Abstract
Background
A proportion of HIV-positive people have condomless sex. Antiretroviral treatment (ART) reduces infectiousness, but a substantial proportion of HIV-diagnosed people are not yet on ART. We describe baseline self-reported risk behaviours in ART-naïve START trial participants.
Methods
All START participants completed a risk behaviour questionnaire. Data were collected on sociodemographics, lifestyle factors, health and wellbeing status, and clinical status. Recent sexual behaviour and HIV transmission beliefs in the context of ART were also assessed. The primary interest was in condomless sex with serodifferent partners (CLS-D).
Results
4601 of 4685 HIV-positive participants (98%) completed the questionnaire (2559 men who have sex with men [MSM]; 803 heterosexual men; 1239 women). Region of recruitment was Europe/Israel 33%, South America/Mexico 25%; Africa 22%; other 21%. Median age was 36 years (IQR 29, 44). 45% reported white ethnicity and 31% black ethnicity. 2% had HIV viral load <50 copies/mL. 17% (767/4601) reported CLS-D; 20% of MSM compared to 10% in heterosexual men and 14% in women. MSM were also more likely to report multiple CLS-D partners. Possible risk limitation measures in MSM included seropositioning. CLS-D was more commonly reported by participants from South America/Mexico compared to Europe. Knowledge of ART impact on transmission risk was low.
Discussion
A substantial minority recruited to the START study reported CLS-D at baseline. CLS-D reporting was higher in MSM than heterosexuals and varied significantly according to region of recruitment. A substantial proportion of MSM reporting CLS-D appear to take transmission risk limitation measures.
Keywords: Condomless sex, Transmission, MSM, Heterosexual, HIV
Background
HIV transmission is largely related to sexual risk behaviours and injecting drug use. Although condom use is generally advised for HIV-positive people to reduce transmission to HIV-negative partners, it is consistently reported that a significant proportion of HIV-positive individuals have condomless sexual intercourse (1, 2). There is now strong evidence that virally suppressive antiretroviral treatment (ART) substantially reduces the infectiousness of people with HIV through heterosexual vaginal intercourse (3-7). Recently reported data help to confirm this and suggest that this may well extend to anal intercourse, including in men who have sex with men (MSM), but further evidence is still required (8).
Globally, there is no clear consensus regarding at what CD4 cell count an HIV-positive person should start ART for their own clinical benefit, with various guidelines giving different recommendations including: ART immediately from diagnosis irrespective of CD4 cell count (9, 10), at a threshold of 500 cells/μL (11, 12) or at 350 cells/μL (13). In virtually all settings, there remains a substantial proportion of HIV-diagnosed people not yet on ART. START is a randomised trial evaluating the risks and benefits of early ART. A total of 4685 HIV-positive participants have been randomly allocated in a 1:1 ratio to the early or deferred ART group (14). Recruitment was completed in 2013, and participants will be followed for a minimum of three years.
The effect of ART at high CD4 cell counts on HIV transmission risk is in part dependent on the extent to which ART use impacts on sexual risk behaviours (both frequency and type of sexual activity) (15). The benefits of ART on infectivity may be lessened or negated if use of ART does not eliminate transmission risk and sexual risk behaviours increase among HIV-positive or -negative people. Such a change in risk behaviours may occur due to knowledge of decreased risk of transmission while on ART, or the perception that, with advances in HIV treatment and near-normal life expectancy in HIV-diagnosed people on ART, HIV infection itself is becoming less of a threat. Evidence predating the HPTN 052 trial (3) suggested that ART use was not associated with increased levels of condomless sex, and indeed ART use has been found to be associated with somewhat reduced levels of condomless sex in some previous cross-sectional studies (2, 16, 17), though not in others (18). However, with growing appreciation that transmission risk is reduced in people with undetectable plasma viral load, levels of condomless sex among those diagnosed with HIV could well increase. A key secondary question for the prospective phase of the START trial is whether early as opposed to deferred initiation of ART (and/or achievement of an undetectable plasma viral load) leads to higher levels of condomless sex with a serodifferent partner (CLS-D), resulting from knowledge of likely reduced transmission risk in people on ART. In the meantime, before the prospective phase is completed, it is informative to assess among START participants, baseline levels of sexual and drug injection-related transmission risk behaviours, in particular CLS-D and reported beliefs expressed at baseline about the infectivity-reducing effects of ART.
It is known that in those diagnosed with HIV, but not yet on ART, the main risk for onward sexual transmission of HIV to partners comes through CLS-D. However, there is evidence that when HIV-positive individuals do not use condoms with sexual partners they may attempt risk reduction through other practices. In HIV-diagnosed MSM in particular it has been reported that those engaging in condomless intercourse may undertake a number of measures in order to reduce the risk of HIV transmission. HIV-positive MSM may have intercourse only with other HIV-positive men (“serosorting”) (19). Among HIV-positive MSM engaging in CLS-D, measures perceived to reduce transmission risk include acting as the receptive and not the insertive partner during anal sex (“seropositioning”) or not ejaculating inside the partner when acting as the insertive partner. Uncertainty remains regarding the extent to which these behaviours may reduce transmission of HIV, although there is some evidence of risk reduction from prospective studies of HIV-negative MSM (20). In addition, such “sero-adaptive” strategies do not protect against transmission of other sexually transmitted infections (STIs) including hepatitis C: one prospective study demonstrated that such strategies increase the risk of urethral and anal STIs compared to not having condomless sex (21). The prevalence of specific potential risk reduction strategies among people engaging in CLS-D is not well described, particularly in different geographical settings. START offers a chance to study the prevalence of CLS-D and specific sexual behaviours in a large group of ART-naïve people across the world, as well as to assess the effect of starting ART on sexual behaviour.
The aim of this analysis is to describe self-reported recent transmission risk behaviours and beliefs at baseline among the HIV-diagnosed individuals enrolled in START, and in particular to assess the prevalence of CLS-D and associations with sociodemographic, lifestyle and other factors.
Methods
Study Design
START is an open-label multicentre international randomised trial comparing serious AIDS and non-AIDS morbidity or mortality between two management strategies for ART-naïve individuals with baseline CD4 cell counts above 500 cells/μL who are randomised to begin ART immediately (early ART) or to defer ART until the CD4 cell count declines to below 350 cells/μL or AIDS occurs (deferred ART).
Study Population
Accrual for the START study started in April 2009 and was completed in December 2013 with 4685 HIV-positive people enrolled from 215 sites in 35 countries. Though all 4685 HIV-positive participants enrolled in START were eligible for inclusion in this analysis, 84 were excluded because either the baseline transmission risk behaviour questionnaire was missing (n=63) or the participant did not want to complete one (n=21), leaving 4601 participants who are included in this analysis. In addition, transmission risk behaviour forms were updated while recruitment was ongoing, so the first 566 (12%) participants completed the first version of the questionnaire that provided less detail in some subsidiary questions. Therefore information on frequency of CLS-D, details concerning type of sex for CLS-D, and transmission risk beliefs is available only for the 4035 participants who completed version 2 of the questionnaire.
Data collection
Baseline and follow-up schedule for data collection is the same in both arms. Study data are collected on standardised case report forms after consent at baseline (within 60 days before randomisation) and then every four months after randomisation until the study closes. Visits include a targeted medical history, a brief medical examination, and determination of CD4 cell count and HIV RNA level. The transmission risk behaviour assessment was conducted at baseline, at month 4, and every 12 months after randomisation by a self-completed questionnaire with participants identified only by a study code. Participants were told that their answers would be kept confidential from clinic staff. This report presents data from the baseline questionnaire only.
Detailed information was collected at baseline on the following: demographic and social factors including gender, year of birth, race/ethnicity, education, likely route of acquisition of HIV; and lifestyle factors including cigarette smoking status, alcohol intake, use of recreational drugs and use of injecting drugs.
Sexual behaviour during the previous two months was ascertained separately for men (sex with women and sex with men) and women (sex with men only). Participants were asked if they had condomless anal or vaginal sex in the past two months, and additionally if they had condomless sex with a person who did not have HIV or whose HIV status they did not know. This was classified as CLS-D (condomless sex with a serodifferent status partner). Participants reporting CLS-D were asked about number of partners, type of intercourse (receptive or insertive, vaginal or anal, and whether ejaculation occurred), and frequency of intercourse for CLS-D during the two-month period. Participants were also asked if they had injected recreational drugs in the previous two months and if needles or syringes or any part of the injection equipment was shared with anyone who did not have HIV or whose HIV status they did not know and, if so, with how many people.
HIV-transmission risk beliefs were also assessed in the context of ART. Each participant was asked to select which of the following options they regarded as true: “A person using HIV treatment who has an undetectable viral load [cannot] or [is much less likely] or [is a little less likely] or [is just as likely] or [is more likely] to pass on (transmit) HIV to someone else compared to someone not on treatment.”
Analysis plan
Participants were classified into one of three gender/sexuality groups based on gender, reported mode of HIV infection, and reported sexual behaviour: 1) MSM (men who were infected via sex with men, or who reported anal sex with men in the past two months), 2) heterosexual men (all men not included in the first group), and 3) women. Respondents were cateogorised as having experienced CLS-D only if they responded affirmatively to the specific CLS-D question, even if they gave inconsistent answers to leader or subsequent questions. The prevalence of CLS-D, number of CLS-D partners, and frequency of CLS-D was assessed overall and for each gender/sexuality group. Partner numbers according to type of sex for CLS-D, and transmission risk beliefs, were also described according to gender/sexuality group. The associations of demographic (age group, race, education), lifestyle (smoking status, alcohol and drug use) and other factors (region and year of recruitment, time since HIV diagnosis, baseline viral load and CD4, transmission risk beliefs) with CLS-D were assessed separately for each gender/sexuality group. Unadjusted associations were assessed by chi-squared tests; adjusted associations were assessed using a modified Poisson regression approach with robust error variance (22) to produce adjusted prevalence ratios with 95% confidence intervals (CI). Multivariable Poisson models included all factors considered in univariable analysis apart from ethnicity (excluded due to the strong association with region of recruitment) and transmission risk beliefs. Multivariable models were additionally adjusted for questionnaire version. In unadjusted and adjusted analyses, tests for trend were used to assess associations for all categorical variables that had ordered categories.
Results
Overall, 4601 people were included in this baseline analysis: 2559 MSM, 803 heterosexual men and 1239 women. The proportion of participants recruited in each geographic region was as follows: Europe/Israel 33%; South America/Mexico 25%; Africa 22%; North America 11%; Asia 8%; Australia 2%. Median age was 36 (IQR 29, 44, range 18-81) years. Overall, 45% of participants were of white ethnicity, 31% were black, 13% Hispanic, 8% Asian, and 4% other ethnicities. In terms of education, 30% were educated to high-school level or less, with 23% achieving a university degree. Overall 32% were current smokers and 10% reported recreational drug use in the past month. The majority of participants had been diagnosed with HIV within the previous two years (66%) with 18% diagnosed in the three months prior to randomisation. As per eligibility criteria, all had a CD4 cell count >500 cells/μL with 37% (1715) having a CD4 cell count >700 cells/μL. 1.9% had an HIV viral load ≤50 copies/mL.
Table 1 shows the prevalence of CLS-D. Overall, 16.7% (767/4601) of participants reported CLS-D in the two months prior to randomisation. MSM were more likely to report CLS-D (20.0%; 513/2559), compared to heterosexual men (9.5%; 76/803) or women (14.4%; 178/1239) (p<0.001 for comparison of MSM with other participants; p=0.001 for comparison of heterosexual men with women, chi-squared tests). Among those reporting CLS-D, MSM were also more likely to report multiple partners, with 16.0% (82/767) reporting more than two CLS-D partners in the previous two months compared to 3.9% (3/76) of heterosexual men and 2.2% (4/178) of women (Table 1). Frequency of CLS-D (available for 697 participants who reported CLS-D on version 2 of the questionnaire) is also given in Table 1. Among those reporting CLS-D, MSM were somewhat more likely to report having CLS-D only once during the two-month period (30.7% versus 23.0% and 25.0% among heterosexual men and women, respectively), but the proportions reporting CLS-D more than 10 times was similar across the groups (13-15%).
Table 1. Condomless sex with HIV-discordant status partner (CLS-D) according to gender/sexuality*.
| Sexual activity in past 2 months | Gender/sexuality group* | |||
|---|---|---|---|---|
|
| ||||
| TOTAL | MSM | Heterosexual Men | Women | |
|
| ||||
| n=4601 | n=2559 | n=803 | n=1239 | |
|
| ||||
| n (%) | n (%) | n (%) | n (%) | |
|
| ||||
| Condomless sex with HIV-discordant status partner (CLS-D) | ||||
| Yes | 767 (16.7) | 513 (20.0) | 76 (9.5) | 178 (14.4) |
| No | 3834 (83.3) | 2046 (80.0) | 727 (90.5) | 1061 (85.6) |
|
| ||||
| Total number CLS-D partners among those reporting CLS-D (N=767) | ||||
| 1 | 525 (68.4) | 310 (60.4) | 61 (80.3) | 154 (86.5) |
| 2 | 119 (15.5) | 98 (19.1) | 9 (11.8) | 12 (6.7) |
| 3-5 | 61 (8.0) | 54 (10.5) | 3 (4.0) | 4 (2.3) |
| >5 | 28 (3.7) | 28 (5.5) | 0 (0) | 0 (0) |
| Not given | 34 (4.4) | 23 (4.5) | 3 (3.9) | 8 (4.5) |
|
| ||||
| Frequency of CLS-D# among those reporting CLS-D (N=697$) | ||||
| Once | 199 (28.6) | 141 (30.7) | 17 (23.0) | 41 (25.0) |
| 2-10 times | 349 (50.1) | 217 (47.3) | 42 (56.8) | 90 (54.9) |
| 11-30 times | 57 (8.2) | 41 (8.9) | 5 (6.8) | 11 (6.7) |
| >30 times | 43 (6.2) | 27 (5.9) | 5 (6.8) | 11 (6.7) |
| Not given | 49 (7.0) | 33 (7.2) | 5 (6.8) | 11 (6.7) |
Defined on the basis of gender, mode of HIV transmission, and sexual behaviour in past two months. Men who reported either MSM transmission or anal sex with another man in past two months were classified as MSM.
Question not asked of 566 participants who did not receive version 2 of the transmission risk behaviour (TRB) questionnaire.
Number reporting CLS-D/Total number, for subgroup who received version 2 of TRB questionnaire: 459/2179 MSM; 74/718 heterosexual men; 164/1138 women.
Table 2 shows the unadjusted associations of factors with CLS-D, separately for MSM, heterosexual men and women. Factors significantly associated with having CLS-D in MSM were: being of younger age (24% age <30 years versus 14% age >50 years, p<0.001 for trend); being of Hispanic (25%), black (23%), or other (29%) ethnicity compared to white (18%) or Asian (18%, p<0.001 for comparison across ethnicities); being from South America/Mexico (28%) or North America (21%) or compared to other regions (≤16%, p<0.001 for comparison across regions). There was also a significant inverse association with time since HIV diagnosis, with 36% of MSM diagnosed with HIV for less than 3 months reporting CLS-D in the previous 2 months, falling to 19% among those diagnosed 3-6 months ago, and 14% among those diagnosed for 5 years or more (p<0.001 for trend). However it is important to note that among participants diagnosed for a few months only it is not known if the CLS-D occurred before or after diagnosis of HIV. MSM with a higher CD4 cell count tended to be more likely to report CLS-D than those with lower CD4 (p=0.045 for trend). Recreational drug use was also strongly associated with CLS-D (29% and 26% among those using drugs ≥1 day per week and <1 day per week, respectively, compared to 19% among those not using drugs, p<0.001 for trend). CLS-D was not significantly associated with education level, viral load level, cigarette smoking status or usual alcohol intake. In addition, there was no significant trend in prevalence of CLS-D with calendar year of randomisation into START. MSM who believed that someone with an undetectable viral load was much less likely to transmit HIV tended to have lower prevalence of CLS-D than MSM who did not concur with this belief (19% versus 23%, p=0.048).
Table 2. Number and percent reporting CLS-D according to baseline characteristics and gender/sexuality.
| TOTAL n=4601 | MSM n=2559 | Heterosexual men n=803 | Women n=1239 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n (%) | n | n (%) CLS-D | p-value | n | n (%) CLS-D | p-value | n | n (%) CLS-D | p-value | |
|
| ||||||||||
| Age group (years) | ||||||||||
| <30 | 1296 (28.2) | 881 | 208 (23.6) | <0.001 | 131 | 15 (11.5) | 0.16 | 284 | 56 (19.7) | <0.001 |
| 30-39 | 1562 (33.9) | 860 | 174 (20.2) | trend | 271 | 27 (10.0) | trend | 431 | 74 (17.2) | trend |
| 40-49 | 1196 (26.0) | 609 | 102 (16.7) | 256 | 25 (9.8) | 331 | 34 (10.3) | |||
| ≥50 | 547 (11.9) | 209 | 29 (13.9) | 145 | 9 (6.2) | 193 | 14 (7.3) | |||
|
| ||||||||||
| Race: | ||||||||||
| White | 2052 (44.6) | 1596 | 284 (17.8) | <0.001 | 267 | 18 (6.7) | 0.052 | 189 | 22 (11.6) | 0.005 |
| Black | 1401 (30.4) | 249 | 58 (23.3) | 366 | 44 (12.0) | 786 | 127 (16.2) | |||
| Hispanic | 602 (13.1) | 450 | 114 (25.3) | 60 | 6 (10.0) | 92 | 14 (15.2) | |||
| Asian | 386 (8.4) | 171 | 30 (17.5) | 78 | 3 (3.9) | 137 | 7 (5.1) | |||
| Other | 160 (3.5) | 93 | 27 (29.0) | 32 | 5 (15.6) | 35 | 8 (22.9) | |||
|
| ||||||||||
| Region: | ||||||||||
| Europe/Israel | 1518 (33.0) | 1173 | 177 (15.1) | <0.001 | 207 | 6 (2.9) | <0.001 | 138 | 14 (10.1) | 0.009 |
| North America | 496 (10.8) | 307 | 64 (20.8) | 83 | 6 (7.2) | 106 | 17 (16.0) | |||
| South America/Mexico | 1135 (24.7) | 813 | 231 (28.4) | 152 | 24 (15.8) | 170 | 31 (18.2) | |||
| Asia | 354 (7.7) | 148 | 24 (16.2) | 72 | 4 (5.6) | 134 | 7 (5.2) | |||
| Africa | 994 (21.6) | 24 | 3 (12.5) | 283 | 36 (12.7) | 687 | 109 (15.9) | |||
| Australia | 104 (2.3) | 94 | 14 (14.9) | 6 | 0 | 4 | 0 | |||
|
| ||||||||||
| Year of randomisation | ||||||||||
| 2009-2010 | 979 (21.3) | 672 | 117 (17.4) | 0.093 | 151 | 9 (6.0) | 0.13 | 156 | 22 (14.0) | 0.64 |
| 2011 | 873 (19.0) | 590 | 135 (22.9) | trend | 128 | 11 (8.6) | trend | 155 | 17 (11.0) | trend |
| 2012 | 1531 (33.3) | 765 | 136 (17.8) | 279 | 31 (11.1) | 487 | 76 (15.6) | |||
| 2013 | 1218 (26.5) | 532 | 125 (23.5) | 245 | 25 (10.2) | 441 | 63 (14.3) | |||
|
| ||||||||||
| Education: | ||||||||||
| Less than high school | 1381 (30.0) | 269 | 55 (20.4) | 0.82 | 353 | 41 (11.6) | 0.013 | 759 | 106 (14.0) | 0.41 |
| High school/A-level equivalent | 994 (21.6) | 546 | 100 (18.3) | trend | 195 | 18 (9.2) | trend | 253 | 35 (13.8) | trend |
| Vocational training/some college | 1187 (25.8) | 882 | 197 (22.3) | 143 | 14 (9.8) | 162 | 26 (16.1) | |||
| University degree or higher | 1039 (22.6) | 862 | 161 (18.7) | 112 | 3 (2.7) | 65 | 11 (16.9) | |||
|
| ||||||||||
| Time since HIV diagnosis: | ||||||||||
| <3 months | 810 (17.6) | 499 | 180 (36.1) | <0.001 | 124 | 21 (16.9) | 0.004 | 187 | 38 (20.3) | 0.001 |
| 3-6 months | 710 (15.4) | 476 | 90 (18.9) | trend | 100 | 12 (12.0) | trend | 134 | 19 (14.2) | trend |
| 6 months - 2 years | 1508 (32.8) | 886 | 135 (15.2) | 261 | 17 (6.5) | 361 | 56 (15.5) | |||
| 2-5 years | 923 (20.1) | 468 | 77 (16.5) | 174 | 18 (10.3) | 281 | 42 (15.0) | |||
| ≥5 years | 650 (14.1) | 230 | 31 (13.5) | 144 | 8 (5.6) | 276 | 23 (8.3) | |||
|
| ||||||||||
| Baseline CD4 cell count (cells/μL): | ||||||||||
| 500-599 | 1450 (31.5) | 862 | 167 (19.4) | 0.045 | 241 | 21 (8.7) | 0.40 | 347 | 43 (12.4) | 0.51 |
| 600-699 | 1436 (31.2) | 846 | 148 (17.5) | trend | 235 | 20 (8.5) | trend | 355 | 58 (16.3) | trend |
| ≥700 | 1715 (37.3) | 851 | 198 (23.3) | 327 | 35 (10.7) | 537 | 77 (14.3) | |||
|
| ||||||||||
| Baseline VL (log10 copies/mL): | ||||||||||
| <3 | 591 (12.9) | 190 | 33 (17.4) | 0.76 | 121 | 9 (7.4) | 0.40 | 280 | 37 (13.2) | 0.88 |
| 3-3.9 | 1467 (31.9) | 766 | 172 (22.5) | trend | 247 | 24 (9.7) | trend | 454 | 66 (14.5) | trend |
| 4-4.9 | 2058 (44.8) | 1279 | 241 (18.8) | 349 | 33 (9.5) | 430 | 69 (16.1) | |||
| ≥5 | 477 (10.4) | 319 | 67 (21.0) | 86 | 10 (11.6) | 72 | 6 (8.3) | |||
|
| ||||||||||
| Current smoker: | ||||||||||
| No | 3147 (68.4) | 1571 | 324 (20.6) | 0.36 | 510 | 50 (9.8) | 0.66 | 1066 | 146 (13.7) | 0.095 |
| Yes | 1454 (31.6) | 988 | 189 (19.1) | 293 | 26 (8.9) | 173 | 32 (18.5) | |||
|
| ||||||||||
| Usual alcohol intake (number of drinks)* | ||||||||||
| 0 | 1248 (27.1) | 365 | 69 (18.9) | 251 | 30 (12.0) | 632 | 78 (12.3) | |||
| 1 | 830 (18.0) | 524 | 97 (18.5) | 0.095 | 130 | 8 (6.2) | 0.42 | 176 | 25 (14.2) | <0.001 |
| 2-4 | 1704 (37.0) | 1192 | 237 (19.9) | trend$ | 266 | 25 (9.4) | trend$ | 246 | 49 (19.9) | trend$ |
| ≥5 | 554 (12.0) | 384 | 91 (23.7) | 106 | 10 (9.4) | 64 | 19 (29.7) | |||
| Missing | 265 (5.8) | 94 | 19 (20.2) | 50 | 3 (6.0) | 121 | 7 (5.8) | |||
|
| ||||||||||
| Days per week used recreational drugs in past month$ | ||||||||||
| None | 3855 (83.8) | 2064 | 386 (18.7) | <0.001 | 701 | 63 (9.0) | 0.103 | 1090 | 16 (14.9) | 0.005 |
| <1 day per week | 335 (7.3) | 298 | 78 (26.2) | trend$ | 24 | 3 (12.5) | trend$ | 13 | 5 (38.5) | trend$ |
| ≥1 days per week | 111 (2.4) | 79 | 23 (29.1) | 21 | 4 (19.1) | 11 | 4 (36.4) | |||
| Missing | 300 (6.5) | 118 | 26 (22.0) | 57 | 6 (10.5) | 125 | 7 (5.6) | |||
|
| ||||||||||
| Transmission beliefs - believes someone with undetectable VL cannot or much less likely to pass on HIV# (N=3902) | ||||||||||
| Yes | 1362 (34.9) | 801 | 151 (18.9) | 0.048 | 210 | 18 (8.6) | 0.34 | 351 | 48 (13.7) | 0.79 |
| No | 2540 (65.1) | 1327 | 298 (22.5) | 464 | 51 (11.0) | 749 | 107 (14.3) | |||
“When you drink alcohol, how many drinks do you normally have?”
Includes amphetamines, methamphetamines, MDMA, powder or crack cocaine, ketamine, and opiates.
Trend across categories, excluding missing category.
Question not asked of 566 participants who did not receive version 2 of transmission risk behaviour questionnaire, missing values for further 133 participants.
CLS-D= condomless sex with one or more serodifferent partners; VL=viral load; MSM=men who have sex with men.
The pattern of associations with CLS-D was broadly similar for heterosexual men. Prevalence of CLS-D decreased with age but this trend was not significant (p=0.16 for trend). CLS-D prevalence differed by region, with highest prevalence in South America/Mexico (16%) and Africa (13%) and lowest in Europe/Israel (3%) (p<0.001 for comparison across regions), and tended to be higher among those of black, Hispanic or other ethnicities (p=0.052 for comparison across ethnicities). Lower level of education was significantly associated with reporting of CLS-D in heterosexual men with 12% of those achieving a less than high-school education reporting CLS-D compared to 3% of those achieving a university degree or higher (p=0.013 for trend). As with MSM, time since HIV diagnosis was inversely associated with CLS-D with 17% of MSM diagnosed with HIV for less than 3 months reporting CLS-D in the previous 2 months, falling to 12% in those diagnosed between 3-6 months ago, and 6% among those diagnosed for at least 5 years (p=0.004 for trend). There was no significant association of CLS-D with CD4 cell count, viral load, smoking status and alcohol intake, or transmission beliefs among heterosexual men. Although there was a tendency for CLS-D prevalence to be higher for those randomised more recently, and higher among those taking recreational drugs, these trends were not statistically significant.
The pattern of association was also similar for women. Among women, younger age was significantly associated with CLS-D, with 20% of women age <30 years reporting CLS-D compared to 7% of those age >50 years (p<0.001 for trend). Ethnicity and region were associated with CLS-D, with those of black, Hispanic and other ethnicity reporting higher prevalences of CLS-D than those of white or Asian ethnicity (p=0.005 for comparison across ethnicities), and women in North America, South America/Mexico and Africa reporting higher prevalence of CLS-D than those in other regions (p=0.009 for comparison across regions). The significant inverse association between time since HIV diagnosis and CLS-D was also apparent in women with 20% of those diagnosed for <3 months reporting CLS-D compared to 14% diagnosed for between 3-6 months and 8% in those diagnosed >5 years (p<0.001 for trend). Increasing alcohol intake and use of recreational drugs were each associated with increased prevalence of CLS-D (p<0.001 and p=0.005 for trend, respectively). There was no significant association with year of randomisation, education, CD4, viral load, smoking status or transmission risk beliefs.
Adjusted associations are shown in Table 3 for MSM, heterosexual men and women separately. Among MSM, region of recruitment, younger age, more recent HIV diagnosis, and recreational drug use were independently associated with CLS-D (p<0.05 for all). Among heterosexual men, region of recruitment and more recent HIV diagnosis were independently associated with CLS-D. Among women, region of recruitment, younger age, more recent HIV diagnosis and greater alcohol intake were independently associated with CLS-D. Some patterns of association were common across gender/sexuality groups. In each gender/sexuality group, the significant association between region and CLS-D remained in multivariable analysis, indicating that this association was not explained by other demographic, lifestyle or HIV-related factors. Compared to Europe/Israel, prevalence of CLS-D tended to be higher among participants from South America/Mexico and North America, and for heterosexual men and women, Africa. In each gender/sexuality group, the trend of decreasing CLS-D prevalence with longer time since diagnosis remained significant in multivariable analysis, mainly due to the higher prevalence of CLS-D among participants diagnosed <3 months prior to randomisation compared to all other groups.
Table 3. Adjusted association of factors with CLS-D, according to gender/sexuality.
| Adjusted prevalence ratios (95% CI) | MSM | Heterosexual Men | Women |
|---|---|---|---|
|
| |||
| n*=2554 | n*=803 | n*=1236 | |
|
| |||
| Age group | |||
| <30 years | 1 | 1 | 1 |
| 30-39 | 0.95 (0.79, 1.13) | 1.02 (0.56, 1.87) | 0.93 (0.68, 1.28) |
| 40-49 | 0.87 (0.71, 1.09) | 1.05 (0.56, 1.95) | 0.54 (0.36, 0.82) |
| ≥50 | 0.70 (0.50, 1.00) | 0.79 (0.34, 1.85) | 0.40 (0.22, 0.74) |
| p=0.037 trend | p=0.68 trend | p<0.001 trend | |
|
| |||
| Region | |||
| Africa | 0.84 (0.28, 2.48) | 3.67 (1.47, 9.13) | 1.53 (0.90, 2.60) |
| Asia/Australia | 0.95 (0.68, 1.32) | 1.41 (0.39, 5.11) | 0.49 (0.19, 1.28) |
| Europe/Israel | 1 | 1 | 1 |
| North America | 1.46 (1.12, 1.91) | 2.27 (0.75, 6.85) | 1.46 (0.78, 2.74) |
| South America/Mexico | 1.68 (1.39, 2.03) | 5.26 (2.17, 12.75) | 1.50 (0.84, 2.68) |
| p<0.001 | p<0.001 | p=0.004 | |
|
| |||
| Time since HIV diagnosis | |||
| <3 months | 1 | 1 | 1 |
| 3-6 months | 0.55 (0.44, 0.69) | 0.79 (0.42, 1.48) | 0.65 (0.39, 1.06) |
| 6 months -2 years | 0.49 (0.40, 0.60) | 0.46 (0.25, 0.86) | 0.73 (0.51, 1.05) |
| 2-5 years | 0.55 (0.43, 0.70) | 0.75 (0.40, 1.40) | 0.81 (0.54, 1.21) |
| ≥5 years | 0.45 (0.31, 0.64) | 0.35 (0.15, 0.79) | 0.51 (0.31, 0.83) |
| p<0.001 trend | p=0.020 trend | p=0.042 | |
|
| |||
| Year of randomisation | |||
| 2009-2010 | 1 | 1 | 1 |
| 2011 | 0.91 (0.71, 1.16) | 0.84 (0.37, 1.91) | 0.74 (0.40, 1.36) |
| 2012 | 0.70 (0.54, 0.91) | 0.91 (0.44, 1.86) | 1.04 (0.63, 1.71) |
| 2013 | 0.87 (0.67, 1.13) | 0.73 (0.34, 1.58) | 1.05 (0.62, 1.78) |
| p=0.13 trend | p=0.45 trend | p=0.46 trend | |
|
| |||
| Education: | |||
| Less than high school | 1 | 1 | 1 |
| High school/A-level equivalent | 0.83 (0.62, 1.11) | 0.89 (0.52, 1.52) | 0.86 (0.59, 1.23) |
| Vocational training/some college | 1.10 (0.84, 1.43) | 1.14 (0.63, 2.09) | 1.01 (0.66, 1.53) |
| University degree or higher | 0.97 (0.73, 1.28) | 0.40 (0.12, 1.34) | 1.23 (0.67, 2.23) |
| p=0.45 trend | p=0.30 trend | p=0.78 trend | |
|
| |||
| Baseline CD4 cell count (cells/μL): | |||
| 500-599 | 1 | 1 | 1 |
| 600-699 | 0.91 (0.75, 1.11) | 1.02 (0.58, 1.78) | 1.23 (0.92, 1.85) |
| ≥700 | 1.15 (0.96, 1.38) | 1.21 (0.73, 2.00) | 1.19 (0.78, 1.58) |
| p=0.13 trend | p=0.45 trend | p=0.69 trend | |
|
| |||
| Baseline VL (log10 copies/mL): | |||
| <3 | 1 | 1 | 1 |
| 3-3.9 | 1.23 (0.88, 1.72) | 1.40 (0.68, 2.88) | 1.07 (0.73, 1.56) |
| 4-4.9 | 1.12 (0.80, 1.56) | 1.50 (0.75, 3.00) | 1.13 (0.77, 1.66) |
| ≥5 | 1.29 (0.88, 1.88) | 1.81 (0.74, 4.40) | 0.59 (0.27, 1.32) |
| p=0.56 trend | p=0.18 trend | p=0.80 trend | |
|
| |||
| Current smoker: | |||
| No | 1 | 1 | 1 |
| Yes | 0.94 (0.80, 1.12) | 0.99 (0.61, 1.61) | 1.04 (0.69, 1.56) |
| p=0.50 | p=0.97 | p=0.86 | |
|
| |||
| Usual alcohol intake (drinks)* | |||
| 0 | 1 | 1 | 1 |
| 1 | 1.06 (0.81, 1.39) | 0.55 (0.27, 1.12) | 1.12 (0.74, 1.70) |
| 2-4 | 1.13 (0.88, 1.44) | 0.85 (0.51, 1.43) | 1.56 (1.10, 2.22) |
| ≥5 | 1.14 (0.86, 1.50) | 0.72 (0.36, 1.46) | 2.07 (1.26, 3.40) |
| Missing | 0.91 (0.51, 1.61) | 0.20 (0.06, 0.70) | 0.87 (0.21, 3.67) |
| p=0.26 trend$ | p=0.44 trend$ | p=0.003 trend$ | |
|
| |||
| Days per week used recreational drugs in past month$ | |||
| None | 1 | 1 | 1 |
| <1 day per week | 1.55 (1.26, 1.93) | 1.86 (0.60, 5.75) | 1.75 (0.88, 3.49) |
| ≥1 days per week | 1.67 (1.18, 2.37) | 2.31 (0.95, 5.62) | 2.09 (0.78, 5.64) |
| Missing | 1.58 (0.98, 2.55) | 3.74 (1.71, 8.19) | 0.65 (0.15, 2.81) |
| p<0.001 trend$ | p=0.14 trend$ | p=0.13 trend$ | |
Adjusted prevalence ratios from modified Poisson regression models.
Models include all factors above, additionally adjusted for questionnaire version.
Number included in model.
Trend across categories, excluding missing category.
CLS-D= condomless sex with one or more serodifferent partners; VL=viral load; MSM=men who have sex with men.
Table 4 shows number of partners according to type of CLS-D among 697 participants reporting CLS-D who completed version 2 of the questionnaire. Among the 459 MSM reporting CLS-D with at least one partner in the previous two months, 40% reported being the insertive partner with ejaculation on at least one occasion, 48% reported being insertive with no ejaculation at least once, and 75% reported being the receptive partner on at least one occasion (Table 4). Of MSM reporting CLS-D, 6% reported anal intercourse with ejaculation with a female partner during the previous two months. Figure 1 reports mutually exclusive categories of the type of intercourse among the 459 MSM who reported CLS-D with male and female partners. Of the 436 MSM reporting anal CLS-D with men, 32% reported receptive anal CLS-D only, and a further 25% reported insertive anal CLS-D always without ejaculation.
Table 4. Numbers of partners according to type of CLS-D, by gender/sexuality among participants reporting at least one episode of CLS-D.
| Type of CLS-D according to gender/sexuality group | Reported type of sex with at least one partner | Number of partners reported | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0 | 1 | 2 | 3-5 | ≥5 | Not answered | ||
|
| |||||||
| MSM (For n=459 reporting CLS-D) | |||||||
|
| |||||||
| Anal sex with men | |||||||
| Insertive with ejaculation | 182 (39.7%) | 213 (46.4) | 144 (31.4) | 20 (4.4) | 11 (2.4) | 7 (1.5) | 64 (13.9) |
| Insertive, no ejaculation | 220 (47.9%) | 184 (40.1) | 164 (35.7) | 27 (5.9) | 14 (3.1) | 15 (3.3) | 55 (12.0) |
| Receptive | 345 (75.2%) | 79 (17.2) | 243 (52.9) | 61 (13.3) | 18 (3.9) | 23 (5.0) | 35 (7.6) |
|
| |||||||
| Vaginal sex with women | |||||||
| Insertive with ejaculation | 22 (4.8%) | 57 (12.4) | 12 (2.6) | 5 (1.1) | 4 (0.9) | 1 (0.2) | 380 (82.8) |
| Insertive, no ejaculation | 32 (7.0%) | 47 (10.2) | 21 (4.6) | 5 (1.1) | 5 (1.1) | 1 (0.2) | 380 (82.8) |
|
| |||||||
| Anal sex with women | |||||||
| Insertive with ejaculation | 26 (5.7%) | 58 (12.6) | 13 (2.8) | 5 (1.1) | 6 (1.3) | 2 (0.4) | 375 (81.7) |
| Insertive, no ejaculation | 34 (7.4%) | 48 (10.5) | 21 (4.6) | 8 (1.7) | 2 (0.4) | 3 (0.7) | 377 (82.1) |
|
| |||||||
| Heterosexual Men (For n=74 reporting CLS-D) | |||||||
|
| |||||||
| Vaginal sex with women | |||||||
| Insertive with ejaculation | 57 (77.0%) | 16 (21.6) | 48 (64.9) | 6 (8.1) | 3 (4.1) | 0 (0) | 1 (1.4) |
| Insertive, no ejaculation | 26 (35.1%) | 37 (50.0) | 23 (31.1) | 1 (1.4) | 1 (1.4) | 1 (1.4) | 11 (14.9) |
|
| |||||||
| Anal sex with women | |||||||
| Insertive with ejaculation | 11 (14.9%) | 54 (73.0) | 7 (9.5) | 3 (4.1) | 1 (1.4) | 0 (0) | 9 (12.2) |
| Insertive, no ejaculation | 12 (16.2%) | 52 (70.3) | 8 (10.8) | 4 (5.4) | 0 (0) | 0 (0) | 10 (13.5) |
|
| |||||||
| Women (For n=164 reporting CLS-D) | |||||||
|
| |||||||
| Vaginal sex with men | 157 (95.7%) | 3 (1.8) | 142 (86.6) | 12 (7.3) | 3 (1.8) | 0 (0) | 4 (2.4) |
|
| |||||||
| Anal sex with men | 18 (11.0%) | 124 (75.6) | 16 (9.8) | 2 (1.2) | 0 (0) | 0 (0) | 22 (13.4) |
Note that no attempt has been made to reconcile partner numbers reported in these subsidiary questions with overall CLS-D partner numbers reported in the main question.
Version 2 of TRB questionnaire ONLY.
CLS-D= condomless sex with one or more serodifferent partners; MSM=men who have sex with men.
Figure 1.
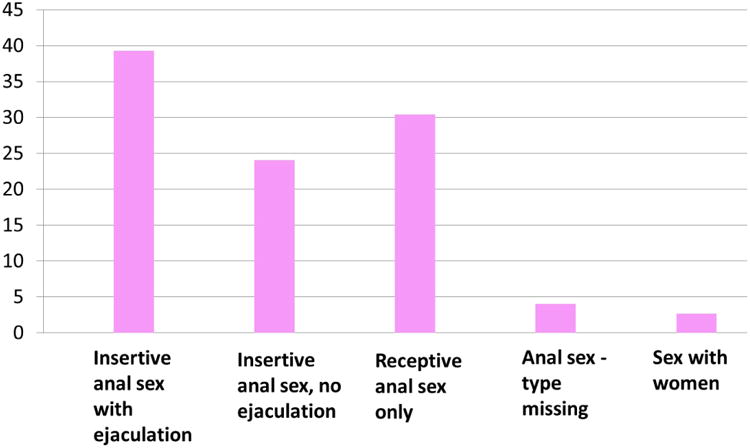
Type of sex among HIV-positive MSM who reported CLS-D. Type of sex for CLS-D, exclusive categories with men being classified according to the first type of sex they reported when moving from left to right on the x axis. Percentages add up to 100. Includes MSM reporting CLS-D using version 2 of questionnaire (N=459).
MSM=Men who have Sex with Men; CLS-D= condomless sex with one or more serodifferent partners
Among 74 heterosexual men who reported CLS-D with a female partner, 77% reported insertive vaginal CLS-D with ejaculation and 35% without ejaculation on at least one occasion (Table 4). A proportion (15%) had insertive anal CLS-D with ejaculation at least once with a female partner. Among 164 women who reported CLS-D with a male partner, the majority (96%) reported that the type of CLS-D was vaginal intercourse; 11% reported anal CLS-D on at least one occasion.
Transmission risk beliefs are shown in Table 5 according to gender/sexuality group, for the subgroup of 3902 participants who completed version 2 of the questionnaire and provided information on this factor. For all gender/sexuality groups, there was a wide range of beliefs regarding the transmission risks of an individual on ART compared to someone not on ART. Overall, just over a third of participants believed that someone with an undetectable viral load was much less likely to, or could not, transmit HIV; this proportion was somewhat higher for MSM (38%) than for heterosexual men (31%) and women (32%) (p<0.001).
Table 5. Transmission risk beliefs according to gender/sexuality.
| Transmission risk beliefs: “A person using HIV treatment who has an undetectable viral load…”* | Gender/sexuality group | |||
|---|---|---|---|---|
|
| ||||
| TOTAL | MSM | Heterosexual Men | Women | |
|
| ||||
| n=3902 | n=2128 | n=674 | n=1100 | |
|
| ||||
| n (%) | n (%) | n (%) | n (%) | |
|
| ||||
| Cannot transmit HIV | 269 (6.9) | 71 (3.3) | 63 (9.4) | 135 (12.3) |
| Is much less likely to transmit HIV | 1093 (28.0) | 730 (34.3) | 147 (21.8) | 216 (19.6) |
| Is a little less likely to transmit HIV | 1050 (26.9) | 490 (23.0) | 199 (29.5) | 361 (32.8) |
| Is just as likely to transmit HIV | 1140 (29.2) | 724 (34.0) | 181 (26.9) | 235 (21.3) |
| Is more likely to transmit HIV | 350 (9.0) | 113 (5.3) | 84 (12.5) | 153 (13.9) |
133 participants who received version 2 of the transmission risk behaviour questionnaire but did not answer the question are excluded.
Version 2 of TRB questionnaire ONLY.
MSM=men who have sex with men.
Very few participants in START reported injecting drug use in the two months prior to randomisation. Overall, among all 4504 participants who responded to questions on recreational drug use, only 1.4% (n=65) reported injecting, and of these 8 had shared injecting equipment with someone of negative or unknown HIV status. Among MSM, 1.9% (47) reported injecting with three men sharing equipment; in heterosexual men 1.3% (10) reported injecting with one of these sharing equipment; and in women 0.7% (8) reported injecting with four women sharing equipment.
Discussion
Ongoing HIV transmission globally is largely due to condomless intercourse between serodifferent partners where the HIV-positive partner has an unsuppressed viral load. Transmissions occur both from those with knowledge of their HIV serostatus and from those without. In the United States it is estimated that over 50% of the approximate 50,000 incident infections per year are from individuals diagnosed with HIV with unsuppressed virus who have CLS-D, resulting in a transmission rate from those with diagnosed HIV of 4.4 per 1000 per year (23). The clinical findings of the START trial will indirectly be very important for public health and transmission. If the study reports an individual health benefit with ART at high CD4 cell counts this will extend the use of ART with subsequent secondary transmission benefits. In this study we report baseline data on risk behaviours for HIV transmission in ART-naïve HIV-diagnosed individuals entering the START trial, prior to being randomised to immediate or deferred ART.
We found in our study that the proportion reporting CLS-D was higher in MSM, at 20%, compared to either heterosexual men (10%) or women (14%). We also found that MSM were more likely to report a greater number of partners among those reporting CLS-D. For example, 6% of MSM reported CLS-D with more than five partners in the previous two months. This is of concern given the high ongoing incidence of HIV (and other STIs) in MSM in particular. In high-income settings, HIV infection continues to affect MSM disproportionately (24, 25). Increases in STIs among both HIV-negative and HIV-positive MSM suggest that a proportion of HIV-positive MSM aware of their diagnosis are not altering or reducing sexual risk behaviour to minimise ongoing HIV transmission (25, 26). Prevalence of condomless intercourse among HIV-diagnosed people has been most widely studied in MSM. A meta-analysis of 30 studies in HIV-diagnosed MSM in the United States that combined studies using different recall periods and a variety of sampling methods, from 2000-2007, found that the prevalence of CLS-D with partners of negative (13%) or unknown (16%) HIV status was 26% overall (1). This is similar to the prevalence of 20% found in our study among MSM using a two-month recall period. In a recent large UK study, the prevalence of CLS-D among a sample of over 2000 HIV-diagnosed MSM recruited from outpatient clinics was 15% overall for a three-month recall period, again similar to our results for the European region (15% for a two-month recall period) (27).
In MSM, factors associated with having CLS-D in our study included younger age, more recent HIV diagnosis, recreational drug use, region of recruitment, and being of Hispanic, black, or other ethnicity compared to being white or Asian. Our results concerning ethnicity appear to contrast with a previous meta-analysis of US MSM that found a lower prevalence of CLS-D in “MSM of colour” compared to “white MSM” (1). Another US study reported high prevalences of CLS-D in HIV-positive bisexual black men, with 26% reporting anal CLS-D with a male partner and 28% reporting anal CLS-D with a female partner (28). In our study, prevalence of CLS-D also varied across geographic regions, being higher in MSM from North and South America/Mexico compared to Europe. This association remained after adjustment for other factors, but could reflect differences in being prepared to report CLS-D as well as real differences in CLS-D prevalence between ethnicities and regions. Prevalence of CLS-D also varied significantly according to region of recruitment among heterosexual men and women, with participants from Africa, in addition to those from North and South America/Mexico, tending to report higher prevalence than those from Europe.
We also found that length of time since HIV diagnosis was significantly associated with prevalence of CLS-D in the past two months, in all gender/sexuality groups. The most marked effect was for those diagnosed for less than three months, who had a considerably higher prevalence of CLS-D than other groups. This suggests that in some cases participants were reporting risk behaviour that occurred before they knew that they were HIV positive. It is not clear how much of the reported risk behaviour occurred in a period prior to receiving an HIV diagnosis among those diagnosed for less than three months. Exclusion of the relatively high proportion of participants who were diagnosed less than three months ago reduced the overall prevalence of CLS-D in the past two months to 16.2%, 8.1%, and 13.3% among MSM, heterosexual men, and women, respectively. However, associations with other factors were similar in this subgroup. Being diagnosed with HIV may have had an impact, at least in the initial stages, to reduce behaviours associated with onward transmission. This reduction in risk behaviours after HIV diagnosis has been reported previously, with some studies indicating that MSM diagnosed with HIV decrease sexual risk behaviour immediately after becoming aware of their HIV-positive status (29). However, a recent longitudinal study in MSM in Amsterdam indicated that the reduction in both condomless anal intercourse and number of sex partners following HIV diagnosis lasted only about one year and then it tended to increase again, until after four years it reached almost the same levels as one year before HIV diagnosis (30). As most men in that study were not on ART this had significant impact for the onward transmission of the virus. In our study, in all gender/sexuality groups, the trend of decreasing prevalence of CLS-D with longer time since HIV diagnosis remained after adjustment for other factors, with the lowest CLS-D prevalence reported in those diagnosed for more than five years. These data suggest that if ART is used for prevention purposes it will have maximum effectiveness if started as soon as possible after diagnosis.
Although education was not associated with CLS-D in MSM or women, there was a significant association in heterosexual men, with prevalence being lower in those who attained higher levels of formal education. However, the association did not remain significant after adjustment for other factors. In contrast to these findings, the baseline results from the SMART study suggested higher levels of education were associated with higher prevalence of high-risk behaviours (2).
We found no association between believing ART rendered individuals less infectious and higher reporting of CLS-D in any group although assessment of this association is more relevant once individuals start ART. Overall, knowledge of the effects of ART on transmission risk was low, particularly in heterosexual men and women. However, two fifths were randomised prior to 2012 and the subsequent changes to guidelines that recommended explaining to all HIV-positive individuals that there is a beneficial effect of ART on infectiousness and that ART should be offered to anyone who wants to take it to reduce transmission risk (9, 13, 32, 33). HPTN 052 data on transmission reduction in the early ART arm were presented in 2011 (3), but it is unclear how long it takes for awareness of biomedical prevention measures to reach the public. Attitudes to taking early ART to prevent transmission may have changed rapidly as the publicity around treatment for prevention gained momentum. However, the prevalence of CLS-D did not increase significantly with calendar year of recruitment in this study, nor did the proportion who believed that ART rendered individuals less infectious (data not shown).
It has been reported that HIV-diagnosed MSM engage in harm-reduction approaches including primarily having condomless sex with other HIV-positive men (serosorting) (19). In terms of reduction of HIV transmission, serosorting has been shown to be effective in established relationships where the HIV status of a regular partner is explicitly known, but is not effective in casual sex settings where HIV status is unknown or only assumed (20). In addition, serosorting on the basis of HIV status does not protect against acquisition of other STIs (21). Some HIV-positive MSM also engage in seropositioning and are reported to be more likely to be the receptive partner during CLS-D because they recognise that the risk of HIV transmission to the negative partner is less when the HIV-negative partner is in the insertive position (19). One study reported that more than half of gay men in serodifferent relationships who reported condomless anal intercourse engaged in strategic positioning, such that the HIV-negative men were insertive and the HIV-positive men were receptive (15). In keeping with this, a substantial proportion of MSM reporting CLS-D in our study appeared to take transmission risk limitation measures with partners of negative or unknown status, reporting a substantially higher prevalence of receptive (75%) than insertive (40%) CLS-D. The relatively high proportion of MSM in our study who reported receptive anal sex only or absence of ejaculation when having insertive anal sex suggests attempts to limit transmission risk rather than sexual preferences alone.
The prevalence of injecting drug use in the two months prior to randomisation was very low, with only 1.4% of START participants reporting injecting, and about 12% of these injecting drug users reporting shared injecting equipment with someone of negative or unknown HIV status. Another study in HIV-positive people who inject drugs, the INSPIRE project, reported that 28% of HIV-positive people who injected drugs also engaged in CLS-D and 12% shared injecting equipment (31).
In this study sexual behaviours were self-reported, which is potentially subject to social desirability bias, although participants were aware that clinic staff would not see their responses. Another limitation is that we did not collect information about condomless sex with partners known to be HIV positive so we cannot assess if the prevalence of insertive or receptive CLS-D differed with HIV-positive partners compared to partners who were HIV negative or whose status was unknown. A further limitation is the inclusion of very few people who injected drugs. Nevertheless, the large geographically diverse sample enables us to compare sexual behaviour across regions using a large sample and a standardised methodology, which has not been done previously.
In summary, we found that a significant minority of HIV-positive people enrolled in the START trial reported recent condomless intercourse with HIV-discordant partner(s) at baseline. The proportion reporting CLS-D was higher in MSM than in heterosexuals. However, a substantial proportion of MSM reporting CLS-D appeared to take transmission risk limitation measures. We also found that CLS-D was more common in North and South America/Mexico than Europe, although this could reflect differences in willingness to report such activity as well as real differences in activity. Overall, knowledge of the effects of ART on transmission risk was low.
An understanding of associations with CLS-D is needed to inform treatment and prevention services, which may include offering ART at high CD4 cell counts to those who may have a high risk of onward transmission of HIV. With the reported baseline information in this study, the longitudinal data on sexual transmission risk in the START trial will provide evidence concerning the effect of early initiation of ART on both individual and population-level HIV transmission risk. This information, combined with the findings of the main START trial, will be important in assessing the impact of early ART and the population-based effect of treatment as prevention on HIV incidence.
Acknowledgments
We would like to thank the START participants without whom this work would not be possible. See INSIGHT START Study Group, 2015, this supplement for a complete list of START investigators.
Funding: The START study is primarily funded by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1-AI068641, the Department of Bioethics at the NIH Clinical Center and five NIH institutes: the National Cancer Institute, the National Heart, Lung, and Blood Institute, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke and the National Institute of Arthritis and Musculoskeletal disorders. Financial support is also provided by the French Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS), the German Ministry of Education and Research, the European AIDS Treatment Network (NEAT), the Australian National Health and Medical Research Council, and the UK Medical Research Council and National Institute for Heath Research. Six pharmaceutical companies (AbbVie, Inc., Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV Healthcare, Janssen Scientific Affairs, LLC, and Merck Sharp and Dohme Corp.) donate antiretroviral drugs to START.
Footnotes
The START study is registered at clinicaltrials.gov (NCT00867048).
Disclosures: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The University of Minnesota, the sponsor of START, receives royalties from the use of abacavir, one of the HIV medicines that can be used in START.
References
- 1.Crepaz N, Marks G, Liau A, et al. Prevalence of unprotected anal intercourse among HIV-diagnosed MSM in the United States: a meta-analysis. AIDS. 2009;23:1617–1629. doi: 10.1097/QAD.0b013e32832effae. [DOI] [PubMed] [Google Scholar]
- 2.Burman W, Grund B, Neuhaus JN, et al. Episodic Antiretroviral Therapy Increases HIV Transmission Risk Compared With Continuous Therapy: Results of a Randomized Controlled Trial. J Acquir Immune Defic Syndr. 2008;49:142–150. doi: 10.1097/QAI.0b013e318183a9ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. for the HPTN 052 Study Team. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23(11):1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds SJ, Makumbi F, Nakigozi G, et al. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS. 2011;25(4):473–477. doi: 10.1097/QAD.0b013e3283437c2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinn TC, Wawer MH, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 7.Fideli US, Allen SA, Musonda R, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses. 2001;17:901–910. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodger A, Bruun T, Weait M, et al. Partners of people on ART - a New Evaluation of the Risks (The PARTNER study): design and methods. BMC Public Health. 2012;12:296. doi: 10.1186/1471-2458-12-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/guidelines (downloaded 5 May 2014)
- 10.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection. 2012 Recommendations of the International Antiviral Society – USA Panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 11. [accessed 14 July 2013]; http://europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/EacsGuidelines-v6.1-2edition.pdf.
- 12.Hirnschall G, Harries AD, Easterbrook PJ, Doherty MC, Ball A. The next generation of the World Health Organization's global antiretroviral guidance. J Int AIDS Soc. 2013;16:18757. doi: 10.7448/IAS.16.1.18757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams I, Churchill D, Anderson J, et al. British HIV Association Guidelines for the Treatment of HIV-1-positive adults with antiretroviral therapy 2012 (Updated November 2013) HIV Med. 2014;15(Suppl 1):1–85. doi: 10.1111/hiv.12119. [DOI] [PubMed] [Google Scholar]
- 14.Babiker AG, Emery S, Fätkenheuer G, et al. Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials. 2013;10(1 Suppl):S5–S36. doi: 10.1177/1740774512440342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van de Ven P, Kippax S, Crawford J, et al. In a minority of gay men, sexual risk practice indicates strategic positioning for perceived risk reduction rather than unbridled sex. AIDS Care. 2002;14:471–480. doi: 10.1080/09540120208629666. [DOI] [PubMed] [Google Scholar]
- 16.Crepaz N, Hart TA, Marks G. HAART and sexual risk behaviour: a meta-analytic review. JAMA. 2004;292:224–236. doi: 10.1001/jama.292.2.224. [DOI] [PubMed] [Google Scholar]
- 17.Stephenson JM, Imrie J, Davis MMD, et al. Is use of antiretroviral therapy among homosexual men associated with increased risk of transmission of HIV infection? Sex Transm Infect. 2003;79(1):7–10. doi: 10.1136/sti.79.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van de Ven P, Mao L, Fogarty A, et al. Undetectable viral load is associated with sexual risk taking in HIV serodiscordant gay couples in Sydney. AIDS. 2005;19(2):179–184. doi: 10.1097/00002030-200501280-00010. [DOI] [PubMed] [Google Scholar]
- 19.Parsons JT, Schrimshaw EW, Wolitski RJ, et al. Sexual harm reduction practices of HIV seropositive gay and bisexual men: serosorting, strategic positioning, and withdrawal before ejaculation. AIDS. 2005;19(Suppl 1):S13–S25. doi: 10.1097/01.aids.0000167348.15750.9a. [DOI] [PubMed] [Google Scholar]
- 20.Jin F, Crawford J, Prestage GP, et al. Unprotected anal intercourse, risk reduction behaviours, and subsequent HIV infection in a cohort of homosexual men. AIDS. 2009;23(2):243–252. doi: 10.1097/QAD.0b013e32831fb51a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin F, Prestage GP, Templeton DJ, et al. The impact of HIV seroadaptive behaviors on sexually transmissible infections in HIV-negative homosexual men in Sydney, Australia. Sex Transm Dis. 2012;39(3):191–194. doi: 10.1097/OLQ.0b013e3182401a2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou AM. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 23.Hall HI, Holtgrave DR, Tang T, Rhodes P. HIV transmission in the United States: considerations of viral load, risk behavior, and health disparities. AIDS Behav. 2013;17(5):1632–1636. doi: 10.1007/s10461-013-0426-z. [DOI] [PubMed] [Google Scholar]
- 24.van Griensven F, de Lind van Wijngaarden JW, Baral S, Grulich A. The global epidemic of HIV infection among men who have sex with men. Curr Opin HIV AIDS. 2009;4(4):300–307. doi: 10.1097/COH.0b013e32832c3bb3. [DOI] [PubMed] [Google Scholar]
- 25.Teague R, Mijch A, Fairley CK, et al. Testing rates for sexually transmitted infections among HIV-infected men who have sex with men attending two different HIV services. Int J STD AIDS. 2008;19(3):200–202. doi: 10.1258/ijsa.2007.007131. [DOI] [PubMed] [Google Scholar]
- 26.Rietmeijer CA, Patnaik JL, Judson FN, Douglas JM., Jr Increases in gonorrhea and sexual risk behaviors among men who have sex with men: a 12-year trend analysis at the Denver Metro Health Clinic. Sex Transm Dis. 2003;30:562–567. doi: 10.1097/00007435-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Lampe F, Speakman A, Phillips A, et al. ART use, viral suppression, and sexual behaviour among HIV-diagnosed MSM in the UK: results from the Antiretrovirals, Sexual Transmission Risk and Attitudes (ASTRA) Study. J Int AIDS Soc. 2012;15:14–15. [Google Scholar]
- 28.Lauby JL, Millett GA, LaPollo AB, Bond L, Murrill CS, Marks G. Sexual risk behaviors of HIV-positive, HIV-negative, and serostatus-unknown Black men who have sex with men and women. Arch Sex Behav. 2008;37(5):708–719. doi: 10.1007/s10508-008-9365-6. [DOI] [PubMed] [Google Scholar]
- 29.Fox J, White PJ, Macdonald N, et al. Reductions in HIV transmission risk behaviour following diagnosis of primary HIV infection: a cohort of high-risk men who have sex with men. HIV Med. 2009;10:432–438. doi: 10.1111/j.1468-1293.2009.00708.x. [DOI] [PubMed] [Google Scholar]
- 30.Heijman T, Geskus RB, Davidovich U, Coutinho RA, Prins M, Stolte IG. Less decrease in risk behaviour from pre-HIV to post-HIV seroconversion among MSM in the combination antiretroviral therapy era compared with the pre-combination antiretroviral therapy era. AIDS. 2012;26(4):489–495. doi: 10.1097/QAD.0b013e32834f9d7c. [DOI] [PubMed] [Google Scholar]
- 31.Mizuno Y, Purcell DW, Metsch LR, et al. Is Injection Serosorting Occurring among HIV Positive Injection Drug Users? Comparison by Injection Partner's HIV Status. J Urban Health. 2011;88:1031–1043. doi: 10.1007/s11524-011-9578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Recommendations for a public health approach. Guidance on Couples HIV Testing and Counseling, including antiretroviral therapy for treatment and prevention in serodiscordant couples. 2012 http://www.who.int/hiv/pub/guidelines/9789241501972/en/ (downloaded 5 May 2014) [PubMed]
- 33.Department of Health. BHIVA and EAGA position statement on the use of antiretroviral therapy to reduce HIV transmission. https://www.gov.uk/government/publications/bhiva-and-eaga-position-statement-on-the-use-of-antiretroviral-therapy-to-reduce-hiv-transmission (downloaded 5 May 2014)


