Abstract
Objective:
Even though there are many drugs for the treatment of gastric ulcers, these drugs sometimes cannot succeed. Since the 1950s, antidepressant drugs have been used for several non-psychiatric indications. Many antidepressant drugs have been shown experimentally to produce antiulcer activity in various ulcer models. Moclobemide is an antidepressant drug which inhibits monoamine oxidase-A (MAO) enzyme selectively. When it is compared to the classic antidepressants drugs, moclobemide is the first choice in depression treatment because of its effectiveness and less side effects. This study aimed to investigate the antiulcer effects of moclobemide and to determine its relationship with antioxidant mechanisms in rat gastric tissue.
Materials and Methods:
The antiulcer activities of 10, 20, 40, 80, 150 mg/kg moclobemide and 20 mg/kg famotidine have been investigated on indomethacin-induced ulcers in rats, and the results have been compared with that of the control group.
Results:
Moclobemide decreased the indomethacin-induced ulcers significantly at all doses used. While used doses of moclobemide increased the glutathione (GSH), nitric oxide (NO) level and superoxide dismutase (SOD) activity, it decreased the malondialdehyde (MDA) level and myeloperoxidase (MPO) activity in stomach tissue when compared to the control group.
Conclusion:
It is determined that an antidepressant drug, moclobemide is a potent anti-ulcer agent. Inhibition of toxic oxidant radicals and activation of antioxidant mechanisms play a role in its anti-ulcer effect mechanisms.
Keywords: Moclobemide, ulcer, antioxidants, indomethacin, rat
Özet
Amaç:
Peptik ülser tedavisinde kullanılan çok sayıda klasik antiülser ilaç grupları bulunsa da, bu ilaçlar ile ülserin kalıcı tedavisi sağlanamamaktadır. 1950’den beri antidepresan ilaçlar non - psikiyatrik bazı hastalıkların tedavisinde kullanılmaktadırlar. Birçok antidepresan ilacın antiülser aktiviteye sahip oldukları deneysel ve klinik çalışmalarla da gösterilmiştir. Moklobemid, monoaminooksidaz - A (MAO - A) enziminin selektif inhibitörü antidepresan bir ilaçtır. Klasik MAO inhibitörleri ile karşılaştırıldığında, etkinliğinin fazla olması ve yan etkilerinin az olması nedeni ile depresyon tedavisinde tercih edilmektedir. Bu çalışmada sıçan mide dokusunda moklobemidin antiülser etkilerinin araştırılması ve oksidan mekanizmalarla ilişkisinin belirlenmesi amaçlanmıştır.
Gereç ve Yöntem:
10, 20, 40, 80 ve 150 mg/kg dozlarında kullanılan moklobemid ve 20 mg/kg dozunda kullanılan famotidinin antiülser aktivitesi sıçanlarda indometazin ile indüklenen ülser modelinde incelenmiş ve sonuçlar kontrol grubu ile karşılaştırılmıştır.
Bulgular:
Moklobemid kullanılan tüm dozlarda indometazin ülserlerinin oluşumunu anlamlı olarak azalttı. Moklobemid, kullanılan bu dozlarda sıçan mide dokusunda glutatyon (GSH), nitrik oksid (NO) seviyeleri ve süperoksid dismutaz (SOD) aktivitesini kontrol grubuna göre artırırken; malondialdehid (MDA) seviyesi ve myeloperoksidaz (MPO) aktivitesini ise azaltmıştır.
Sonuç:
Antidepresan bir ilaç olan moklobemidin, güçlü bir antiülser ajan olduğu tespit edilmiştir. Moklobemidin antiülser etki mekanizmasında toksik oksidan radikallerin baskılanması ve antioksidan mekanizmaların aktivasyonu rol oynamaktadır.
Introduction
Moclobemide is an antidepressant drug which inhibits monoamine oxidase A (MAO-A) enzyme selectively [1]. In depression therapy, it is strongly preferred over classical MAO inhibitors, because it is effective and has fewer adverse effects [2]. Antidepressant drugs are also used for many non-psychiatric disease treatments [3].
There have been several studies on the use of antidepressants in diseases of the gastrointestinal system (GIS) [4]. It has been shown in some experimental and clinical studies that some of the antidepressant drugs have anti-ulcer activity [5, 6].
Gastric ulcers are well known as a polyetiologic chronic disease [7]. It has been shown that various factors such as stress, trauma, sepsis, haemorrhagic shock, pulmonary and liver diseases, use of cigarettes and alcohol, and steroidal and non-steroidal drugs all have a role in the impairment of the balance between aggressive and protective factors in ulcer etiopathogenesis [8, 9]. Apart from these factors, depression with psychotic and somatic symptoms has been seen in patients with GIS disease [10]. Clinical studies on this issue have demonstrated that anxiolytic and antidepressant drug therapy is useful in ulcerative patients with depression [11]. Because antidepressant drugs are useful for clinical ulcer treatment, one may think that ulcer pathogenesis is particularly connected with depression. However, etiologic factors are ambiguous; ulcer diseases and the physiopathological conditions are similar in the illness process [12]. For example, increased levels of reactive oxygen species (ROS) are indicated in the mechanism of various gastric damages, no matter what the aggressive factor that caused the ulcer [13]. This supports the theory that ROS and ulcer pathogenesis are closely connected [14].
A significant difference between the oxidant and anti-oxidant parameters of damaged and healthy tissues indicates their importance in ulcer formation and treatment [15]. Although many classic drugs are available to treat gastric ulcers, these drugs cannot have permanent treatment, and researches to find such a treatment are ongoing. In recent years, experimental studies have shown that the antidepressant drug mirtazapine is better than famotidine on the indomethacin-induced ulcer model and that its gastroprotective effect originates from its antioxidant property [16].
However, no such information about the anti-ulcer effects of moclobemide exists in the literature on antidepressant drugs. This study therefore aims to investigate the anti-ulcer activity of moclobemide in the indomethacin-induced ulcer model in rats.
Material and Methods
Animals
48 male albino Wistar rats, obtained from the Medical Experimental Research Centre, Atatürk University, were used in the study. Each weighed between 190 and 200 g and all were fed under normal conditions (22°C) in separate groups. Animal experiments were performed in accordance with national guidelines for the use and care of laboratory animals and were approved by the local Animal Care Committee of Atatürk University (Approval number: 2009.2.1/3).
Chemicals
Whole biochemical assay compounds were purchased from SIGMA (Germany) and MERCK (Germany). In addition, indomethacin and moclobemide were bought from Deva Drugs (Istanbul, Turkey) and thiopental was obtained from IE Ulagay (Istanbul, Turkey).
Moclobemide Effects on Indomethacin-Induced Ulcers in Rats
This experiment examined the anti-ulcer activity of different doses of moclobemide on indomethacin-induced ulcers in rats [17]. These 48 rats were separated into 8 groups, each consisting of 6 rats. All fasted for 24 hours, and then doses of 10, 20, 40, 80, 150 mg/kg moclobemide and 20 mg/kg famotidine [16, 18] were administered by oral gavage to six of the groups. The other two groups, indomethacin control and healthy intact, were administered an equal volume of water. Five minutes later, 25 mg/kg dose of indomethacin were administered to all groups except healthy intact group. The healthy intact rats were given the same volume of distilled water orally. Six hours after the administration of indomethacin, all rat groups were sacrificed using a high dose of sodium thiopental (50 mg/kg). The stomachs of all the rats were then excised. Ulcer areas on the stomachs’ surface were examined macroscopically and measured on millimetre-square paper. After this assessment, the stomachs were forwarded to biochemistry laboratory for the determination of oxidantantioxidant parameters. Effects of moclobemide on ulcer formation and oxidative stress parameters were evaluated and compared to that indomethacin control rat group.
Experimental groups:
Moclobemide 10 mg/kg + indomethacin 25 mg/kg (5 min after moclobemide)
Moclobemide 20 mg/kg + indomethacin 25 mg/kg (5 min after moclobemide)
Moclobemide 40 mg/kg + indomethacin 25 mg/kg (5 min after moclobemide)
Moclobemide 80 mg/kg + indomethacin 25 mg/kg (5 min after moclobemide)
Moclobemide 150 mg/kg + indomethacin 25 mg/kg (5 min after moclobemide)
Famotidine 20 mg/kg + indomethacin 25 mg/kg (5 min after famotidine)
Healthy intact rats that administered Distilled Water.
Control group rats that administered distilled water + indomethacin 25 mg/kg (5 min after distilled water)
Biochemical Analysis of the Gastric Tissue
In this part, 0.2 mg of whole gastric tissue (damaged and healthy parts together) was weighed for each stomach. The samples were homogenized in ice with 2-mL buffers (consisting of 0.5% HDTMAB [0.5% hexa desil tri methyl ammonium bromide] pH=6 potassium phosphate buffer for myeloperoxidase analysis, consisting of 1.15% potassium chloride solution for malondialdehyde analysis and pH=7.5 phosphate buffer for the other analyses). Then, they were centrifuged at 4°C, 10000 rpm for 15 minutes. The supernatant part was used as the analysis sample.
Malondialdehyde (MDA) Analysis
The concentrations of gastric mucosal lipid peroxidation were determined by estimating MDA using the thio barbituric acid test [19]. The rat stomachs were promptly excised and rinsed with cold saline. To minimize the possibility of interference of haemoglobin with free radicals, any blood adhering to the mucosa was carefully removed. The corpus mucosa was scraped, weighed, and homogenized in 10mL of 100 g/L KCl. The homogenate (0.5 mL) was added to a solution containing 0.2 mL of 80 g/L sodium lauryl sulphate, 1.5 mL of 200 g/L acetic acid, 1.5 mL of 8 g/L 2-thiobarbiturate, and 0.3 mL distilled water. The mixture was incubated at 98°C for 1 h. Upon cooling, 5 mL of n-butanol: pyridine (15:l) was added. The mixture was vortexed for 1 min and centrifuged for 30 min at 4000 rpm. The absorbance of the supernatant was measured at 532 nm. The standard curve was obtained by using 1,1,3,3-tetramethoxypropane.
Total Glutathione (tGSH) Analysis
The amount of GSH in the total homogenate was measured according to the method of Sedlak and Lindsay with some modifications [20]. The sample was weighed and homogenized in 2 mL of 50 mM Tris–HCl buffer containing 20 mM EDTA and 0.2 mM sucrose at pH 7.5. The homogenate was immediately precipitated with 0.1 mL of 25% trichloroacetic acid, and the precipitate was removed after the centrifugation at 4200 rpm for 40 min at 4°C. The supernatant was used to determine GSH using 5,5-dithiobis (2-nitrobenzoic acid) (DTNB). Absorbance was measured at 412 nm using a spectrophotometer.
NO (Nitric Oxide) Analysis
Serum nitric oxide levels were measured by the Griess reaction [21]. Nitric oxide measurement is difficult because of its brief half-life. Therefore, nitrate and nitrite levels, which are stable end products of nitric oxide metabolism, were used as markers. The Griess reagent consists of sulphanilamide and N-(1-napthyl)-ethylenediamine. The method is based on a two-step process. The first step is the conversion of nitrate into nitrite using a nitrate reductase. The second step is the addition of the Griess reagent, which converts nitrite into a deep purple azo compound; photometric measurement of absorbance at 540 nm wavelength is due to the fact that this azo chromophore accurately determines the nitrite concentration.
Myeloperoxidase (MPO) Analysis
The activity of MPO in the total homogenate was measured according to the method of Wei and Frenkel with some modifications. The sample was weighed and homogenized in 2 mL of 50 mM phosphate buffer containing HDTMAB (0.5%) and centrifuged at 3500 rpm for 60 minutes at 4°C. The supernatant was used to determine the MPO activity using 4-aminoantipyrine-2% phenol solution. 4-aminoantipyrine-2% phenol solution and H2O2 were added and equilibrated for 3–4 min. After establishing the basal rate, a 0.2 mL sample suspension was added and quickly mixed. Increases in absorbance at 510 nm for 4 min at 0.1-min intervals were recorded. Protein concentration was assayed with bicinchoninic acid. Absorbance was measured at 412 nm using a spectrophotometer [22].
Superoxide Dismutase (SOD) Analysis
Measurements were performed according to Sun et al. [23]. SOD estimation was based on the generation of super-oxide radicals produced by xanthine and xanthine oxidase, which react with nitro blue tetrazolium (NTB) to form purple coloured-formazan dye. The sample was centrifuged at 6000 rpm for 10 minutes and then the brilliant supernatant was used as assay sample. The supernatant was immediately reacted with xanthine oxidase. The assay tubes incubated for 1 minute and formazan was then measured at 560 nm. As more of the enzyme exists, the least O2•− radical that reacts with NBT occurs.
Statistical Analysis
All data were subjected to one-way ANOVA using IBM SPSS statistics software package, version 13.0. Differences among groups were attained using the LSD option and significance was declared at p<0.05. Results are means ± SEM.
Results
Effects of Moclobemide on Indomethacin-Induced Ulcers in Rats
Macroscopic analyses showed that every rat that had received indomethacin (25 mg/kg) had an ulcerated stomach. Fewer ulcers were found in the groups that were given 20, 40, 80 and 150 mg/kg doses of moclobemide. In the indomethacin control group, ulcer areas of different forms and sizes were dispersed over all stomach surfaces. Hyperaemia was more evident in the control group. As seen in Table 1, the control group rats (indomethacin only) showed ulcerated areas measuring 54.3±3.2 mm2. In the 10 mg/kg moclobemide group, it was 22.5±5.9 mm2, and the other moclobemide groups were measured at 0.3±0.2, 0.3±0.2, 1.3±0.8 and 0.5±0.4 mm2 respectively. Rats who received 20 mg/kg famotidine had ulcer areas of 0.5±0.4. mm2.
Table 1.
The effect of moclobemide in indomethacin induced ulcers in rat gastric tissue.
| Drugs | Dose mg/kg | Number of animals | Ulcer area mm2 | Antiulcer effect (%) | P |
|---|---|---|---|---|---|
| Moclobemide | 10 | 6 | 22.5±5.9 | 58.4 | <0.001 |
| Moclobemide | 20 | 6 | 0.3±0.2 | 99.4 | <0.001 |
| Moclobemide | 40 | 6 | 0.3±0.2 | 99.4 | <0.001 |
| Moclobemide | 80 | 6 | 1.3±0.8 | 97.6 | <0.001 |
| Moclobemide | 150 | 6 | 0.5±0.4 | 99.1 | <0.001 |
| Famotidine | 20 | 6 | 0.5±0.4 | 99.1 | <0.001 |
| Control | - | 6 | 54.3±3.3 | - | |
| Healthy Intact | - | 6 | - | - | - |
Biochemical Analyses
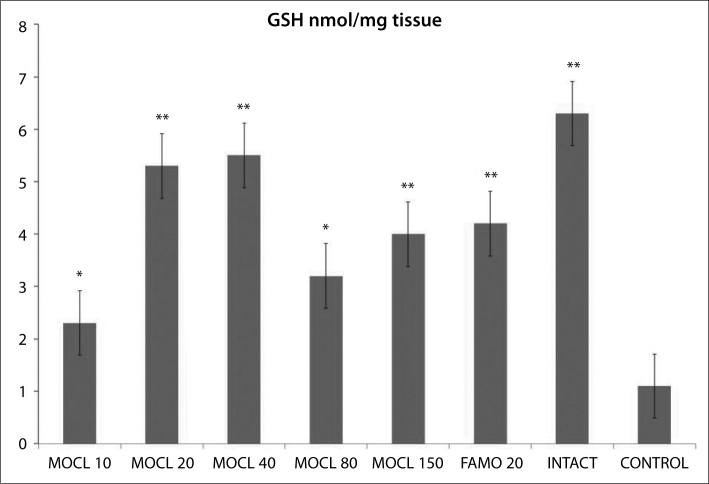
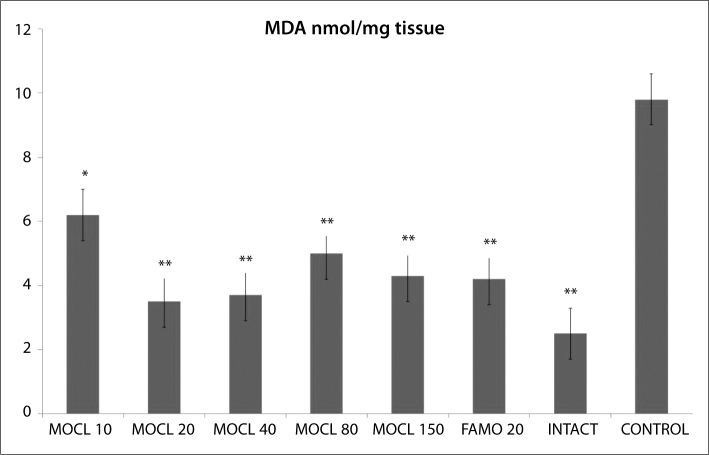
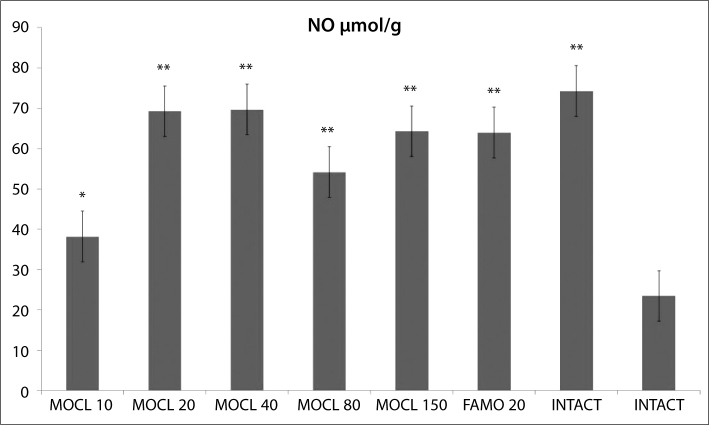
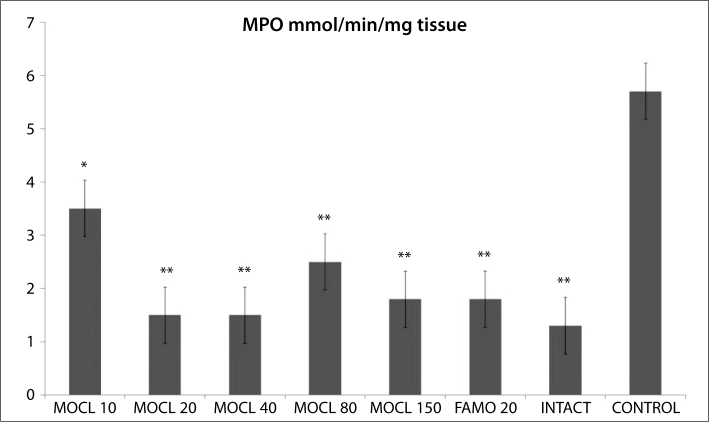
As seen in Figures 1, 2, 3, 4 and 5, the gastric tissue of the control groups rats (administered indomethacin only) contained an average 1.1±0.3 nmol/mg tissue of tGSH, 9.8±1.4 nmol/mg tissue MDA, 77.3±1.6 mmol/minute/mg tissue SOD, 23.5±2.9 μmol/g NO and 5.7±1.0 mmol/minute/mg tissue MPO. For intact rats the measurements were 6.3±0.6 nmol/mg tissue tGSH, 2.5±0.4 nmol/mg tissue MDA, 97.7±2.8 mmol/minute/mg tissue SOD, 74.3±2.0 μmol/g NO and 1.3±0.2 mmol/minute/mg tissue MPO. In the gastric tissue of rats administered 10 mg/kg moclobemide, the levels were 2.3±0.3 nmol/mg tissue tGSH, 6.2±0.6 nmol/mg tissue MDA, 85.2±2.3 mmol/minute/mg tissue SOD, 38.2±2.5 μmol/g NO and 3.5±0.4 mmol/minute/mg tissue MPO. For rats given 20 mg/kg moclobemide, the measurements were 5.3±0.5 nmol/mg tissue tGSH, 3.5±0.6 nmol/mg tissue MDA, 96.7±3.0 mmol/minute/mg tissue SOD, 69±4.2 μmol/g NO and 1.5±0.3 mmol/minute/mg tissue MPO. In the gastric tissue of rats administered 40 mg/kg moclobemide, there were 5.5±0.4 nmol/mg tissue tGSH, 3.7±0.6 nmol/mg tissue MDA, 96.8±2.2 mmol/minute/mg tissue SOD, 69.7±3.9 μmol/g NO and 1.5±0.2 mmol /minute/mg tissue MPO. For rats given 80 mg/kg moclobemide, the levels were 3.2±0.3 nmol/mg tissue tGSH, 5.0±0.7 nmol/mg tissue MDA, 88.7±1.5 mmol/minute/mg tissue SOD, 54.2±3.4 μmol/g NO and 2.5±0.4 mmol/minute/mg tissue MPO. At 150 mg/kg moclobemide, gastric tissues contained 4.0±0.5 nmol/mg tissue tGSH, 4.3±0.6 nmol/mg tissue MDA, 93.5±1.7 mmol/minute/mg tissue SOD, 64.3±2.1 μmol/g NO and 1.8±0.4 mmol/minute/mg tissue MPO. For those rats given 20 mg/kg famotidine, the results were 4.2±0.4 nmol/mg tissue tGSH, 4.2±0.5 nmol/mg tissue MDA, 93.5±1.9 mmol/minute/mg tissue SOD, 64.0±1.9 μmol/g NO and 1.8±0.5 mmol /minute/mg tissue MPO.
Figure 1.
The effect of various doses of moclobemide (MOCL) and famotidine (FAMO) treatment on total glutathione (GSH) level in indomethacin-induced ulcer model in rat gastric tissue. Results are means ±SEM. N: 6.* p<0.05, ** p<0.0001.
Figure 2.
The effect of various doses of moclobemide (MOCL) and famotidine (FAMO) treatment on malondialdehyde (MDA) level in rat gastric tissue. Results are means ±SEM. N: 6.* p<0.05, ** p<0.0001.
Figure 3.
The effect of various doses of moclobemide (MOCL) and famotidine (FAMO) treatment on nitric oxide (NO) level in rat gastric tissue. Results are means ±SEM. N: 6.* p<0.05, ** p<0.0001.
Figure 4.
The effect of various doses of moclobemide (MOCL) and famotidine (FAMO) treatment on myeloperoxidase (MPO) activity in rat gastric tissue. Results are means ± SEM. N: 6.* p<0.05, ** p<0.0001.
Figure 5.
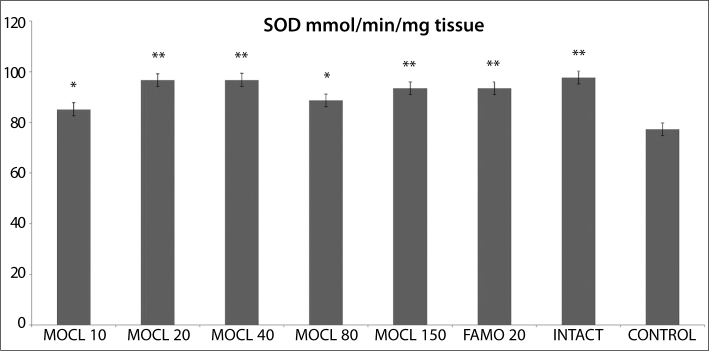
The effect of various doses of moclobemide (MOCL) and famotidine (FAMO) treatment on super-oxide dismutase (SOD) activity in rat gastric tissue. Results are means ± SEM. N: 6.* p<0.05, ** p<0.0001.
Discussion
In this study, the anti-ulcer effect of moclobemide was investigated in rats, using an indomethacin-induced ulcer model. In addition, the effect of moclobemide on oxidant and antioxidant parameters in rat stomach tissue was evaluated. Experimental results of this study show that moclobemide decreased the indomethacin-induced ulcers significantly at all doses used (10, 20, 40, 80 and 150 mg/kg doses). The anti-ulcer effect of famotidine, a strong H2 receptor antagonist, was compared to that of moclobemide. Famotidine (20 mg/kg dose) is a significant inhibitor of indomethacin-induced ulcers, as shown in earlier studies [24]. The results of this study show that moclobemide, administered in 20, 40, 80 and 150 mg/kg doses protects against gastric ulcers as much as famotidine. Non-steroidal anti-inflammatory drugs (NSAIDs) caused damage on rat gastric tissue by inhibiting cytoprotective prostaglandins which is cyclooxygenase (COX) pathway product [25]. Indomethacin is non-selective cyclooxygenase enzyme inhibitor and has highly harmful gastrointestinal side effects [25]. Indomethacin has been shown to produce higher gastric damage in rats when compared to other NSAIDs [26]. For this reason, it has become the preferred drug for inducing ulcer models [24, 25].
It is known that moclobemide is an antidepressant drug which inhibits monoaminoxidase A (MAO-A) enzyme selectively. Compared to other MAO inhibitors, it does not cause tyramine intoxication [27], and is therefore considered a safer drug. Many antidepressant drugs have anti-ulcer effects and reported gastroprotective effects. A tricyclic antidepressant drug, Amitriptyline has antiulcer effects in various ulcer models by different ways; for example it shows its antiulcer effect by inhibiting lipooxygenase enzyme in indomethacin-induced ulcers and by an anti-leukotriene effect both peripheral and central areas in cold-stress induced ulcers [28]. In many experimental studies, antidepressant drugs have been shown to produce anti-ulcer effects by reducing histamine secretion from mast cells, inhibiting gastric acid secretion, and blocking leukotriene (LTC4, D4, E4) receptors [28].
To support this opinion, COX-2 is responsible for indomethacin’s anti-inflammatory effects and COX-1 is responsible for indomethacin’s GIS-adverse effects [9]. However, while a less COX-2 selective drug, nimesulide has a protective effect in indomethacin induced gastric ulcer; more COX-2 selective drugs such as celecoxib and rofecoxib does not have a gastroprotective effect [29]. This shows that indomethacin’s ulcerative effects on GIS are not explained by a single COX inhibition. The etiopathogenesis of indomethacin-induced gastric damage demonstrates that, in addition to inhibiting the synthesis of cytoprotective PG, ROS have been involved. Yoshikawa et al. [30] stated that indomethacin causes an increase in ROS formation and lipid peroxidation via blocked mucosal cells’ antioxidant systems. Lipid peroxidation in stomach tissues and membranes results in gastric damage [30]. It has been reported in the literature that indomethacin has been reduced antioxidant parameters in stomach tissue [30]. Experimental results of this study are in line with these previous data.
This study shows that all treatments with famotidine (20mg/kg) and moclobemide (10, 20, 40, 80, 150 mg/kg) also increased the GSH content significantly. When administered in the absence of an indomethacin dose, this affected the antioxidant defence system positively and reduced gastric damage. For moclobemide doses with the highest anti-ulcer activity (20–40 mg/kg), the gastric GSH level is higher than for doses with lower activity (80 mg/kg). The highest GSL level was measured in the intact group. GSH protects gastrointestinal tissue lipids from oxidative damage. As shown in many studies, in gastric tissue damaged by indomethacin, GSH level is lowered [16, 30]. GSH detoxifies hydrogen peroxide and organic acids chemically; hydrogen peroxide accumulates in the absence of GSH [31]. In the presence of transition metals such as Fe and Cu, hydrogen peroxide reacts with superoxide, resulting in the formation of the highly reactive and cytotoxic hydroxyl radical [32]. Both experimental results of this study and the literature have shown the important relationship between gastric GSH levels and ulcers.
All the doses of moclobemide that we used significantly decreased the MDA content, compared to the control group. An increase in MDA content is correlated to an increase in tissue damage. The most effective dose of moclobemide also decreased the MDA content more than the other doses did. MDA is the final product of lipid peroxidation and is used to determine the lipid peroxidation levels [33]. MDA content increases correlated to lipid peroxidation if the oxidants were not detoxicated enough and increased MDA content reflects tissue damage degree [33]. Increasing lipid peroxidation production is an important cause of NSAIDs’ damaging effects on stomach tissue [33]. Earlier studies have shown that gastric damage causes an increased MDA level [34].
Indomethacin significantly increased the MPO activities in rat stomach tissue, compared to intact rats. All the doses of moclobemide decreased the MPO activity significantly. Previous study has determined that MPO activity increases in NSAID-damaged stomach tissue [35]. As well as MPO catalyses production of ROS which damages cell membranes; it starts proinflammatory cell activation [36]. A high level of MPO enzyme activity in gastric tissue indicates increased neutrophil secretion [36]. Neutrophil activation causes excessive secretions of radicals such as O2, H2O2, and OH. These radicals composes products such as hypochlorous acid and N-chloramine that cause tissue damage, reacting with MPO [37].
In this study, all doses of moclobemide also increased gastric NO levels significantly. NO’s actions are to increase the PG levels in the stomach, to regulate gastric blood flow, to decrease neutrophil adhesion, to regulate mucosal protection mechanism and to suppress the increased MPO levels after the administration of indomethacin [38]. These actions of NO play a role in the gastroprotection. The amount of NO reduces thr gastric tissue damage caused by indomethacin [39].
In intact rat stomach tissue SOD activity was significantly lower than that of indomethacin control rats. All the doses of moclobemide that we used significantly increased the SOD content, compared to the control group. SOD activity is least in damaged tissue and at its highest in healthy tissue, according to the literature [40]. Different studies have pointed out the relationship between SOD activity and prostaglandin synthesis, and this relationship is suggested as a possible mechanism of indomethacin-induced ulcers [41]. This enzyme’s physiological function is to protect the cells from the adverse effects of free radicals via inhibited lipid peroxidation [42].
In conclusion, it is determined that a powerful antidepressant drug, moclobemide, is a potent anti-ulcer agent. Inhibition of toxic oxidant radicals and activation of anti-oxidant mechanisms play a role in its anti-ulcer effect mechanisms. These effects are seen even at low doses.
Acknowledgments
This research was conducted in the Laboratory of Pharmacology at Ataturk University, School of Medicine, Department of Pharmacology, 25240, Erzurum, Turkey.
Footnotes
Ethics Committee Approval: Animal experiments were performed in accordance with national guidelines for the use and care of laboratory animals and were approved by the local Animal Care Committee of Atatürk University (Approval number: 2009.2.1/3).
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.A., H.S.; Design - A.A.; Supervision - H.S.; Funding - A.A.; Materials - A.A.; Data Collection and/or Processing - A.A.; Analysis and/or Interpretation - A.A., H.H.A; Literature Review - A.A.; Writing - A.A.; Critical Review - H.S.
Conflict of Interest: No conflict of interest declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Berlin I, Zimmer R, Thiede HM, et al. Comparison of the monoamine oxidase inhibiting properties of two reversible and selective monoamine oxidase-A inhibitors moclobemide and toloxatone, and assessment of their effect on psychometric performance in healthy subjects. Br J Clin Pharmacol. 1990;30:805–16. doi: 10.1111/j.1365-2125.1990.tb05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egrilmez A, Aydemir O, Onal C, Kultur S. The Efficacy of Moclobemide in Treatment of Major Depression. Bull Clin Psychopharmacol. 1995;5:46–50. [Google Scholar]
- 3.Olden KW. The use of antidepressants in functional gastrointestinal disorders: new uses for old drugs. CNS Spectr. 2005;10:891–6. doi: 10.1017/s1092852900019866. [DOI] [PubMed] [Google Scholar]
- 4.Ruud TE, Hoff GS, Tonder M, Holter O. Doxepin and cimetidine in the treatment of duodenal ulcer: an open clinical and endoscopic study. J Clin Psychiatry. 1982;43:56–60. [PubMed] [Google Scholar]
- 5.Gabry KE, Chrousos GP, Rice KC, et al. Marked suppression of gastric ulcerogenesis and intestinal responses to stress by a novel class of drugs. Mol Psychiatry. 2002;7:474–83. doi: 10.1038/sj.mp.4001031. [DOI] [PubMed] [Google Scholar]
- 6.Keshavarzian A, Wibowo A, Gordon JH, Fields JZ. MPTP-induced duodenal ulcers in rat. Prevention by reuptake blockers for serotonin and norepinephrine, but not dopamine. Gastroenterology. 1990;98:554–60. [PubMed] [Google Scholar]
- 7.Suleyman H, Demirezer LO, Buyukokuroglu ME, et al. Antiulcerogenic effect of Hippophae rhamnoides L. Phytother Res. 2001;15:625–7. doi: 10.1002/ptr.831. [DOI] [PubMed] [Google Scholar]
- 8.Soll AH, Isenberg J. Peptic ulcer disease: epidemiology, pathophisiology, clinical manifestations and diagnosis. In: Drazen JM, Gill GN, Griggs RC, et al., editors. Goldman Bennett Cecil Texbook of Medicine. 21st Ed ed. Philadelphia: Saunders; 2000. pp. 671–5. [Google Scholar]
- 9.Hooderwerf WA, Pasricha PJ. Pharmacotherapy of gastric acidity, peptic ulcers, and gastroesophageal reflux disease. In: Brunton L, editor. Goodman and Gilman’s the pharmacological basis of therapeutics. New-York: Mc Graw-Hill; 2006. pp. 967–81. [Google Scholar]
- 10.Guldahl M. The effect of trimipramine (Surmontil r) on masked depression in patients with duodenal ulcer. A double-blind study. Scand J Gastroenterol Suppl. 1977;43:27–31. [PubMed] [Google Scholar]
- 11.Sen T, Abdulsalam CA, Pal S, et al. Effect of amitriptyline on gastric ulceration. Fundam Clin Pharmacol. 2002;16:311–5. doi: 10.1046/j.1472-8206.2002.00092.x. [DOI] [PubMed] [Google Scholar]
- 12.Mozsik G, Javor T. A biochemical and pharmacological approach to the genesis of ulcer disease. I. A model study of ethanol-induced injury to gastric mucosa in rats. Dig Dis Sci. 1988;33:92–105. doi: 10.1007/BF01536637. [DOI] [PubMed] [Google Scholar]
- 13.Itoh M, Guth PH. Role of oxygen-derived free radicals in hemorrhagic shock-induced gastric lesions in the rat. Gastroenterology. 1985;88:1162–7. doi: 10.1016/s0016-5085(85)80075-5. [DOI] [PubMed] [Google Scholar]
- 14.Salim AS. Scavenging free radicals to prevent stress-induced gastric mucosal injury. Lancet. 1989;2:1390. doi: 10.1016/s0140-6736(89)91991-0. [DOI] [PubMed] [Google Scholar]
- 15.Kisaoglu A, Borekci B, Yapca OE, Bilen H, Suleyman H. Tissue Damage and Oxidant/Antioxidant Balance. Eurasian J Med. 2013;45:47–9. doi: 10.5152/eajm.2013.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilici M, Ozturk C, Dursun H, et al. Protective effect of mirtazapine on indomethacin-induced ulcer in rats and its relationship with oxidant and antioxidant parameters. Dig Dis Sci. 2009;54:1868–75. doi: 10.1007/s10620-008-0560-z. [DOI] [PubMed] [Google Scholar]
- 17.Guidobono F, Pagani F, Ticozzi C, Sibilia V, Pecile A, Netti C. Protection by amylin of gastric erosions induced by indomethacin or ethanol in rats. Br J Pharmacol. 1997;120:581–6. doi: 10.1038/sj.bjp.0700941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suleyman H, Cadirci E, Albayrak A, et al. Comparative study on the gastroprotective potential of some antidepressants in indomethacin-induced ulcer in rats. Chem Biol Interact. 2009;180:318–24. doi: 10.1016/j.cbi.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 20.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 21.Bories PN, Bories C. Nitrate determination in biological fluids by an enzymatic one-step assay with nitrate reductase. Clin Chem. 1995;41:904–7. [PubMed] [Google Scholar]
- 22.Wei H, Frenkel K. In vivo formation of oxidized DNA bases in tumor promoter-treated mouse skin. Cancer Res. 1991;51:4443–9. [PubMed] [Google Scholar]
- 23.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 24.Suleyman H, Halici Z, Cadirci E, Hacimuftuoglu A, Keles S, Gocer F. Indirect role of alpha2-adrenoreceptors in anti-ulcer effect mechanism of nimesulide in rats. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:189–98. doi: 10.1007/s00210-007-0151-0. [DOI] [PubMed] [Google Scholar]
- 25.Suleyman H, Demircan B, Karagoz Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol Rep. 2007;59:247–58. [PubMed] [Google Scholar]
- 26.Sigthorsson G, Crane R, Simon T, et al. COX-2 inhibition with rofecoxib does not increase intestinal permeability in healthy subjects: a double blind crossover study comparing rofecoxib with placebo and indomethacin. Gut. 2000;47:527–32. doi: 10.1136/gut.47.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finberg JP. Pharmacology of reversible and selective inhibitors of monoamine oxidase type A. Acta Psychiatr Scand Suppl. 1995;386:8–13. doi: 10.1111/j.1600-0447.1995.tb05918.x. [DOI] [PubMed] [Google Scholar]
- 28.Ogle CW, Cho CH. Effects of sulphasalazine on stress ulceration and mast cell degranulation in rat stomach. Eur J Pharmacol. 1985;112:285–6. doi: 10.1016/0014-2999(85)90512-6. [DOI] [PubMed] [Google Scholar]
- 29.Suleyman H, Cadirci E, Albayrak A, Halici Z. Nimesulide is a selective COX-2 inhibitory, atypical non-steroidal anti-inflammatory drug. Curr Med Chem. 2008;15:278–83. doi: 10.2174/092986708783497247. [DOI] [PubMed] [Google Scholar]
- 30.Yoshikawa T, Naito Y, Kishi A, et al. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut. 1993;34:732–7. doi: 10.1136/gut.34.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 32.Malo C, Wilson JX. Glucose modulates vitamin C transport in adult human small intestinal brush border membrane vesicles. J Nutr. 2000;130:63–9. doi: 10.1093/jn/130.1.63. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–14. [PubMed] [Google Scholar]
- 34.Kumtepe Y, Borekci B, Karaca M, Salman S, Alp HH, Suleyman H. Effect of acute and chronic administration of progesterone, estrogen, FSH and LH on oxidant and antioxidant parameters in rat gastric tissue. Chem Biol Interact. 2009;182:1–6. doi: 10.1016/j.cbi.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Whittle BJ. Mechanisms underlying intestinal injury induced by anti-inflammatory COX inhibitors. Eur J Pharmacol. 2004;500:427–39. doi: 10.1016/j.ejphar.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 36.Lau D, Baldus S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol Ther. 2006;111:16–26. doi: 10.1016/j.pharmthera.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Karmeli F, Okon E, Rachmilewitz D. Sulphydryl blocker induced gastric damage is ameliorated by scavenging of free radicals. Gut. 1996;38:826–31. doi: 10.1136/gut.38.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512–20. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- 39.Lanas A, Bajador E, Serrano P, et al. Nitrovasodilators, low-dose aspirin, other nonsteroidal antiinflammatory drugs, and the risk of upper gastrointestinal bleeding. N Engl J Med. 2000;343:834–9. doi: 10.1056/NEJM200009213431202. [DOI] [PubMed] [Google Scholar]
- 40.Cadirci E, Suleyman H, Aksoy H, et al. Effects of Onosma armeniacum root extract on ethanol-induced oxidative stress in stomach tissue of rats. Chem Biol Interact. 2007;170:40–8. doi: 10.1016/j.cbi.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 41.El-Missiry MA, El-Sayed IH, Othman AI. Protection by metal complexes with SOD-mimetic activity against oxidative gastric injury induced by indomethacin and ethanol in rats. Ann Clin Biochem. 2001;38:694–700. doi: 10.1258/0004563011900911. [DOI] [PubMed] [Google Scholar]
- 42.Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344:721–4. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]