Abstract
BMS-433771 is a potent inhibitor of respiratory syncytial virus (RSV) replication in vitro. Mechanism of action studies have demonstrated that BMS-433771 halts virus entry through inhibition of F protein-mediated membrane fusion. BMS-433771 also exhibited in vivo efficacy following oral administration in a mouse model of RSV infection (C. Cianci, K. Y. Yu, K. Combrink, N. Sin, B. Pearce, A. Wang, R. Civiello, S. Voss, G. Luo, K. Kadow, E. Genovesi, B. Venables, H. Gulgeze, A. Trehan, J. James, L. Lamb, I. Medina, J. Roach, Z. Yang, L. Zadjura, R. Colonno, J. Clark, N. Meanwell, and M. Krystal, Antimicrob. Agents Chemother. 48:413-422, 2004). In this report, the in vivo efficacy of BMS-433771 against RSV was further examined in the BALB/c mouse and cotton rat host models of infection. By using the Long strain of RSV, prophylactic efficacy via oral dosing was observed in both animal models. A single oral dose, administered 1 h prior to intranasal RSV inoculation, was as effective against infection as a 4-day b.i.d. dosing regimen in which the first oral dose was given 1 h prior to virus inoculation. Results of dose titration experiments suggested that RSV infection was more sensitive to inhibition by BMS-433771 treatment in the BALB/c mouse host than in the cotton rat. This was reflected by the pharmacokinetic and pharmacodynamic analysis of the efficacy data, where the area under the concentration-time curve required to achieve 50% of the maximum response was ∼7.5-fold less for mice than for cotton rats. Inhibition of RSV by BMS-433771 in the mouse is the result of F1-mediated inhibition, as shown by the fact that a virus selected for resistance to BMS-433771 in vitro and containing a single amino acid change in the F1 region was also refractory to treatment in the mouse host. BMS-433771 efficacy against RSV infection was also demonstrated for mice that were chemically immunosuppressed by cyclophosphamide treatment, indicating that compound inhibition of the virus did not require an active host immune response.
Respiratory syncytial virus (RSV), a single-stranded RNA virus of negative genome polarity, is a member of the Pneumovirus genus of the Paramyxovirus family. RSV was first described as occurring in humans in 1957, after being recovered from two infants hospitalized with severe lower respiratory tract infections (7). Today, RSV is recognized as a leading agent involved in lower respiratory tract disease in infants, as well as a significant respiratory tract pathogen in the elderly. In humans, RSV-induced disease typically begins in the nasopharynx after a 4- to 5-day incubation period (19, 32). Upper respiratory tract infection proceeds with severe nasal congestion and profuse rhinorrhea, advancing to a cough and pharyngitis. Progression to lower respiratory tract infection may follow, leading to pneumonia in the most serious cases.
In efforts to understand RSV pathogenesis and treat the infection, several animal models have been established (5). Although no animal model exactly reproduces the viral disease states of infected humans and it is unclear whether efficacy in animals will translate to efficacy in humans, each animal species does offer unique advantages for in vivo experimentation (5, 9). Bovine and ovine RSV are common pathogens of cattle and sheep, respectively, and share some common disease characteristics with human RSV (16, 37). However, human RSV does not infect these species. Primate models, including chimpanzee, rhesus monkey, and African green monkey, provide genetically related hosts that are permissive to human RSV infection, but their high maintenance costs prohibit the use of statistically significant numbers of animals (5). Small-rodent models provide for the practical concerns of maintenance cost, ease of handling, and statistically significant cohorts. Studies of rodent models of RSV infection (28, 29), chiefly using inbred BALB/c mouse and cotton rat (Sigmodon hispidus), have contributed to the understanding of the pathogenesis and immunobiology of human RSV disease (1, 11, 15). The BALB/c mouse host model of RSV infection has been examined extensively, foremost to provide insights into the immunology of RSV disease (16). The establishment of highly characterized inbred mouse strains and an extensive repertoire of specific immunological reagents make the murine model attractive for experimental infection. However, no RSV antiviral formulations have been licensed based upon mouse studies alone. As a host species for RSV, the cotton rat was estimated to be 100-fold more permissive for infection per inoculum dose of virus and immunologically 10-fold more responsive to the virus than the BALB/c murine host (22, 25). Cotton rat studies were used to demonstrate the efficacy of ribavirin and RSV immunoglobulin against RSV infection; these were prerequisite studies to the subsequent clinical trials leading to their licensure as therapeutic and prophylactic agents, respectively (17, 27).
Antiviral in vivo efficacy in the cotton rat or BALB/c host models of RSV infection was reported for several RSV fusion inhibitors, although oral dosing was not used in those studies (2, 18, 24, 36). The discovery of BMS-433771, a novel azabenzimidazolone small-molecule RSV fusion inhibitor, which in preliminary experiments exhibited in vivo efficacy against RSV infection in the BALB/c mouse host model by the oral route of administration, was reported previously (8). This report presents results of experiments that further explore the in vivo antiviral activity of BMS-433771 in the BALB/c mouse and cotton rat host models of RSV infection.
MATERIALS AND METHODS
Viruses.
The Long strain of RSV was obtained from the American Type Culture Collection (Rockville, Md.). A variant of the Long strain of RSV, designated K394R, was also used to infect mice. K394R was obtained in vitro by selection for resistance to our panel of RSV fusion inhibitors and has a lysine-to-arginine change at amino acid position 394 within the F1 region of the RSV fusion protein. K394R was shown to be 1,250-fold more resistant to BMS-433771 in vitro than the wild-type, parental Long strain (8). All viruses were propagated in HEp-2 cells, and infectious RSV titers were determined for the viral stocks by 50% infective cytopathic end point tissue culture dose (TCID50), as described previously (8).
Animal host models of RSV infection.
The in vivo antiviral efficacy of BMS-433771 by oral administration in the BALB/c mouse host model (14, 28, 35) and in the cotton rat (Sigmodon hispidus) host model (29) of RSV infection was examined. In addition, chemically immunosuppressed BALB/c mice were also used as RSV-infected hosts (34). These mice were chemically immunosuppressed by intraperitoneal injection of 100 mg of cyclophosphamide (Sigma Chemical, St. Louis, Mo.)/kg of body weight 5 days and 1 day prior to RSV inoculation. Female BALB/c mice were purchased from Charles River Laboratories (Wilmington, Mass.) and used at a body weight of 16 to 20 g at 12 to 16 weeks of age. Cotton rats (either male or female) were purchased from Harlan Sprague Dawley (Indianapolis, Ind.) and used at a body weight of 90 to 100 g at 14 to 18 weeks of age. All animals were housed in certified specific-pathogen-free rooms in cages covered with barrier filters, with cages of sentinel animals to monitor for contagious agents. All animals were fed and watered ad libitum. Protocols for use of animals were approved by the Bristol-Myers Squibb Pharmaceutical Research Institute Animal Use and Care Committee, according to National Institutes of Health Guidelines (USPHS) as spelled out in the Guide for the Care and Use of Laboratory Animals (25a).
For oral administration to all animals, BMS-433771 was dissolved in sterile water, and the solution was adjusted to pH 2 to 3.5 with HCl (0.1 N). In some studies, the compound was dissolved in a solution of 50% PEG400 (Sigma) in water. All animals were treated with 0.2 ml of dissolved BMS-433771, delivered by oral gavage. Unless indicated otherwise, oral compound treatments were usually given 1 h prior to RSV inoculation. For virus infection, mice were anesthetized by an intraperitoneal injection of ketamine (70 mg/kg) and xylazine (20 mg/kg) and inoculated by the intranasal route, drop-wise, with 105 TCID50 of RSV in a 50-μl cell culture medium. Cotton rats were anesthetized by methoxyflurane gas inhalation and were inoculated by the intranasal instillation of 2 × 105 TCID50 of the Long strain of RSV in 100-μl cell culture media drop-wise.
Assay for determination of infectious RSV titers.
On day 4 after RSV inoculation, all test animals were euthanized by CO2 gas asphyxiation, and the lungs were excised, weighed, and prepared as homogenates for viral titration. Lungs were homogenized (10%, wt/vol) in a Hanks balanced salt solution containing 0.21 M sucrose, 25 mM HEPES, and 5 mM sodium l-glutamate, supplemented with 20 U of penicillin G/ml, 20 μg of streptomycin/ml, and 0.05 μg of amphotericin B (GIBCO/BRL, Carlsbad, Calif.)/ml. Lung homogenates were frozen on dry ice, thawed to release cell-associated virus, and then held on ice until clarification by centrifugation at 300 × g for 10 min at 4°C. The resulting supernatant samples were immediately titrated for RSV infectivity in HEp-2 cells as described previously (8). Final RSV lung titers for each animal were calculated as the reciprocal of the log10 dilution of TCID50 and were expressed as log10 TCID50 per gram of lung. Infectious RSV lung titers for animal treatment groups, or cohorts, were calculated as the geometric mean titers and also expressed as log10 TCID50 per gram of lung. The lower limit of infectious RSV detection for this assay was ∼2.3 log 10 TCID50 per gram of lung.
Pharmacokinetic studies of cotton rats and mice.
The systemic exposure of BMS-433771 was evaluated for nonfasted cotton rats following oral administration of 25, 50, 100, and 200 mg/kg. Serum samples were obtained by cardiac puncture before dosing and 15, 60, 240, and 420 min after dosing. There were three cotton rats per time point for oral doses of 50, 100, and 200 mg/kg and one cotton rat per time point for the 25-mg/kg oral dose. Samples were stored at −20°C until analysis.
In order to evaluate the systemic exposure of BMS-433771 in BALB/c mice, the compound was administered to nonfasted animals (n = 2 per time point) at oral (p.o.) doses of 1, 10, and 50 mg/kg. Pharmacokinetic doses were lower in mice than in cotton rats because it was observed that efficacy was obtained at lower doses in the BALB/c mouse host model. Serum samples were taken by cardiac puncture before dosing and 15, 30, 60, 120, and 240 min after dosing and were stored at −20°C until analysis.
Serum samples from mouse and cotton rat studies were treated with 2 volumes of acetonitrile containing an internal standard. After centrifugation to remove precipitated proteins, a 10-μl portion of the clear supernatant was injected onto a liquid chromatography (LC)-tandem mass spectrometer for analysis. The LC system, which consisted of two Shimadzu LC10AD pumps (Columbia, Mass.), a Perkin-Elmer Series 200 autosampler (Norwalk, Conn.), and a Hewlett-Packard Series 1100 column compartment (Palo Alto, Calif.), was interfaced to a Micromass Quattro LC tandem mass spectrometer (Beverly, Mass.) equipped with an electrospray interface. The column used was a Keystone Hypersil C18, 2 by 20 mm, 3-μm-diameter particles (Bellefonte, Pa.), maintained at 60°C with a flow rate of 0.3 ml/min. The mobile phase consisted of 10 mM ammonium acetate in water-methanol (75:25), with pH adjusted to 5.5 with glacial acetic acid (hereafter in this paragraph referred to as A) and 10 mM ammonium acetate in methanol (hereafter in this paragraph referred to as B). The initial mobile phase composition was 95% A and 5% B. After injection of the sample, the mobile phase was changed to 15% A and 85% B over 0.1 min and held at that composition for one additional minute. The mobile phase was then returned to the initial conditions and the column was reequilibrated for 0.9 min. The total analysis time was 2 min. An 11-point standard curve was constructed from 3.9 to 4,000 ng/ml and was fitted with a quadratic regression weighted by reciprocal concentration (1/x). Standards were analyzed in duplicate. Quality control samples at three concentrations within the range of the calibration curve were analyzed in triplicate with each analytical set. For BMS-433771, the observed concentrations of at least two-thirds of quality control samples were generally within 20% of theory, indicating acceptable assay performance.
The area under the concentration-time curve (AUC) following oral administration of BMS-433771 was calculated by using the linear trapezoidal rule. A pharmacokinetic-pharmacodynamic (PK-PD) correlation between the viral titer reduction (measured as log10 TCID50 in lung homogenates) and systemic exposure (plasma AUCs) in mouse and cotton rat was established by using the Emax model equation, E = (Emax × AUC)/(EAUC50 + AUC), where E is a pharmacologic response (i.e., viral titer reduction), Emax is the maximum pharmacologic response, and EAUC50 is the plasma AUC that achieves 50% of Emax. Some oral AUC data for mice (i.e., 1.5, 5, and 15 mg/kg) were obtained by interpolation from a curve fit of the dose-AUC relationship established from the oral doses of 1, 10, and 50 mg/kg. Curve fitting was performed by using KaleidaGraph software version 3.51 (Reading, Pa.). Goodness of fit was assessed by the coefficient of determination (R2), chi-square value (χ2), the standard error associated with the parameter estimated (Error), and visual inspection of the fitting and residual plots.
RESULTS
Single-dose and multiple-dose prophylactic efficacy of BMS-433771 in rodent models of RSV infection.
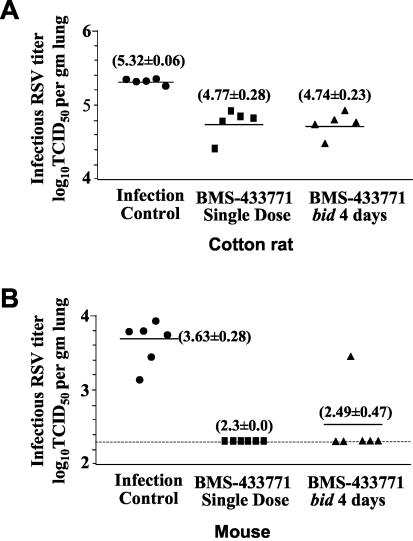
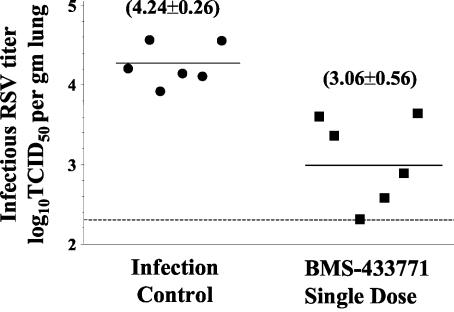
The effects of BMS-433771 were examined in both models of infection to compare the 4-day b.i.d. dosing regimen to the single-dose treatment. In one group of cotton rats, BMS-433771 was administered as a single oral 50-mg/kg dose given 1 h prior to inoculation of virus. For another group, it was administered b.i.d. at a 50-mg/kg/dose for 4 days, with the first dose given 1 h before virus inoculation. Animals were sacrificed 4 days later, and their lungs were assayed for infectious RSV (Fig. 1A). A single-dose treatment, given orally 1 h prior to RSV inoculation, was as effective against infection as the multiple-dose b.i.d. regimen. In this experiment, the single and multiple b.i.d. dose treatment regimens achieved >0.5 log10 TCID50 reduction in the infective RSV titers in cotton rat lungs (Fig. 1A).
FIG. 1.
Oral treatment efficacy of BMS-433771 against RSV in the cotton rat (A) and in the mouse BALB/c (B) host models of infection. Animals were inoculated intranasally with the Long strain of RSV. The compound (50 mg/kg) was administered by oral gavage, either as a single dose given 1 h before RSV inoculation or as a 4-day b.i.d. regimen (25 mg/kg/dose) in which the first dose was given 1 h before virus inoculation. Treatment cohorts are shown on the abscissa; animals of the infection control group were virus inoculated but not treated with BMS-433771. The infectious RSV lung titers are shown on the ordinate as log10 TCID50 per gram of lung. Each data point symbol represents the RSV titer for each individual animal of the respective treatment cohort. The horizontal line, drawn in each cohort, marks the geometric mean RSV titer of the group, with the viral titer number in parentheses. The horizontal hatched line, across the graph, represents the RSV titer at the limit of assay detection.
BMS-433771 also exhibited efficacy against RSV in the BALB/c mouse host model of infection (Fig. 1B). Similar results were observed with the treatment consisting of a single oral 50-mg/kg/dose given 1 h prior to virus inoculation and with a 4-day b.i.d. oral treatment regimen using a 50-mg/kg/dose, in which the first dose was given 1 h prior to RSV inoculation. The magnitude of RSV inhibition in the mice was greater than that in the cotton rats. The RSV lung titers of all but one of the treated mice were below the detection limit for this assay, i.e., ∼2.3 log10 TCID50 per gram (Fig. 1B). As there was no significant difference in antiviral efficacy between the single-oral-dose and the multiple-b.i.d.-dose treatment regimens, the single dose BMS-433771 treatment, given 1 h prior to virus inoculation, was used as the standard dosing routine for subsequent studies.
Dose range titration of BMS-433771 against RSV in rodent host models.
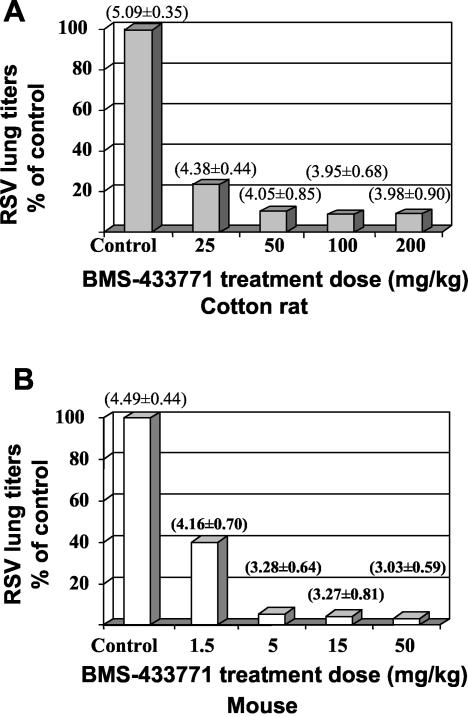
In order to examine whether inhibition of RSV by BMS-433771 in the cotton rat was dose dependent, a dose-ranging study was performed (Fig. 2A). Cohorts of five cotton rats were administered single oral doses of 25, 50, 100, or 200 mg of BMS-433771/kg 1 h prior to virus inoculation, and 4 days later, homogenized lung samples of sacrificed animals were assayed for RSV titers. Compared to the titers of untreated, control animals, reductions of ∼0.5 to ∼1.2 log10 TCID50 in infective RSV titers were noted in lung homogenates of cotton rats treated with any dose of BMS-433771 (Fig. 2A). For the BMS-433771 treatment cohorts, at the doses of 25, 50, 100, and 200 mg/kg respective reductions of 80, 91, 93, and 92% in geometric mean titers of RSV were noted. The reductive antiviral effect of BMS-433771 in the cotton rats appeared to be limited to slightly more than 1 log10 TCID50 per g of lung, as the treatments at the higher dose ranges from 50 to 200 mg/kg were comparably active.
FIG. 2.
Oral treatment dose titration of BMS-433771 in cotton rats (A) and BALB/c mice (B). Single oral doses of BMS-433771 were given by gavage to cohorts of six animals 1 h prior to intranasal inoculation of Long strain. The cohorts are shown on the abscissa; the bar for the animal group inoculated with virus but not treated with BMS-433771 is labeled Control. Infectious RSV titers of each respective cohort are presented as bars on the ordinate, as percentages of the geometric mean viral titer (log10 TCID50 per gram of lung) of the control group. The geometric mean RSV titer for each respective cohort is listed as the number in parentheses.
In order to determine the dose of BMS-433771 required for efficacy against RSV in the BALB/c host model of infection, cohorts of six animals were treated with single oral doses of 1.5, 5, 15, and 50 mg/kg. Reductions in RSV lung titers in treated mice were dose dependent (Fig. 2B). The most significant reductions in infectious RSV titers were evident at the 50-, 15-, and 5-mg/kg doses of BMS-433771. At these doses, respective reductions in the geometric means of treated cohorts compared to those of untreated animals were 97, 96, and 95%, respectively, with two animals in each of the 50- and 15-mg/kg cohorts exhibiting virus levels below the detectable limit (<2.3 log10 TCID50). The 1.5-mg/kg dose of BMS-433771 reduced geometric mean viral titers by 60%. Thus, in the BALB/c mouse host model, the effective dose range of BMS-433771 needed to significantly reduce infectious RSV lung titers by ∼1.0 log10 TCID50 per gram was estimated to be ≥5 mg/kg when administered 1 h prior to virus inoculation.
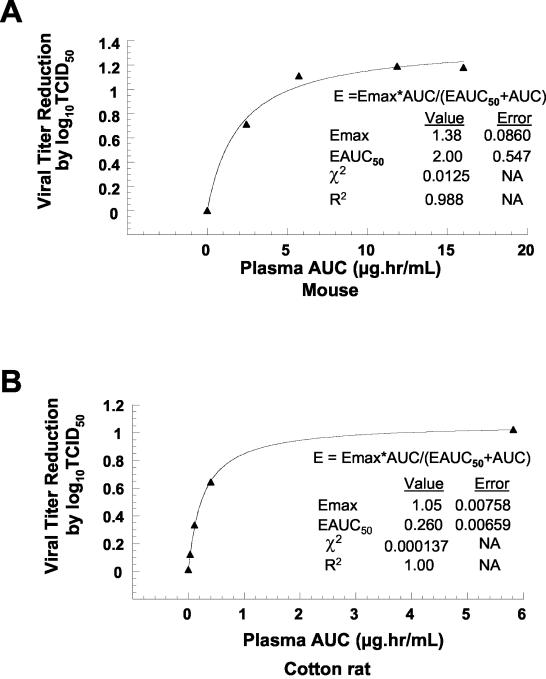
For mice, a single 5-mg/kg treatment dose of BMS-433771 caused a ≥1.0 log10 TCID50 reduction in RSV lung titers, whereas in the cotton rat model this level of inhibition was observed at or above the 50-mg/kg dose (Fig. 2). In order to understand this differential response to BMS-433771, a pharmacokinetic and pharmacodynamic analysis of the viral titer reduction (TCID50) and oral systemic exposure (plasma AUCs) was performed with the RSV-infected mouse and cotton rat models (Fig. 3). The maximum pharmacological response (Emax) of cotton rats and that of mice were comparable (1.05 log10 TCID50 and 1.38 log10 TCID50, respectively). However, the EAUC50 (a parameter that measures in vivo potency of compounds' values) of the two species were significantly different (2.0 and 0.26 μg-h/ml for cotton rats and mice, respectively). The data suggest that there are considerable pharmacodynamic differences between the two rodent models, which may explain the difference in dose requirement for efficacy.
FIG. 3.
Pharmacokinetic-pharmacodynamic correlation between viral titer reduction and systemic exposure (plasma AUC) of BMS-433771 in cotton rats (A) and mice (B). See Materials and Methods for an explanation of parameters and symbols.
Antiviral mechanism of RSV inhibition by BMS-433771 in vivo.
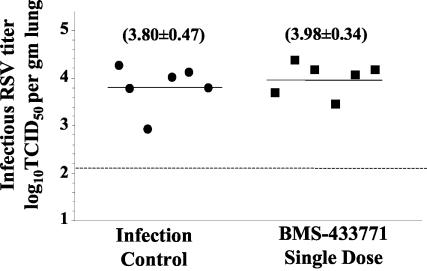
RSV Long strain variants resistant to BMS-433771 were previously identified through in vitro selection in cell culture (8). One of these resistant viruses contained a mutation in the F gene corresponding to a single lysine-to-arginine change in amino acid 394 of the F protein. Proof that this amino acid change in F1 alone induced resistance to BMS-433771 was demonstrated through the use of reverse genetics by showing that wild-type virus altered only at this position was resistant to inhibition by the compound (8). This finding verified that the mechanism of action of BMS-433771 in vitro is through inhibition of viral fusion and involves the F1 polypeptide. However, in vivo, the antiviral mechanism of BMS-433771 treatment has not been elucidated. Thus, an experiment using the K394R Long strain virus variant for mouse infection was conducted. In this study, mice were treated orally with BMS-433771 for 4 days by a b.i.d. dosing regimen at 50 mg/kg/day, with the first dose given 1 h before virus inoculation. Whereas this treatment regimen was effective against infection by wild-type virus (Fig. 1B), infection by the K394R variant was refractory to this treatment (Fig. 4). The results of this experiment support the hypothesis that the antiviral effect of BMS-433771 functions in vivo through the same mechanism of virus fusion inhibition as that observed in vitro.
FIG. 4.
Mechanism of action of BMS-433771 against RSV in vivo. Mice were treated orally with a single dose of BMS-433771 at 50 mg/kg 1 h prior to the intranasal inoculation of the K394R Long virus variant. There were six mice for each cohort group. Symbols and lines are as defined in the legend to Fig. 1.
Efficacy of BMS-433771 against RSV in immunosuppressed mice.
In the BALB/c mouse host model, it has been shown that an intact immune response mediates viral clearance (6, 13, 26, 41). Without antiviral treatment, healthy immunosufficient BALB/c mice clear RSV by 8 days postinoculation (14). Experiments were conducted to determine if an active host immune response was required for the antiviral efficacy of BMS-433771 against RSV infection in mice (Fig. 5). To this end, BALB/c mice were rendered immunosuppressed by cyclophosphamide treatment. Immunosuppressed mice were treated with a single oral 50-mg/kg dose of BMS-433771 administered 1 h prior to RSV inoculation and were sacrificed after 4 days, and lung homogenates were used to determine RSV titer. Infectious RSV titers from the lung homogenates of immunosuppressed mice treated with BMS-433771 were reduced by >1.2 log10 TCID50 compared to the titers from immunosuppressed, but untreated, infection control mice (Fig. 5). These results indicated that the antiviral effect of BMS-433771 in mice did not depend on an active host immune response.
FIG. 5.
Efficacy of BMS-433771 against RSV in immunosuppressed mice. BALB/c mice, chemically immunosuppressed by cyclophosphamide treatment, were given BMS-433771 in a single oral dose of 50 mg/kg 1 h prior to intranasal inoculation with the Long strain of RSV. There were six mice per cohort group. Symbols and lines are as defined in the legend to Fig. 1.
Evaluation of BMS-433771 for therapeutic efficacy against RSV in mice.
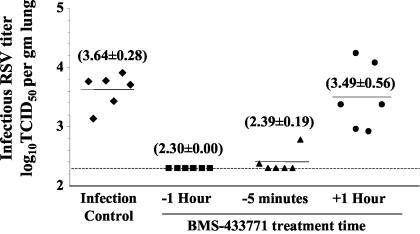
An inhibitory effect on RSV growth by BMS-433771 in mice was clearly observed when the compound was dosed in a prophylactic regimen. Experiments were then conducted to determine the therapeutic potential of BMS-433771 against RSV in the BALB/c mouse model of infection. The compound was administered at 50 mg/kg in a single oral dose either 1 h or 5 min before virus inoculation or 1 h postinoculation (Fig. 6). Prophylactic treatment with BMS-433771, either at 5 min or 1 h prior to infection, was efficacious, reducing infectious lung titers in most animals to the assay detection limit. However, when BMS-433771 treatment was administered 1 h after virus inoculation, only a small reduction in infectious titers (∼0.2 log10 TCID50) compared to those of untreated, infection control mice was observed (Fig. 6).
FIG. 6.
Evaluation of BMS-433771 treatment of BALB/c mice for therapeutic efficacy against RSV infection. Mice were treated with a single oral dose of BMS-433771 at 50 mg/kg 1 h before (−1 h) RSV inoculation, 5 min before (−5 min), or 1 h after (+1 h) RSV inoculation. Mice were inoculated intranasally with the Long strain of RSV. Symbols and lines are as defined in the legend to Fig. 1.
DISCUSSION
BMS-433771, an azabenzimidazolone derivative, is a novel small-molecule inhibitor of RSV fusion with oral efficacy in two rodent models of RSV infection. The compound was effective in reducing viral titers in the lungs of both cotton rats and BALB/c mice when administered 1 h before inoculation with RSV (Fig. 1 and 2). Interestingly, with regard to oral treatment dosing regimens in both rodent host species, BMS-433771 was just as effective when administered in a single oral dose 1 h before virus inoculation as it was when administered in a b.i.d. regimen for 4 days, with the first dose administered 1 h before virus (Fig. 1). Single-dose prophylactic efficacy in animal models was reported for a number of antiviral agents. These include the RSV inhibitor RFI-641, administered intranasally in cotton rats (18), and the viral attachment inhibitor PAMPS, administered intranasally against both RSV and measles virus in cotton rats (39). Furthermore, the nucleoside analogs cidofovir (31) and acyclovir (30) were effective against cowpox virus and herpes simplex virus, respectively, in mouse models of infection using a single-dose regimen.
BMS-433771 oral treatment dose titration studies performed with mice showed that a measurable effect upon RSV replication was observed at a dose as low as 1.5 mg/kg when given 1 h before virus inoculation (although ranges overlapped with those of the control cohort). However, significant reductions (>1.0 log10 TCID50) in viral titers were repeatedly observed at the 5.0-mg/kg dose (Fig. 2 and unpublished observations). In similar treatment experiments performed with cotton rats, higher doses of BMS-433771 were needed to obtain a >1.0 log10 TCID50 reduction in viral lung titers and the results were more variable (Fig. 2 and unpublished observations). There could be several reasons for the compound dose range differences between the two rodent host species. Cotton rats have been reported to be more permissive to RSV infection and produce higher titers than those observed in the BALB/c mouse host model (Fig. 1) (14, 28). The lower level of replication in BALB/c mice than in cotton rats may result in an increased antiviral effect in mice. This difference between the two animal models may also be reflected by the pharmacokinetic and pharmacodynamic data analysis of the efficacy results. The AUC that is required to achieve 50% of the maximum response was ∼7.5-fold lower for mice than for cotton rats. This is probably not due to the difference in protein binding between the two species, since the levels of serum protein binding were similar across species (data not shown). Differences in the pathogenesis and compartments of RSV infection in the respiratory tracts of these animals may also be relevant factors in the prophylactic efficacy of BMS-433771. The cotton rat is more susceptible to RSV infection in both the upper and lower portions of the respiratory tract, where the virus replicates in the nasal epithelium and the bronchiolar epithelium but not in the trachea or the alveoli of the lungs (20, 29). For mice, RSV replication was reported to be predominate in the alveolar cells of the lung (15, 35). Exposure to BMS-433771 could vary in the different compartments of the lung. Accordingly, the dynamics of compound interaction and exposure within the different tissues and cell types of the respiratory tract of these animals may be important variables affecting the antiviral efficacy of BMS-433771.
The mechanism of action for BMS-433771 in vitro was proven to be fusion inhibition through a variety of tests, including time of addition studies, temperature shift experiments, and generation of resistant viruses (8). BMS-433771 inhibited two fusion protein-mediated functions: (i) early-stage fusion between the cell membrane and viral envelope during entry and (ii) late-stage syncytia formation. To confirm that the mechanism of action in vivo was also through inhibition of fusion, the K394R virus variant, selected for resistance to BMS-433771 in vitro (8), was tested in the BALB/c mouse host model for sensitivity to antiviral inhibition by compound treatment. In vivo infection by K394R was refractory to BMS-433771 treatment in a dose regimen that effectively suppressed viral infection by the parental wild-type virus (Fig. 4). This result provided evidence that in vivo, the mechanism of action of BMS-433771 was consistent with the in vitro antiviral mechanism of RSV fusion inhibition. BMS-433771 was also found to be effective against RSV in mice that were chemically immunosuppressed, indicating that an active host immune response was not a factor involved in the antiviral efficacy observed with BMS-433771 (Fig. 5).
BMS-433771 was effective when orally administered up to 5 min before RSV inoculation but was generally ineffective when given 1 h after inoculation (Fig. 6). Possible explanations could be related to the pathogenesis of the virus in this animal model and/or the mechanism of action of BMS-433771 consisting of inhibition of the early step of membrane fusion. Interestingly, similar findings were obtained for RFI-641 (CL387626), another small-molecule RSV fusion inhibition that targets the F protein (3, 10, 12). Although it was effective when administered intranasally to cotton rats 1 h prior to virus inoculation, there was no demonstrable therapeutic effect by RFI-641 when administered postinfection in this small animal model (40). However, evidence for the therapeutic efficacy of RFI-641 against RSV was obtained in studies with experimentally infected African green monkeys, whereby intranasal administration of this compound at 12 and 24 h postinfection reduced viral titers in both nasal and throat washings (18). With consideration of the in vivo findings with RFI-641, the lack of an antiviral therapeutic effect by BMS-433771 against RSV in mice may reflect limitations by poorly understood factors involved in the dynamics of host species-virus interactions during the course of infection at the cellular and/or physiologic level. The lack of therapeutic efficacy was consistent with the results of treatment experiments in which a single oral prophylactic dose of BMS-433771 was as effective in reducing RSV lung titers as a multiple 4-day b.i.d. dosing regimen in which only one dose was given before virus inoculation (Fig. 1). In addition, no evidence of selection of virus resistant to BMS-433771 was observed when samples obtained from infected and treated animals were examined in cell culture (data not shown). However, it should be noted that in a recent study prophylactic and therapeutic efficacy against RSV in cotton rats was demonstrated by using a different fusion inhibitor, JNJ 248068, delivered by small-particle aerosol (38).
The availability of animal models for RSV infection enables evaluation of potential inhibitor compounds in vivo. However, the uniquely predictive value of these models for human infections is not proven and may have inherent limitations (5). Nonetheless, for a new chemical entity with inhibitory activity against RSV in vitro, the mouse and cotton rat models of RSV infection can provide in vivo proof of principle for the antiviral efficacy of compounds, spurring further interest towards clinical development. BMS-433771 is a potent, selective, small-molecule inhibitor of RSV fusion in vitro and in two rodent host models of RSV infection. Although a number of small-molecule RSV fusion inhibitors have been disclosed recently (2, 18, 23, 24, 33, 38), BMS-433771 is distinguished from these compounds by its property of efficacy after oral administration in rodent hosts of RSV infection. Currently, Synagis, an injectable humanized monoclonal antibody, is approved as a prophylactic agent against RSV (21), whereas the only antiviral compound available for the treatment of hospitalized patients with RSV infection is ribavirin. The therapeutic administration of ribavirin to patients is clinically cumbersome, as it is delivered in aerosol formulation. Furthermore, such treatment by ribavirin has been at best clinically supportive, as it has been difficult to clearly assess its efficacy (4). There is a need for effective, easy-to-administer prophylactic and therapeutic agents against RSV infections in humans, and BMS-433771 may be a candidate for this unmet medical need, pending additional assessments.
REFERENCES
- 1.Anderson, J. J., J. Norden, D. Saunders, G. L. Toms, and R. Scott. 1990. Analysis of the local and systemic immune responses induced in BALB/c mice by experimental respiratory syncytial virus infection. J. Gen. Virol. 71:1561-1570. [DOI] [PubMed] [Google Scholar]
- 2.Andries, K., M. Moeremans, T. Gevers, R. Willebrords, C. Sommen, J. Lacrampe, F. Janssens, and P. R. Wyde. 2003. Substituted benzimidazoles with nanomolar activity against respiratory syncytial virus. Antivir. Res. 60:209-219. [DOI] [PubMed] [Google Scholar]
- 3.Aulabaug, A., W. Ding, G. A. Ellestad, A. Gazumyan, C. D. Hess, G. Krishnamurthy, B. Mitsner, and J. Zaccardi. 2000. Inhibition of respiratory syncytial virus by a new class of chemotherapeutic agents. Drugs of the Future 25:287-294. [Google Scholar]
- 4.Broughton, S., and A. Greenough. 2003. Effectiveness of drug therapies to treat or prevent respiratory syncytial virus infection-related morbidity. Expert Opin. Pharmacother. 4:1801-1808. [DOI] [PubMed] [Google Scholar]
- 5.Byrd, L. G., and G. A. Prince. 1997. Animal models of respiratory syncytial virus infection. Clin. Infect. Dis. 25:1363-1368. [DOI] [PubMed] [Google Scholar]
- 6.Cannon, M. J., E. J. Stott, G. Taylor, and B. A. Askonas. 1987. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells. Immunology 62:133-138. [PMC free article] [PubMed] [Google Scholar]
- 7.Chanock, R., B. Roizman, and R. Myers. 1957. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). Am. J. Hyg. 66:281-290. [DOI] [PubMed] [Google Scholar]
- 8.Cianci, C., K. Y. Yu, K. Combrink, N. Sin, B. Pearce, A. Wang, R. Civiello, S. Voss, G. Luo, K. Kadow, E. Genovesi, B. Venables, H. Gulgeze, A. Trehan, J. James, L. Lamb, I. Medina, J. Roach, Z. Yang, L. Zadjura, R. Colonno, J. Clark, N. Meanwell, and M. Krystal. 2004. In vitro characterization of an orally active fusion inhibitor of respiratory syncytial virus. Antimicrob. Agents Chemother. 48:413-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, P. L., K. McIntosh, and C. R. M. 2001. Respiratory syncytial virus, p. 1443-1486. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 3rd ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 10.Ding, W., B. Mitsner, G. Krishnamurthy, A. Aulabaugh, C. D. Hess, J. Zaccardi, M. Cutler, B. Feld, A. Gazumyan, Y. Raifeld, A. Nikitenko, S. A. Lang, Y. Gluzman, B. O'Hara, and G. A. Ellestad. 1998. Novel and specific respiratory syncytial virus inhibitors that target virus fusion. J. Med. Chem. 41:2671-2675. [DOI] [PubMed] [Google Scholar]
- 11.Franke-Ullmann, G., C. Pfortner, P. Walter, C. Steinmuller, M. L. Lohmann-Matthes, L. Kobzik, and J. Freihorst. 1995. Alteration of pulmonary macrophage function by respiratory syncytial virus infection in vitro. J. Immunol. 154:268-280. [PubMed] [Google Scholar]
- 12.Gazumyan, A., B. Mitsner, and G. A. Ellestad. 2000. Novel anti-RSV dianionic dendrimer-like compounds: design, synthesis and biological evaluation. Curr. Pharm. Des. 6:525-546. [DOI] [PubMed] [Google Scholar]
- 13.Graham, B. S., L. A. Bunton, J. Rowland, P. F. Wright, and D. T. Karzon. 1991. Respiratory syncytial virus infection in anti-mu-treated mice. J. Virol. 65:4936-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham, B. S., M. D. Perkins, P. F. Wright, and D. T. Karzon. 1988. Primary respiratory syncytial virus infection in mice. J. Med. Virol. 26:153-162. [DOI] [PubMed] [Google Scholar]
- 15.Graham, B. S., J. A. Rutigliano, and T. R. Johnson. 2002. Respiratory syncytial virus immunobiology and pathogenesis. Virology 297:1-7. [DOI] [PubMed] [Google Scholar]
- 16.Grubbs, S. T., S. A. Kania, and L. N. Potgieter. 2001. Prevalence of ovine and bovine respiratory syncytial virus infections in cattle determined with a synthetic peptide-based immunoassay. J. Vet. Diagn. Investig. 13:128-132. [DOI] [PubMed] [Google Scholar]
- 17.Hruska, J. F., P. E. Morrow, S. C. Suffin, and R. G. Douglas. 1982. In vivo inhibition of respiratory syncytial virus by ribavirin. Antimicrob. Agents Chemother. 21:125-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huntley, C. C., W. J. Weiss, A. Gazumyan, A. Buklan, B. Feld, W. Hu, T. R. Jones, T. Murphy, A. A. Nikitenko, B. O'Hara, G. Prince, S. Quartuccio, Y. E. Raifeld, P. Wyde, and J. F. O'Connell. 2002. RFI-641, a potent respiratory syncytial virus inhibitor. Antimicrob. Agents Chemother. 46:841-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, K. M., R. M. Chanock, D. Rifkind, H. M. Kravetz, and V. Knight. 1961. Correlation of virus shedding, serologic response and illness in adult volunteers. JAMA 176:663-667. [PubMed] [Google Scholar]
- 20.Johnson, R. A., G. A. Prince, S. C. Suffin, R. L. Horswood, and R. M. Chanock. 1982. Respiratory syncytial virus infection in cyclophosphamide-treated cotton rats. Infect. Immun. 37:369-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, S., C. Oliver, G. A. Prince, V. G. Hemming, D. S. Pfarr, S.-C. Wang, M. Dormitzer, J. O'Grady, S. Koenig, J. K. Tamura, R. Woods, G. Bansal, D. Couchenour, E. Tsao, W. C. Hall, and J. F. Young. 1997. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 176:1215-1224. [DOI] [PubMed] [Google Scholar]
- 22.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422-434. [DOI] [PubMed] [Google Scholar]
- 23.Kimura, K., S. Mori, K. Tomita, K. Ohno, K. Takahashi, S. Shigeta, and M. Terada. 2000. Antiviral activity of NMSO3 against respiratory syncytial virus infection in vitro and in vivo. Antivir. Res. 47:41-51. [DOI] [PubMed] [Google Scholar]
- 24.McKimm-Breschkin, J. 2000. VP-14637 ViroPharma. Curr. Opin. Investig. Drugs 1:425-427. [PubMed] [Google Scholar]
- 25.Murphy, B. R., G. A. Prince, L. A. Lawrence, K. D. Croen, and P. L. Collins. 1990. Detection of respiratory syncytial virus (RSV) infected cells by in situ hybridization in the lungs of cotton rats immunized with formalin-inactivated virus or purified RSV F and G glycoprotein subunit vaccine and challenged with RSV. Virus Res. 16:153-162. [DOI] [PubMed] [Google Scholar]
- 25a.National Research Council Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 26.Openshaw, P. J. 1995. Immunity and immunopathology to respiratory syncytial virus. The mouse model. Am. J. Respir. Crit. Care Med. 152:S59-62. [DOI] [PubMed] [Google Scholar]
- 27.Prince, G. A., V. G. Hemming, R. L. Horswood, and R. M. Chanock. 1985. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 3:193-206. [DOI] [PubMed] [Google Scholar]
- 28.Prince, G. A., R. L. Horswood, J. Berndt, S. C. Suffin, and R. M. Chanock. 1979. Respiratory syncytial virus infection in inbred mice. Infect. Immun. 26:764-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prince, G. A., A. B. Jenson, R. L. Horswood, E. Camargo, and R. M. Chanock. 1978. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am. J. Pathol. 93:771-791. [PMC free article] [PubMed] [Google Scholar]
- 30.Sawtell, N. M., D. I. Bernstein, and L. R. Stanberry. 1999. A temporal analysis of acyclovir inhibition of induced herpes simplex virus type 1 in vivo reactivation in the mouse trigeminal ganglia. J. Infect. Dis. 180:821-823. [DOI] [PubMed] [Google Scholar]
- 31.Smee, D. F., K. W. Bailey, M. Wong, and R. W. Sidwell. 2000. Intranasal treatment of cowpox virus respiratory infections in mice with cidofovir. Antivir. Res. 47:171-177. [DOI] [PubMed] [Google Scholar]
- 32.Sterner, G., S. Wolontis, B. Bloth, and G. de Hevesy. 1966. Respiratory syncytial virus. An outbreak of acute respiratory illnesses in a home for infants. Acta Paediatr. Scand. 55:273-279. [DOI] [PubMed] [Google Scholar]
- 33.Sudo, K., K. Konno, W. Watanabe, S. Shigeta, and T. Yokota. 2001. Mechanism of selective inhibition of respiratory syncytial virus by a benzodithiin compound (RD3-0028). Microbiol. Immunol. 45:531-537. [DOI] [PubMed] [Google Scholar]
- 34.Sudo, K., W. Watanabe, M. Shichi, K. Konno, S. Shigeta, and T. Yokota. 1999. Mouse model of respiratory syncytial virus infection to evaluate antiviral activity in vivo. Antivir. Chem. Chemother. 10:135-139. [DOI] [PubMed] [Google Scholar]
- 35.Taylor, G., E. J. Stott, M. Hughes, and A. P. Collins. 1984. Respiratory syncytial virus infection in mice. Infect. Immun. 43:649-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tidwell, R. R., J. D. Geratz, W. A. Clyde, K. U. Rosenthal, and E. J. Dubovi. 1984. Suppression of respiratory syncytial virus infection in cotton rats by bis(5-amidino-2-benzimidazolyl)methane. Antimicrob. Agents Chemother. 26:591-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van der Poel, W. H., A. Brand, J. A. Kramps, and J. T. Van Oirschot. 1994. Respiratory syncytial virus infections in human beings and in cattle. J. Infect. 29:215-228. [DOI] [PubMed] [Google Scholar]
- 38.Wyde, P. R., S. N. Chetty, P. Timmerman, B. E. Gilbert, and K. Andries. 2003. Short duration aerosols of JNJ 2408068 (R170591) administered prophylactically or therapeutically protect cotton rats from experimental respiratory syncytial virus infection. Antivir. Res. 60:221-231. [DOI] [PubMed] [Google Scholar]
- 39.Wyde, P. R., D. K. Moore-Poveda, E. De Clercq, J. Neyts, A. Matsuda, N. Minakawa, E. Guzman, and B. E. Gilbert. 2000. Use of cotton rats to evaluate the efficacy of antivirals in treatment of measles virus infections. Antimicrob. Agents Chemother. 44:1146-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyde, P. R., D. K. Moore-Proveda, B. O'Hara, W.-D. Ding, B. Mitsner, and B. E. Gilbert. 1998. CL387626 exhibits marked and unusual antiviral activity against respiratory syncytial virus in tissue culture and in cotton rats. Antivir. Res. 38:31-42. [DOI] [PubMed] [Google Scholar]
- 41.Wyde, P. R., C. S. Sun, and V. Knight. 1983. Replication of respiratory syncytial virus in lungs of immunodeficient mice. J. Reticuloendothel. Soc. 34:125-129. [PubMed] [Google Scholar]