Abstract
Background
In adults, vitamin D deficiency is common in patients with nonalcoholic fatty liver disease (NAFLD) and has been associated with the severity of histology. There are known differences between adult and pediatric NAFLD, with little data regarding the relationship between vitamin D and pediatric NAFLD.
Aim
To examine the relationship between vitamin D levels and NAFLD in children.
Methods
Clinical and histological data was utilized from children aged 2–18 years with biopsy proven NAFLD enrolled in the Nonalcoholic Steatohepatitis Clinical Research Network studies. 25(OH) vitamin D levels were measured from serum. Data examined included demographics, anthropometrics, laboratory markers and liver histology. Data were analyzed using 3 categories of vitamin D level: deficient (≤ 20 ng/mL), insufficient (21–29 ng/mL), and sufficient (≥ 30 ng/mL).
Results
102 children were studied. There was a high prevalence (80/102, 78%) of vitamin D deficiency or insufficiency. However, there were no significant associations between vitamin D level and the histological characteristics or severity of NAFLD. Significantly higher levels of triglycerides were found in those with vitamin D deficiency (p=0.004), but there was no association with other features of the metabolic syndrome.
Conclusions
There is a high prevalence of vitamin D deficiency and insufficiency in children with biopsy-proven NAFLD, however no association was found between vitamin D deficiency and the severity of disease on biopsies. This differs from adult NAFLD studies where vitamin D deficiency correlates with histological severity, potentially suggesting differences in the risk factors for or consequences of pediatric NAFLD.
Keywords: Vitamin D, Non-alcoholic Fatty Liver Disease, children, pediatrics
INTRODUCTION
Vitamin D has been shown to have a role in many disease processes including autoimmune disease, infectious disease, cardiovascular disease and common cancers (1), inflammatory processes (2) and liver diseases (3).
The prevalence of 25(OH) vitamin D insufficiency or deficiency is high in both adults (4) and children (5) and has been associated with obesity in adults (6) and adolescents (7,8). Additionally, low levels of vitamin D in adolescents have been associated with the metabolic syndrome independently of adiposity (9).
Nonalcoholic fatty liver disease (NAFLD) is a hepatic manifestation of the metabolic syndrome (10, 11), is increasing in prevalence in the US and is reported to affect nearly 11% of adolescents (12). Animal models have shown that vitamin D deficiency in obese rats exacerbates NAFLD histology (13). Furthermore, a meta-analysis of studies of adults with NAFLD have shown an association between decreased vitamin D levels and NAFLD diagnosed on biopsies, radiology and liver enzymes (14) with studies showing low vitamin D levels correlating with the severity of steatosis independent of other components of the metabolic syndrome (15,16,17). The pathogenesis of the association between low vitamin D levels and NAFLD is unclear, but protective anti-inflammatory, anti-fibrotic and metabolic effects of vitamin D on both parenchymal hepatocytes and non-parenchymal hepatic cells have been suggested (18).
Although pediatric and adult NAFLD share many features, there are known differences in histology, for example location of inflammation and fibrosis (19), with some suggestion that there is more aggressive disease progression and potentially different NAFLD etiologies in the young obese population (20). Although adult data suggest there is an association between low vitamin D levels and NAFLD, there is a paucity of pediatric data regarding vitamin D status and NAFLD. Data regarding the relationship between vitamin D levels and pediatric NAFLD could potentially lead to better insight into the pathogenesis of NAFLD in children and may be actionable in conjunction with increased outdoor physical activity recommendations.
The primary aim of our study was to examine the relationship between 25(OH) vitamin D levels and biopsy proven NAFLD in children, including the degree of inflammation and fibrosis on biopsies.
Secondary aims were to examine the relationship between 25(OH) vitamin D levels with transaminases, dietary history, BMI z-score, insulin resistance and serum inflammatory markers in children with biopsy proven NAFLD.
MATERIALS AND METHODS
Study sample
This study utilized pediatric clinical and histological data obtained from the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) funded Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN), a multicenter, collaborative of 8 pediatric clinical centers to assess the etiology, natural history and therapy of NAFLD. Between 2004 and 2008, the NASH CRN enrolled children age ≥2 years with NAFLD into the observational NAFLD Database study (NAFLD DB) (21). Additionally, children ≥7 years with biopsy proven NAFLD, were randomized into the Treatment of NAFLD in Children (TONIC) trial (22). All participating centers’ Institutional Review Boards and an NIDDK appointed Data and Safety Monitoring Board approved all NASH CRN study protocols.
The current investigation included all children from either NASH CRN study, ages 2–18 years, with biopsy proven NAFLD, and a baseline serum sample within 31 days of biopsy.
Vitamin D determination
25(OH) vitamin D levels were measured from serum samples using the FDA approved, validated (23) chemiluminescent LIAISON® 25OH vitamin D assay (DiaSorin, Stillwater, MN) on the LIAISON® analyzer according to the manufacturer's instructions.
Histology
All liver histology was centrally reviewed by the Pathology Committee of the NASH CRN (24). NAFLD was defined as the presence of at least 5% steatosis and the absence of evidence for other etiologies of chronic liver disease. Biopsies were scored according to the NASH CRN criteria (23).
To characterize associations between vitamin D level and liver histology severity, some grades or stages of a histological feature were combined as follows: steatosis (2 levels: grade 1 (5–33%) vs. 2–3 (>33%)), lobular inflammation (2 levels: grades 0–1 (< 2 on 20× magnification) vs. 2–3 (≥2 on 20×)), portal chronic inflammation (2 levels: grade 0 (none) vs. 1–2 (mild or more)), hepatocellular ballooning injuring (2 levels: grades 0–1 (none/few) vs. 2 (many)), Mallory-Denk bodies (2 levels: many vs. rare/absent), fibrosis (4 levels: stages 0 (none), 1 (1a,b,c-mild/moderate), 2 (moderate), 3–4 (bridging/cirrhosis)). Advanced fibrosis was defined as bridging fibrosis or cirrhosis. The diagnostic categorization of steatohepatitis was grouped into 4 levels for analyses (NAFLD only, borderline steatohepatitis zone 3, borderline steatohepatitis zone 1, definite NASH). The NAFLD Activity Score (NAS), which ranges from 0 to 8, was analyzed as a binary variable: 0–4 vs. 5–8.
Study variables
Demographic information was collected via structured interview and questionnaires. Anthropometric measures were determined by the study physician at enrollment. Body mass index (BMI) z-score was determined according to age and gender based on data from the CDC(25) and categorized into 2 groups: <2 (overweight or less) vs. ≥2 (moderately or severely obese). Tanner stage was defined as early (stages 1–2) vs. late (stages 3–5).
Fasting whole blood samples were obtained via venipuncture and were processed for glucose, insulin, triglycerides and cholesterol (total, HDL and LDL). The homeostatic model assessment of insulin resistance (HOMA-IR) was determined from fasting glucose and insulin values. Other laboratory assays included were alanine aminotransferase, aspartate aminotransferase, uric acid, C-reactive protein, iron, ferritin, gamma glutamyl trans-peptidase and platelets.
Three variables for physical activity levels were created by using available self-reported information from the Modifiable Activity Questionnaire (26): (1) screen time (< 2 hrs/day vs. ≥ 2 hrs/day), (2) any exercise (<1 hr/day vs. ≥1 hr/day) (3) an average of all physical activity metabolic equivalent tasks (MET)-hours/week). Average daily dietary and supplemental vitamin D and calcium intake, and daily servings of dairy products was collected via the Block Brief 2000 Food Frequency Questionnaire (27).
Children were classified as having the metabolic syndrome if they met ≥3 of the 5 individual components from criteria previously published (28).
Statistical Analyses
For univariable analyses, 3 categories of vitamin D level were created: deficient (≤ 20 ng/mL), insufficient (21–29 ng/mL), and sufficient (≥ 30 ng/mL)(1). Categorical data were compared across vitamin D groups using either Fisher’s exact test or a Pearson’s Chi-square test. The P value for trend was determined from a Cochran-Armitage trend test, Mantel-Haenszel Chi-square test or the exact version of the test (29). Continuous data were compared across vitamin D groups using a Kruskal-Wallis non-parametric test due to non-normality of the measures. The P value for trend was determined from a non-parametric score test for trend (30).
Univariable and multivariable analyses were used to determine associations between vitamin D deficiency (compared to insufficiency and sufficiency) and clinical and histological characteristics. Unadjusted odds ratios (ORs), 95% confidence intervals and P’s were determined from simple logistic regression of vitamin D severity on each characteristic. Adjusted ORs, 95% confidence intervals and P’s were determined from multiple logistic regression of vitamin D severity on each characteristic with adjustment for sex (boys vs. girls), ethnicity (Hispanic vs. non-Hispanic), Tanner stage (late vs. early) and season of sample collection (spring/summer vs. fall/winter). P values were determined from a Wald test. For Mallory bodies, the adjusted model was assessed using exact logistic regression due to small numbers. Age 2 was excluded from the analyses of age due to being an outlier; however, the inference did not change with inclusion of the data point.
For all analyses, either SAS (version 9.3; SAS Institute, Inc., Cary, NC) or Stata (release 12; Stata Corps LP, College Station, TX) statistical software was utilized. We considered differences statistically significant when P values were < 0.05. Nominal two-sided P values were used.
RESULTS
106 children with biopsy proven NAFLD age 2 to 18 years were identified with histology from centrally reviewed biopsies and a baseline serum sample within 31 days of biopsy. 102 of these children had a vitamin D level obtained and were included in this analysis; 4 had insufficient serum. Demographics and characteristics of these children are summarized in table 1. Overall, 78% (80/102) of subjects were vitamin D deficient or insufficient, with 35% (36/102) having insufficiency and 43% (44/102) having deficiency. Baseline demographics of subjects in the study were not significantly different in any of the vitamin D groups (table 2) including age, gender, race and ethnicity. Anthropometric measures of Tanner stage, BMI z-score, weight and waist circumference also did not significantly differ between the three groups.
Table 1.
Demographic, anthropometric and laboratory values for children with NAFLD in the Vitamin D study
| Characteristics | All Children (N=102) |
|---|---|
| Specimen characteristic: | |
| Vitamin D level | 23.1 ± 8.8 |
| Season: Spring/summer (Apr–Sept) | 68 (67%) |
| Demographics: | |
| Gender: Boys | 83 (81%) |
| Age at enrollment (years), mean±SD | 12.9 ± 2.7 |
| Caucasian: Yes | 74 (73%) |
| Hispanic: Yes | 71 (70%) |
| Region: South | 57 (56%) |
| Anthropometric: | |
| Tanner stage: early (1–2) | 63 (62%) |
| Tanner stage, mean±SD | 1.41 ± 2.4 |
| Body Mass Index (Z-score): | |
| < 2.0 (overweight or less) | 15 (15%) |
| 2.0 – (moderately or severely obese ) | 87 (85%) |
| Mean±SD | 2.40 ± 0.50 |
| Weight, kg, (mean±SD) | 86 ± 23 |
| Waist circumference, cm, (mean±SD) | 107 ± 14 |
| Laboratory values† (mean±SD): | |
| ALT (U/L) | 115 ± 68 |
| AST (U/L) | 67 ± 37 |
| GGT (U/L) | 48.5 ± 36.7 |
| Glucose (mg/dL) | 88.8 ± 8.4 |
| Insulin (mcU/mL) | 32.2 ± 17.6 |
| HOMA-IR (mg/dL × mcU/mL/405) | 7.1 ± 4.1 |
| Triglycerides (mg/dL) | 141.5 ± 89.5 |
| Cholesterol, total (mg/dL) | 168.6 ± 31.6 |
| Cholesterol, HDL (mg/dL) | 36.7 ± 7.2 |
| Cholesterol, LDL (mg/dL) | 105.3 ± 26.0 |
106 participants age 2 through 18 at enrollment in NAFLD DB or TONIC with histology from centrally reviewed biopsies within 31 days of blood draw were analyzed for Vitamin D level. A total of 102 participants had a Vitamin D level.
No. (%) or mean±SD are presented.
Variable definitions:
ALT: alanine aminotransferase, AST: aspartate aminotransferase, GGT: gamma glutamyl trans-peptidase, HOMA-IR: homeostatis model assessment of insulin resistance index
Table 2.
Baseline characteristics by 3 levels of Vitamin D for pediatric participants in the NASH CRN Database studies
| Vitamin D Deficiency (N=102)* | |||||
|---|---|---|---|---|---|
| Sufficient ≥ 30 ng/mL (N=22) |
Insufficient 21 – 29 ng/mL (N=36) |
Deficient ≤ 20 ng/mL (N=44) |
P† |
P trend‡ |
|
| Percent in each group | 22% | 35% | 43% | ||
| Specimen characteristic: | |||||
| Vitamin D level | 36.1 ± 6.6 | 24.3 ± 2.2 | 15.7 ± 3.8 | ||
| Season: Fall/winter (Oct-Mar) | 5 (15%) | 8 (24%) | 21 (62%) | 0.03 | 0.02 |
| Spring/summer (Apr–Sept) | 17 (25%) | 28 (41%) | 23 (34%) | ||
| Demographics: | |||||
| Gender: Girls | 4 (21%) | 6 (32%) | 9 (47%) | 0.91 | 0.77 |
| Boys | 18 (22%) | 30 (36%) | 35 (42%) | ||
| Age at enrollment (years), mean±SD | 12.3 ± 3.0 | 12.9 ± 2.0 | 13.2 ± 3.0 | 0.31 | 0.13 |
| Caucasian: Yes | 17 (23%) | 22 (30%) | 35 (47%) | 0.21 | 0.54 |
| Non-white | 4 (22%) | 7 (39%) | 7 (39%) | ||
| Hispanic: Yes | 16 (23%) | 28 (39%) | 27 (38%) | 0.27 | 0.23 |
| Not Hispanic | 6 (19%) | 8 (26%) | 17 (55%) | ||
| Region: South | 11 (19%) | 19 (33%) | 27 (47%) | 0.61 | 0.34 |
| North | 11 (24%) | 17 (38%) | 17 (38%) | ||
| Anthropometric: | |||||
| Tanner stage: early (1–2) | 15 (24%) | 23 (37%) | 25 (40%) | 0.64 | 0.35 |
| Late (3 – 5) | 7 (18%) | 13 (33%) | 19 (49%) | ||
| Tanner stage, mean±SD | 2.1 ± 1.5 | 2.3 ± 1.4 | 2.6 ± 1.5 | 0.38 | 0.17 |
| Body Mass Index (Z-score): | 0.92 | 0.93 | |||
| < 2.0 (overweight or less) | 3 (20%) | 6 (40%) | 6 (40%) | ||
| 2.0 – (moderately or severely obese ) | 19 (22%) | 30 (34%) | 38 (44%) | ||
| Mean±SD | 2.41 ± 0.31 | 2.34 ± 0.32 | 2.45 ± 0.68 | 0.74 | 0.85 |
| Weight, kg, (mean±SD) | 84 ± 26 | 86 ± 22 | 87 ± 23 | 0.93 | 0.73 |
| Waist circumference, cm, (mean±SD) | 105 ± 14 | 108 ± 15 | 108 ± 13 | 0.86 | 0.69 |
| Laboratory values (mean±SD): | |||||
| ALT (U/L) | 120 ± 69 | 120 ± 76 | 109 ± 62 | 0.57 | 0.34 |
| AST (U/L) | 75 ± 42 | 70 ± 41 | 62 ± 30 | 0.45 | 0.21 |
| Uric Acid (mg/dL) | 5.9 ± 1.3 | 5.9 ± 1.3 | 6.3 ± 1.6 | 0.54 | 0.35 |
| C-reactive protein (CRP) (mg/dL)§ | 0.83 ± 1.13 | 0.65 ± 0.49 | 1.06 ± 1.70 | 0.82 | 0.63 |
| Iron (mcg/dL) | 84.8 ± 31.3 | 87.4 ± 34.0 | 87.3 ± 26.9 | 0.95 | 0.80 |
| Ferritin (ng/mL) | 80.8 ± 52.4 | 91.4 ± 75.2 | 109.8 ± 104.8 | 0.35 | 0.19 |
| GGT (U/L) | 40.7 ± 18.9 | 61.3 ± 54.8 | 42.0 ± 18.5 | 0.61 | 0.94 |
| Glucose (mg/dL) | 87.8 ± 8.1 | 89.9 ± 8.1 | 88.6 ± 8.8 | 0.79 | 0.86 |
| Insulin (mcU/mL) | 26.8 ± 14.7 | 33.2 ± 18.2 | 34.2 ± 18.3 | 0.24 | 0.12 |
| HOMA-IR (mg/dL X mcU/mL/405) | 5.9 ± 3.5 | 8.8 ± 9.0 | 7.6 ± 4.3 | 0.20 | 0.12 |
| Triglycerides (mg/dL) | 98.9 ± 36.7 | 128.1 ± 53.1 | 173.4 ± 116.7 | 0.004 | 0.001 |
| Cholesterol, total (mg/dL) | 149.2 ± 32.7 | 171.4 ± 31.6 | 176.0 ± 27.6 | 0.007 | 0.003 |
| Cholesterol, HDL (mg/dL) | 35.1 ± 7.7 | 37.9 ± 7.7 | 36.6 ± 6.7 | 0.27 | 0.43 |
| Cholesterol, LDL (mg/dL) | 94.9 ± 26.8 | 108.1 ± 27.9 | 108.2 ± 23.1 | 0.09 | 0.05 |
| Metabolic syndrome: | |||||
| Metabolic syndrome: | |||||
| Yes (3 or more factors) | 3 (13%) | 7 (30%) | 13 (57%) | 0.30 | 0.12 |
| No (0, 1, or 2 factors) | 19 (24%) | 29 (37%) | 31 (39%) | ||
| Number of factors (0–5): | 1.7 ± 1.0 | 1.7 ± 1.2 | 1.7 ± 1.2 | 0.88 | 0.85 |
| Percent with each factor: | |||||
| Central obesity | 15 (21%) | 24 (34%) | 31 (44%) | 0.98 | 0.83 |
| High Triglycerides | 2 (9%) | 9 (41%) | 11 (50%) | 0.26 | 0.19 |
| Low HDL cholesterol | 10 (37%) | 8 (30%) | 9 (33%) | 0.08 | 0.05 |
| High Blood pressure | 9 (21%) | 14 (33%) | 20 (47%) | 0.83 | 0.66 |
| Impaired Glucose | 1 (11%) | 5 (56%) | 9 (33%) | 0.48 | 0.98 |
| Activity level: | |||||
| Screen time: < 2 hrs/day | 2 (12%) | 4 (24%) | 11 (65%) | 0.13 | 0.07 |
| ≥ 2 hrs/day | 18 (22%) | 32 (40%) | 31 (38%) | ||
| Exercise: <1 hr/day | 4 (33%) | 3 (25%) | 5 (42%) | 0.49 | 0.53 |
| ≥ 1 hr/day | 18 (20%) | 33 (37%) | 39 (43%) | ||
| 2-week activity survey: MET-hrs/week | 45.57 ± 24.2 | 38.7 ± 21.1 | 39.7 ± 19.1 | 0.43 | 0.55 |
| Diet: | |||||
| Vitamin D intake: | |||||
| Vit D supplementation | 0.27 | 0.10 | |||
| No | 21 (23%) | 34 (37%) | 37 (40%) | ||
| Yes | 1 (10%) | 2 (20%) | 7 (70%) | ||
| Dietary daily Vitamin D (IU) | 250 ± 308 | 208 ± 195 | 194 ± 124 | 0.90 | 0.95 |
| Supplemental daily Vitamin D (IU) | 21.1 ± 91.8 | 27.6 ± 103.2 | 51.3 ± 129.0 | 0.40 | 0.21 |
| Total daily Vitamin D intake (IU) | 271 ± 329 | 236 ± 202 | 245 ± 152 | 0.68 | 0.41 |
| Calcium intake: | |||||
| Calcium supplementation | |||||
| No | 21 (23%) | 33 (37%) | 36 (40%) | 0.27 | 0.08 |
| Yes | 1 (3%) | 3 (25%) | 8 (67%) | ||
| Dietary daily Calcium (mg) | 1017 ± 542 | 998 ± 578 | 991 ± 438 | 0.99 | 0.91 |
| Supplemental daily Calcium (mg) | 6.8 ± 29.82 | 33.6 ± 135 | 24.0 ± 60.0 | 0.39 | 0.17 |
| Total daily Calcium intake (mg) | 1024 ± 546 | 1031 ± 619 | 1015 ± 419 | 0.99 | 0.80 |
| Daily servings of dairy products | 2.4 ± 1.2 | 2.3 ± 1.6 | 2.2 ± 1.2 | 0.85 | 0.65 |
| Histologic characteristics: | |||||
| Steatosis grade: | 0.24 | 0.73 | |||
| 5 – 33% | 8 (25%) | 12 (38%) | 12 (38%) | ||
| 34% – 66% | 3 (10%) | 14 (47%) | 13 (43%) | ||
| > 66% | 11 (28%) | 10 (25%) | 19 (48%) | ||
| Lobular inflammation, 20× mag: | 0.86 | 0.80 | |||
| < 2 | 10 (20%) | 19 (37%) | 22 (43%) | ||
| ≥ 2 | 12 (24%) | 17 (33%) | 22 (43%) | ||
| Portal chronic inflammation: | 0.63 | 0.28 | |||
| None | 1 (12%) | 2 (25%) | 5 (62%) | ||
| Mild/More than mild | 21 (22%) | 34 (36%) | 39 (41%) | ||
| Ballooning injury: | 0.57 | 0.68 | |||
| None/few | 15 (19%) | 29 (38%) | 33 (43%) | ||
| Many | 7 (28%) | 7 (28%) | 11 (44%) | ||
| Mallory-Denk bodies: | 0.72 | 0.26 | |||
| Rare or absent | 22 (23%) | 34 (35%) | 41 (42%) | ||
| Many | 0 (0%) | 2 (40%) | 3 (60%) | ||
| Fibrosis stage: | 0.75 | 0.25 | |||
| None (0) | 5 (16%) | 10 (31%) | 17 (53%) | ||
| Mild/ moderate (1a,1b,1c) | 11 (27%) | 14 (34%) | 16 (39%) | ||
| Moderate (2) | 3 (17%) | 7 (39%) | 8 (44%) | ||
| Bridging/cirrhosis (3, 4) | 3 (27%) | 5 (45%) | 3 (27%) | ||
| Steatohepatitis diagnosis: | 0.23 | 0.61 | |||
| NAFLD, not NASH | 3 (14%) | 10 (45%) | 9 (41%) | ||
| Suspicious/borderline Zone 3 | 5 (23%) | 4 18%) | 13 (59%) | ||
| Suspicious/borderline Zone 1 | 6 (29%) | 10 (48%) | 5 (24%) | ||
| Definite | 8 (22%) | 12 (32%) | 17 (46%) | ||
| NAFLD Activity Score (NAS): | 0.96 | ||||
| 0 – 4 | 9 (17%) | 23 (44%) | 20 (38%) | 0.15 | 0.96 |
| 5 – 8 | 13 (26%) | 13 (26%) | 24 (48%) | ||
| Mean±SD | 4.7 ± 1.5 | 4.1 ±1.3 | 4.5 ±1.5 | 0.24 | 0.94 |
No. (%) or mean±SD are presented.
P (2-sided) to ascertain the significance of differences across the 3 Vitamin D level groups was determined using either a Pearson’s chi-square test or Fisher’s exact test for categorical variables, and a Kruskal-Wallis test for continuous variables.
P to ascertain the significance of a trend across the 3 Vitamin D groups was determined using Mantel-Haenszel chi-square test (or exact test) for categorical variables, and a non-parametric score test for trend for continuous variables.
CRP unavailable for 17 subjects in NAFLD DB.
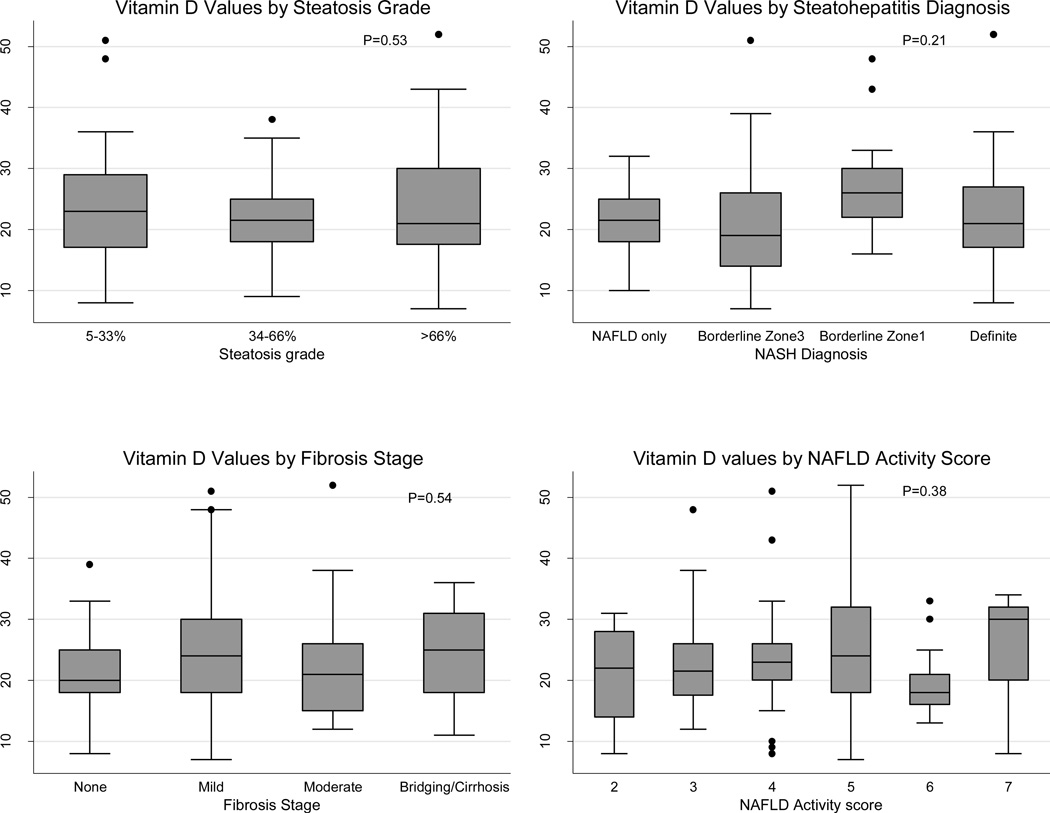
There was no significant difference between the three vitamin D groups and the distributions of histological characteristics or severity of NAFLD (table 2, figure 1), including steatosis grade, lobular inflammation, portal chronic inflammation, ballooning injury, Mallory-Denk bodies, fibrosis stage, steatohepatitis diagnosis and NAS. Having vitamin D deficiency was not significantly associated with these histological features of NAFLD (table 3) after adjustment for gender, ethnicity, tanner stage and season.
Figure 1.
Vitamin D level (ng/mL) by Steatosis grade, Fibrosis stage, Steatohepatitis diagnosis, and NAFLD activity score. P (2-sided) derived from linear regression of Vitamin D value on each categorical histological feature.
Table 3.
Association of Vitamin D Deficiency with metabolic syndrome, laboratory, dietary, and histological characteristics of pediatric participants in the NASH CRN Database studies
| Odds of Vitamin D deficiency (≤ 20 ng/mL) | ||||
|---|---|---|---|---|
| Unadjusted* | Adjusted† | |||
| (N=102) | (N=102) | |||
| Characteristics | OR (95% C.I.) | P | OR (95% C.I.) | P |
| Demographics and physical: | ||||
| Region: South vs. north | 1.48 (0.69, 3.29) | 0.33 | 1.98 (0.81, 4.83) | 0.13 |
| Age at enrollment (years)‡ | 1.14 (0.97, 1.34) | 0.12 | 1.15 (0.88, 1.51) | 0. |
| Waist circumference, cm | 1.01 (0.98, 1.04) | 0.65 | 1.00 (0.96, 1.03) | 0.81 |
| Laboratory values: | ||||
| ALT (U/L) | 0.99 (0.98, 1.00) | 0.19 | 1.00 (0.99, 1.01) | 0.79 |
| AST (U/L) | 0.99 (0.98, 1.00) | 0.19 | 1.00 (0.98, 1.01) | 0.48 |
| Uric Acid (mg/dL) | 1.21 (0.91, 1.61) | 0.19 | 1.07 (0.76, 1.51) | 0.69 |
| C-reactive protein (CRP) (mg/dL)§ | 1.01 (0.99, 1.03) | 0.48 | 1.01 (0.99, 1.03) | 0.49 |
| Iron (mcg/dL) | 1.00 (0.99, 1.01) | 0.89 | 1.00 (0.98, 1.01) | 0.82 |
| Ferritin (ng/mL) | 1.00 (1.00, 1.01) | 0.22 | 1.00 (1.00, 1.01) | 0.14 |
| GGT (U/L) | 0.99 (0.98, 1.00) | 0.13 | 0.99 (0.97, 1.01) | 0.20 |
| Glucose (mg/dL) | 0.99 (0.95, 1.04) | 0.75 | 1.00 (0.95, 1.05) | 0.98 |
| Insulin (mcU/mL) | 1.01 (0.99, 1.03) | 0.34 | 1.01 (0.99, 1.04) | 0.37 |
| HOMA-IR (mg/dL × mcU/405) | 1.05 (0.95, 1.16) | 0.35 | 1.05 (0.94, 1.17) | 0.38 |
| Triglycerides (mg/dL) | 1.01 (1.00, 1.02) | 0.006 | 1.01 (1.00, 1.02) | 0.006 |
| Cholesterol, total (mg/dL) | 1.01 (1.00, 1.03) | 0.04 | 1.01 (1.00, 1.03) | 0.05 |
| Cholesterol, HDL (mg/dL) | 0.99 (0.94, 1.05) | 0.84 | 1.00 (0.94, 1.06) | 0.89 |
| Cholesterol, LDL (mg/dL) | 1.01 (0.99, 1.02) | 0.32 | 1.01 (0.99, 1.03) | 0.32 |
| Metabolic syndrome§: | ||||
| Metabolic syndrome (yes vs. no) | 2.01 (0.79, 5.15) | 0.15 | 1.86 (0.67, 5.16) | 0.23 |
| Number of factors (0–5): | 1.02 (0.72, 1.43) | 0.93 | 0.99 (0.68, 1.45) | 0.96 |
| Presence metabolic component (yes vs. no): | ||||
| Central obesity | 1.10 (0.47, 2.59) | 0.83 | 1.09 (0.42, 2.83) | 0.86 |
| High Triglycerides | 1.39 (0.54, 3.60) | 0.49 | 1.30 (0.45, 3.74) | 0.63 |
| Low HDL cholesterol | 0.56 (0.22, 1.40) | 0.21 | 0.56 (0.21, 1.50) | 0.25 |
| High Blood pressure | 1.27 (0.57, 2.80) | 0.56 | 1.28 (0.56, 2.95) | 0.56 |
| Impaired Glucose | 0.63 (0.15, 2.69) | 0.54 | 0.48 (0.10, 2.34) | 0.36 |
| Activity level: | ||||
| Screen time: ≥ 2 hrs/day vs.< 2 hrs/day | 0.34 (0.11, 1.01) | 0.05 | 0.25 (0.07, 0.91) | 0.04 |
| Exercise: ≥ 1 hr/day vs.<1 hr/day | 1.07 (0.32, 3.63) | 0.91 | 1.51 (0.38, 6.07) | 0.56 |
| 2-week activity survey, MET-hrs/week | 1.00 (0.98, 1.02) | 0.73 | 1.00 (0.98, 1.02) | 0.91 |
| Diet: | ||||
| Vitamin D intake: | ||||
| Vitamin D supplementation (yes vs. no) | 3.47 (0.84, 14.28) | 0.09 | 2.40 (0.50, 11.54) | 0.27 |
| Total daily Vitamin D intake (IU) | 1.00 (1.00, 1.00) | 0.92 | 1.00 (1.00, 1.00) | 0.93 |
| Calcium intake | ||||
| Calcium supplementation (yes vs. no) | 3.00 (0.84, 10.71) | 0.09 | 1.92 (0.47, 7.94) | 0.36 |
| Total daily Calcium intake (mg) | 1.00 (1.00, 1.00) | 0.90 | 1.00 (1.00, 1.00) | 0.78 |
| Daily servings of dairy products | 0.95 (0.69, 1.31) | 0.77 | 1.01 (0.71, 1.45) | 0.94 |
| Histologic characteristics: | ||||
| Steatosis grade: ≥ 34% vs. 5–33% | 1.40 (0.60, 3.30) | 0.44 | 1.85 (0.70, 4.92) | 0.22 |
| Lobular inflammation, 20× mag: ≥ 2 vs. <2 | 1.00 (0.46, 2.19) | 1.00 | 1.05 (0.45, 2.43) | 0.91 |
| Portal chronic inflammation: ≥ mild vs. none | 0.43 (0.10, 1.89) | 0.26 | 0.38 (0.08, 1.92) | 0.24 |
| Ballooning injury: Many vs. none/few | 1.35 (0.61, 2.97) | 0.46 | 1.69 (0.70, 4.05) | 0.24 |
| Mallory-Denk bodies: Many vs. rare/absent | 2.05 (0.33, 12.82) | 0.44 | 2.15 (0.30, 15.24) | 0.44 |
| Advanced fibrosis: Advanced vs. not | 0.46 (0.11, 1.84) | 0.27 | 0.56 (0.13, 2.43) | 0.44 |
| Definite NASH: Definite vs. not | 1.20 (0.53, 2.70) | 0.67 | 1.58 (0.64, 3.94) | 0.32 |
| NAFLD Activity Score: 5–8 vs. 0–4 | 1.48 (0.67, 3.25) | 0.33 | 2.14 (0.86, 5.36) | 0.10 |
| Demographic adjustment characteristics: | ||||
| Gender: Boys vs. girls | 0.81 (0.30, 2.20) | 0.68 | -- | |
| Ethnicity: Hispanic vs. not Hispanic | 0.51 (0.22, 1.19) | 0.12 | -- | |
| Tanner stage: late (3–5) vs. early (1–2) | 1.44 (0.65, 3.23) | 0.37 | -- | |
| BMI Z-score | 1.16 (0.38, 3.55) | 0.79 | -- | |
| Season: Spring/summer vs. fall/winter | 0.32 (0.13, 0.74) | 0.008 | -- | |
Estimates determined from logistic regression of each characteristic on severe Vitamin D deficiency (≤ 20 ng/mL). Overall P (2-sided) determined from a Wald test.
Number of children’s samples with: severe deficiency=44; mild deficiency or sufficiency (> 20 ng/mL)=58
Estimates determined from logistic regression of each characteristic on severe Vitamin D deficiency adjusted for sex (boys vs. girls), Hispanic (yes vs. no), Tanner stage (3–5 vs. 1–2), BMI Z-score and season of sample collection (spring/summer vs. fall/winter). Overall P (2-sided) determined from a Wald test.
Excluded the sample of the child age 2 as an outlier and given that the inference did not change from when the sample was included. The vitamin D category for that participant was severe (vitamin D level=18 ng/mL)
For metabolic syndrome and its components, estimates were determined from multiple logistic regression adjusted for sex, Hispanic, Tanner stage and season, but not BMI Z-score, due to high correlation with one of the components of metabolic syndrome.
Significantly higher levels of triglycerides were found in those with vitamin D deficiency and insufficiency (173.4±116.7 mg/dL in deficient group vs. 128.1±53.1 mg/dL in insufficient group vs. 98.9±36.7 mg/dL in sufficient group, p=0.004) (table 2). However, no significant difference between Vitamin D groups was found between the percentage of children who met the criteria for high triglycerides as a feature of the metabolic syndrome (defined as > 95th percentile for age and sex), or other individual features of the metabolic syndrome of central obesity, high blood pressure and impaired glucose, or having metabolic syndrome (table 2). The percentage of patients with a low HDL cholesterol, however, trended towards increased numbers in the vitamin D sufficient group (33% in deficient group vs. 30% in insufficient group vs. 37% in sufficient group, p=0.08, p trend = 0.05).
Children with vitamin D deficiency or insufficiency had higher total cholesterol levels than those who were sufficient (176.0±27.6 mg/dL in deficient group vs. 171.4±31.6 mg/dL in insufficient group vs. 149.2±32.7 mg/dL in sufficient group, p=0.007, table 2) and a trend towards higher LDL cholesterol levels (p trend=0.05). There were no differences seen between the three vitamin D groups and other laboratory parameters analyzed (ALT, AST, uric acid, CRP, Iron, Ferritin, GGT, Insulin, HOMA-IR, HDL cholesterol, see table 2).
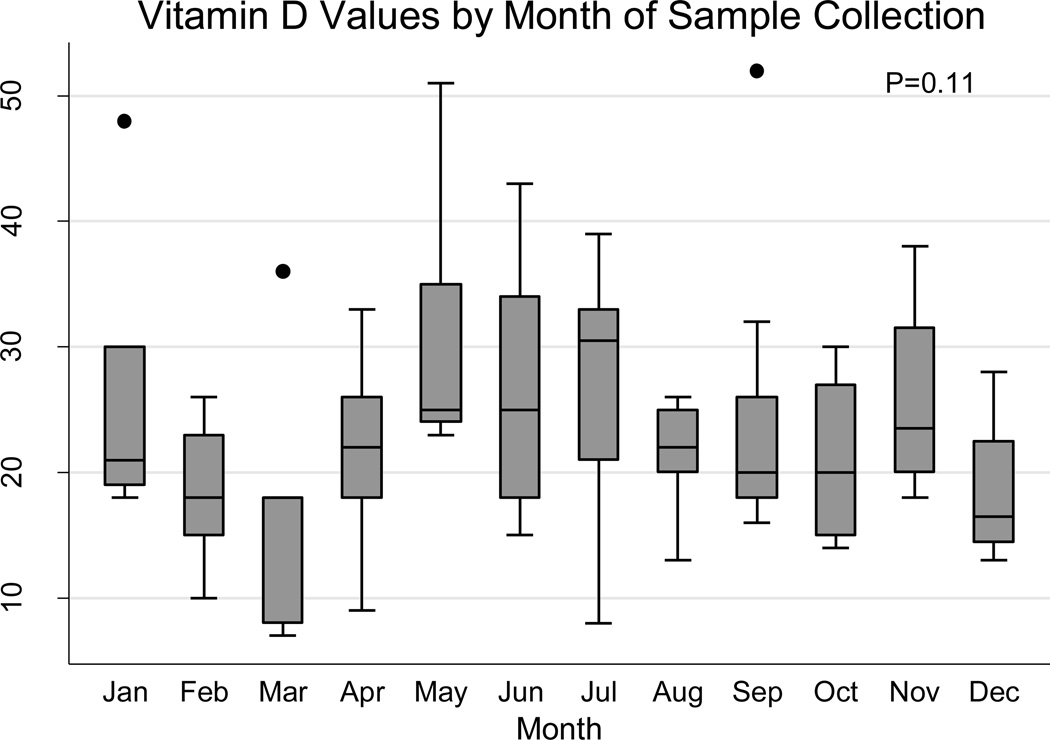
Vitamin D levels did significantly vary with the time of year in which they were collected, with higher levels of deficiency being found in fall/winter (62%) than spring/summer(34%)(p=0.03), with an OR of having vitamin D deficiency in the spring/summer vs. fall/winter of 0.32 (95% CI 0.13–0.74, p=0.008). However, there was non-significant variation in vitamin D level from month to month (p=0.11, Figure 2). There was no significant association between vitamin D levels and geographical region of the USA (north/south), dietary intake of vitamin D and calcium and activity level, including exercise and (MET)-hours/week. There was no significant difference between screen time and vitamin D group (p=0.07), however the adjusted OR of being vitamin D deficient with higher screen time was 0.27, p=0.04 (table 3), suggesting decreased screen time was associated with vitamin D deficiency.
Figure 2.
Vitamin D level by month of sample collection
The 102 children included in this vitamin D study (17 from DB, 85 from TONIC) were compared by logistic regression to the children not included in the study (178 from DB, 88 from TONIC), due to not having biopsy data or labs drawn within 31 days of biopsy, by basic demographics, laboratory and histology characteristics. Those included in the vitamin D analyses were more likely to be Hispanic, in earlier Tanner stages and had higher BMI z-scores. None of these characteristics were associated with vitamin D level. No histological features were associated with being in the vitamin D analyses.
DISCUSSION
This is the largest and most comprehensive pediatric study to date to examine the relationship between 25(OH) vitamin D status and the degree of inflammation and fibrosis on biopsies in NAFLD. In this study no statistically significant association was found between vitamin D deficiency and the histological severity of NAFLD. In addition, no association was found between vitamin D deficiency and aminotransferase levels. Our findings agree with a large pediatric study by Katz (31) using National Health and Nutrition Examination Survey (NHANES) data where vitamin D levels were not found to be independently associated with suspected NAFLD based on elevated ALT levels in adolescents, and a smaller study by Ashraf (32) where no significant differences were found in AST and ALT when comparing adolescents with and without adequate levels of vitamin D. Conversely, Manco (33) reported that low 25(OH) vitamin D increased the likelihood of fibrosis and necroinflammation in pediatric NAFLD; however this was in a small case series published as a letter without methodology of the study included.
Our findings of a lack of association with vitamin D status and histological severity of NAFLD in children differ from findings of adult studies. Targher reported a correlation between low vitamin D levels and severity of steatosis, necroinflammation and fibrosis on biopsy (15), and low vitamin D levels have been reported to correlate with the severity of steatosis independent of other components of the metabolic syndrome (16,17). These adult studies may possibly differ from our pediatric findings for a number of reasons. One hypothesis is that 25-hydroxylation of vitamin D may be reduced in pre-existing liver damage rather than leading to the liver damage, so adults who may have had the disease for longer in addition to accumulating a number of other insults to the liver over their life spans, may have lower levels of vitamin D correlating to their liver histology. Another possibility is that although there is much overlap, there are known differences in the histology (19) and course (20) of pediatric NAFLD compared with adult NAFLD, possibly suggesting that different etiologies and risk factors may be responsible for the disease.
In our study a striking number of subjects had vitamin D deficiency or insufficiency (78%). A meta-analysis (14) of published studies to date assessing the relationship of vitamin D concentrations and NAFLD diagnosed on biopsies, radiology and liver enzymes showed that NAFLD patients compared with controls were 1.26 times more likely to be vitamin D deficient (OR:1.26, 95% CI:1.17, 1.35). Our study did not have a control group without NAFLD to make this comparison. However, population based data from NHANES III reveals a prevalence of vitamin D insufficiency or deficiency as high as 63% in children aged 1–5 years and 73% in those 6–11 years (5).
In previous studies, low levels of vitamin D have been associated with obesity (6), with vitamin D insufficiency rates in obese adolescents reported ranging between 29% to more than 90% (7,8) influenced by ethnicity and climate. However in our study, a statistically significant difference was not found between the three vitamin D groups and obesity. Although no statistically significant differences were found, the majority of the patients in this study had vitamin D deficiency or insufficiency (78%) and were moderately to severely obese with a BMI z-score ≥2 (87/102, 85%), so it may be possibly underpowered to detect an association with BMI z-score and vitamin D sufficiency/deficiency. Low levels of vitamin D have been associated with features of the metabolic syndrome independent of adiposity (9), however in our study vitamin D deficiency was associated with higher triglyceride levels, but not other features of the metabolic syndrome.
Many factors can contribute towards vitamin D deficiency. Vitamin D levels did vary with season, with higher levels in the spring/summer and lower levels in the fall/winter as previously described in the United States (34). 68/102 (67%) of samples in this study were collected during the spring/summer months and 34/102 (33%) in the winter months; however, samples were collected during every month of the year (Figure 2), with a range of 4 to 17, and an average of 6 samples per month, likely providing an adequate sampling across the entire year to not skew the results found. Moreover, there were no differences in the distribution of the month of sample collection between the three vitamin D groups. Vitamin D levels were not associated with dietary intake of vitamin D; however all three groups had a mean calculated intake of less than 600IU, which is the current recommended dietary allowance (RDA) (35), potentially contributing to the high levels of vitamin D deficiency seen overall. Additionally 70% (71/102) of children in this study were Hispanic, with data from NHANES reporting high levels of vitamin D deficiency (80%) in the Hispanic pediatric population (36).
Limitations of this study include that data was cross-sectional and hence, causal inferences cannot be drawn. Longitudinal pediatric studies would be a better way to define the relationship between vitamin D deficiency as an etiology or consequence of NAFLD. All children in this study had NAFLD; a control group who are obese or have metabolic syndrome may have better been able to define the relationship between vitamin D deficiency and NAFLD. In addition, given vitamin D levels are frequently insufficient in the Hispanic population and NAFLD is frequent in this same population, a study including non-NAFLD Hispanic participants may be necessary to better define the relationship between Vitamin D deficiency and NAFLD. Additionally, this study was unable to assess the relationship between “very low” levels of vitamin D and NAFLD histology as only 7 patients had vitamin D levels ≤12 ng/mL and so was underpowered to do so. There were also small numbers of patients with vitamin D sufficiency (n=22), which limits the power to detect differences, and this may be important given the negative findings of this study. However, this is the largest study to date looking at the relationship between vitamin D deficiency and biopsy proven NAFLD in children while taking into account seasonal variation of sampling over a time span of 4 years.
CONCLUSION
There is a high prevalence of 25(OH) vitamin D deficiency and insufficiency in children with biopsy-proven NAFLD. However, no association was found between vitamin D deficiency and the severity of inflammation and fibrosis on the biopsies. This differs from adult NAFLD studies where vitamin D deficiency correlates with histological severity on biopsies, potentially suggesting differences in the risk factors for or consequences of pediatric NAFLD. Controlled longitudinal studies are needed to better define the relationship between vitamin D status and pediatric NAFLD.
ACKNOWLEDGEMENTS
We would like to thank the NASH CRN clinical center. The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713), and the National Institute of Child Health and Human Development (NICHD).
Several clinical centers use support from the National Center for Advancing Translational Sciences (NCATS) in conduct of NASH CRN Studies (grants UL1TR000439, UL1TR000077,UL1TR000436, UL1TR000150, UL1TR000424, UL1TR000006, UL1TR000448, UL1TR000040, UL1TR000100, UL1TR000004, UL1TR000423, UL1TR000058, UL1TR000067, UL1TR000454).
Funding Source: The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713), and the National Institute of Child Health and Human Development (NICHD).
Abbreviations
- NAFLD
nonalcoholic fatty liver disease nonalcoholic fatty liver disease.
- NASH CRN
Nonalcoholic Steatohepatitis Clinical Research Network.
- NHANES
National Health and Nutrition Examination Survey.
Footnotes
Financial Disclosure: No authors have financial relationships relevant to this article to disclose.
Conflict of Interest: No authors have conflicts of interest to disclose.
Contributors’ Statement Page:
Suchitra K Hourigan: Dr. Hourigan conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted.
Stephanie Abrams: Dr. Abrams conceptualized and designed the study, enrolled many patients included, critically revised manuscript and approved the final manuscript as submitted.
Katherine Yates: Ms. Yates conducted all statistical analyses for this study, critically revised manuscript and approved the final manuscript as submitted.
Kim Pfeifer:Ms. Pfeifer worked directly to obtain 25(OH) vitamin D levels from the serum samples, coordinated data collection from patients, critically revised manuscript and approved the final manuscript as submitted.
Michael Torbenson: Dr. Torbenson reviewed liver histology as part of the Pathology Committee of the NASH CRN, provided expertise regarding pathology for this manuscript, critically revised manuscript and approved the final manuscript as submitted.
Karen Murray: Dr. Murray designed the study, enrolled many patients included, critically revised manuscript and approved the final manuscript as submitted.
Christian L. Roth: Dr. Roth designed the study, enrolled many patients included, critically revised manuscript and approved the final manuscript as submitted.
Kris Kowdley: Dr. Kowdley designed the study, enrolled many patients included, critically revised manuscript and approved the final manuscript as submitted.
Ann O Scheimann: Dr. Scheimann conceptualized and designed the study, enrolled many patients included, critically revised manuscript and approved the final manuscript as submitted.
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Eng J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Guillot X, Semerano L, Saidenberg-Kermanac'h N, et al. Vitamin D and inflammation. Joint Bone Spine. 2010;77(6):552–557. doi: 10.1016/j.jbspin.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Kitson MT, Roberts SK. D-livering the message: the importance of vitamin D status in chronic liver disease. J Hepatol. 2012;57(4):897–909. doi: 10.1016/j.jhep.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 5.Mansbach JM, Ginde AA, Camargo CA., Jr Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics. 2009;124(5):1404–1410. doi: 10.1542/peds.2008-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellia A, Garcovich C, D'Adamo M, et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern Emerg Med. 2013;8(1):33–40. doi: 10.1007/s11739-011-0559-x. [DOI] [PubMed] [Google Scholar]
- 7.Harel Z, Flanagan P, Forcier M, et al. Low vitamin D status among obese adolescents: prevalence and response to treatment. J Adolesc Health. 2011;48(5):448–452. doi: 10.1016/j.jadohealth.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Lenders CM, Feldman HA, Von Scheven E, et al. Relation of body fat indexes to vitamin D status and deficiency among obese adolescents. Am J Clin Nutr. 2009;90(3):459–467. doi: 10.3945/ajcn.2008.27275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reis JP, von Mühlen D, Miller ER, 3rd, et al. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 2009;124(3):e371–e379. doi: 10.1542/peds.2009-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 11.Sung KC, Kim SH. Interrelationship between fatty liver and insulin resistance in the development of type 2 diabetes. J Clin Endocrinol Metab. 2011;96(4):1093–1097. doi: 10.1210/jc.2010-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr. 2013;162(3):496–500. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roth CL, Elfers CT, Figlewicz DP, et al. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. Hepatology. 2012;55(4):1103–1111. doi: 10.1002/hep.24737. [DOI] [PubMed] [Google Scholar]
- 14.Eliades M, Spyrou E, Agrawal N, et al. Meta-analysis: vitamin D and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38(3):246–254. doi: 10.1111/apt.12377. [DOI] [PubMed] [Google Scholar]
- 15.Targher G, Bertolini L, Scala L, et al. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17(7):517–524. doi: 10.1016/j.numecd.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Abawi M, Birerdinc A, Baranova A, et al. Vitamin D levels in non-alcoholic fatty liver disease (NAFLD) patients correlate with apoptosis and serum levels of M30. Am J Gastroenterol. 2011;106(Suppl 2):S121. [Google Scholar]
- 17.Dasarathy J, Periyalwar P, Allampati S, et al. Hypovitaminosis D associated with more advanced non alcoholic fatty liver disease. Hepatology. 2012;56(S1):889A–890A. [Google Scholar]
- 18.Geier A. Shedding new light on vitamin D and fatty liver disease. J Hepatol. 2011;55(2):273–275. doi: 10.1016/j.jhep.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 19.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42(3):641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 20.Holterman AX, Guzman G, Fantuzzi G, et al. Nonalcoholic fatty liver disease in severely obese adolescent and adult patients. Obesity. 2013;21(3):591–597. doi: 10.1002/oby.20174. [DOI] [PubMed] [Google Scholar]
- 21.Lavine JE, Schwimmer JB. Nonalcoholic Steatohepatitis-Clinical Research Network. Pediatric initiatives within the Nonalcoholic Steatohepatitis-Clinical Research Network (NASH CRN) J Pediatr Gastroenterol Nutr. 2003;37(3):220–221. doi: 10.1097/00005176-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Lavine JE, Schwimmer JB, Van Natta ML, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Effect of vitamin E or met for min for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305(16):1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ersfeld DL, Rao DS, Body JJ, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem. 2004;37(10):867–874. doi: 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Kleiner DE, Brunt EM, Van Natta M, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 25.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 26.Kriska AM. Modifiable activity questionnaire. In: A collection of physical activity questionnaires for health-related research. In: Pereira MA, FitzGerald SJ, Gregg EW, editors. Med. Sci. Sports Exerc. Suppl. 6. Vol. 29. 1997. pp. S73–S78. [PubMed] [Google Scholar]
- 27.Nutrition Quest. Assessment and analysis services. Nutrition Quest Web site. http://nutritionquest.com/assessment/. Published 2009. [Google Scholar]
- 28.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 29.Agresti A. An Introduction to Categorical Data Analysis. Second Edition. New York: John Wiley & Sons, Inc.; 2007. [Google Scholar]
- 30.Cuzick J. A Wilcoxon-type test for trend. Statistics in Medicine. 1985;4(1):87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 31.Katz K, Brar PC, Parekh N, et al. Suspected nonalcoholic Fatty liver disease is not associated with vitamin d status in adolescents after adjustment for obesity. J Obes. 2010:496–829. doi: 10.1155/2010/496829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.A, Alvarez J, Saenz K, Gower B, et al. Threshold for effects of vitamin D deficiency on glucose metabolism in obese female African-American adolescents. J Clin Endocrinol Metab. 2009;94(9):3200–3206. doi: 10.1210/jc.2009-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manco M, Ciampalini P, Nobili V. Low levels of 25-hydroxyvitamin D(3) in children with biopsy-proven nonalcoholic fatty liver disease. Hepatology. 2010;51(6):2229. doi: 10.1002/hep.23724. author reply 2230. [DOI] [PubMed] [Google Scholar]
- 34.Kasahara AK, Singh RJ, Noymer A. Vitamin D (25OHD) Serum Seasonality in the United States. PLoS One. 2013;8(6):e65785. doi: 10.1371/journal.pone.0065785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calicum and Vitamin D. Washington, DC: National Academy Press; 2010. [Google Scholar]
- 36.Mansbach JM, Ginde AA, Camargo CA., Jr Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics. 2009;124(5):1404. doi: 10.1542/peds.2008-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]