Abstract
Objective
Determine whether early weight gain predicts full remission at end-of-treatment (EOT) and follow-up in two different treatments for adolescent anorexia nervosa, and to track the rate of weight gain throughout treatment and follow-up.
Method
Participants were 121 adolescents with AN (mean age = 14.4 years, SD = 1.6), from a two-site (Chicago and Stanford) randomized controlled trial. Adolescents were randomly assigned to family-based treatment (FBT) (n=61) or individual adolescent supportive psychotherapy (AFT) (n=60). Treatment response was assessed using percent of expected body weight (EBW) and the global score on the Eating Disorder Examination (EDE). Full remission was defined as having achieved ≥95% EBW and within one standard deviation of the community norms of the EDE. Full remission was assessed at EOT as well as 12-month follow-up.
Results
Receiver operating characteristic analyses showed that the earliest predictor of remission at EOT was a gain of 5.8 pounds (2.65 kg) by session 3 in FBT (AUC = .670; p=.043), and a gain of 7.1 pounds (3.20 kg) by session 4 in AFT (AUC=0.754, p=.014). Early weight gain did not predict remission at follow-up for either treatment. A survival analysis showed that weight was marginally superior in FBT as opposed to AFT (Wald chi-square=3.692, df=1, p=.055).
Conclusion
Adolescents with AN who receive either FBT or AFT, and show early weight gain, are likely to remit at EOT. However, FBT is superior to AFT in terms of weight gain throughout treatment and follow-up.
Keywords: adolescent anorexia nervosa, family-based treatment, adolescent focused therapy, early treatment response
Early change in treatment is a promising clinical indicator of treatment outcome in patients with eating disorders. For instance, examining weight gain trajectories for adult patients with anorexia nervosa (AN) in an inpatient setting, demonstrated that gains at weeks 3 and 4 accurately classified a majority of treatment responders vs. non-responders (1). For bulimia nervosa (BN), it has been shown that early change in binge eating and purging among adolescents receiving FBT or individual supportive therapy is a good predictor of response at the end of treatment (2). Early reduction in these behaviors among adults who receive cognitive behavior therapy (CBT) is also a good predictor of end of treatment response (3). In this study, Fairburn and colleagues demonstrated that early change in treatment is a robust predictor of immediate outcome and long-term outcome in BN. In adolescent AN, two published reports have investigated early response to outpatient treatment with family-based treatment (FBT) as a predictor of outcome. In the first, Lock and colleagues (4) showed that weight gain by sessions 2, 9, or 10 in a sample of 86 adolescents was predictive of a positive outcome. Participants received one of two doses of FBT delivered in the context of a randomized controlled trial (RCT). In the second, Doyle and colleagues (5) expanded these findings and examined the utility of early weight response in predicting outcome for a clinic sample of adolescents with AN. They showed that weight gain of 4 pounds by session 4 best predicted outcome (>95% of expected body weight [EBW]) at the end of a course of FBT.
Hence, to date, early change in weight has only been examined for FBT. Therefore, the primary objective of the present study was to determine whether early weight gain predicts full remission at the end of treatment and at follow-up for both FBT and an individual treatment in a sample of adolescents who participated in a recently published RCT (6). A secondary objective was to examine the rate of weight change during treatment and the follow-up period.
METHODS
Data are from a two-site RCT (The University of Chicago and Stanford University) that compared family-based treatment (FBT) and individual adolescent focused therapy (AFT). Adolescents (n=121) meeting DSM-IV criteria for AN, excluding criterion D, were randomly assigned to either FBT or AFT. Both treatments lasted 12 months, and all participants received 24 hours of therapy, regardless of treatment modality. Assessments were conducted at baseline, end-of-treatment (EOT), and at 6- and 12-month follow-up. The Institutional Review Boards of The University of Chicago and Stanford University approved this study, and all participants and participation involved informed consent. Details of this design, main outcome findings, and moderator/mediator analyses are reported elsewhere (6,7).
Statistical Analyses
IBM SPSS Statistics 19 was used for all analyses. To examine if early weight gain in treatment was predictive of remission status, we conducted receiver operating characteristic (ROC) analyses with a nonparametric distribution assumption and choosing cut-points to achieve equal sensitivity and specificity. We evaluated the association between weight gain, i.e., the difference in pounds between baseline and sessions 1 through 8, and remission status at EOT and at 12-month follow-up. The ROC area under the curve (AUC) and corresponding 95% confidence interval were utilized to evaluate the relationship between weight gain and remission. The AUC represents the probability that a randomly selected participant who achieved remission, would have reached a higher weight at that session than a randomly selected participant who had not remitted. The main outcome analyses are originally reported elsewhere but will be briefly reiterated here. Details of the mixed-effects modeling analyses and number needed to treat (NNT) effect size calculations are available in the original reports (6,7). Using the cut-points determined in the ROC, independent t- and chi-square tests were used to compare individuals who had achieved the ROC weight gain cut-point versus those who had not.
Evaluating the time to achieve at least 95% EBW was performed using survival analyses (8,9). Weight data were available at 3 months, 6 months, 9 months, EOT, 6-month follow-up (18 months), and 12-month follow-up (24 months). Individuals with no follow-up data (n=8, 6.6%), those with limited within-treatment data due to treatment drop-out (n=11, 9.1%), and those who completed follow-up assessments but did not achieve 95% EBW by the final time point (i.e., 12-month follow-up; n=29, 24.0%) were treated as “censored” observations, indicating that treatment response did not occur prior to termination of the measurement period. A Cox proportional hazard model was then fitted using a log logistic distribution. Indicator variables, coded as −.5 and +.5 were created to represent treatment group, treatment center, and treatment by center interaction (10); an overall coefficient for treatment group was obtained. Finally, for individuals who had achieved 95% EBW at any point in the 24 month period, independent t- and chi-square tests were used to compare those who achieved this weight within 6 months versus those achieved it after 6 months.
RESULTS
Participant Characteristics
Participants were predominantly female (90.9%) with a mean age of 14.4 years (SD = 1.6). Race/ethnicity was largely non-Hispanic White (76%, n=92), but also Hispanic White (7.4%, n=9), Asian (10.7%, n=13), Black (0.8%, n=1), and ‘other’ (5.0%, n=6). Mean %EBW was 80.4% (SD = 3.6) with a mean BMI of 16.1 (SD = 1.1) using the Centers for Disease Control and Prevention growth charts; 17.4% (n=21) reported purging behavior. Average duration of illness was 11.3 months (SD=8.6), with 44.6% (n=54) of the sample reporting prior hospitalization for AN or medical problems associated with AN. Approximately one quarter of participants (24.5%, n=29) met criteria for a current comorbid psychiatric disorder by the Schedule for Affective Disorders and Schizophrenia for School-Aged Children, and 16.5% (n=20) were taking psychotropic medications at baseline. Families were mostly intact (78.9%, n=95) and most parents had attended some higher education (means of 15.7 and 16.2 years of education for mothers and fathers, respectively). Remission data were available at end of treatment (n=103, 85.5%), 6-month follow-up (n=88, 72.7%), and 12-month follow-up (n=93, 76.9%). Compared to those with data at EOT, adolescents with missing data were more likely to take a psychotropic medication at baseline (33.3% v. 13.6%; φ =−.19, p=.037). There were no other differences for those with missing data at EOT, 6-month follow-up, or 12-month follow-up.
Treatment Outcomes
Remission was defined as reaching at least 95% EBW and scoring within 1 SD of community norms on the EDE (6,7). Briefly, there were no statistical differences in remission between treatments at EOT (FBT = 42% vs. AFT = 23%, p=.055; NNT=5). However, at both 6-month follow-up (FBT = 40% vs. AFT = 18%, p=.029; NNT=5), and 12-month follow-up (FBT = 49% vs. AFT = 23%, p=.024; NNT=4), FBT was significantly superior to AFT on this measure.
Early Weight Gain and Remission
ROC curves for adolescents receiving FBT (n=50) revealed that weight gain at sessions 3 through 8 was significantly associated with EOT remission status (p<.05), with AUC values ranging from 0.670 to 0.929 (see Table 1a). The earliest predictor of EOT remission was gaining at least 5.8 pounds (2.65 kg) by session 3 (AUC=0.670, p=.043; 42.6% met threshold). The strongest predictor of EOT remission was gaining at least 11.2 pounds by session 8 (AUC=0.929, p=.010; 13.1%). ROC curves for adolescents receiving AFT (n=53) revealed that weight gain at sessions 4 through 6 was significantly associated with EOT remission status (p< 0.05), with AUC values for those sessions ranging from 0.739 to 0.791 (see Table 1a). The earliest predictor of EOT remission was gaining at least 7.1 pounds (3.20 kg) by session 4 (AUC=0.754, p=.014; 35.0%), although weight gain of at least 5.1 pounds by session 3 was marginally significant (AUC=0.687, p=.058; 38.3%). The strongest predictor of EOT remission was gaining at least 7.8 pounds by session 5 (AUC=0.791, p=.007; 35.0%). The earliest session for which ROC curves were significant across treatment types was session 4. None of the ROC curves were significantly associated with remission at 12-month follow-up for adolescents in FBT (n=44) or AFT (n=49) (see Table 1b).
Table 1a.
ROC analyses of weight gain to predict remission at end of treatment.
| Session | AUC (95% CI) | Significance | Weight gain cutpoint a | ||
|---|---|---|---|---|---|
| Pounds | Kilograms | ||||
| FBT | 1 | .661 (.501–.821) | .056 | 4.1 | 1.85 |
| 2 | .655 (.498–.812) | .066 | 3.9 | 1.75 | |
| 3 | .670 (.515–.825) | .043 | 5.8 | 2.65 | |
| 4 | .725 (.580–.870) | .009 | 6.5 | 2.95 | |
| 5 | .778 (.638–.917) | .002 | 7.6 | 3.45 | |
| 6 | .807 (.660–.953) | .002 | 8.7 | 3.95 | |
| 7 | .909 (.773–1.000) | .002 | 10.0 | 4.55 | |
| 8 | .929 (.790–1.000) | .010 | 11.2 | 5.10 | |
| AFT | 1 | .470 (.292–.648) | .762 | 2.4 | 1.10 |
| 2 | .608 (.445–.770) | .277 | 4.4 | 2.00 | |
| 3 | .687 (.532–.843) | .058 | 5.1 | 2.30 | |
| 4 | .754 (.604–.904) | .014 | 7.1 | 3.20 | |
| 5 | .791 (.653–.928) | .007 | 7.8 | 3.55 | |
| 6 | .739 (.586–.893) | .037 | 7.6 | 3.45 | |
| 7 | .727 (.564–.890) | .066 | 9.0 | 4.10 | |
| 8 | .667 (.372–.961) | .371 | 8.3 | 3.75 | |
Table 1b.
ROC analyses of weight gain to predict remission at 12-month follow-up.
| Session | AUC (95% CI) | Significance | Weight gain cutpointa | ||
|---|---|---|---|---|---|
| Pounds | Kilograms | ||||
| FBT | 1 | .573 (.397–.750) | .405 | 4.1 | 1.85 |
| 2 | .557 (.385–.729) | .519 | 3.9 | 1.75 | |
| 3 | .517 (.342–.691) | .851 | 5.5 | 2.50 | |
| 4 | .558 (.382–.734) | .521 | 6.7 | 3.05 | |
| 5 | .675 (.502–.848) | .070 | 7.6 | 3.45 | |
| 6 | .608 (.410–.806) | .299 | 8.5 | 3.85 | |
| 7 | .600 (.328–.872) | .477 | 10.0 | 4.55 | |
| 8 | .444 (.081–.807) | .749 | 10.9 | 4.95 | |
| AFT | 1 | .538 (.364–.712) | .713 | 2.4 | 1.10 |
| 2 | .633 (.466–.800) | .200 | 4.6 | 2.10 | |
| 3 | .663 (.482–.845) | .115 | 5.2 | 2.35 | |
| 4 | .627 (.413–.840) | .245 | 6.4 | 2.90 | |
| 5 | .612 (.399–.826) | .326 | 7.3 | 3.30 | |
| 6 | .510 (.257–.763) | .938 | 6.0 | 2.70 | |
| 7 | .496 (.215–.777) | .978 | 8.0 | 3.65 | |
| 8 | .554 (.208–.899) | .750 | 7.8 | 3.55 | |
Key: FBT = Family-Based Treatment; AFT = Adolescent Focused Therapy;
Cutpoint to achieve equal sensitivity and specificity.
Early Treatment Responders versus Non-early Responders
Early treatment responders (i.e., those gaining ≥5.8 pounds at FBT session 3, or ≥7.1 pounds at AFT session 4) were compared to non-early responders. Participants who had missing weight data due to psychotherapy non-attendance were assumed to not have met the weight gain criterion (n=6 in FBT, n=5 in AFT). Compared to non-early FBT responders, early FBT responders (n=27, 44.3%) differed at baseline only in their parents’ education level, such that early FBT responders had parents with fewer years of education (15.9 vs. 17.2; t58.3=−2.1, p=.045). There were no other baseline differences between non-early and early FBT responders (ps > .10). Compared to adolescents without early response to AFT, early AFT responders (n=21, 35.0%) were more likely to have no comorbidity (85.7% vs. 59.0%; φ=−.27, p=.034), and although not statistically significant, appeared to be more likely to have an intact family (90.5% vs. 69.2%; φ=.24, p=.063). There were no other baseline differences between early AFT responders v. non-early responders (ps > .10). Treatment group (AFT vs. FBT) was not significantly associated with early treatment-specific response.
Timely versus Slow Weight Gain
A majority of participants achieved 95% EBW at one point within two years of initiating treatment (n=74, 61.2%) - approximately two-thirds (67.2%, n=41) of those who received FBT and more than half (55.0%, n=33) of those who received AFT. Baseline characteristics were compared between adolescents who achieved 95% EBW within the first 6 months of treatment (timely: n=55, 45.5%) to those who achieved 95% EBW at any point between 6 months and one-year follow-up (slow: n=19, 15.7%). Participants who failed to achieve 95% EBW (n=47, 38.8%) were excluded from this analysis. At baseline, adolescents with timely weight restoration differed from those with slow weight restoration in that the former were more likely to be male (81.8% vs. 41.8%; φ=.23, p=.037), and had more problematic family functioning (2.1 vs. 1.8; t71=2.1, p=.036).
Rate of Weight Gain Throughout Treatment and Follow-up
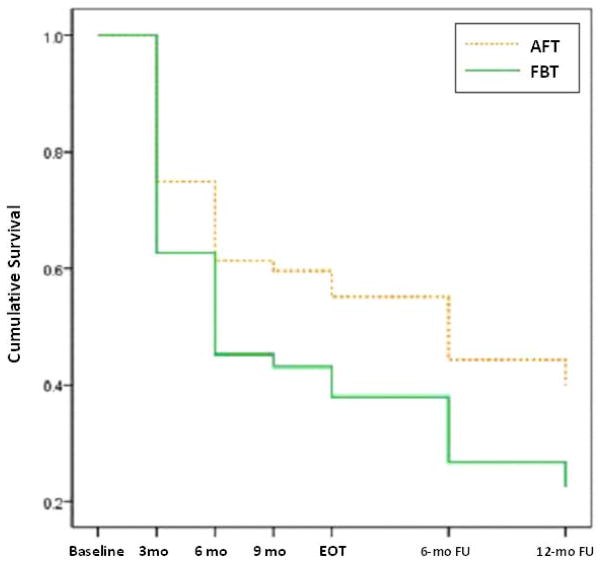
Weight data were available at 3 months, 6 months, 9 months, EOT, 6-month follow-up (18 months), and 12-month follow-up (24 months). Controlling for center and center by treatment interaction, time to 95% EBW was marginally associated with treatment group, such that adolescents receiving FBT achieved 95% EBW sooner than adolescents receiving AFT (Wald chi-square=3.692, df=1, p=.055, OR=1.6, 95% CI=1.0–2.5). At all within-treatment and follow-up points, a greater percentage of individuals who received FBT achieved 95% EBW compared to AFT. Figure 1 depicts time to reach 95% EBW, where the vertical axis represents the survival function, or the proportion of non-responders as defined by achieving less than 95% EBW.
Figure 1.
Survival analysis comparing treatment conditions to predict weight restoration.
DISCUSSION
Early weight gain in both treatments predicted remission at EOT. ROC analyses showed that weight gain of more than 5 pounds by session 3 of FBT predicted remission at EOT, and weight gain of more than 7 pounds by session 4 of AFT predicted remission at EOT. Early weight gain was not predictive of remission at 12-month follow-up in either treatment. This suggests that early weight gain is not essential for remission at follow-up, although it is likely to minimize the medical and psychological consequences resulting from acute starvation. However, in a recent study, achievement of 95% EBW at the end FBT was predictive of remission (11). Taken together, these findings support the notion that early weight gain predicts EOT weight restoration to 95% EBW, while EOT weight restoration predicts long-term recovery. Few baseline variables distinguished early weight responders from non-responders, regardless of treatment. However, some variables predicted achievement of 95% EBW within 6 months (timely) as compared to a slow weight response, that is, male gender, greater family disadvantages and engaging in purging.
A survival analysis of the time to achieve at least 95% EBW showed that FBT added more participants to this marker at every time point throughout treatment and follow-up. In other words, adolescents in FBT tended to gain weight more rapidly and more consistently than their counterparts in AFT. Nevertheless, both treatments were limited to some extent in their efficacy across individuals, with more than one third of adolescents failing to reach 95% EBW at any time point assessed in a two-year period. While weight gain is only one component of treatment and does not equate with remission, these findings add to the growing body of literature showing that patients with early weight gain are more likely to be responders at post-treatment (4,5). It also extends the limited prior work in adolescents with AN in that it offers a comparison between FBT and AFT, showing that early weight gain in both treatments predicts remission at EOT, but that weight gain is both quicker and more consistent in FBT than is the case for AFT.
The mechanisms of change in either FBT of AFT for adolescents with AN remain unclear. However, our findings do lend further support to our previous hypothesis regarding possible treatment mechanisms of action (5). For FBT, the active facilitation of early weight gain through parental efforts seems to have a positive impact on remission status. In AFT, the focus is on supporting the adolescent achieving self-efficacy and thereby reversing self-starvation. It would appear that even in AFT, early weight gain through the adolescent’s own efforts can exert a positive impact of remission. Current research to improve therapy focuses on FBT, and whether adolescents with AN who fail to respond early on in FBT will benefit from this treatment if it is adapted to address the potential barriers to early response (12). This study is a first step to help clarify whether there are specific family or patient behaviors that complicate treatment from the get-go. Developing targeted treatment strategies early on in treatment in FBT, such as helping parents achieve a united effort toward weight restoration, or repeating the family meal early on in treatment to enhance parental re-feeding skills, may be particularly helpful and could prove outcome. What may help in AFT remains unexplored.
More research is also needed to describe whether early non-responders show certain characteristic profiles that are different from responders. Our study highlighted two factors, i.e., parental education for FBT and comorbid psychiatric diagnosis for AFT, which could potentially guide us in future efforts in this domain. Early responders in FBT have parents with relatively fewer years of education, whereas early responders in AFT are more likely to have no psychiatric comorbidity. It may be that higher parental education increases parents’ worry about psychological causes and the need to focus on them; or that parents with higher levels of education may have less time, because of work commitments, to focus on the tasks inherent to FBT. Also speculatively, the absence of psychiatric comorbidity may have enabled adolescents to more effectively engage in weight gain behaviors in AFT, given that this treatment may typically have a broader focus on issues other than the eating disorder. Therefore, this finding may suggest that AFT is less suitable for adolescents with complex psychiatric comorbidity, and with higher levels of impairment, which would render early weight gain difficult to achieve.
This study was limited by a relatively modest sample size. Still, it was the first to utilize data from a RCT of two psychosocial treatments for adolescent AN to examine early response to treatment and remission at EOT and 12-month follow-up. The definition of remission took both weight (% EBW) and concomitant psychological markers (EDE global score) into account. The importance of psychological change for long-term recovery (EDE) is important and consistent with our prior work on predictors of recovery (11). However, this study only examined the time to initial achievement of weight restoration and remission, and treatment gains were not always maintained across time. The results of this study may not generalize to clinical populations because all participants received one of two manualized treatments provided at two specialist treatment centers.
This study underscores that rapid and early weight gain in the treatment of adolescents with AN is associated with the likelihood of good outcome at EOT, and in a related study (11), EOT weight restoration predicts long term outcome in adolescent AN. However, other factors appear to be important for long-term remission. Future research might examine alternative factors identifiable early in treatment that are associated with good outcome in the months following treatment completion. In particular, factors that can be directly modified or those that inform specific adaptations to treatment components could prove to be beneficial.
Acknowledgments
This study was supported by NIH grants R01-MH-070620 (Dr. Le Grange) and R01-MH-070621 (Dr. Lock). Drs Le Grange and Lock receive honoraria from the Training Institute for Child and Adolescent Eating Disorders, LLC, and royalties from Guilford Press. Dr. Le Grange also receives royalties from Routledge, and Drs Lock and Agras receive royalties from Oxford University Press.
References
- 1.Hartmann A, Wirth C, Zeeck A. Prediction of failure of inpatient treatment of anorexia nervosa from early weight gain. Psychotherapy Res. 2007;17:218–229. [Google Scholar]
- 2.Le Grange D, Doyle P, Crosby R, Chen E. Early response to treatment in adolescent bulimia nervosa. Int J Eat Disord. 2008;41:755–757. doi: 10.1002/eat.20566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fairburn CG, Agras WS, Walsh BT, Wilson GT, Stice E. Prediction of outcome in bulimia nervosa by early change in treatment. Am J Psychiatry. 2004;161:2322–2324. doi: 10.1176/appi.ajp.161.12.2322. [DOI] [PubMed] [Google Scholar]
- 4.Lock J, Couturier J, Bryson S, Agras WS. Predictors of dropout and remission in family therapy for adolescent anorexia nervosa in a randomized clinical trial. Int J Eat Disord. 2006;39:639–647. doi: 10.1002/eat.20328. [DOI] [PubMed] [Google Scholar]
- 5.Doyle P, Le Grange D, Celio-Doyle A, Loeb K, Crosby R. Early response to family-based treatment for adolescent anorexia nervosa. Int J Eat Disord. 2010;43:659–662. doi: 10.1002/eat.20764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lock J, Le Grange D, Agras S, Bryson S, Booil J. Randomized clinical trial comparing family-based treatment to adolescent focused individual therapy for adolescents with anorexia nervosa. Arch Gen Psychiatry. 2010;67:1025–1032. doi: 10.1001/archgenpsychiatry.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Grange D, Lock J, Agras WS, Moye A, Bryson S, Jo B, Kraemer H. Moderators and mediators of remission in family-based treatment and adolescent focused therapy for anorexia nervosa. Beh Res Therapy. 2012;50:85–92. doi: 10.1016/j.brat.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox DR, Oakes D. Analysis of survival data. New York: Chapman & Hall/CRC; 1984. [Google Scholar]
- 9.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New Jersey: John Wiley & Sons; 1980. [Google Scholar]
- 10.Kraemer HC, Blasey CM. Centering in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatric Res. 2004;13:141–151. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lock J, Agras WS, Le Grange D, Couturier J, Safer D, Bryson SW. Predicting recovery in eating disorders. Int J Eat Disord. doi: 10.1002/eat.22175. in press. [DOI] [PubMed] [Google Scholar]
- 12.Darcy A, Bryson S, Agras S, Fitzpatrick K, Le Grange D, Lock J. Do in-vivo behaviors predict early response in family-based treatment for anorexia nervosa? Beh Res Therapy. doi: 10.1016/j.brat.2013.09.003. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]