Abstract
OBJECTIVES
Current staging systems for perihilar cholangiocarcinoma (pCCA) are inadequate, as they are based on surgical pathology and therefore not relevant to unresectable patients. Clinical trials for potential targeted therapies for pCCA are hampered by the lack of an accurate, nonoperative staging system for predicting survival. We aimed at developing a clinical staging system for pCCA, which would be of prognostic relevance for all pCCA patients and help stratify patients for clinical trials.
METHODS
Clinical information at the time of pCCA diagnosis of 413 patients seen at Mayo Clinic, Rochester, MN between 2002 and 2010 was retrospectively analyzed. A survival predictive model was developed using Cox proportional hazards analysis. The performance of the staging system was compared with the current AJCC/UICC (the American Joint Committee on Cancer/the Union for International Cancer Control) 7th tumor-node-metastasis (TNM) staging system.
RESULTS
Eastern Cooperative Oncology Group (ECOG) status, tumor size and number, vascular encasement, lymph node and peritoneal metastasis and CA 19-9 level were grouped into a four-tier staging system. The median survivals of stages I, II, III, and IV patients were 48.6, 21.8, 8.6, and 2.8 months, with hazard ratios (95% confidence interval) of 1.0 (reference), 1.7 (1.1–2.6), 3.1 (2.0–4.7), and 8.7 (5.2–14.5), respectively (P<0.0001). This staging system had greater concordance statistics (standard error) than the TNM staging system (0.725 (0.018) vs. 0.614 (0.017)), indicating better performance in predicting survival.
CONCLUSIONS
This staging system, based on nonoperative information at the time of pCCA diagnosis, has excellent discriminatory power to classify patients into four prognostic stages. It could be useful to clinicians and for the design of clinical trials.
INTRODUCTION
Cholangiocarcinoma (CCA) is a hepatobiliary cancer that displays features of bile ductular differentiation. The overall incidence of CCA has been on the rise. These tumors are highly lethal, as the 5-year survival rate has remained low at approximately 10% since the 1980s (1). CCA is currently classified on the basis of anatomic location into three distinct subsets: intrahepatic, perihilar, and distal CCA (2,3). These subsets are different in their genetics, clinical presentation, management, and patient outcomes. Each subset therefore requires its own staging system for predicting survival.
Perihilar CCA (pCCA), which includes tumors arising from the secondary branches of the right and left hepatic ducts extending to just above the site of cystic duct origin, is the most common subset, accounting for 50% of all CCAs (4). Surgical resection is the mainstay of potentially curative treatment for pCCA, but less than 25% of patients present with early-stage disease that can be treated with curative resection (5). Excellent outcomes have been reported following liver transplantation with neoadjuvant chemoradiation in a subset of patients with early-stage disease (6). For patients who are not eligible for resection or transplantation, treatment options are limited, and the current practice standard is chemotherapy with gemcitabine plus cisplatin. However, this systemic chemotherapeutic approach is only palliative, and median life expectancy is extended a mere 3 months (7). Novel therapies for pCCA are therefore urgently needed. The design of clinical trials for pCCA is hampered by the lack of a nonoperative clinical staging system for pCCA. Such a staging system is needed to appropriately stratify patients within clinical trial designs.
Currently, there are three commonly used pCCA classification systems: the Bismuth-Corlette classification, the Memorial Sloan-Kettering Cancer Center staging system, and the American Joint Committee on Cancer/Union for International Cancer Control tumor-node-metastasis (TNM) system (8-10). All of these are operative staging systems; thus, none can be used to predict survival of patients with unresectable disease. The Bismuth-Corlette classification and the Memorial Sloan-Kettering Cancer Center staging system are primarily used for guiding surgical approach in patients who are eligible for surgical resection. The TNM staging system is specifically designated for predicting prognosis; however, it is based solely on information from resected specimens, and therefore it is not applicable to most pCCA patients who have unresectable disease and must be staged on clinical, rather than pathologic, criteria (10).
Because none of the current staging systems are relevant to the entire cohort of pCCA patients, a broader staging system based on nonoperative information is required for prognostication in clinical practice and for adequate stratification of patients in clinical trials of novel therapies (11,12). To address this deficiency, we constructed a new staging system based on independent predictors of survival at the time of pCCA diagnosis and assessed the ability of the new staging system to predict survival in a large cohort of pCCA patients seen at a single referral center.
METHODS
Patients and procedures
The study was approved by the Mayo Clinic Institutional Review Board. All patients diagnosed with pCCA seen at Mayo Clinic, Rochester, MN between January 1, 2002 and December 31, 2010 were identified (n=524). We searched for pCCA cases in the Mayo Clinic Life Sciences System using the ICD-9-CM code of “156.1” and/or the keywords “cholangiocarcinoma” and “bile duct cancer” to identify all potential pCCA patients. We then reviewed the medical record. Patients who received any treatments for pCCA before their initial Mayo visit, except for biliary stenting, e.g., surgical resection or exploration (n=54), or chemotherapy (n=24), those who did not have radiologic imaging available for review (n=25), and those who did not provide authorization for research (n=8) were excluded. The diagnosis of CCA was established by any of the following six criteria: (i) surgical histopathology from resected tumor or residual tumor in the explanted liver (n=102); (ii) histology from intraluminal endoscopic core biopsy, percutaneous needle biopsy, or intraoperative biopsy (n=105); (iii) intraluminal brush cytology (111 positive cytology and 12 suspicious cytology; suspicious cytology at Mayo has a specificity of >95% for the diagnosis of CCA in the absence of primary sclerosing cholangitis (PSC)) (13-15), (iv) polysomy demonstrated by fluorescence in situ hybridization of intraluminal brush cytology specimens in combination with a malignant-appearing biliary tract stricture demonstrated by radiologic imaging or a mass lesion on cross-sectional imaging studies (n=23) (3,16,17), (v) a CA-19-9 level >100 U/ml in the setting of a malignant-appearing biliary tract stricture demonstrated by radiologic imaging and without bacterial cholangitis (n=43) (3,13,18), or (vi) a perihilar mass lesion with a malignant-appearing biliary tract stricture on cross-sectional imaging studies with evidence of malignant clinical progression during the follow-up period (n=17, the median follow-up period was 9.3 months).
Baseline characteristics, including Eastern Cooperative Oncology Group (ECOG) performance status defined on the basis of patient report of the level of activity and symptoms, CA 19-9 level, and radiographic findings at the time of diagnosis were retrospectively abstracted. Tumor extent, vascular encasement (defined as tumor soft tissue partially or completely surrounding a vessel), lymph node metastasis, and metastatic spread to liver (indicating intrahepatic metastasis) or peritoneum (or other organs) at the time of pCCA diagnosis, before any operative procedures, were ascertained on the basis of the hepatobiliary radiologist report in the medical record (computerized tomography scan and/or magnetic resonance imaging). Tumor size was determined by the maximum radial diameter of the largest tumor.
In the diagnostic and treatment algorithm for pCCA at Mayo Clinic, all patients were evaluated for resectibility, and if deemed ineligible, liver transplantation was considered. Patients who were not candidates for either surgical resection or transplantation were considered for systemic chemotherapy or best supportive care. Palliative biliary drainage with biliary tract stenting (either endoscopic or percutaneous) was performed to relieve cholestasis in patients with jaundice. Patients with potentially resectable disease underwent chest imaging (X-ray and/or computerized tomography scan) to rule out lung metastases. Those patients with either unresectable pCCA or pCCA arising in the setting of PSC who were potentially eligible for liver transplantation underwent endoscopic ultrasound as part of the pretransplant evaluation protocol, to assess for regional lymph node involvement.
Statistical analysis
Data analysis was performed using SAS 9.1 (SAS Institute, Cary, NC). Baseline characteristics were summarized as frequency (categorical variables) and mean±s.d. or median (range) as appropriate (continuous variables). Death was the primary end point. Median survival, defined as time from the first visit date at Mayo Clinic to the last follow-up or death date, was estimated using the Kaplan-Meier method. For the main analysis, all patients were followed up from treatment assignment to death, ignoring any treatment changes, with the primary goal of assessing the patients’ overall survival. We also performed a second analysis of patients receiving liver transplantation by treating transplant and death as competing risks in order to better understand their trajectories. Follow-up was censored on May 31, 2013. The median follow up of the entire cohort was 78.2 months.
A total of 413 pCCA patients were included in the analysis (Supplementary Figure S1 online). Patients were categorized into four groups on the basis of initial intended treatment as follows: (i) transplant group including patients listed for the protocol of neoadjuvant chemoradiation, followed by liver transplantation (i.e., including both intended and actual transplantation); (ii) resection group including those who underwent resection with curative intent (i.e., including both aborted and completed resection); (iii) palliative biliary drainage group including those who received stenting in combination with nonsurgical therapies, e.g., systemic chemotherapy, and (iv) best supportive care (BSC) group. There were 92, 82, 224, and 15 patients in the transplant, resection, palliative biliary drainage, and BSC groups, respectively (Supplementary Figure S1).
To identify predictors of survival at the time of pCCA diagnosis, hazard ratios (HRs) and 95% confidence intervals (95% CIs) were calculated using Cox proportional hazards regression analysis. The treatment type was included as both an ordinary covariate and as a stratification factor, to verify that the results were robust to the manner of inclusion. The first model included the targeted treatment group as a covariate, because that is one of the strongest predictors of later survival. The second model included the treatment group as a stratification factor. When stratifying on treatment, other covariates in the risk model examined the risk of each subject relative to others in the same treatment strata. For the continuous variable tumor size, we first assessed the overall shape of the relationship between tumor size and survival of patients using a smoothing spline function—e.g., does risk increase linearly or have a threshold?—and used the observed pattern to guide its inclusion in the final model. Owing to the large sample size, we were able to test all of the predictors in both a univariate and a multivariate model. P <0.05 was considered significant.
Significant variables identified by the multivariate analyses were incorporated into a four-level staging system. We also included three a priori candidate predictors proposed in our previous publication, i.e., multicentric tumors (indicating intrahepatic metastasis), vascular encasement, and lymph node metastasis, as they are major determinants of surgical resectability and eligibility for liver transplantation (3,12). Patients were categorized on the basis of this staging system, and survival was compared using the log-rank test.
The predictive performance of the proposed staging system was examined along with the current 7th TNM staging system using the concordance score. It is important to note that the patients in our cohort were categorized by TNM stage on the basis of radiologic findings instead of the surgical pathologic findings required for the bona fide TNM stage. Because pathologic information on tumor depth (T1) and extension of tumor into the surrounding tissues (T2) was not available in 75% of our cohort, stages I (T1N0M0) and II (T2N0M0) were grouped together.
RESULTS
Baseline characteristics
Table 1 displays demographic, clinical, and tumor characteristics. A total of 113 (27%) patients had PSC (of whom 17 had clinical features of portal hypertension, as assessed by ascites, jaundice, and esophageal varices) and 9 (2%) had non-PSC-related cirrhosis as their underlying risk factors.
Table 1.
Baseline characteristics of pCCA patient cohorta
| Transplant (n=92) | Resection (n=82) | Stentingb (n=224) | BSC (n=15) | |
|---|---|---|---|---|
| Age, years (mean±s.d., range) | 50±11 (25–73) | 65±12 (31–85) | 66±14 (29–100) | 67±17 (23–88) |
| Male | 71 (77%) | 44 (54%) | 140 (63%) | 9 (60%) |
| Whitec | 87 (97%) | 73 (95%) | 188 (94%) | 14 (100%) |
| PSC | 58 (63%) | 3 (4%) | 46 (21%) | 6 (40%) |
| Non-PSC cirrhosis | 1 (1%) | 0 (0%) | 7 (3%) | 1 (7%) |
| ECOG statusd | ||||
| 0 | 54 (59%) | 54 (66%) | 80 (36%) | 2 (13%) |
| 1 | 31 (34%) | 24 (29%) | 92 (41%) | 8 (53%) |
| 2 | 7 (7%) | 4 (5%) | 35 (16%) | 3 (20%) |
| 3 | 0 (0%) | 0 (0%) | 13 (6%) | 1 (7%) |
| 4 | 0 (0%) | 0 (0%) | 3 (1%) | 1 (7%) |
| Tumor location | ||||
| Common hepatic duct | 8 (9%) | 10 (12%) | 9 (4%) | 1 (7%) |
| Hepatic duct confluence | 8 (9%) | 18 (22%) | 34 (15%) | 2 (13%) |
| Right or left hepatic duct | 39 (42%) | 54 (66%) | 89 (40%) | 7 (47%) |
| Right and left hepatic duct | 37 (40%) | 0 (0%) | 92 (41%) | 5 (33%) |
| Maximum tumor diameter (cm; median, range) | 2.8 (1.4–5.0) | 2.4 (1.0–7.0) | 3.7 (1.1–10.9) | 4.5 (2.0–11.0) |
| Number of tumors | ||||
| Nonidentified | 47 (51%) | 38 (46%) | 69 (31%) | 2 (13%) |
| Single | 45 (49%) | 35 (43%) | 110 (49%) | 8 (53%) |
| Multiple (≥2) | 0 (0%) | 9 (11%) | 45 (20%) | 5 (34%) |
| Vascular encasement | 30 (33%) | 19 (23%) | 113 (50%) | 10 (67%) |
| Lymph node metastasisd | ||||
| No | 92 (100%) | 81 (99%) | 145 (65%) | 6 (40%) |
| Hilar lymph node | 0 (0%) | 1 (1%) | 24 (11%) | 1 (7%) |
| Distant lymph node | 0 (0%) | 0 (0%) | 54 (24%) | 8 (53%) |
| Peritoneal (or other organ) metastasis | 0 (0%) | 0 (0%) | 42 (19%) | 3 (20%) |
| Median (range) CA 19-9 (U/ml)e | 83 (1–11,937) | 76 (1–9,970) | 436 (1–216,300) | 336 (5–143,540) |
| CA 19–9 ≥1,000 U/ml | 13 (14%) | 7 (10%) | 81 (39%) | 5 (39%) |
BSC, best supportive care; pCCA, perihilar cholangiocarcinoma; PSC, primary sclerosing cholangitis.
All variables except for age, tumor diameter, and CA 19-9 level are shown in number (%).
Palliative biliary tract drainage for jaundice.
2, 5, 24, and 1 patient in the transplant, resection, stenting, and BSC groups had missing data.
One patient in the stenting group had missing data.
11, 18, and 2 patients in resection, stenting, and BSC groups had missing data.
Survival predictors of pCCA patients
As expected, initial intended treatment type was independently associated with survival (Supplementary Table S1). To assess the impact of potential nonproportional hazards on the treatment arm, treatment type was treated as strata in the model determining the impact of other variables on survival (Supplementary Table S2). Of the variables assessed at the time of diagnosis, age, ECOG status, tumor size, peritoneal (or other organ) metastasis diagnosed by either biopsy or imaging, and CA 19-9 were independently associated with survival, suggesting that these variables have an impact on survival after accounting for treatment modality (Supplementary Table S2). Different cutoff s of CA 19-9, i.e., ≥100, ≥500, and ≥1,000 U/ml, were further examined to identify the optimum cutoff that best discriminated survival outcomes. We found that the median survivals were 27.4, 13.3, 12.9, and 5.9 months, with HRs (95% CI) of 1 (reference), 1.8 (1.3–2.5), 2.1 (1.3–3.3), and 4.8 (3.5–6.7) for patients with CA 19-9 <100, 100–499, 500–999, and ≥1,000 U/ml, respectively. The CA 19-9 cutoff of ≥1,000 U/ml was therefore chosen for the multivariate analysis. The adjusted HR (95% CI) was 1.2 (1.1–1.4) for age per 10 years; 1.6 (1.2–2.1) for ECOG 1 or 2 and 10.6 (5.1–22.00) for ECOG 3 or 4 when compared with ECOG 0; 1.2 (1.1–1.4) for tumor size; 3.0 (1.8–4.9) for peritoneal metastasis; and 1.8 (1.3–2.5) for CA 19–9≥1,000 U/ml, P=0.0037, 0.0012, <0.0001, 0.0029, <0.0001, and 0.0002, respectively. Interestingly, tumor number, vascular encasement, and lymph node metastasis were not statistically significant in the treatment strata model, suggesting that the impact of these variables on survival may be different among the treatment groups.
Because ECOG status, tumor size, peritoneal metastasis, and CA 19-9≥1,000 U/ml were independently associated with survival, they were included in the proposed staging system. We selected a tumor size cutoff of 3 cm for two reasons: first, the median tumor size was 3 cm for the entire cohort, and having a tumor size greater than 3 cm was associated with a HR (95% CI) of death of 2.8 (2.2–3.6), P<0.0001. The median survival was 18.2 vs. 5.3 months for those with tumor size ≤3 cm vs. >3 cm, respectively. Second, it was shown that the 5-year survival after liver transplantation was more than 80% for pCCA patients who had tumor size <3 cm, and a tumor size cutoff of a radial diameter of 3 cm has been established as the optimal cutoff for patient selection for liver transplantation for pCCA (6,18).
Proposed pCCA staging system
Table 2 summarizes the proposed staging system based on independent and a priori candidate variables. Figure 1 shows the staging algorithm. On the basis of this algorithm, 76, 101, 164, and 58 patients were classified as stages I, II, III, and IV, respectively (14 patients could not be classified owing to the lack of information on ECOG status (n=1) or CA 19-9 (n=13)).
Table 2.
Proposed staging system for pCCA
| Variables | Stage I (n=76) | Stage II (n=101) | Stage III (n=164) | Stage IV (n=58) |
|---|---|---|---|---|
| Mass lesiona | Unicentric ≤3cm | Unicentric ≤3cm | Unicentric >3cm or multicentric | NA |
| Vascular encasement | No | Yes | NA | NA |
| Metastasis | No | No | Lymph node metastasisb | Peritoneal (or other organ) metastasis |
| ECOG performance status | 0 | 1–2 | 0–2 | 3–4 |
| CA 19-9 level (U/ml) | <1,000 | <1,000 | ≥1,000 | NA |
NA, not applicable; pCCA, perihilar cholangiocarcinoma.
A tumor size cutoff of 3 cm was used in the proposed staging system.
Hazard ratios of hilar and distant lymph node metastasis were not significantly different and were grouped as lymph node metastasis.
Figure 1.
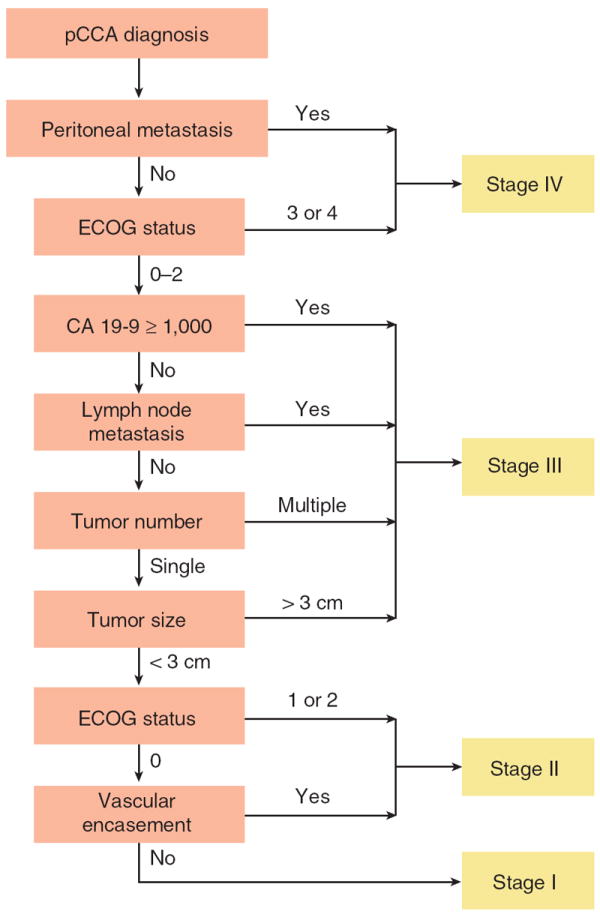
Staging algorithm for the proposed staging system. Stage IV includes patients with peritoneal or other organ metastasis or with ECOG status 3 or 4. Stage III includes patients with ECOG status 0, 1, or 2 and any of the following: CA 19-9 ≥1,000 U/ml, lymph node metastasis, multiple lesions (i.e., intrahepatic metastasis), or single lesion >3 cm, regardless of the presence or absence of vascular encasement. Stage II includes patients with single lesions up to 3 cm and either vascular encasement or ECOG status 1 or 2. Stage I includes ECOG status 0 patients with single lesions up to 3 cm without vascular encasement. pCCA, perihilar cholangiocarcinoma.
Survival of pCCA patients classified by the proposed staging system
At the time the data were censored, 281 (70%) of 399 patients classified by the proposed staging algorithm had died. The median survival of the entire cohort was 12.2 months, with 1- and 5-year survival rates of 51% and 18%, respectively.
Survival of pCCA patients was clearly distinguished by the proposed four stages (Figure 2a). The median survivals of stages I, II, III, and IV patients were 48.6, 21.8, 8.6, and 2.8 months, with HRs (95% CI) of 1.0 (reference), 1.8 (1.2–2.7), 5.1 (3.5–7.5), and 16.6 (10.5–26.2), respectively (overall P<0.0001; Table 3). After adjusting for treatment using the treatment strata model, the HRs of stages I, II, III, and IV were 1.0 (reference), 1.7 (1.2–2.6), 3.1 (2.0–4.6), and 8.7 (5.2–14.6), respectively (P<0.0001).
Figure 2.
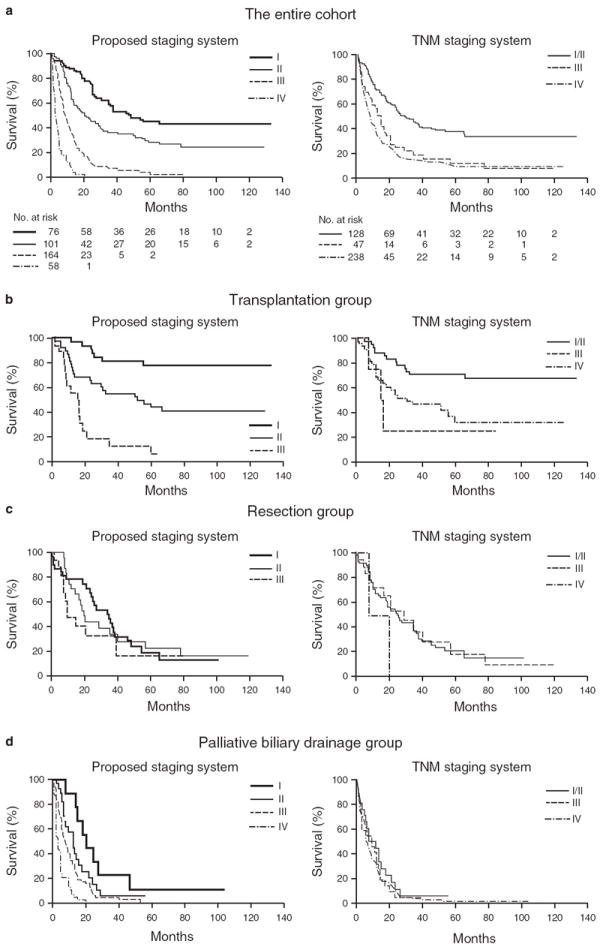
Survival of patients classified by the proposed staging system and the tumor-node-metastasis (TNM) staging system. (a) The entire cohort. (b) Transplantation group. (c) Resection group. (d) Palliative biliary drainage group.
Table 3.
Kaplan–Meier estimate of median survival and survival rate of pCCA patients classified by the proposed staging system
| Stage | Number of death/total | Median survival (months) | 1-Year Kaplan–Meier estimate (95% CI) | 5-Year Kaplan–Meier estimate (95% CI) | Univariate HR (95% CI) | P | Univariate HR (95% CI) from treatment strata model | P |
|---|---|---|---|---|---|---|---|---|
| I | 39/76 | 48.6 | 87.9% (80.8–95.6) | 45.3% (34.6–58.9) | 1.00 (Reference) | <0.0001 | 1.00 (Reference) | <0.0001 |
| II | 62/101 | 21.8 | 69.4% (60.4–79.8) | 27.9% (19.6–39.8) | 1.80 (1.20–2.68) | 0.0043 | 1.72 (1.15–2.58) | 0.0087 |
| III | 129/164 | 8.6 | 36.1% (28.9–45.0) | 2.8% (0.8–9.9) | 5.12 (3.52–7.45) | <0.0001 | 3.08 (2.03–4.57) | <0.0001 |
| IVa | 51/58 | 2.8 | 8.1% (3.2–20.6) | — | 16.60 (10.52–26.22) | <0.0001 | 8.67 (5.20–14.56) | <0.0001 |
HR, hazard ratio; pCCA, perihilar cholangiocarcinoma; 95% CI, 95% confidence interval.
3-Month and 6-month survival rates of stage IV patients were 46.4% and 22.2%, respectively.
Survival of pCCA patients receiving different treatments
The median survival of pCCA patients was significantly different among treatment groups, i.e., 60.2, 25.3, 5.8, and 5.6 months for the transplant, resection, palliative biliary drainage, and BSC groups, respectively (P<0.0001). When transplantation and death were treated as competing risks (i.e., to estimate survival of patients who received complete transplantation), the patients did not reach median survival with 1- and 5-year survival of 96% and 91%, respectively. Survival of patients in the palliative biliary drainage group was not statistically different from that of patients in the BSC group (P=0.17).
Survival of pCCA patients in each treatment group classified by the proposed staging system
Survival for patients within each treatment group was discriminated by the proposed stages (3-month, 6-month, 1-year, and 5-year Kaplan–Meier estimates, and HRs (95% CI) are shown in Supplementary Tables S3–6). Of the 92 patients in the transplant group, 33, 40, and 19 were classified as stage I, II, and III, respectively. Stage I patients did not reach median survival, whereas stage II and stage III patients had median survivals of 52.1 and 15.5 months, respectively (P<0.0001; Figure 2b ; Supplementary Table S3).
Of the 82 patients in the resection group, 32, 26, and 16 patients were classified as stage I, II, and III, respectively; 8 patients had missing CA 19-9 values for staging. Although the overall median survivals of stages I, II, and III patients in the resection group were not statistically different, i.e., 35.2, 20.2, and 9.7 months, respectively (P=0.41; Figure 2c), stage I patients had a better 2-year survival rate than stage II and stage III patients, i.e., 68%, 44%, and 33%, respectively (P=0.41; Supplementary Table S4).
Of the 224 patients in the palliative biliary drainage group, 11, 34, 119, and 54 were classified as stage I, II, III, and IV, respectively; 6 had missing CA 19-9 values for staging. The median survivals were 20.8, 12.4, 7.7, and 3.0 months, respectively (P<0.0001; Figure 2d ; Supplementary Table S5). Reasons for the selection of stenting alone for patients with early-stage disease, i.e. stage I, included technical reasons which made surgery not feasible (n=6), comorbid diseases (n=3), and other patient factors (n=2).
In the BSC group, 1, 10, and 4 patients were classified as stage II, III, and IV, respectively. The median survivals of stage III and stage IV patients were 6.1 and 1.0 months, respectively (P=0.02; Supplementary Table S6).
Predictive performance of the proposed staging system vs. the TNM staging system
Figure 2a shows survival of patients classified by the TNM staging system using radiologic information. The median survivals of radiologic TNM stages I/II, III, and IV patients were 27.7, 13.4, and 8.6 months, with HRs (95% CI) of 1.0 (reference), 2.3 (1.6–3.5), and 2.8 (2.1–3.7), respectively (overall P<0.0001; Table 4). After adjusting for treatment in the treatment strata model, the HRs of TNM I/II, III, and IV were 1.0 (reference), 1.5 (0.99–2.30), and 1.8 (1.2–2.5), respectively, P=0.01. Despite statistical significance, the HRs of the TNM stages were close to each other, suggesting that the discriminative power of the TNM staging system was low.
Table 4.
Kaplan–Meier estimate of median survival and survival rate of pCCA patients classified by the radiologic TNM staging system
| Stage | Number of death/total | Median survival (months) | 1-Year Kaplan–Meier estimate (95% CI) | 5-Year Kaplan–Meier estimate (95% CI) | Univariate HR (95% CI) | P | Univariate HR (95% CI) from treatment strata model | P |
|---|---|---|---|---|---|---|---|---|
| I/II | 75/128 | 27.7 | 70.9% (63.2–79.5) | 36.8% (28.6–47.1) | 1.00 (Reference) | <0.0001 | 1.00 (Reference) | 0.0100 |
| III | 39/47 | 13.4 | 56.5% (43.9–72.8) | 11.1% (4.3–29.1) | 2.31 (1.55–3.45) | <0.0001 | 1.50 (0.99–2.26) | 0.0575 |
| IV | 178/238 | 8.6 | 38.0% (31.8–45.4) | 8.5% (5.2–13.8) | 2.80 (2.10–3.73) | <0.0001 | 1.76 (1.22–2.54) | 0.0026 |
HR, hazard ratio; pCCA, perihilar cholangiocarcinoma; TNM, tumor-node-metastasis; 95% CI, 95% confidence interval.
When patients were categorized by treatment, the TNM stages were associated with survival of patients only in the transplant group (P=0.004) but not in the other groups (P>0.05; Figure 2b–d ; Supplementary Tables S7–10). Further, the median survivals of TNM I/II, III, and IV patients in the transplant group did not decrease in order as anticipated, instead, they were median survival not reached, 15.5, and 31.1 months, with HRs (95% CI) of 1.0 (reference), 4.1 (1.2–14.4), and 2.8 (1.5–5.5), respectively (overall P=0.004).
To assess the performance of the staging systems, we estimated the concordance scores of both staging systems. The concordance score (standard error) of the proposed staging system, 0.725 (0.018), was better associated with the outcome of patient death than that of the TNM staging system: 0.614 (0.017). When patients were categorized by treatments, the concordance score of the proposed staging system was higher than that of the radiologic TNM staging system for all treatment types (Table 5).
Table 5.
Concordance score (standard error) of the proposed staging system and the current TNM staging systema
| Patient group | Proposed staging system | TNM staging system |
|---|---|---|
| Entire cohort | 0.725 (0.018) | 0.614 (0.017) |
| Transplantation | 0.716 (0.041) | 0.624 (0.039) |
| Resection | 0.568 (0.041) | 0.506 (0.032) |
| Palliative biliary drainage | 0.661 (0.023) | 0.516 (0.019) |
| Best supportive care | 0.727 (0.067) | 0.515 (0.061) |
TNM, tumor-node-metastasis.
The analysis included 399 patients who had complete data for both the proposed staging system and the TNM staging system.
DISCUSSION
The main limitation of currently available classifications of pCCA is that they are not applicable to the entire cohort of pCCA patients seen in the clinic. The Bismuth-Corlette classification, including tumor location and extent, was designed to guide surgical treatment (8). It is not designed as a staging system and does not relate to survival (20-22). The Memorial Sloan-Kettering Cancer Center (MSKCC) staging system, including tumor location and extent classified by the Bismuth-Corlette classification, vascular involvement, and lobar atrophy, is a preoperative staging system that has been mainly used to determine potential surgical resectability (9). The MKSCC system was developed using a cohort of 225 patients with both unresectable and resectable disease. Although it was originally shown to predict survival of pCCA patients, this finding was not replicated in other studies (23,24). The patient cohort had an ascertainment bias toward surgically resectable patients, as 71% of the cohort underwent surgery with curative intent and only 29% presented with unresectable disease. The main utility of the MKSCC system therefore remains for defining surgical resectability. In addition, when the MKSCC system was developed, liver transplantation was not an established treatment modality for pCCA. The TNM staging system is the only pCCA classification that is specifically developed for predicting survival (10). It was updated in 2010 and shown to have better predictive performance than the prior version (25) ; however, other studies reported that the current TNM staging system was not associated with the overall survival of pCCA patients undergoing surgical resection (24,26). Recently, a new staging system for pCCA has been proposed, which includes consideration of the severity of underlying liver disease and detailed information on tumor extent and vascular involvement, but again it relies on findings from surgical pathology, and its correlation with survival has not been examined (27).
On the basis of analysis of 413 pCCA patients, we have developed a staging system for pCCA, which provides patient homogeneity in terms of survival. The staging system takes into account both patient and tumor characteristics, including ECOG status, tumor size and number, vascular encasement, presence and location of metastases, and CA 19-9 level. The staging system, developed using nonpathologic information, has excellent discriminatory power to predict survival of patients in each stage and had better prognostic performance, demonstrated by the higher concordance score, than the current TNM staging system.
The major advantage of our proposed staging system over the current staging systems is that our system can provide prognostication and stratify both surgical and nonsurgical patients. All variables in our system are obtained preoperatively at the time of diagnosis and are therefore relevant to all pCCA patients, not only those eligible for surgery. All variables are easily obtained, as they are components of the routine clinical evaluation of patients with suspected pCCA. The new staging system includes cases from the more recent era of liver transplantation for pCCA, and it also allows prognostication for patients with advanced disease. It therefore covers the full breadth of presentation of pCCA patients.
As expected, age, ECOG status, tumor size, peritoneal metastasis, and CA 19-9 levels were independent predictors of survival in the current study. ECOG status 3–4 was the strongest predictor of survival, followed by peritoneal metastasis. Interestingly, we found that CA 19-9 was an independent predictor of survival. Although CA 19-9 has been widely used as a diagnostic marker for CCA, whether it can be used as a biomarker for predicting survival has not been well studied. A CA 19-9 of >100 U/ml was shown to be a predictor for recurrence in a subgroup of pCCA patients treated with liver transplantation (13). In this study, CA 19-9 ≥1,000 U/ml was significantly associated with worse survival regardless of stage or treatment received. Although a number of biomarkers were recently shown to be associated with prognosis of pCCA patients, these biomarkers remain in phase 1 or 2 studies of cancer biomarker development, i.e., most biomarkers were obtained from surgical specimens, the studies were conducted in a small cohort, mainly in patients undergoing surgery, no validation studies are available, and no comparisons of the predictive performance with the CA 19-9 have been made (28-34).
Although tumor number, vascular encasement, and lymph node metastasis have been shown to predict pCCA survival in previous studies (20-22,24,35-37), we did not find independent associations between these variables and survival, likely owing to correlations between individual tumor characteristics, for example, tumor size and number (data not shown).
There are a number of strengths of our study. Mayo Clinic is a national and international referral center for patients with PSC and/or CCA. As such, we have a large cohort of pCCA patients presenting with all disease stages. The follow-up duration was long enough to have an adequate number of patients with the outcome of interest (i.e., death), allowing us to generate statistically robust models predicting mortality. Importantly, including treatments as strata is the more robust choice for analysis. In this data set, however, the coefficients for all of the other variables in the model including treatment as covariate (data not shown) hardly changed with the treatment strata model, indicating the robustness of our analyses. The patients are well distributed among the proposed stages, i.e., 19, 25, 41, and 15%, and likely reflect the proportions of pCCA patients typically seen in practice.
There are several limitations to the study. The staging system was developed using a cohort of patients seen in a single center, albeit referred from throughout North America, where the specialized treatment modalities, including liver transplantation, for pCCA are available. External validation of the new proposed staging system in an independent cohort is required to determine whether the proposed staging system can be generalized to other institutions. However, owing to the relatively small number of patients with pCCA seen at even high volume hepatobiliary cancer centers, we were not able to include a validation cohort in the present study. We are initiating an eff ort to validate the proposed staging system in a multicenter cohort of pCCA patients of whom a reasonable proportion has been treated with liver transplantation. Because there is no standard clinical staging system for pCCA currently available for us to determine whether the proposed staging system is superior to the current standard clinical staging system, the radiologic TNM staging system was used instead of the classical pathologic TNM staging as the gold standard for comparison, in order to allow us to include all patients in the comparison analysis. We did attempt to compare the performance of the proposed staging system to that of the classical TNM staging system in patients undergoing resection. However, the number of patients treated with resection was too small to draw any conclusions. Another limitation was that this staging system was developed from a Western population, in which PSC is a major risk factor for CCA. Whether this staging system is applicable to other populations with different ethnic backgrounds and risk factors is unknown.
In summary, a major benefit of this proposed staging system is that it can be applied to all pCCA patients regardless of the treatment received, and it will permit the reliable stratification of patients for phase 2 and 3 clinical trials into more homogeneous cohorts with similar expected survival outcomes.
Supplementary Material
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Currently available staging systems for perihilar cholangiocarcinoma (pCCA) rely solely on pathologic information.
-
✓
The application of these staging systems is therefore restricted to a small subset of pCCA patients with relatively early-stage disease.
WHAT IS NEW HERE
-
✓
A new staging system was developed on the basis of five nonoperative variables obtained at the time of diagnosis of pCCA.
-
✓
This staging system has excellent discriminatory power to classify patients into four discrete stages with different survival outcomes.
-
✓
The performance of the clinical staging system in predicting survival of pCCA patients was superior to that of the current TNM staging system.
Acknowledgments
Financial support: This work was supported by Grants CA100882, CA128633, and CA165076 from the National Cancer Institute (NCI) and the Cholangiocarcinoma Foundation (to L.R.R.), DK59427 from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK; to G.J.G.); the Mayo Clinic Center for Cell Signaling in Gastroenterology (NIDDK P30DK084567); the Mayo Clinic Cancer Center (NCI CA15083); and the Mayo Clinic Center for Clinical and Translational Science (NCATS UL1 TR000135). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The sponsors had no role in study design; data abstraction, analysis, and interpretation; or writing of the manuscript.
Footnotes
Guarantor of the article: Lewis R. Roberts, MB, ChB, PhD.
Specific author contributions: Study design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis, and critical revision of the manuscript for important intellectual content: Roongruedee Chaiteerakij; study design, acquisition of data, analysis and interpretation of data, statistical analysis, and critical revision of the manuscript for important intellectual content: William S. Harmsen; acquisition of data and drafting of the manuscript: Carlos Romero Marrero; acquisition of data: Mohammed M. Aboelsoud, Albert Ndzengue, and Joseph Kaiya; study concept and design, analysis and interpretation of data, statistical analysis, and critical revision of the manuscript for important intellectual content: Terry M. Therneau; study concept and design, acquisition of data, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content: William Sanchez; study concept and design, analysis and interpretation of data, statistical analysis, critical revision of the manuscript for important intellectual content, obtained funding, and study supervision: Gregory J. Gores; study concept and design, analysis and interpretation of data, statistical analysis, critical revision of the manuscript for important intellectual content, obtained funding, administrative support, and study supervision: Lewis R. Roberts.
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
CONFLICT OF INTEREST
Potential competing interests: None.
References
- 1.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–44. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 2.Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11:13–21. doi: 10.1016/j.cgh.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gores GJ, Darwish Murad S, Heimbach JK, et al. Liver transplantation for perihilar cholangiocarcinoma. Digest Dis. 2013;31:126–9. doi: 10.1159/000347207. [DOI] [PubMed] [Google Scholar]
- 4.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–62. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakeeb A, Tran KQ, Black MJ, et al. Improved survival in resected biliary malignancies. Surgery. 2002;132:555–63. doi: 10.1067/msy.2002.127555. [DOI] [PubMed] [Google Scholar]
- 6.Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88–98. doi: 10.1053/j.gastro.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 8.Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstetr. 1975;140:170–8. [PubMed] [Google Scholar]
- 9.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–17. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NCCN Guidelines Version 1. 2013 http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf.
- 11.Hiriart JB, Laurent C, Blanc JF. Perihilar cholangiocarcinoma, the need for a new staging system. Clin Res Hepatol Gastroenterol. 2011;35:697–8. doi: 10.1016/j.clinre.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Blechacz BR, Sanchez W, Gores GJ. A conceptual proposal for staging ductal cholangiocarcinoma. Curr Opin Gastroenterol. 2009;25:238–9. doi: 10.1097/MOG.0b013e3283292383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heimbach JK, Gores GJ, Haddock MG, et al. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. 2006;82:1703–7. doi: 10.1097/01.tp.0000253551.43583.d1. [DOI] [PubMed] [Google Scholar]
- 14.Fritcher EG, Kipp BR, Slezak JM, et al. Correlating Routine Cytology, Quantitative Nuclear Morphology by Digital Image Analysis, and Genetic Alterations by Fluorescence In Situ Hybridization to Assess the Sensitivity of Cytology for Detecting Pancreatobiliary Tract Malignancy. Am J Clin Pathol. 2007;128:272–9. doi: 10.1309/BC6DY755Q3T5W9EE. [DOI] [PubMed] [Google Scholar]
- 15.Fritcher EG, Kipp BR, Halling KC, et al. A multivariable model using advanced cytologic methods for the evaluation of indeterminate pancreatobiliary strictures. Gastroenterology. 2009;136:2180–6. doi: 10.1053/j.gastro.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 16.Kipp BR, Stadheim LM, Halling SA, et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol. 2004;99:1675–81. doi: 10.1111/j.1572-0241.2004.30281.x. [DOI] [PubMed] [Google Scholar]
- 17.Moreno Luna LE, Kipp B, Halling KC, et al. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064–72. doi: 10.1053/j.gastro.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassoun Z, Gores GJ, Rosen CB. Preliminary experience with liver transplantation in selected patients with unresectable hilar cholangiocarcinoma. Surg Oncol Clin N Am. 2002;11:909–21. doi: 10.1016/s1055-3207(02)00036-4. [DOI] [PubMed] [Google Scholar]
- 19.Heimbach JK, Haddock MG, Alberts SR, et al. Transplantation for hilar cholangiocarcinoma. Liver Transpl. 2004;10:S65–S68. doi: 10.1002/lt.20266. [DOI] [PubMed] [Google Scholar]
- 20.Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258:129–40. doi: 10.1097/SLA.0b013e3182708b57. [DOI] [PubMed] [Google Scholar]
- 21.de Jong MC, Marques H, Clary BM, et al. The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: a multi-institutional analysis of 305 cases. Cancer. 2012;118:4737–47. doi: 10.1002/cncr.27492. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo K, Rocha FG, Ito K, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg. 2012;215:343–55. doi: 10.1016/j.jamcollsurg.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Zervos EE, Osborne D, Goldin SB, et al. Stage does not predict survival aft er resection of hilar cholangiocarcinomas promoting an aggressive operative approach. Am J Surg. 2005;190:810–5. doi: 10.1016/j.amjsurg.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Cho MS, Kim SH, Park SW, et al. Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J Gastrointest Surg. 2012;16:1672–9. doi: 10.1007/s11605-012-1960-0. [DOI] [PubMed] [Google Scholar]
- 25.Juntermanns B, Sotiropoulos GC, Radunz S, et al. Comparison of the sixth and the seventh editions of the UICC classification for perihilar cholangiocarcinoma. Ann Surg Oncol. 2013;20:277–84. doi: 10.1245/s10434-012-2486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami Y, Uemura K, Sudo T, et al. Perineural invasion in extrahepatic cholangiocarcinoma: prognostic impact and treatment strategies. J Gastrointest Surg. 2013;17:1429–39. doi: 10.1007/s11605-013-2251-0. [DOI] [PubMed] [Google Scholar]
- 27.Deoliveira ML, Schulick RD, Nimura Y, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53:1363–71. doi: 10.1002/hep.24227. [DOI] [PubMed] [Google Scholar]
- 28.Ruzzenente A, Iacono C, Conci S, et al. A novel serum marker for biliary tract cancer: diagnostic and prognostic values of quantitative evaluation of serum mucin 5AC (MUC5AC) Surgery. 2014;155:633–9. doi: 10.1016/j.surg.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Han Y, Zhang W, Liu Y. Identification of hepatoma-derived growth factor as a potential prognostic and diagnostic marker for extrahepatic cholangiocarcinoma. World J Surg. 2013;37:2419–27. doi: 10.1007/s00268-013-2132-4. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe A, Suzuki H, Yokobori T, et al. Forkhead box protein C2 contributes to invasion and metastasis of extrahepatic cholangiocarcinoma, resulting in a poor prognosis. Cancer Sci. 2013;104:1427–32. doi: 10.1111/cas.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabris L, Cadamuro M, Moserle L, et al. Nuclear expression of S100A4 calcium-binding protein increases cholangiocarcinoma invasiveness and metastasization. Hepatology. 2011;54:890–9. doi: 10.1002/hep.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian F, Li D, Chen J, et al. Aberrant expression of GATA binding protein 6 correlates with poor prognosis and promotes metastasis in cholangiocarcinoma. Eur J Cancer. 2013;49:1771–80. doi: 10.1016/j.ejca.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Itatsu K, Zen Y, Yamaguchi J, et al. Expression of matrix metalloproteinase 7 is an unfavorable postoperative prognostic factor in cholangiocarcinoma of the perihilar, hilar, and extrahepatic bile ducts. Hum Pathol. 2008;39:710–9. doi: 10.1016/j.humpath.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Tolek A, Wongkham C, Proungvitaya S, et al. Serum alpha1beta-glycoprotein and afamin ratio as potential diagnostic and prognostic markers in cholangiocarcinoma. Exp Biol Med. 2012;237:1142–9. doi: 10.1258/ebm.2012.012215. [DOI] [PubMed] [Google Scholar]
- 35.Patel SH, Kooby DA, Staley CA, 3rd, et al. The prognostic importance of lymphovascular invasion in cholangiocarcinoma above the cystic duct: a new selection criterion for adjuvant therapy? HPB (Oxford) 2011;13:605–11. doi: 10.1111/j.1477-2574.2011.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu YF, Yang XQ, Lu XF, et al. Fibroblast growth factor receptor 4 promotes progression and correlates to poor prognosis in cholangiocarcinoma. Biochem Biophys Res Commun. 2014;446:54–60. doi: 10.1016/j.bbrc.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 37.Ebata T, Nagino M, Kamiya J, et al. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg. 2003;238:720–7. doi: 10.1097/01.sla.0000094437.68038.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


