Abstract
We investigated in three groups of awake and sleeping goats whether there are differences in ventilatory responses after injections of Ibotenic acid (IA, glutamate receptor agonist and neurotoxin) into the pre-Bötzinger complex (preBötC), lateral parabrachial (LPBN), medial (MPBN) parabrachial, or Kölliker-Fuse nuclei (KFN). In one group, within minutes after bilateral injection of 10 µl IA (50 mM) into the preBötC, there was a 10-fold increase in breathing frequency, but 1.5 h later, the goats succumbed to terminal apnea. These data are consistent with findings in reduced preparations that the preBötC is critical to sustaining normal breathing. In a second group, increasing volumes (0.5–10 µl) of IA injected at weekly intervals into the preBötC elicited a near-dose-dependent tachypnea and irregular breathing that lasted at least 5 h. There were apneas restricted to wakefulness, but none were terminal. Postmortem histology revealed that the preBötC was 90% destroyed, but there was a 25–40% above normal number of neurons in the presumed parafacial respiratory group that may have contributed to maintenance of arterial blood gas homeostasis. In a third group, bilateral injections (1 and 10 µl) of IA into the LPBN, MPBN, or KFN did not significantly increase breathing in any group, and there were no terminal apneas. However, 3–5 h after the injections into the KFN, breathing frequency was decreased and the three-phase eupneic breathing pattern was eliminated. Between 10 and 15 h after the injections, the eupneic breathing pattern was not consistently restored to normal, breathing frequency remained attenuated, and there were apneas during wakefulness. Our findings during wakefulness and NREM sleep warrant concluding that (a) the preBötC is a primary site of respiratory rhythm generation; (b) the preBötC and the KFN are determinants of respiratory pattern generation; (c) after IA-induced lesions, there is time-dependent plasticity within the respiratory control network; and (d) ventilatory control mechanisms are state dependent.
Keywords: respiratory rhythm and pattern generation, pre-Bötzinger complex, pontine respiratory group, neural plasticity, wakefulness, NREM sleep
1 INTRODUCTION
The site(s) and mechanism(s) of respiratory rhythm and pattern generation have been studied for over a century and they have been considered particularly controversial over the last 20 years. For example, St John and Bledsoe (1985) found in decerebrate cats that the pons alone is capable of generating a respiratory rhythm; therefore, they proposed that the pontine pneumotaxic center “may underlie the neurogenesis of eupnea.” This view seems contradictory to the findings of Smith et al. (1991) in a neonatal rat brainstem–spinal cord in vitro preparation in which the pre-Bötzinger complex (preBötC) was critical for respiratory rhythmogenesis. However, the findings of Smith et al. do not eliminate a role for the rostral pontine nuclei. Indeed, recent studies in which transections were made through the pons and medulla in an arterially perfused brainstem–spinal cord preparation of a juvenile rat found that the pons is necessary for the eupneic three-phase (inspiration, post inspiration, and expiration) respiratory pattern and that the preBötC in isolation mediates a gasp-like breathing pattern (Abdala et al., 2009; Smith et al., 2007). In general, these recent data agree with the results of lesion studies performed on adult anesthetized cats by Lumsden (1923) nearly 100 years ago.
Most studies on respiratory rhythm and pattern generation have been performed using reduced preparations; thus, there has been uncertainty whether the intact ventilatory control systems function as suggested by these studies. Accordingly, we (Bonis et al., 2010; Krause et al., 2009; Wenninger et al., 2004b) and others (McKay et al., 2005; Tan et al., 2008) have recently carried out studies on awake and sleeping mammals to test hypotheses generated from studies on reduced preparations. Herein, we summarize our findings in awake and sleeping adult goats studied before and after neurotoxic lesions of either the preBötC or the pontine respiratory group.
2 EXPERIMENTAL DESIGN AND METHODOLOGY
All studies were reviewed and approved by the Medical College of Wisconsin Animal Care Committee. The experimental design and methodology were essentially the same for each of three studies. Initially, the goats underwent surgery to (a) elevate the carotid arteries subcutaneously, which permitted subsequent catheterization for monitoring of blood pressure and sampling of blood for measurement of PaCO2, PaO2, and arterial pH; (b) place electrodes in the skull for sleep scoring; and (c) place electrodes in the upper airway, diaphragm, and abdominal muscles for assessment of changes in the respiratory pattern. After a 2-week period for recovery from surgery, a series of control studies were completed. Thereafter, using a dorsal craniotomy, microtubules were chronically implanted bilaterally just dorsal to the preBötC, lateral parabrachial (LPBN), medial parabrachial (MPBN), or Kölliker-Fuse nuclei (KFN). In some goats, in this second surgery, we also created a tracheostomy, which permitted mechanical ventilation when required for survival after a neurotoxin-induced reduction of breathing. The goats were then allowed at least 2 weeks for recovery before control studies were repeated. For all studies, we injected in the awake state the neurotoxin ibotenic acid (IA), which irreversibly binds to glutamate receptors resulting in sustained neuronal activation and eventual neuronal death because of disruption of intracellular homeostasis (Michelot and Melendez-Howell, 2003).
RESULTS
3.1 Study 1: Abrupt Destruction of the PreBötC
Prior to our studies, Gray et al. (2001) had shown that injection of the neurotoxin saporin conjugated to substance P into the preBötC of rats destroyed neurons expressing neurokinin-1 receptors, resulting in an ataxic or irregular breathing pattern days later. Subsequently, we found that the same injections in goats also induced an irregular breathing pattern (Fig. 1), but in spite of the loss of about 30% of pre- BötC neurons, the goats remained healthy and maintained normal arterial blood gases (Wenninger et al., 2004a). We therefore sought to determine the effects of an even greater destruction of the preBötC. Accordingly, we injected into the preBötC 10 µl of IA (50 mM) unilaterally followed by a 10 µl injection on the contralateral side 1 h later (Wenninger et al., 2004b). Within minutes of each injection, there was a marked increase in breathing frequency, which, after the contralateral injection, was at least 10-fold above control. The increase was sustained for about 30 min after the contralateral injection, but then the frequency and duration of apneas (Fig. 1) began to increase. In each of the three goats, terminal apnea occurred about 40 min after the contralateral IA injection. In four subsequent goats, a chronic tracheostomy permitted mechanical ventilation of the animals beginning about 30 min after the contralateral IA injection. At 30 min intervals over the subsequent 3.5 h, we stopped the mechanical ventilator to assess spontaneous breathing. We consistently found a markedly reduced breathing, nearly absent diaphragm muscle activity, and abdominal muscle activity that appeared to be causing a passive inspiration by driving lung volume below functional residual capacity (Fig. 2). Because of the marked persistent arterial hypercapnia and hypoxemia during spontaneous breathing (Fig. 2), the goats were euthanized 5 h after the first IA injection. Subsequent histology revealed an estimated 70% loss of all preBötC neurons. We concluded that in the awake state, an intact preBötC is critical to sustaining sufficient breathing for survival (Wenninger et al., 2004b).
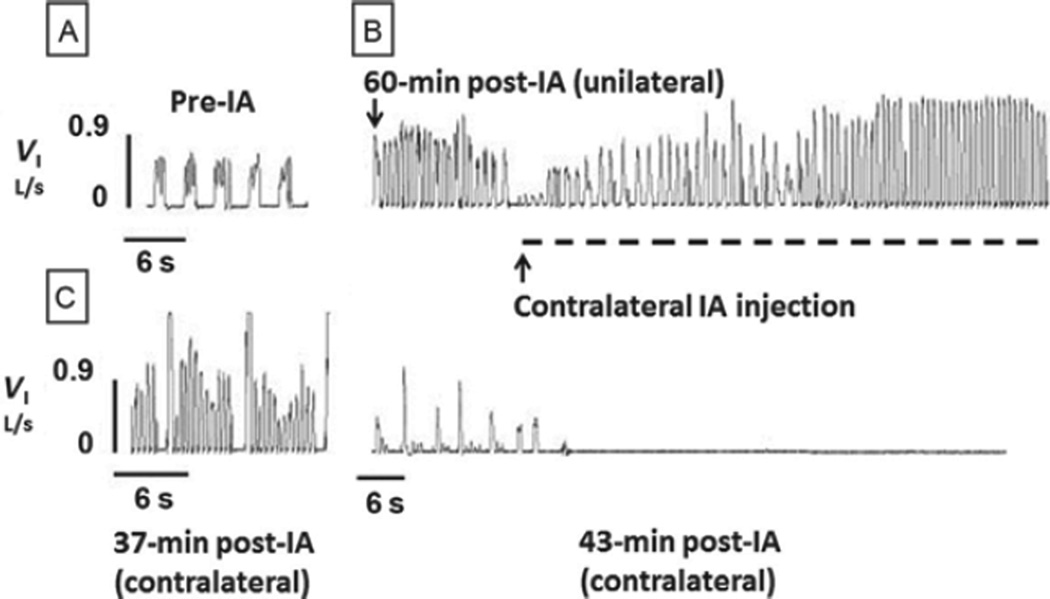
FIGURE 1. Bilateral injection of Ibotenic acid (IA; 10 µl) into the preBötC of awake goats results in terminal apnea.
Inspiratory flow (VI) is shown for a goat: (A) before (pre-IA) injections, (B) 60-min postunilateral IA injection, and (C) during (dashed line) and 37- to 43-min post-IA injection in the contralateral preBötC. Note the tachypnea 60 min after the unilateral injection, which was accentuated by the contralateral IA injection, but 37 min later, apneas became evident, which 6 min later were terminal.
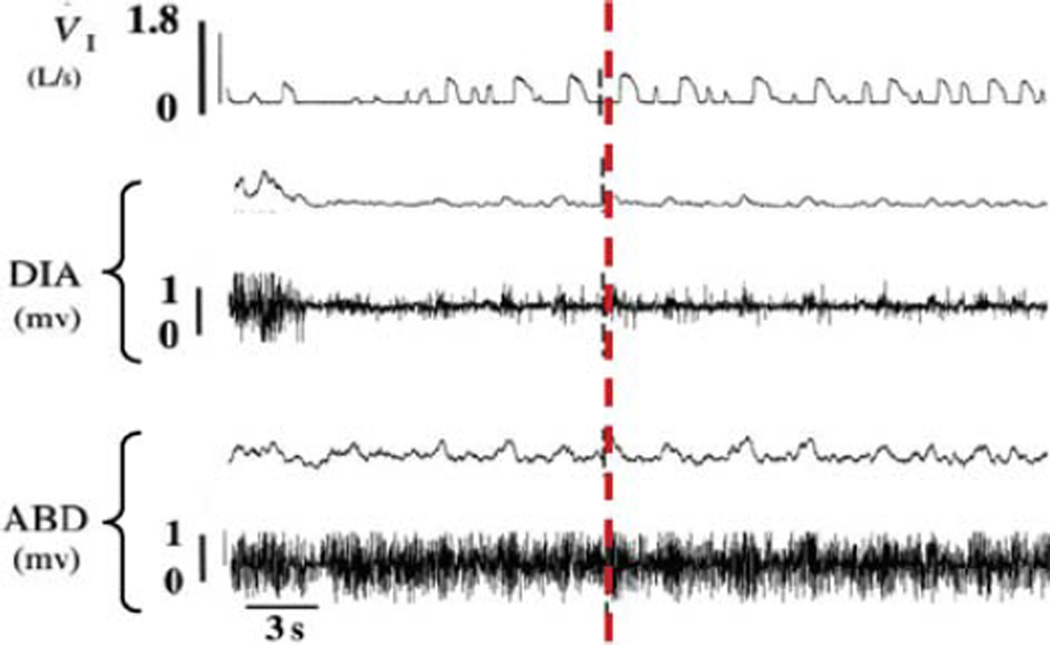
FIGURE 2. When awake goats are mechanically ventilated between 1.5 and 5 h after bilateral injection of Ibotenic acid into the preBötC, temporary removal of mechanical ventilation results in minimal diaphragm activity and spontaneous breathing.
Shown are inspiratory flow (VI), raw and integrated diaphragm (DIA), and abdominal (ABD) muscle activities during spontaneous breathing after interruption of mechanical ventilation 3 h following bilateral injection of Ibotenic acid into the preBötC. Note the minimal diaphragm activity. The vertical dashed line emphasizes that inspiratory flow occurred after contraction of the abdominal muscle, suggesting inspiration was passive. Between 60 and 90 s of spontaneous breathing, PaCO2 and PaO2 were 56.9 and 39.7 mmHg, respectively, indicating marked hypoventilation.
3.2 Study 2: Incremental Destruction of the PreBötC
We (Bisgard et al., 1980; Forster, 2003; Pan et al., 1998) and others (Feldman et al., 2003; Mitchell et al., 1993, 2000) have documented recovery or plasticity within many components of the ventilatory control system after lesion-induced attenuation of breathing. We therefore hypothesized that plasticity would also occur within the respiratory rhythm and pattern generating network if destruction of the preBötC was incremental, allowing for the time required for the development of cellular changes at other sites within (or outside) the respiratory network. Accordingly, in seven awake goats instrumented as previously described, we injected increasing volumes of IA (0.5, 1, 5, 10 µl) into the preBötC at weekly intervals (Krause et al., 2009). All injections except the 10 µl were bilateral and were completed a week apart. In an eighth goat, the injections were made lateral to the nucleus of the solitary tract (NTS).
Each injection of IA into the preBötC resulted in an increase in breathing frequency that tended to be dose dependent and was sustained for at least 5 h (Fig. 3). Injections of IA lateral to the NTS did not affect breathing frequency. In addition to the tachypnea, the IA injections resulted in a highly irregular breathing pattern (Fig. 4) that differed between goats and between volumes of IA injected. The coefficient of variation of expiratory time was significantly (P < 0.05) increased from control only after the 1 µl injection. The goats were also studied at night between 10 and 15 h after the injections. Data were obtained during wakefulness and NREM sleep, but insufficient data were obtained during REM sleep to discern the effect of the IA injections on breathing during REM sleep. At night after the IA injections, breathing frequency had returned to control levels, but the goats’ breathing remained highly irregular. Most notable were central apneas after the 10 µl injections that were predominately during the awake state. However, there was a remarkable recovery after each injection; the coefficient of variation for pulmonary ventilation, breathing frequency, tidal volume, and inspiratory and expiratory (TE) times did not differ (P > 0.10) between values obtained before any injections and values obtained 1 week after the last injection. Arterial blood gases also were not chronically affected by the injections, but pulmonary ventilation and breathing frequency were reduced and TE was increased 1 week after the last injections. Our conclusion was that plasticity had occurred within the respiratory rhythm and pattern generating network to maintain a near normal level of breathing and maintain arterial blood gas homeostasis.
FIGURE 3. Ibotenic acid (IA) injection into the preBötC of awake goats results in a tachypnea sustained for at least 5 h.
Presented are the average (±SEM) percent increases from control of seven goats after a 1-µl bilateral injection and 2 and 3 weeks later after unilateral 10 µl injections into the left and right preBötC. The injections were made at time zero. Note that the tachypnea after the 10 µl injections was greater than that after the 1 µl injections, but the tachypnea after the 10 µl injection on the right was less than that after the 10 µl injection on the left. This difference in response to 10 µl injections could be because the previous injections on the left had nearly destroyed the preBötC; thus, it could not be activated by the injection on the right.
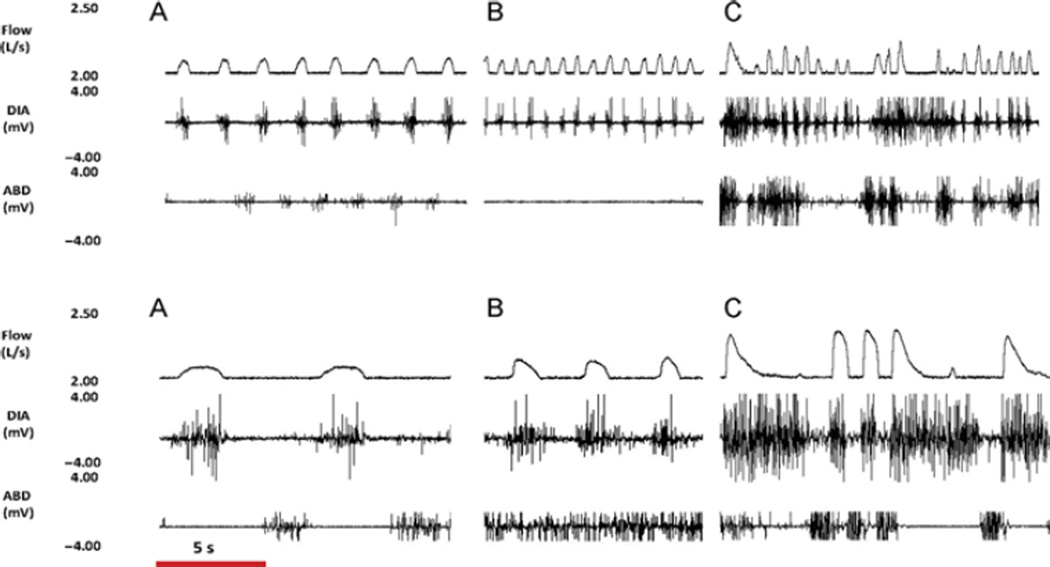
FIGURE 4. Ibotenic acid (IA) injection into the preBötC of an awake goat results in tachypnea and irregular breathing.
Shown for an awake goat are inspiratory flow (Flow) and raw diaphragm, (DIA) and abdominal (ABD) muscle activities (A) before, (B) 1h after, and (C) 3 h after 1 µl (upper panel) and 10 µl (bottom panel) IA injections into the preBötC. The time bar shown at the bottom applies to all traces. Note that both injections initially increased breathing frequency, but eventually both injections caused a highly irregular breathing. Note also the regular but reduced flow before the 10 µl injection indicating that the goat had recovered from the previous injections, which had chronically reduced breathing frequency.
Postmortem histology indicated that, on average, in the preBötC region and the regions caudal and rostral, there were, respectively, only 10%, 11%, and 18% of total living neurons remaining, compared with control unlesioned goats (Krause et al., 2009). On the other hand, in the region ventral to the facial nucleus (FN), the number (+25%, P < 0.01) and density of neurons (+14–30%, P < 0.01) were greater in preBötC lesioned goats than in control goats (Neumueller et al., 2011). In contrast, the number and density of neurons in the LPBN, MPBN, and KFN did not differ between preBötC lesioned and control goats (Neumueller et al., 2011). Ventral to FN in goats (Miller et al., 2011), there is a high concentration of Phox2b neurons, which are a marker of the parafacial respiratory nucleus/retrotrapezoid nucleus (pFRG/RTN) hypothesized to be a site required for normal respiratory rhythm and pattern generation (Guyenet, 2008). Our data suggest that the pFRG/RTN could have been an anatomic site of plasticity after near-complete destruction of the preBötC.
3.3 Study 3: Effects of Lesions of the LPBN, MPBN, and KFN
Since the pioneering studies of Lumsden nearly a century ago (Lumsden, 1923), it has been accepted that neurons in the rostral pons (PRG) are part of a network controlling breathing. Data from many subsequent studies using reduced preparations have led to different conclusions regarding the specific role of the PRG. For example, it has been concluded that the PRG is a site (a) that underlies the neurogenesis of eupnea (St John and Bledsoe, 1985), (b) that is critical to the eupneic three-phase respiratory pattern (Abdala et al., 2009), (c) that has postinspiratory premotor neurons controlling upper airway resistance during reflex control and vocalization (Dutschmann and Herbert, 2006), and (d) that comprises heterogeneous populations of neurons facilitating or depressing other respiratory neurons within the network controlling breathing (Chamberlin and Saper, 1994). Accordingly, we hypothesized that (1) neurotoxic lesions in the PRG would disrupt eupneic respiratory rhythm and pattern in a site- and state-specific manner and (2) there would be recovery from the acute effects of lesions in the PRG. The goats were studied during wakefulness and NREM sleep at weekly intervals after no injections or after 1 or 10 µl injections of IA (50 mM) into the LPBN (n = 3), MPBN (n = 4), or KFN (n = 4).
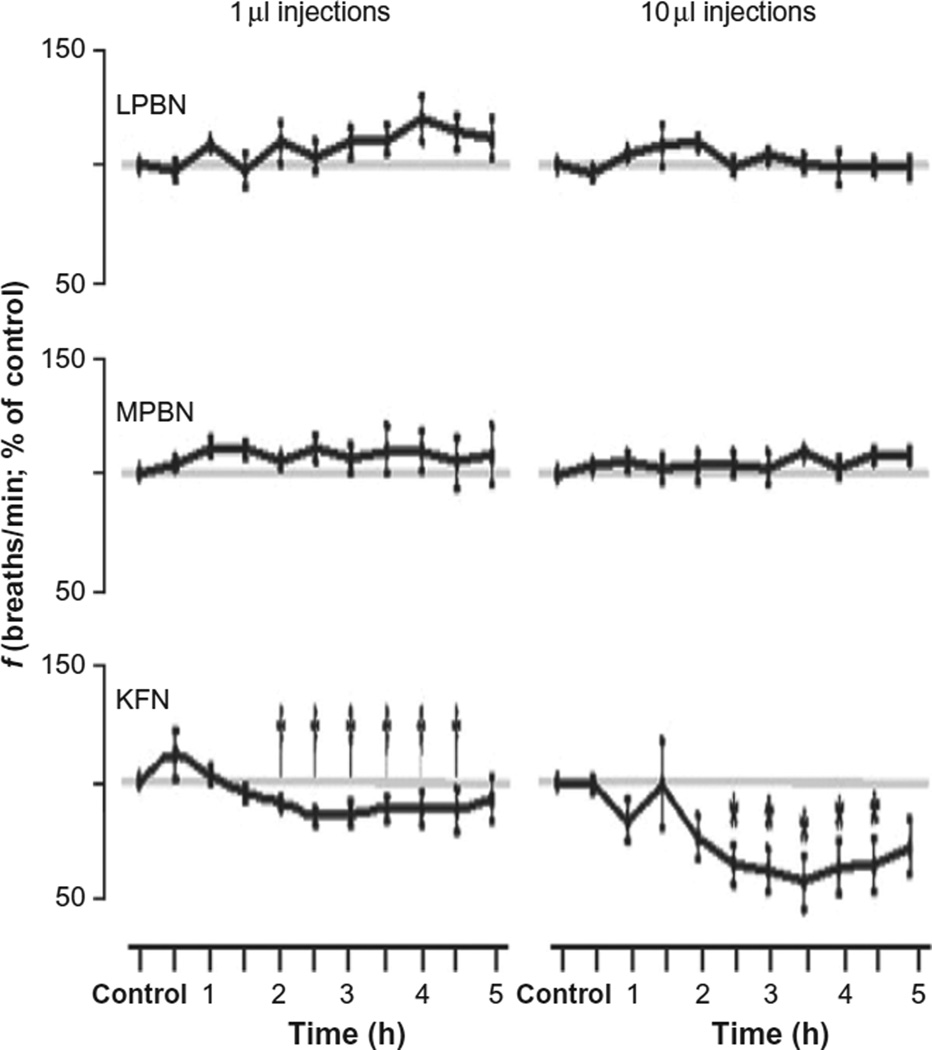
Neither the 1 nor the 10 µl IA injections significantly (P > 0.10) increased breathing frequency when injected into any of the three pontine nuclei (Fig. 5), but during the first hour after the 10 µl injection into the KFN, breathing frequency transiently increased at different times in all four goats. Between 2 and 5 h after both IA injections into the KFN, breathing frequency was significantly (P < 0.01) below the control period (Fig. 5). During the latter part of this period, tidal volume in the KFN goats was significantly increased (P < 0.05).
FIGURE 5. Bilateral injections of Ibotenic acid (IA) into the KFN of awake goats significantly decrease breathing frequency about 2 h after the injection, but injections into LPBN and MPBN do not significantly affect breathing frequency.
The left and right panels represent data after ibotenic acid injections of 1 and 10 µl, respectively. Values are the mean (±SEM) of four, three, and four goats, respectively, of the three groups. The symbols * and † denote statistically significant differences from control (P < 0.05).
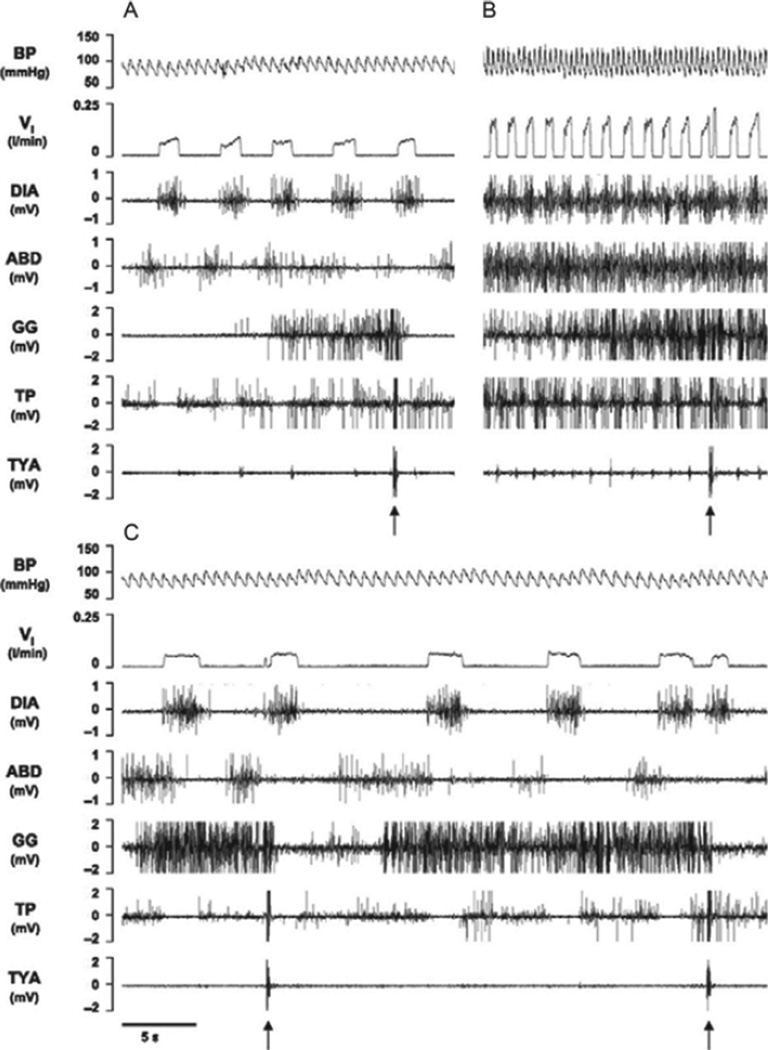
The 10 µl IA injections changed the pattern of breathing in all four KFN goats. An initial mild tachypnea in all goats was associated with an augmented incrementing inspiratory flow and increased diaphragm, abdominal, and upper airway muscle activities (Fig. 6). Notable was the increased activity of the laryngeal constrictor, the thyroid arytenoid (TYA), whose activity is a marker of the postinspiratory period of the three-phase eupneic respiratory cycle (Dutschmann and Herbert, 2006; Fig. 6). However, at variable times after the injections in these goats, breathing frequency decreased to below control. In three of the goats, the inspiratory flow pattern became square-waved, and respiratory-related muscle activity was reduced and TYA activity was eliminated (Fig. 6). This temporal pattern in the TYA is similar to what Dutschmann and Herbert (2006) recently found in an intra-arterially perfused brainstem preparation of juvenile rats. They found that glutamate microinjections into distinct parts of the KFN evoked sustained laryngeal constriction. Subsequently, isoguvacine (GABA receptor agonist) microinjections into the KFN abolished laryngeal constriction and decreased breathing frequency. In other words, both the isoguvacine injections into the KFN of rats and the delayed effect of IA in awake goats had the effect of abolishing phase 2 of the normal three-phase respiratory pattern.
FIGURE 6. In a KFN goat, bilateral injection of IA (10 µl) during the day caused a biphasic response in inspiratory flow (VI), blood pressure (BP), and heart rate compared with control conditions (A), with a transient (20 min) hyperpnea (B) immediately after the injection, followed by a prolonged (>3 h) hypopnea (C).
Arrows indicate typical swallow patterns as identified by the abrupt maximal contraction of airway thyropharyngeal (TP) and thyroid arytenoid (TYA) muscles. Electromyograms are also shown for the diaphragm (DIA), abdominal (ABD), and genioglossal (GG) muscles. Note the incrementing flow pattern in A and B, which in C is square wave. Note also the increased TYA activity in B and its absence in C, which suggests that the three-phase eupneic flow pattern was eliminated in C.
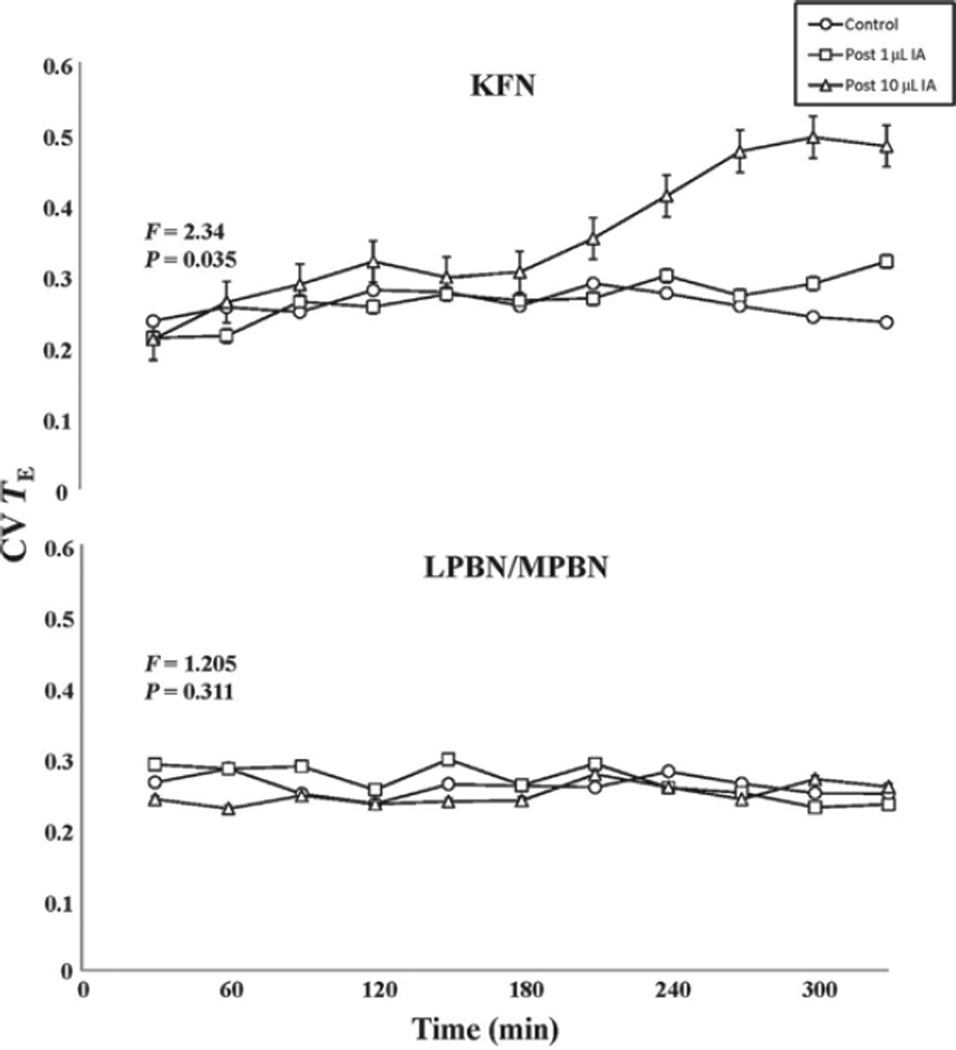
In the fourth KFN goat, the initial tachypnea and augmented inspiratory flow and muscle activity switched to a decrementing inspiratory flow and genioglossus muscle activity pattern and a prolonged apneustic-like contraction of the diaphragm (Fig. 7). The secondary effect in this goat is also similar to that of the isoguvacine injections in rats, which triggered apneusis and a variable and decreased breathing frequency (Dutschmann and Herbert, 2006; Fig. 7). Indeed, in our KFN goats, respiratory activity became more variable as, for example, the coefficient of variation in TE significantly (P < 0.01) increased between 3 and 5 h after the 10 µl IA injection (Fig. 8). In the LPBN and MPBN goats, there were no significant disruptions of the respiratory pattern (as indicated by the unchanged coefficient of variation of TE) (Fig. 8).
FIGURE 7. In another KFN goat, 10 µl bilateral injection of Ibotenic acid (IA) during the day also caused a biphasic response in VI, BP, and heart rate compared to control conditions (A), with a transient (<20 min) hyperpnea (B) followed by hypopnea and an apneustic-like breathing pattern(C).
Arrows denote typical swallow patterns. Note the incrementing flow pattern in A and B and the decrementing flow pattern in C. Also note the prolonged contraction of the diaphragm (DIA) in C followed by prolonged abdominal (ABD) activity, which are clear indicators of disruption of the eupneic respiratory pattern.
FIGURE 8. Bilateral injection of Ibotenic acid (IA) into the KFN of awake goats disrupts normal respiratory pattern generation as indicated by a significantly (P < 0.01) increased coefficient of variation of expiratory time (CV TE) more than 2 h after the injection.
Over a 5-h control period and after 1 µl IA injection into the KFN, the CV TE did not change nor were there any changes with injections into the LPBN or MPBN. Data are the mean (±SEM) of three or four goats. The F and P values listed in the figures are from two-way repeated measures ANOVA, comparing for each group the CV TE for the three conditions (control, and 1 and 10 µl IA injections). A two ANOVA comparing values of CV TE for the 10 µl injections of KFN versus LPBN/MPBN groups yielded F and P values of 21.5 and <0.001, respectively.
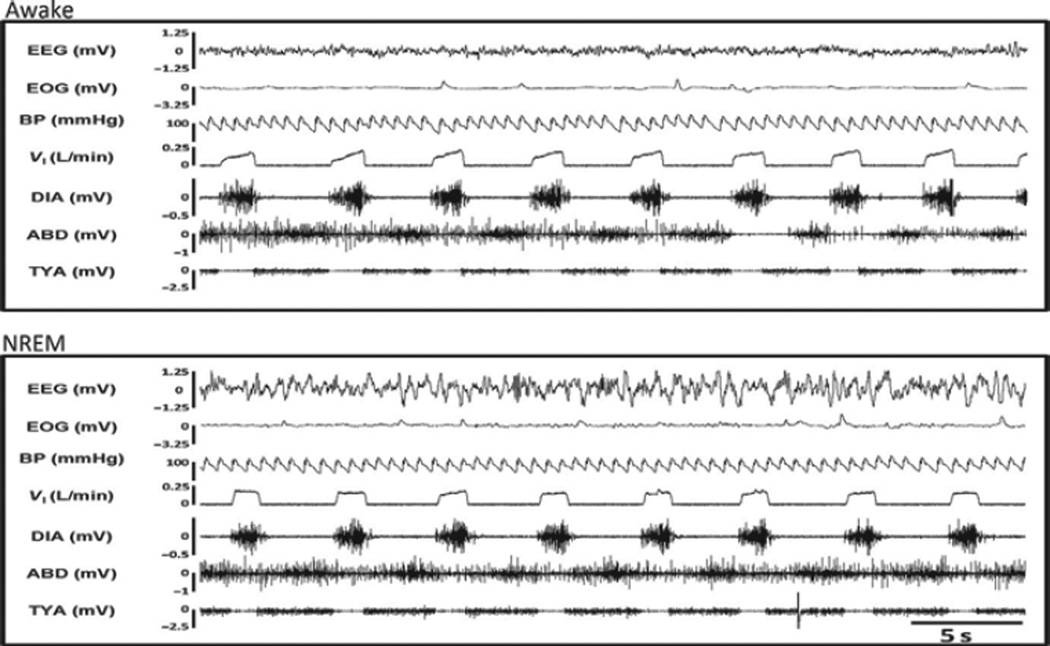
Between 10 and 15 h after the IA injections, the goats were studied during wakefulness and NREM sleep. In the KFN goats, the breathing patterns had recovered in most breaths to an augmenting inspiratory flow pattern and normal diaphragm muscle activity during both wakefulness and NREM sleep (Fig. 9). However, abdominal and, particularly, TYA activity were tonic, which contrasted with the burst of TYA activity shown in Fig. 6; thus, these data suggest that there was incomplete recovery of the normal three-phase respiratory pattern. Moreover, even though the coefficients of variation (as shown in Fig. 10 for TE) were not different from any other night studies, the breathing frequency remained reduced and TE remained prolonged (Fig. 10). In addition, there was a significantly (P < 0.05) increased number of central apneas between 10 and 15 h after the IA injections (primarily during wakefulness) which further suggest incomplete recovery.
FIGURE 9. There was substantial recovery of ventilation at night between 10 and 15 h after the bilateral 10 µl IA injections into the KFN.
Inspiratory flow (VI), heart rate, blood pressure (BP), and respiratory muscle activity pattern had returned to almost control levels during both awake (top panel) and NREM (bottom panel) sleep states. Diaphragm (DIA), abdominal (ABD), and genioglossus (GG) muscle activity are shown.
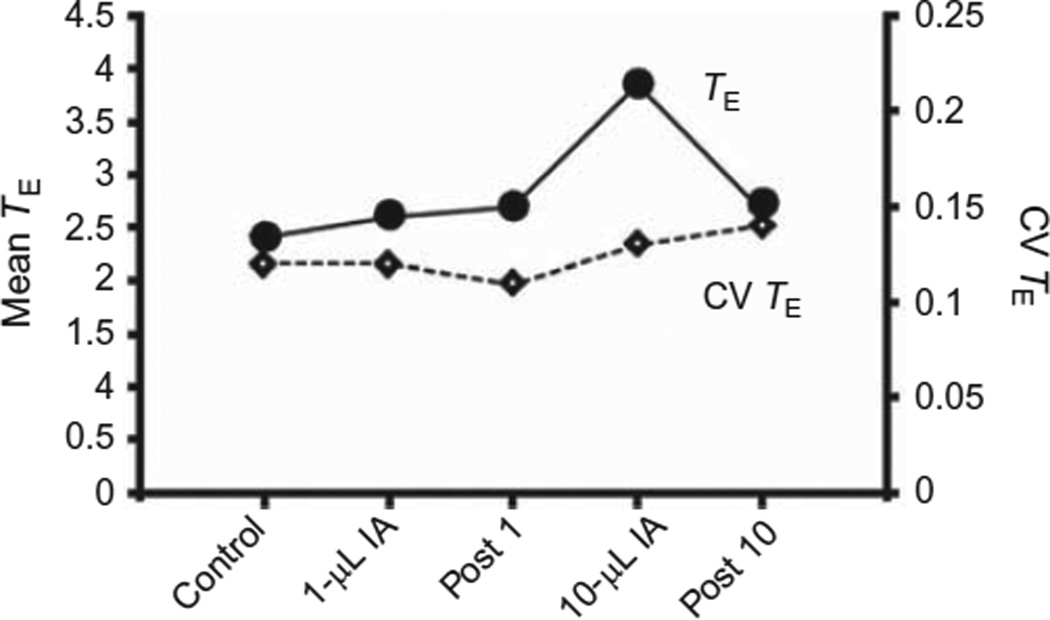
FIGURE 10. After Ibotenic acid (IA) injection into the KFN of goats, there is a dissociation of recovery of respiratory frequency and pattern.
Shown for a single goat (same as Figs. 7 and 9) are the average expiratory times (TE) (closed circles, index of breathing frequency) and the coefficient of variation of TE (CV TE) (diamond, index of pattern of breathing) over five night studies that included the control study, 10–15 h after injecting 1 and 10 µl of IA into the KFN, and 7 days after these two injections. Note that by 10–15 h after the 10 µl injection, CV TE was normal but TE remained elevated.
Postmortem histology indicated that in the KFN group of goats, the number of LPBN, MPBN, and KFN neurons remaining was about 50% of normal (Bonis et al., 2010). For the MPBN goats, the number of neurons remaining was 60–80% of normal. Finally, for the LPBN goats, the number of neurons remaining was 73–92% of normal. Thus, the neuronal deficit was not consistent over all three nuclei, and it was much less than for the preBötC studies described earlier.
The findings that IA injections into the KFN altered respiratory rhythm and pattern but IA injections into the LPBN and MPBN had no effects are consistent with our hypothesis and with the findings of others that there is a heterogeneous population of neurons in the pontine respiratory group (Chamberlin, 2004; Chamberlin and Saper, 1994). Furthermore, a heterogeneous population of neurons could explain the time-dependent changes we found with IA injections into KFN as IA may have initially stimulated medullary inspiratory neurons, but in time diffusion of IA may have reached neurons stimulating expiratory neurons. Our second hypothesis of state-dependent effects was also validated as we found central apneas in the night study that were restricted to the awake state (Bonis et al., 2010).
We were unable to obtain a clear insight into plasticity or recovery after KFN IA injection-induced changes in ventilatory control. Of note was the recovery of the variability of respiratory parameters 10–15 h after IA injections into the KFN, but there seemed to be incomplete recovery of the normal TYA activity of the three-phase respiratory pattern generation (Fig. 9). In addition, breathing frequency had not recovered, which suggests that rhythm-generating mechanisms or their modulation remained altered. One week after the IA injections, both parameters had recovered (Fig. 10).
4 MAJOR CONCLUSIONS COMPARING DATA OF THE THREE STUDIES
The major strengths of our studies are as follows: (1) the data were obtained under physiologic wakefulness and NREM sleep and (2) there was uniformity across all studies including the species studied, the choice of neurotoxin to create lesions, the experimental design and protocols, and the variables measured. A potential limitation of our studies is that the extent of the lesions was not uniform over preBötC and the three rostral pontine nuclei. Future studies with more extensive lesions of the pontine nuclei will be required to completely delineate their functional role in regulating respiratory rhythm and pattern generation. Nevertheless, we conclude that there is a clear difference in the effects of IA injections into the preBötC and the pontine respiratory group. These differences point to distinct roles for these sites in the control of breathing. The marked initial tachypnea and eventual terminal apneas after IA injections into the preBötC versus no, or minimal, tachypnea or terminal apneas after injections into the PRG provide strong evidence to conclude that there is a clear difference in the role of these sites in respiratory rhythm generation during wakefulness in an intact mammal. One possible conclusion is that the preBötC is normally the primary site of rhythm generation receiving input from multiple sources including the rostral pontine nuclei. This conclusion is consistent with that of other investigators using different reduced preparations (Abdala et al., 2009; Feldman et al., 2003; Ramirez et al., 1998; Smith et al., 1991; Tan et al., 2008). However, there has been concern whether findings in these reduced preparations are indeed representative of the fully intact awake and asleep condition. The data from our studies should mitigate these concerns.
Our findings warrant additional conclusions regarding the preBötC. First, the role of the preBötC does not seem restricted to rhythm generation as we found clear disruptions in the normal pattern of breathing after lesions of the preBötC (Fig. 4). Second, the preBötC is not exclusively responsible for respiratory rhythm as in both studies 1 and 2, rhythmic respiration continued after near total destruction of the pre- BötC albeit time-dependent plasticity was required to reestablish a normal rhythm and pattern. Third, IA-induced apneas restricted to the awake state suggest the state dependency of rhythm generating mechanisms (Rybak et al., 2008).
The KFN can affect breathing frequency and therefore likely respiratory rhythm generation. Of note is that the time course of the IA effects on breathing frequency is the same as the time course on heart rate (Figs. 6 and 7; Bonis et al., 2010) and the occurrence of swallows (Bonis et al., 2011, 2013). It seems then that IA injection into the KFN has a unique effect among the rostral pontine nuclei of stimulating or depressing these three physiologic functions. However, the KFN also serves a prominent role in respiratory pattern generation, that is, the sequential activation pattern of respiratory pump and airway muscles. Indeed, our findings confirm data from reduced preparations that the three-phase eupneic respiratory pattern is dependent on an intact KFN (Abdala et al., 2009; Dutschmann and Herbert, 2006). Moreover, our finding that apneas 10–15 h after the IA injections into the KFN were restricted to the awake state provides evidence that the KFN’s role in rhythm generation is state dependent, similar to the preBötC (Rybak et al., 2008).
The KFN and MPBN also have important roles in coordinating breathing and swallowing. In intact goats, swallows do not cause a resetting of the respiratory rhythm generator (Bonis et al., 2013). However, when we perturbed the KFN or MPBN by implanting microtubules, swallows during both inspiration and expiration caused a resetting of the respiratory rhythm (Bonis et al., 2011). These findings suggest that the KFN and MPBN are part of a network that establishes the priority among physiologic functions that utilize common anatomical structures (Bolser et al., 2006).
In summary, our findings during wakefulness and NREM sleep warrant concluding that (a) the preBötC is a primary site of respiratory rhythm generation; (b) the preBötC and the KFN are determinants of respiratory pattern generation; (c) after IA-induced lesions, there is time-dependent plasticity within the respiratory control network; and (d) ventilatory control mechanisms are state dependent.
Acknowledgment
This work was supported by Department of Veterans Affairs and National Heart, Lung and Blood Institute Grants HL25739 and HL007852
References
- Abdala AP, Rybak IA, Smith JC, Zoccal DB, Machado BH, St-John WM, Paton JF. Multiple pontomedullary mechanisms of respiratory rhythmogenesis. Respir. Physiol. Neurobiol. 2009;168:19–25. doi: 10.1016/j.resp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgard GE, Forster HV, Klein JP. Recovery of peripheral chemoreceptor function after denervation in ponies. J. Appl. Physiol. 1980;49:964–970. doi: 10.1152/jappl.1980.49.6.964. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Poliacek I, Jakus J, Fuller DD, Davenport PW. Neurogenesis of cough, other airway defensive behaviors and breathing: a holarchical system? Respir. Physiol. Neurobiol. 2006;152:255–265. doi: 10.1016/j.resp.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonis JM, Neumueller SE, Krause KL, Kiner T, Smith A, Marshall BD, Qian B, Pan LG, Forster HV. Site-specific effects on respiratory rhythm and pattern of ibotenic acid injections in the pontine respiratory group of goats. J. Appl. Physiol. 2010;109:171–188. doi: 10.1152/japplphysiol.00934.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonis JM, Neumueller SE, Marshall BD, Krause KL, Qian B, Pan LG, Hodges MR, Forster HV. The effects of lesions in the dorsolateral pons on the coordination of swallowing and breathing in awake goats. Respir. Physiol. Neurobiol. 2011;175:272–282. doi: 10.1016/j.resp.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonis JM, Neumueller SE, Krause KL, Pan LG, Hodges MR, Forster HV. Contributions of the Kolliker-Fuse nucleus to coordination of breathing and swallowing. Respir. Physiol. Neurobiol. 2013;189:10–21. doi: 10.1016/j.resp.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL. Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respir. Physiol. Neurobiol. 2004;143:115–125. doi: 10.1016/j.resp.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J. Neurosci. 1994;14:6500–6510. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. The Kolliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur. J. Neurosci. 2006;24:1071–1084. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu. Rev. Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster HV. Plasticity in the control of breathing following sensory denervation. J. Appl. Physiol. 2003;94:784–794. doi: 10.1152/japplphysiol.00602.2002. [DOI] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires pre-Botzinger complex neurokinin-1 receptor-expressing neurons. Nat. Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J. Appl. Physiol. 2008;105:404–416. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KL, Forster HV, Kiner T, Davis SE, Bonis JM, Qian B, Pan LG. Normal breathing pattern and arterial blood gases in awake and sleeping goats after near total destruction of the presumed pre-Botzinger complex and the surrounding region. J. Appl. Physiol. 2009;106:605–619. doi: 10.1152/japplphysiol.90966.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden T. Observations on the respiratory centres in the cat. J. Physiol. 1923;57:153–160. doi: 10.1113/jphysiol.1923.sp002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of pre-Botzinger complex neurons. Nat. Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelot D, Melendez-Howell LM. Amanita muscaria: chemistry, biology, toxicology, and ethnomycology. Mycol. Res. 2003;107:131–146. doi: 10.1017/s0953756203007305. [DOI] [PubMed] [Google Scholar]
- Miller J, Neumueller S, Qian B, Hodges MR, Pan L, Forster HV. Hypoventilation and reduced CO2 sensitivity with sustained expiratory muscle activity in the awake goat. FASEB J. 2011;25:847. [Google Scholar]
- Mitchell G, Bach K, Martin P, Foley K. Modulation and plasticity of the exercise ventilatory response. Respiration in Health and Disease. 1993:269–277. [Google Scholar]
- Mitchell GS, Bach KB, Martin PA, Foley KT, Olson EB, Brownfield MS, Miletic V, Behan M, McGuirk S, Sloan HE. Increased spinal monoamine concentrations after chronic thoracic dorsal rhizotomy in goats. J. Appl. Physiol. 2000;89:1266–1274. doi: 10.1152/jappl.2000.89.4.1266. [DOI] [PubMed] [Google Scholar]
- Neumueller S, Hodges MR, Krause K, Marshall B, Bonis J, Qian B, Pan LG, Forster HV. Anatomic changes in multiple brainstem nuclei after incremental, near-complete neurotoxic destruction of the pre-Botzinger Complex in adult goats. Respir. Physiol. Neurobiol. 2011;175:1–11. doi: 10.1016/j.resp.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Pan LG, Forster HV, Martino P, Strecker PJ, Beales J, Serra A, Lowry TF, Forster MM, Forster AL. Important role of carotid afferents in control of breathing. J. Appl. Physiol. 1998;85:1299–1306. doi: 10.1152/jappl.1998.85.4.1299. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Botzinger complex in vivo eliminates breathing but not gasping. J. Physiol. 1998;507(Pt 3):895–907. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak IA, O’Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Morris KF, Lindsey BG. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J. Neurophysiol. 2008;100:1770–1799. doi: 10.1152/jn.90416.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J. Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John WM, Bledsoe TA. Genesis of rhythmic respiratory activity in pons independent of medulla. J. Appl. Physiol. 1985;59:684–690. doi: 10.1152/jappl.1985.59.3.684. [DOI] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing pre-Botzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat. Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah T, Davis S, Forster HV. Small reduction of neurokinin-1 receptor-expressing neurons in the pre-Botzinger complex area induces abnormal breathing periods in awake goats. J. Appl. Physiol. 2004a;97:1620–1628. doi: 10.1152/japplphysiol.00952.2003. [DOI] [PubMed] [Google Scholar]
- Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. Large lesions in the pre-Botzinger complex area eliminate eupneic respiratory rhythm in awake goats. J. Appl. Physiol. 2004b;97:1629–1636. doi: 10.1152/japplphysiol.00953.2003. [DOI] [PubMed] [Google Scholar]