Abstract
Susceptibilities of Madurella mycetomatis against amphotericin B and itraconazole in vitro were determined by protocols based on NCCLS guidelines (visual reading) and a 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) assay for fungal viability. The XTT assay was reproducible and sensitive for both antifungals. Itraconazole (MIC at which 50% of the isolates tested are inhibited [MIC50]) of 0.06 to 0.13 mg/liter) was superior to amphotericin B (MIC50 of 0.5 to 1.0 mg/liter).
Little is known about the susceptibility of the fungus Madurella mycetomatis, the major cause of eumycetoma, to antifungal agents (6). In the past, ketoconazole was promoted as the drug of choice (4, 6, 8, 10, 15, 16), but clinical response to ketoconazole is often poor (5, 17, 18, 25, 26). Other studies show that early treatment with itraconazole (ITC) seems to be optimal (5, 10, 17). Here we evaluate the in vitro activities of ITC and amphotericin B (AMB) against 36 clinical isolates of M. mycetomatis. MICs were determined visually by a method based on the NCCLS (approved standard M38-A) (20). In addition, a quantitative colorimetric method using the dye2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) was used (12, 13, 24).
Independent clinical isolates (n = 34) obtained from Sudanese mycetoma patients visiting the Mycetoma Research Clinic (University of Khartoum, Khartoum, Sudan) during the year 1999 were included. Two additional clinical isolates were derived from patients from Mali (2). Strains were cultivated on Sabouraud dextrose agar with or without 80 mg of gentamicin (Centrafarm, Etten-Leur, The Netherlands) per liter or on malt extract agar (Difco Laboratories, Paris, France). Species were identified as described previously (1, 9).
ITC was obtained from Janssen Pharmaceutica Products, Ghent, Belgium, and AMB was obtained from Bristol-Myers Squibb, Woerden, The Netherlands. The protocol for susceptibility testing (broth macrodilution) was based on the NCCLS procedure for filamentous fungi (approved standard M38-A [20]. To prepare inocula from cultures in RPMI 1640 with l-glutamine (0.3 g/liter) and 20 mM morpholinepropanesulfonic acid, mycelia were harvested by 5 min of centrifugation at 2,158 × g and washed once with sterile saline. After sonification (20 s at 28-μm maximum power; Soniprep, Beun de Ronde, The Netherlands) of the hyphal suspension, Tween 60 was added at 0.05% (vol/vol), and the transmissions were adjusted to 70% at 660 nm (Novaspec II; Pharmacia Biotech). The inoculated tubes were incubated at 37°C for 7 days. Inoculum reading controls (hyphal suspension in saline without antifungals) were included, as were growth controls without antifungals.
The viable fungal mass was determined colorimetrically with XTT as the substrate as described previously (19). Tubes containing final concentrations of 250 μg of XTT/ml and menadione (58 μM) were incubated for 2 h at 37°C and for another 3 h at room temperature. The tubes were then centrifuged, and the extinction coefficient of the supernatant was measured at 450 nm in a microplate reader.
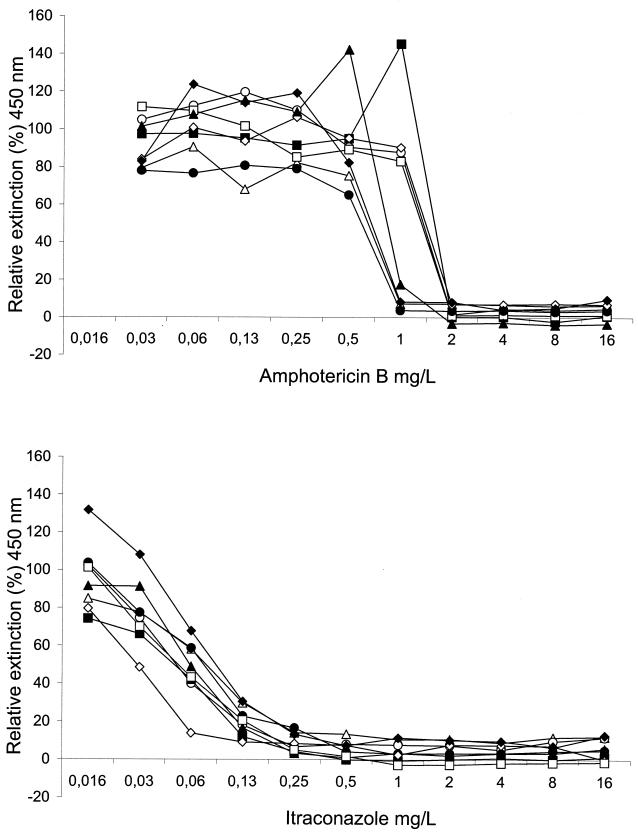
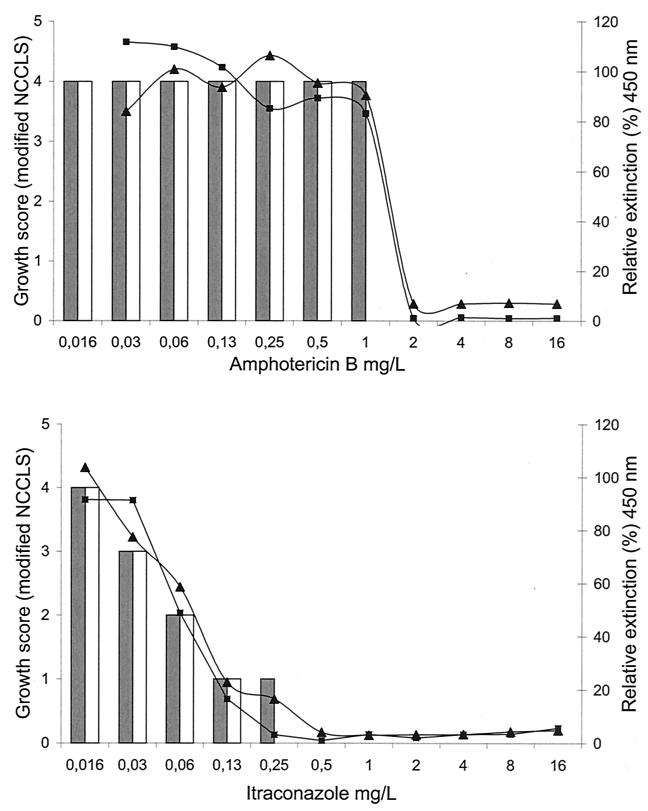
Figure 1 shows the reproducibility of antifungal susceptibility testing of an M. mycetomatis strain (mm55) according to the XTT assay. For AMB, a sudden switch to full reduction in viable fungal mass was observed. Exposure to ITC resulted in a concentration-dependent gradual decrease. In Fig. 2, the results for the XTT assay and the modified NCCLS method were compared for M. mycetomatis strain mm55. A concentration-dependent pattern of antifungal activity, each being different for AMB and ITC, was observed. The MICs of AMB and ITC for M. mycetomatis mm-55 were 1 to 2 and 0.25 to 0.5 mg/liter, respectively.
FIG. 1.
Reproducibility of susceptibility testing of M. mycetomatis strain mm-55 against AMB and ITC by the XTT method. Curves represent the relative extinction at 450 nm for each drug concentration compared to the growth control (100%). Assays were repeated eight times.
FIG. 2.
Antifungal susceptibility testing of M. mycetomatis strain mm-55 against AMB and ITC in duplicate. Curves represent the relative extinction at 450 nm for each drug concentration compared to the growth control (100%) obtained by the XTT assay. Bars represent the growth levels obtained by the modified NCCLS method as determined in a separate experiment.
Table 1 shows the susceptibilities of 36 M. mycetomatis clinical isolates determined by both methods. Often, high susceptibility to ITC is accompanied by relatively low susceptibility to AMB. For AMB, the MIC values ranged from 0.13 to 4 mg/liter (MIC at which 50% of the isolates tested are inhibited [MIC50] of 0.5 to 1 mg/liter). For ITC, the MIC values ranged from 0.016 to 1 mg/liter (MIC50 of 0.06 to 0.13 mg/liter). With the XTT assay, 100% reduction in viable fungal mass could not be determined as a number of strains produced pigments that influenced the color intensity. For all 36 M. mycetomatis isolates, 80% reduction was obtained with AMB ranging from 0.13 to 8 mg/liter and with ITC from 0.016 to 1 mg/liter. A wide range of MICs was obtained for AMB as well as ITC for our clinical isolates, irrespective of their clonal relatedness (3). This finding implies that gene expression levels rather than differential gene presence are a driving factor in the development of resistance.
TABLE 1.
Antifungal susceptibilities of 36 M. mycetomatis clinical isolates
| Isolate | MIC (mg/liter)a:
|
|||||
|---|---|---|---|---|---|---|
| AMB
|
ITC
|
|||||
| XTT assay at reduction indicated
|
NCCLS modified | XTT assay at reduction indicated
|
NCCLS modified | |||
| >50% | >80% | >50% | >80% | |||
| mm71 | 0.13 | 0.13 | 0.13 | 0.03 | 0.06 | 0.13 |
| p2 | 0.13 | 0.13 | 0.13 | 0.06 | 0.13 | 0.25 |
| mm46 | 0.13 | 0.13 | 0.25 | 0.01 | 0.01 | 0.01 |
| mm54 | 0.13 | 0.25 | 0.5 | 0.01 | 0.01 | 0.03 |
| mm30 | 0.13 | 0.5 | 0.5 | 0.13 | 1 | 0.5 |
| mm49 | 0.13 | 0.5 | 0.5 | 0.01 | 0.01 | 0.06 |
| mm58 | 0.13 | 0.5 | 1 | 0.06 | 0.13 | 0.06 |
| mm63 | 0.25 | 0.25 | 0.25 | 0.03 | 0.03 | 0.06 |
| mm39 | 0.25 | 0.5 | 0.25 | 0.06 | 0.06 | 0.06 |
| mm36 | 0.25 | 0.5 | 0.5 | 0.06 | 0.06 | 0.06 |
| mm41 | 0.25 | 0.5 | 0.5 | 0.06 | 0.13 | 0.13 |
| mm83 | 0.25 | 0.5 | 0.5 | 0.03 | 0.06 | 0.06 |
| mm22 | 0.25 | 0.5 | 1 | 0.06 | 0.13 | 0.13 |
| mm14 | 0.5 | 0.5 | 0.5 | 0.06 | 0.13 | 0.13 |
| mm28 | 0.5 | 0.5 | 0.5 | 0.06 | 0.13 | 0.06 |
| mm10 | 0.5 | 0.5 | 1 | 0.25 | 1 | 1 |
| mm29 | 0.5 | 0.5 | 1 | 0.03 | 0.06 | 0.06 |
| mm25 | 0.5 | 1 | 0.5 | 0.06 | 0.13 | 0.06 |
| mm18 | 1 | 1 | 1 | 0.01 | 0.03 | 0.01 |
| mm55 | 1 | 1 | 1 | 0.06 | 0.25 | 0.25 |
| mm50 | 1 | 1 | 2 | 0.01 | 0.03 | 0.03 |
| mm52 | 1 | 1 | 4 | 0.06 | 0.5 | 0.5 |
| mm26 | 1 | 2 | 1 | 0.06 | 0.25 | 0.25 |
| mm72 | 1 | 2 | 1 | 0.06 | 0.06 | 0.03 |
| mm78 | 1 | 2 | 1 | 0.06 | 0.06 | 0.06 |
| mm43 | 1 | 2 | 2 | 0.13 | 0.25 | 0.25 |
| mm64 | 1 | 2 | 2 | 0.03 | 0.03 | 0.06 |
| mm68 | 1 | 2 | 2 | 0.06 | 0.5 | 0.5 |
| p1 | 1 | 2 | 2 | 0.25 | 0.5 | 1 |
| mm45 | 1 | 4 | 2 | 0.25 | 0.5 | 0.5 |
| mm73 | 2 | 2 | 1 | 0.06 | 0.06 | 0.25 |
| mm56 | 2 | 2 | 2 | 0.03 | 0.06 | 0.06 |
| mm13 | 2 | 4 | 4 | 0.5 | 1 | 0.5 |
| mm35 | 4 | 4 | 4 | 0.5 | 1 | 0.5 |
| mm16 | 4 | 8 | 4 | 0.25 | 0.5 | 0.5 |
| mm31 | 4 | 4 | 4 | 0.13 | 0.5 | 0.25 |
For the XTT assay, the concentrations resulting in more than 50% or 80% reduction in viable fungal mass are shown. For the NCCLS modified, the concentrations resulting in complete inhibition of fungal growth (MIC) are shown. Data are median values for three determinations.
The filamentous nature of M. mycetomatis frustrates the straightforward use of the standardized NCCLS protocols since a conidial suspension is usually used as an inoculum (7, 11, 20). Preparing a standardized inoculum for the poorly differentiating and nonsporulating fungal species is problematic (9). To standardize the inoculum, the harvested hyphae were first homogenized. These inocula were found to be within the recommended range of 0.4 × 104 to 5 × 104 CFU per ml (20).
As the initial hyphal suspension already shows significant turbidity, which complicates visual reading of the antifungal activity, the XTT assay was also used. It generated reproducible data and showed good agreement with the MIC data for AMB. This overall agreement was also documented for various other fungal species (19).
The antifungal effect of ITC is superior to that of AMB. Approximately 45% of the 36 M. mycetomatis isolates showed susceptibility to ITC concentrations of less than 0.13 mg/liter, whereas AMB was not effective at those concentrations. Prevention of growth of all isolates was obtained with ITC at 1 mg/liter or less and with AMB at 8 mg/liter or less; these results are in agreement with earlier findings for other filamentous ascomycetes (14, 18, 20). Activities of ITC against dermatophytes (11) and agents of hyalohyphomycosis, phaeohyphomycosis, chromoblastomycosis, and mycetoma were also demonstrated (18). Recently, ITC has been effectively used for the treatment of a case of fungal mycetoma due to Fusarium solani (27). Compared to ITC, voriconazole showed comparable or increased in vitro activity against a number of emerging and less common mold pathogens (21). The high in vitro susceptibility of the M. mycetomatis isolates may nominate ITC as the drug of choice for treatment. About 33% of the M. mycetomatis isolates included in this study had an AMB MIC that is higher than the average peak level in plasma of 1.5 mg/liter (22). In addition to the relatively low antifungal activity, the requirement for long-term treatment of mycetoma patients together with the potential toxic side effects of AMB further limits its use as a first-line therapeutic agent.
In conclusion, the XTT assay is optimal for antifungal susceptibility testing of M. mycetomatis since it avoids visual reading. It also provides additional insight into the dynamics of killing. The assessment of antifungal susceptibility may be particularly useful for patients not responding to initial medical treatment.
REFERENCES
- 1.Ahmed, A. O., M. M. Mukhtar, M. Kools-Sijmons, A. H. Fahal, S. de Hoog, B. Gerrits Van den Ende, E. E. Zijlstra, H. Verbrugh, E. S. Abugroun, A. M. Elhassan, and A. van Belkum. 1999. Development of a species-specific PCR-restriction fragment length polymorphism analysis procedure for identification of Madurella mycetomatis. J. Clin. Microbiol. 37:3175-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, A. O., N. Desplaces, P. Leonard, F. Goldstein, S. De Hoog, H. Verbrugh, and A. van Belkum. 2003. Molecular detection and identification of agents of eumycetoma: detailed report of two cases. J. Clin. Microbiol. 41:5813-5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed, A. O., W. van de Sande, H. Verbrugh and A. van Belkum. Madurella mycetomatis strains from mycetoma lesions in Sudanese patients are clonal. J. Clin. Microbiol. 41:4537-4541. [DOI] [PMC free article] [PubMed]
- 4.Andreu, J. M. 1986. Actual treatment of fungus mycetoma: interest in associating ketoconazole and conservative surgery. Med. Trop. (Mars) 46:293-297. [PubMed] [Google Scholar]
- 5.Bayles, M. A. 1992. Tropical mycoses. Chemotherapy 38:27-34. [DOI] [PubMed] [Google Scholar]
- 6.Boiron, P., R. Locci, M. Goodfellow, S. A. Gumaa, K. Isik, B. Kim, M. M. McNeil, M. C. Salinas-Carmona, and H. Shojaei. 1998. Nocardia, nocardiosis and mycetoma. Med. Mycol. 36:26-37. [PubMed] [Google Scholar]
- 7.Capilla, J., M. Ortoneda, F. J. Pastor, and J. Guarro. 2001. In vitro antifungal activities of the new triazole UR-9825 against clinically important filamentous fungi. Antimicrob. Agents Chemother. 45:2635-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuce, L. C., E. L. Wroclawski, and S. A. Sampaio. 1980. Treatment of paracoccidioidomycosis, candidiasis, chromomycosis, lobomycosis, and mycetoma with ketoconazole. Int. J. Dermatol. 19:405-408. [DOI] [PubMed] [Google Scholar]
- 9.de Hoog, G. S., J. Guarro, J. Gene, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Universitat Rovira i Virgili, Reus, Spain.
- 10.Fahal, A. H. December2002, posting date. Mycetoma updates. Mycetoma Research Center, University of Khartoum, Khartoum, Sudan. [Online.] www.mycetoma.org.
- 11.Fernandez-Torres, B., A. J. Carrillo, E. Martin, A. Del Palacio, M. K. Moore, A. Valverde, M. Serrano, and J. Guarro. 2001. In vitro activities of ten antifungal drugs against 508 dermatophyte strains. Antimicrob. Agents Chemother. 45:2524-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawser, S. P., C. Jessup, J. Vitullo, and M. A. Ghannoum. 2001. Utility of 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenyl-amino)carbonyl]-2H-tetrazolium hydroxide (XTT) and minimum effective concentration assays in the determination of antifungal susceptibility of Aspergillus fumigatus to the lipopeptide class compounds. J. Clin. Microbiol. 39:2738-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawser, S. P., H. Norris, C. J. Jessup, and M. A. Ghannoum. 1998. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5- [(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards methods of testing clinical yeast isolates for susceptibility to antifungal agents. J. Clin. Microbiol. 36:1450-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, R. K., M. A. Ciblak, N. Nordoff, L. Pasarell, D. W. Warnock, and M. R. McGinnis. 2000. In vitro activities of voriconazole, itraconazole, and amphotericin B against Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum. Antimicrob. Agents Chemother. 44:1734-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahgoub el, S. 1994. Medical treatment of mycetoma. Sudan Med. J. 32:88-97. [Google Scholar]
- 16.Mahgoub, E. S., and S. A. Gumaa. 1984. Ketoconazole in the treatment of eumycetoma due to Madurella mycetomi. Trans. R. Soc. Trop. Med. Hyg. 78:376-379. [DOI] [PubMed] [Google Scholar]
- 17.McGinnis, M. R. 1996. Mycetoma Dermatol. Clin. 14:97-104. [DOI] [PubMed] [Google Scholar]
- 18.McGinnis, M. R., and L. Pasarell. 1998. In vitro testing of susceptibilities of filamentous ascomycetes to voriconazole, itraconazole, and amphotericin B, with consideration of phylogenetic implications. J. Clin. Microbiol. 36:2353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meletiadis, J., J. W. Mouton, J. F. G. M. Meis, B. A. Bouman, P. J. Donnelly, P. E. Verweij, and EUROFUNG Network. 2001. Comparison of spectrophotometric and visual readings of NCCLS method and evaluation of a colorimetric method based on reduction of a soluble tetrazolium salt, 2,3-bis [2-methoxy-4-nitro-5-[(sulfenylamino)Carbonyl]-2H-tetrazolium-hydroxide], for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:4256-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 21.Radford, S. A., E. M. Johnson, and D. W. Warnock. 1997. In vitro studies of activity of voriconazole (UK-109,496), a new triazole antifungal agent against emerging and less-common mold pathogens. Antimicrob. Agents Chemother. 41:841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rippon, J. W. 1988. Medical mycology, the pathogenic fungi and the pathogenic actinomycetes, 3rd ed. The W.B. Saunders Co., Philadelphia, Pa.
- 23.Smith, E. L., and S. Kutbi. 1997. Improvement of eumycetoma with itraconazole. J. Am. Acad. Dermatol. 36:279-280. [DOI] [PubMed] [Google Scholar]
- 24.Tellier, R., M. Krajden, G. Grigoriew, and I. Campbell. 1992. Innovative endpoint determination system for antifungal susceptibility testing of yeasts. Antimicrob. Agents Chemother. 36:1619-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venugopal, P. V., and T. V. Venugopal. 1993. Treatment of eumycetoma with ketoconazole. Australas. J. Dermatol. 34:27-29. [DOI] [PubMed] [Google Scholar]
- 26.Venugopal, P. V., T. V. Venugopal, E. S. Ramakrishna, and S. Ilavarasi. 1993. Antimycotic susceptibility testing of agents of black grain eumycetoma. J. Med. Vet. Mycol. 31:161-164. [DOI] [PubMed] [Google Scholar]
- 27.Yera, H., M. E. Bougnoux, C. Jeanrot, M. T. Baixench, G. De Pinieux, and J. Dupouy-Camet. 2003. Mycetoma of the foot caused by Fusarium solani: identification of the etiologic agent by DNA sequencing. J. Clin. Microbiol. 41:1805-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]