 95% CI = 1.10–1.86 in CCR5−/−) post-surgery. A maximum score (F, H, J) representing highest score from all sectioned levels for a given knee indicates that DMM knees have significant more OA score in both CCR5−/− and WT mice compared to sham knees. It was also shown that there was no significant difference between CCR5−/− mice and WT mice for maximum OA score at any time point post-surgery. MFC = medial femoral condyle; LFC = lateral femoral condyle; MM = medial meniscus; LM = lateral meniscus; T = Tibia. Images in the left panel have magnification of 40× and scale bar = 300 µm while images in the right panel have magnification of 100× and scale bar = 100 µm. Higher magnification images of the selected box showed right to the lower magnification images. Asterisks (*) indicate P < 0.05. Graphs represent mean ± 95% CI.
Statistical analysis of summed OA scores (across all four quadrants) showed that there is no significant genotype-by-procedure interaction effect at 4-weeks after surgery. However, this effect reached a borderline significance (P = 0.058) at the 8-week and formal significance (P = 0.039) at the 12-week time point (Table II). Pair-wise comparison showed that the summed OA score was found to be significantly lower in DMM knees of CCR5−/− compared to WT mice at 8- (P < 0.01; 95% CI = 5.52–20.02 for CCR5−/−) and 12-weeks (P < 0.01; 95% CI = 1.10–1.86 for CCR5−/−) post-surgery [Fig. 1(G–I)]. The maximum OA score that represents the highest score in any of the knee cartilage compartments was not significantly different between the two genotypes [Fig. 1(F, H and J)].
Table II.
F ratios and associated P values for various parameters studies for CCR5−/− and WT mice by time points
| Time point | Effects | Summed OA score |
Max OA score |
Synovium | BV/TV | CDI | SMI | Tb.N. | Tb.Th. | Tb.Sp. | Subchondral bone thickness |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 weeks | Genotype | F = 0.016 | F = 0.618 | F = 0.716 | F = 0.036 | F = 0.141 | F = 0.249 | F = 0.385 | F = 0.840 | F = 0.026 | F = 1.870 |
| P = 0.900 | P = 0.439 | P = 0.405 | P = 0.848 | P = 0.708 | P = 0.619 | P = 0.537 | P = 0.363 | P = 0.870 | P = 0.182 | ||
| Procedure | F = 17.124 | F = 8.584 | F = 0.373 | F = 0.022 | F = 2.504 | F = 2.866 | F = 0.003 | F = 1.518 | F = 0.034 | F = 6.112 | |
| P < 0.001 | P = 0.006 | P = 0.546 | P = 0.880 | P = 0.119 | P = 0.095 | P = 0.953 | P = 0.223 | P = 0.852 | P = 0.019 | ||
| Genotype*procedure | F = 1.689 | F = 1.939 | F = 0.247 | F = 1.057 | F = 2.267 | F = 0.083 | F = 0.179 | F = 5.241 | F = 0.233 | F = 0.015 | |
| P = 0.205 | P = 0.175 | P = 0.623 | P = 0.308 | P = 0.137 | P = 0.774 | P = 0.673 | P = 0.025 | P = 0.630 | P = 0.901 | ||
| 8 weeks | Genotype | F = 7.267 | F = 1.531 | F = 1.680 | F = 5.022 | F = 1.127 | F = 4.149 | F = 0.722 | F = 1.487 | F = 0.919 | F = 9.173 |
| P = 0.011 | P = 0.224 | P = 0.203 | P = 0.029 | P = 0.292 | P = 0.046 | P = 0.398 | P = 0.227 | P = 0.341 | P = 0.005 | ||
| Procedure | F = 63.105 | F = 75.004 | F = 14.046 | F = 0.282 | F = 5.633 | F = 1.116 | F = 0.497 | F = 5.185 | F = 0.225 | F = 19.570 | |
| P < 0.001 | P < 0.001 | P = 0.001 | P = 0.597 | P = 0.021 | P = 0.295 | P = 0.483 | P = 0.026 | P = 0.636 | P < 0.001 | ||
| Genotype*procedure | F = 3.820 | F = 1.808 | F = 0.678 | F = 2.151 | F = 0.127 | F = 1.026 | F = 0.344 | F = 0.110 | F = 0.145 | F = 0.756 | |
| P = 0.058 | P = 0.187 | P = 0.415 | P = 0.148 | P = 0.722 | P = 0.315 | P = 0.559 | P = 0.740 | P = 0.704 | P = 0.391 | ||
| 12 weeks | Genotype | F = 1.662 | F = 0.030 | F = 0.584 | F = 5.959 | F = 0.002 | F = 12.731 | F = 0.141 | F = 8.047 | F = 0.151 | F = 0.596 |
| P = 0.222 | P = 0.865 | P = 0.458 | P = 0.040 | P = 0.958 | P = 0.007 | P = 0.716 | P = 0.021 | P = 0.707 | P = 0.462 | ||
| Procedure | F = 5.306 | F = 5.435 | F = 14.428 | F = 0.092 | F = 6.802 | F = 3.337 | F = 2.907 | F = 7.316 | F = 1.648 | F = 25.507 | |
| P = 0.040 | P = 0.038 | P = 0.002 | P = 0.768 | P = 0.031 | P = 0.105 | P = 0.126 | P = 0.026 | P = 0.235 | P < 0.001 | ||
| Genotype*procedure | F = 5.304 | F = 1.453 | F = 3.180 | F = 0.403 | F = 0.023 | F = 0.000 | F = 0.133 | F = 2.302 | F = 0.077 | F = 0.001 | |
| P = 0.040 | P = 0.251 | P = 0.097 | P = 0.543 | P = 0.881 | P = 0.984 | P = 0.724 | P = 0.167 | P = 0.787 | P = 0.972 |
BV/TV = trabecular bone volume fraction, the ratio of trabecular bone volume over endocortical total volume; CDI = connectivity density index, SMI = structure model index, Tb.N. = trabecular number, Tb.Th. = trabecular thickness, Tb.Sp. = trabecular separation.
Bold values indicate statistical significance at P < 0.
Synovium analysis in CCR5−/− and WT mice
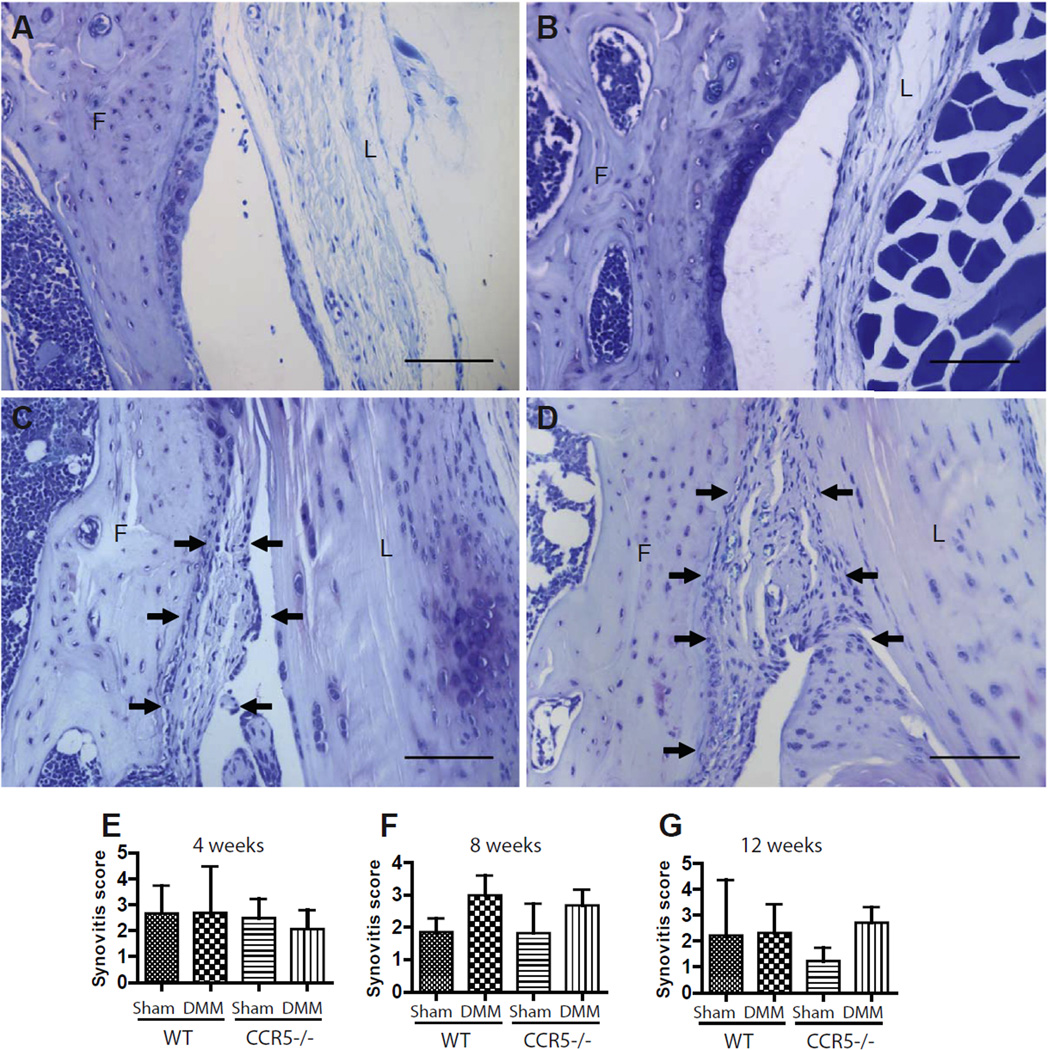
No significant differences were detected for synovial score (Table II) at any time point post-surgery. Synovitis scores for WT and CCR5−/− DMM-operated knees were not significantly influenced by variation in genotype [Fig. 4(A–G)]. Post-surgical inflammation of synovium (i.e., synovitis) appeared to decrease in shams for 8- and 12-week time points compared to 4-week time point, whereas that of DMM limb persisted. This was to be expected given that post-surgical inflammation was higher at earlier time points. We failed to find any significant difference in degree of synovitis between the two genotypes/procedure combinations at any of the time points studied.
Fig. 4. Degree of synovitis in CCR5−/− and WT mice.
The sham-operated side of both CCR5−/− (A) and WT (B) mice after DMM showed normal plasticity and cellularity at 8-weeks after DMM. The DMM sides in CCR5−/− mice (C) and WT mice (D) showed more hyperplasia and increased cell density compared to sham-operated knees following induction of DMM at 8-weeks. The synovitis score at 4-weeks (N = 16 for WT, N = 14 for CCR5−/−) (E), 8-weeks (N = 14 for WT, N = 16 for CCR5−/−) (F) and at 12-weeks (N = 6 each genotype) (G) from all the four quadrants of three sections from each knee was based on the synovial lining thickness and the synovial stromal cell density. We observed no statistically significant differences in degree of synovitis between the two genotypes. Arrows show synovial hyperplasia and increased cell density. F = femur; L = ligament. Magnification 200× (Scale bar = 100 µm). Graphs represent mean ± 95% CI.
Analysis of bone phenotype
We examined bone of the distal tibia by comparing values for these parameters: BV/TV, connectivity density index (CDI), SMI, subchondral bone plate thickness, Tb.N., Tb.Th., and Tb.Sp. We used analysis of variance to find significant genotype-by-procedure interactions that would indicate CCR5-ablation causes a different surgical response compared to wild type. By this analysis, we found very few significant differences by genotype (BV/TV, SMI, subchondral bone thickness, and Tb.Th.) and by procedure (subchondral bone thickness, CDI, Tb.Th., BV/TV, and SMI). We did observe a significant interaction at 4 weeks for trabecular thickness (Table II), but it disappeared by 8- and 12-weeks. These results suggest no differences in bone quality between CCR5−/− and WT in response to traumatic injury (DMM-surgery).
Discussion
Chemokines exert their effects through binding to specific cell membrane receptors9,11. Functionally, they can be divided into inflammatory, homeostatic, and angiogenic/angiostatic chemokines8. Several chemokines are expressed at elevated levels in OA chondrocytes, proposed to be due to an abnormal mechanical stress8,35. The role of chemokines in post-traumatic OA has not been investigated. Molecular studies demonstrate that cartilage chondrocytes produce substantial quantities and varieties of CCR ligands and chemokines in response to cytokines14,15,19,20, and the genes for CCL3 and CCL4 are regulated by the transcription factors NF-κB and C/EBPβ known to be involved in OA13. Therefore, we took the approach to study post-traumatic OA in mice deficient in CCR5.
This study shows that mice lacking CCR5 are less susceptible to post-traumatic OA as evidenced by a significant reduction in cartilage degeneration in CCR5−/− mice after DMM surgery compared to WT control mice. There were no significant differences in the degree of synovitis and bone metabolic parameters between CCR5−/− and WT mice in response to DMM surgery. In addition, there was no difference in osteophyte formation between CCR5−/− and WT mice, which reflects a normal anabolic process in both genotypes.
We observed that CCR5−/− mice exhibit significantly less cartilage degeneration than WT mice, strongly suggesting involvement of CCR5 in cartilage degeneration. While differences in articular cartilage degeneration were not seen between CCR5−/− and WT mice at the early 4-week time point, they were observed at the later 8- and 12-week time-points, a time line that has been shown previously for other mouse strains29,32. While degeneration of articular cartilage is considered a hallmark of end-stage OA36,37, observing less degeneration in CCR5−/− mice suggests some degree of protection from developing post-traumatic OA.
We found a significant genotype-by-procedure interaction effect for the summed OA score but not for maximum OA score. The absence of significance for maximum OA score is attributable to the nature of scoring system: any out of range score in summed OA scoring system is covered by the calculation of means across all the sectioned levels. In contrast, for maximum OA scoring, one has to take into consideration the maximum score in any one of the sections without calculating means across all sections. Thus, the CCR5−/− mice showed less widespread areas of cartilage destruction compared to WT control mice.
Under normal physiological conditions the synovial lining is made up of a thin layer of cells35, while in OA the synovium shows hypertrophy and hyperplasia with an increased number of lining cells38. In addition, activated synovium may produce proteases, catabolic mediators, and cytokines that accelerate the progression of OA35,38. It has been reported that CCR5 is expressed in OA synovial fibroblasts, therefore, we expected to see less synovitis in CCR5−/− mice than WT. While we were unable to observe a statistically significant difference among the genotypes/procedure combinations for hyperplasia or synovial cell density, we did see a slightly higher overall synovitis in DMM knees of WT compared to CCR5−/− at 4- and 8-weeks [Fig. 2(E and F)] that resolved at 12- weeks. Other studies17,39 in rats have shown blockade of CCR5 in an adjuvant-induced arthritis model resulted in reduced inflammation of synovium17. In addition, CCR5 antagonists have been reported to reduce joint destruction and synovitis in collagen-induced arthritis, both in monkeys39 and mice40. A possible explanation for the differences in the effect on synovitis between our findings and those reported above may reside in the nature of the arthritis models: collagenase-induced arthritis (as well as adjuvant-induced arthritis) is more inflammatory41,42 than the slowly eroding OA induced by DMM surgery28. Our findings suggest that cartilage degeneration can proceed without significant synovitis. Despite the evidence for the pro-inflammatory role of CCR5, several studies suggest its anti-inflammatory role27,43. Therefore, we suggest that further mechanistic studies are needed to investigate the roles of CCR5 and its ligands in modulating changes in cartilage following a traumatic joint injury.
Our findings indicate that there are small baseline differences in few bone parameters between the two genotypes (but not in response to surgery). However, the biological significance of these differences is difficult to predict with these data. In this study, measurements from the lateral and medial compartments of the tibial plateau were combined. In others when lateral and medial compartments were analyzed separately, medial subchondral bone thickening was correlated with the degree of cartilage damage29,32. The lack of significant genotype-by-procedure interaction effect suggests that the response of the bone to DMM in CCR5-deficient mice is not different from that of WT mice. This finding is in line with a previous study that has shown that expression of RANTES, an inducer of CCR519, is not upregulated as much in traumatic injury as is in OA and rheumatoid arthritis44.
A limitation associated with the current study is the use of contralateral limb as sham-operated because this approach could pose issues such as gait modification and movement provoked pain. In spite of studies that report use of sham and operated knees in different mice32,45, there are also reports using sham and surgery-operated knees within the same animal29,46. Interestingly, in unpublished observations, we noted that the measurements of bone parameters of sham-operated knees differed depending on whether the contralateral knee was itself a sham or DMM-operated knee. Although debatable, combining sham and DMM operations in the same mouse provides a better control for the effect of the surgery up to but not including transection of the MMTL.
Taken together, our findings demonstrate a protective role of the CCR5-ablation in post-traumatic OA development through modulating changes in cartilage (less degeneration), without involving changes in bone and synovium. Therefore, CCR5 may be a target for intervention after traumatic insult to the articular joint.
Acknowledgments
The authors would like to thank Crystal Idleburg, Tarpit Patel, and Elizabeth DeLassus (Department of Orthopaedic Surgery, Washington University in St. Louis, MO) for valuable assistance with histology and micro-CT analyses.
Role of the funding source
This study was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01-AR050847 (Sandell, PI) and R01-AR045550 (Sandell, PI). Drs Rai and Schmidt were supported by Ruth L Kirschstein National Research Service Award Fellowship from National Institute of Arthritis and Musculoskeletal and Skin Diseases through grant T32-AR060719. Dr Rai is currently supported by NIH Pathway to Independence Award (1K99AR064837). Histology and micro-CT were available through the Washington University Musculoskeletal Research Center grant P30-AR057235 (Sandell, PI). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases or the National Institutes of Health.
Footnotes
Contributions
All authors contributed to the conception and design of the study, analysis, and interpretation of data, and preparation and approval of the manuscript. In addition, MFR, and KT contributed to data acquisition, MFR, KT, and EJS analyzed the data, MFR, KT, EJS and LJS wrote the manuscript, and MFR, KT, and LJS take responsibility for the integrity of the work.
Conflict of interest
The authors have no conflict of interest.
Contributor Information
K. Takebe, Email: takebek@wudosis.wustl.edu.
M.F. Rai, Email: raim@wudosis.wustl.edu.
E.J. Schmidt, Email: schmidtej@wudosis.wustl.edu.
L.J. Sandell, Email: sandelll@wudosis.wustl.edu.
References
- 1.Sniekers YH, Intema F, Lafeber FP, van Osch GJ, van Leeuwen JP, Weinans H, et al. A role for subchondral bone changes in the process of osteoarthritis; a micro-CT study of two canine models. BMC Musculoskelet Disord. 2008;9:20. doi: 10.1186/1471-2474-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 3.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–246. [PubMed] [Google Scholar]
- 4.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorenz H, Richter W. Osteoarthritis: cellular and molecular changes in degenerating cartilage. Prog Histochem Cytochem. 2006;40:135–163. doi: 10.1016/j.proghi.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Attur MG, Dave M, Akamatsu M, Katoh M, Amin AR. Osteoarthritis or osteoarthrosis: the definition of inflammation becomes a semantic issue in the genomic era of molecular medicine. Osteoarthritis Cartilage. 2002;10:1–4. doi: 10.1053/joca.2001.0488. [DOI] [PubMed] [Google Scholar]
- 7.Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2:459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 8.Vergunst CE, van de Sande MG, Lebre MC, Tak PP. The role of chemokines in rheumatoid arthritis and osteoarthritis. Scand J Rheumatol. 2005;34:415–425. doi: 10.1080/03009740500439159. [DOI] [PubMed] [Google Scholar]
- 9.Borzi RM, Mazzetti I, Marcu KB, Facchini A. Chemokines in cartilage degradation. Clin Orthop Relat Res. 2004:S53–S61. doi: 10.1097/01.blo.0000143805.64755.4f. [DOI] [PubMed] [Google Scholar]
- 10.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 11.Balistreri CR, Caruso C, Grimaldi MP, Listi F, Vasto S, Orlando V, et al. CCR5 receptor: biologic and genetic implications in age-related diseases. Ann N Y Acad Sci. 2007;1100:162–172. doi: 10.1196/annals.1395.014. [DOI] [PubMed] [Google Scholar]
- 12.Weiss ID, Shoham H, Wald O, Wald H, Beider K, Abraham M, et al. Ccr5 deficiency regulates the proliferation and trafficking of natural killer cells under physiological conditions. Cytokine. 2011;54:249–257. doi: 10.1016/j.cyto.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Bryan JL, DeLassus E, Chang LW, Liao W, Sandell LJ. CCAAT/Enhancer-binding protein {beta} and NF-{kappa}B mediate high level expression of chemokine genes CCL3 and CCL4 by human chondrocytes in response to IL-1{beta} J Biol Chem. 2010;285:33092–33103. doi: 10.1074/jbc.M110.130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandell LJ, Xing X, Franz C, Davies S, Chang LW, Patra D. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta. Osteoarthritis Cartilage. 2008;16:1560–1571. doi: 10.1016/j.joca.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Xing X, Hensley G, Chang LW, Liao W, Abu-Amer Y, et al. Resistin induces expression of proinflammatory cytokines and chemokines in human articular chondrocytes via transcription and messenger RNA stabilization. Arthritis Rheum. 2010;62:1993–2003. doi: 10.1002/art.27473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas CS, Martinez RJ, Attia N, Haines GK, 3rd, Campbell PL, Koch AE. Chemokine receptor expression in rat adjuvant-induced arthritis. Arthritis Rheum. 2005;52:3718–3730. doi: 10.1002/art.21476. [DOI] [PubMed] [Google Scholar]
- 17.Shahrara S, Proudfoot AE, Woods JM, Ruth JH, Amin MA, Park CC, et al. Amelioration of rat adjuvant-induced arthritis by Met-RANTES. Arthritis Rheum. 2005;52:1907–1919. doi: 10.1002/art.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamoto H, Kamatani N. A CCR-5 antagonist inhibits the development of adjuvant arthritis in rats. Rheumatology (Oxford) 2006;45:230–232. doi: 10.1093/rheumatology/kei213. [DOI] [PubMed] [Google Scholar]
- 19.Yuan GH, Masuko-Hongo K, Sakata M, Tsuruha J, Onuma H, Nakamura H, et al. The role of C-C chemokines and their receptors in osteoarthritis. Arthritis Rheum. 2001;44:1056–1070. doi: 10.1002/1529-0131(200105)44:5<1056::AID-ANR186>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 20.Borzi RM, Mazzetti I, Cattini L, Uguccioni M, Baggiolini M, Facchini A. Human chondrocytes express functional chemokine receptors and release matrix-degrading enzymes in response to C-X-C and C-C chemokines. Arthritis Rheum. 2000;43:1734–1741. doi: 10.1002/1529-0131(200008)43:8<1734::AID-ANR9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 21.Alaaeddine N, Olee T, Hashimoto S, Creighton-Achermann L, Lotz M. Production of the chemokine RANTES by articular chondrocytes and role in cartilage degradation. Arthritis Rheum. 2001;44:1633–1643. doi: 10.1002/1529-0131(200107)44:7<1633::AID-ART286>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 22.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haringman JJ, Smeets TJ, Reinders-Blankert P, Tak PP. Chemokine and chemokine receptor expression in paired peripheral blood mononuclear cells and synovial tissue of patients with rheumatoid arthritis, osteoarthritis, and reactive arthritis. Ann Rheum Dis. 2006;65:294–300. doi: 10.1136/ard.2005.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang CH, Hsu CJ, Fong YC. The CCL5/CCR5 axis promotes interleukin-6 production in human synovial fibroblasts. Arthritis Rheum. 2010;62:3615–3624. doi: 10.1002/art.27755. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki N, Nakajima A, Yoshino S, Matsushima K, Yagita H, Okumura K. Selective accumulation of CCR5+ T lymphocytes into inflamed joints of rheumatoid arthritis. Int Immunol. 1999;11:553–559. doi: 10.1093/intimm/11.4.553. [DOI] [PubMed] [Google Scholar]
- 26.Yamada H, Nakashima Y, Okazaki K, Mawatari T, Fukushi J, Oyamada A, et al. Preferential accumulation of activated Th1 cells not only in rheumatoid arthritis but also in osteoarthritis joints. J Rheumatol. 2011;38:1569–1575. doi: 10.3899/jrheum.101355. [DOI] [PubMed] [Google Scholar]
- 27.Doodes PD, Cao Y, Hamel KM, Wang Y, Rodeghero RL, Kobezda T, et al. CCR5 is involved in resolution of inflammation in proteoglycan-induced arthritis. Arthritis Rheum. 2009;60:2945–2953. doi: 10.1002/art.24842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto S, Rai MF, Janiszak KL, Cheverud JM, Sandell LJ. Cartilage and bone changes during development of post-traumatic osteoarthritis in selected LGXSM recombinant inbred mice. Osteoarthritis Cartilage. 2012;20:562–571. doi: 10.1016/j.joca.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Lewis JS, Hembree WC, Furman BD, Tippets L, Cattel D, Huebner JL, et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthritis Cartilage. 2011;19:864–873. doi: 10.1016/j.joca.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Botter SM, Glasson SS, Hopkins B, Clockaerts S, Weinans H, van Leeuwen JP, et al. ADAMTS5−/− mice have less subchondral bone changes after induction of osteoarthritis through surgical instability: implications for a link between cartilage and subchondral bone changes. Osteoarthritis Cartilage. 2009;17:636–645. doi: 10.1016/j.joca.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 34.Patel V, Issever AS, Burghardt A, Laib A, Ries M, Majumdar S. Micro CT evaluation of normal and osteoarthritic bone structure in human knee specimens. J Orthop Res. 2003;21:6–13. doi: 10.1016/S0736-0266(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 35.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howell DS. Pathogenesis of osteoarthritis. Am J Med. 1986;80:24–28. doi: 10.1016/0002-9343(86)90075-6. [DOI] [PubMed] [Google Scholar]
- 37.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Attur M, Samuels J, Krasnokutsky S, Abramson SB. Targeting the synovial tissue for treating osteoarthritis (OA): where is the evidence? Best Pract Res Clin Rheumatol. 2010;24:71–79. doi: 10.1016/j.berh.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Vierboom MP, Zavodny PJ, Chou CC, Tagat JR, Pugliese-Sivo C, Strizki J, et al. Inhibition of the development of collagen-induced arthritis in rhesus monkeys by a small molecular weight antagonist of CCR5. Arthritis Rheum. 2005;52:627–636. doi: 10.1002/art.20850. [DOI] [PubMed] [Google Scholar]
- 40.Yang YF, Mukai T, Gao P, Yamaguchi N, Ono S, Iwaki H, et al. A non-peptide CCR5 antagonist inhibits collagen-induced arthritis by modulating T cell migration without affecting anti-collagen T cell responses. Eur J Immunol. 2002;32:2124–2132. doi: 10.1002/1521-4141(200208)32:8<2124::AID-IMMU2124>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 41.Whiteley PE, Dalrymple SA. Models of inflammation: adjuvant-induced arthritis in the rat. Curr Protoc Pharmacol. 2001;Chapter 5(Unit 5.5) doi: 10.1002/0471141755.ph0505s13. [DOI] [PubMed] [Google Scholar]
- 42.Williams RO. Collagen-induced arthritis as a model for rheumatoid arthritis. Methods Mol Med. 2004;98:207–216. doi: 10.1385/1-59259-771-8:207. [DOI] [PubMed] [Google Scholar]
- 43.Moreno C, Nicaise C, Gustot T, Quertinmont E, Nagy N, Parmentier M, et al. Chemokine receptor CCR5 deficiency exacerbates cerulein-induced acute pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1089–G1099. doi: 10.1152/ajpgi.00571.2005. [DOI] [PubMed] [Google Scholar]
- 44.Lisignoli G, Toneguzzi S, Pozzi C, Piacentini A, Grassi F, Ferruzzi A, et al. Chemokine expression by subchondral bone marrow stromal cells isolated from osteoarthritis (OA) and rheumatoid arthritis (RA) patients. Clin Exp Immunol. 1999;116:371–378. doi: 10.1046/j.1365-2249.1999.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valverde-Franco G, Pelletier JP, Fahmi H, Hum D, Matsuo K, Lussier B, et al. In vivo bone-specific EphB4 overexpression in mice protects both subchondral bone and cartilage during osteoarthritis. Arthritis Rheum. 2012;64:3614–3625. doi: 10.1002/art.34638. [DOI] [PubMed] [Google Scholar]
- 46.Fahlgren A, Messner K, Aspenberg P. Meniscectomy leads to an early increase in subchondral bone plate thickness in the rabbit knee. Acta Orthop Scand. 2003;74:437–441. doi: 10.1080/00016470310017758. [DOI] [PubMed] [Google Scholar]