Abstract
BACKGROUND
Reproductive-age women need effective interventions to prevent the acquisition of human immunodeficiency virus type 1 (HIV-1) infection.
METHODS
We conducted a randomized, placebo-controlled trial to assess daily treatment with oral tenofovir disoproxil fumarate (TDF), oral tenofovir–emtricitabine (TDF-FTC), or 1% tenofovir (TFV) vaginal gel as preexposure prophylaxis against HIV-1 infection in women in South Africa, Uganda, and Zimbabwe. HIV-1 testing was performed monthly, and plasma TFV levels were assessed quarterly.
RESULTS
Of 12,320 women who were screened, 5029 were enrolled in the study. The rate of retention in the study was 91% during 5509 person-years of follow-up. A total of 312 HIV-1 infections occurred; the incidence of HIV-1 infection was 5.7 per 100 person-years. In the modified intention-to-treat analysis, the effectiveness was −49.0% with TDF (hazard ratio for infection, 1.49; 95% confidence interval [CI], 0.97 to 2.29), −4.4% with TDF-FTC (hazard ratio, 1.04; 95% CI, 0.73 to 1.49), and 14.5% with TFV gel (hazard ratio, 0.85; 95% CI, 0.61 to 1.21). In a random sample, TFV was detected in 30%, 29%, and 25% of available plasma samples from participants randomly assigned to receive TDF, TDF-FTC, and TFV gel, respectively. Independent predictors of TFV detection included being married, being older than 25 years of age, and being multiparous. Detection of TFV in plasma was negatively associated with characteristics predictive of HIV-1 acquisition. Elevations of serum creatinine levels were seen more frequently among participants randomly assigned to receive oral TDF-FTC than among those assigned to receive oral placebo (1.3% vs. 0.2%, P = 0.004). We observed no significant differences in the frequencies of other adverse events.
CONCLUSIONS
None of the drug regimens we evaluated reduced the rates of HIV-1 acquisition in an intention-to-treat analysis. Adherence to study drugs was low.
Daily oral preexposure prophylaxis with 300 mg of tenofovir disoproxil fumarate (TDF), alone or in combination with 200 mg of emtricitabine (FTC) (TDF-FTC [Truvada, Gilead Sciences]), reduces the risk of acquisition of human immunodeficiency virus type 1 (HIV-1) by 50% or more among persons with high adherence to the regimen, with demonstrated efficacy in men who have sex with men, heterosexuals, and injection-drug users.1–4 On the basis of these observations, in July 2012 the Food and Drug Administration approved daily treatment with Truvada for the prevention of HIV-1 acquisition, and the Centers for Disease Control and Prevention has issued guidelines for its use.5 However, Truvada was found to be ineffective in preventing HIV-1 acquisition among women in the Preexposure Prophylaxis Trial for HIV Prevention among African Women (FEM-PrEP), whose rate of adherence, as assessed on the basis of plasma tenofovir (TFV) levels, was less than 40%.6
The topical application of antiretroviral agents, including TFV, is effective in preventing rectal and cervicovaginal infection with simian immunodeficiency virus in macaques.7,8 Participants in the Centre for the AIDS Programme of Research in South Africa (CAPRISA) 004 trial who were assigned to receive pericoital treatment with 1% TFV gel had a 39% reduction in the risk of HIV-1 acquisition relative to women assigned to receive placebo, and greater protection was observed with higher adherence.9
The potential for protection with oral or topical antiretroviral agents, which we anticipated would have distinct safety, acceptability, and pharmacokinetic profiles, informed the design of the Vaginal and Oral Interventions to Control the Epidemic (VOICE) trial (MTN-003), which was initiated while the trials described above were under way. The aim of the VOICE trial was to estimate the effectiveness of daily treatment with vaginal TFV gel, as compared with placebo gel, and of oral TDF and oral TDF-FTC, as compared with oral placebo, in preventing sexually acquired HIV-1 infection in women and to assess the safety profiles of each of the active treatments.
METHODS
STUDY POPULATION
From September 2009 through June 2011, we screened 12,320 women at 15 sites in South Africa, Uganda, and Zimbabwe (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). We enrolled women 18 to 45 years of age who were neither pregnant nor breast-feeding and who reported recent vaginal intercourse, were using effective contraception, and had normal renal, hematologic, and hepatic function (Table S2 in the Supplementary Appendix).
RANDOMIZATION AND STUDY PROCEDURES
Participants were randomly assigned in a 1:1:1:1:1 ratio to one of five regimens: oral TDF (300 mg) and TDF-FTC placebo, oral TDF-FTC (300 mg of TDF and 200 mg of FTC) and TDF placebo, oral TDF placebo and oral TDF-FTC placebo, vaginal 1% TFV gel, or vaginal placebo gel.10 Participants were counseled to use the products daily. All participants were tested for sexually transmitted infections at the time of enrollment, annually, and when indicated. Standard HIV risk-reduction counseling, individualized adherence counseling, condoms, and hepatitis B immunization were provided. The study product was withheld if the woman became pregnant, began breast-feeding, and had clinical or laboratory adverse events. Site-based community educators and advisory boards provided input into the study design and conduct. All procedures are described in Table S3 in the Supplementary Appendix and in the study protocol (available at NEJM.org).
STUDY END POINTS
The primary effectiveness end point was HIV-1 infection, identified by seroconversion as determined with the use of a standard algorithm (Fig. S2 in the Supplementary Appendix). HIV-1 testing was performed monthly, and the study product was immediately withheld if any rapid HIV assay was positive, pending confirmation by means of an enzyme-linked immunosorbent assay and subsequent Western blotting. Participants who became infected with HIV-1 were offered enrollment in a seroconversion study and were referred for care. All end points were reviewed by an HIV-1 end-point adjudication committee, the members of which were unaware of the study-group assignments. Participants were followed for 8 weeks after the last visit at which empty containers and unused study product were returned, so that any delayed HIV-1 seroconversions could be detected.
Safety monitoring included monthly interviews and pregnancy testing, quarterly serum chemical testing and urine dipstick analysis of protein and glucose levels, and twice-yearly pelvic examinations. Adherence to the study product was assessed by means of a questionnaire administered by interview monthly; by monthly in-clinic counts of returned pills, empty pill bottles, or unused vaginal applicators; and by a quarterly audio computer-assisted self-interview (ACASI). Condom use and sexual practices were also assessed with an ACASI.
STUDY OVERSIGHT
The study was funded by the National Institutes of Health. Study products were donated by Gilead Sciences (TDF, TDF-FTC, and oral placebo) and CONRAD (TFV and placebo gels); neither CONRAD nor Gilead played any additional role in the study or had access to the data presented here. Written informed consent was obtained from all participants. The study was designed and conducted by members of the protocol team, who collected data, monitored conduct, and performed statistical analyses. The study was approved annually by the institutional review boards and ethics committees at all participating institutions (Table S4 in the Supplementary Appendix). Progress was reviewed every 3 to 6 months by an independent data and safety monitoring board. The investigators, participating institutions, and sponsor agreed to maintain the confidentiality of data. All the authors had access to the data and assume responsibility for the integrity and completeness of the results and the fidelity to the protocol. The manuscript was prepared by a designated writing team of investigators, all of whom are listed authors, who collectively agreed to submit it for publication.
DRUG LEVEL AND VIRAL RESISTANCE ANALYSES
Plasma TFV concentrations were determined with the use of a validated ultra-performance liquid chromatographic–tandem mass spectrometric (UPLC-MS/MS) method11; the limit of TFV and FTC quantification was 0.31 ng per milliliter. TFV and FTC were extracted from cervicovaginal fluid collected on Dacron swabs by means of solidphase extraction, and analyte concentrations were determined with UPLC-MS/MS. The lower limits of quantification were 0.625 ng per swab for TFV and 2.5 ng per swab for FTC. Plasma HIV-1 genotyping (codons 1 through 99 of protease and codons 1 through 350 of reverse transcriptase) was performed, with the use of the ViroSeq HIV-1 Genotyping System, version 2.0 (Celera), in patients who underwent seroconversion and who had plasma HIV-1 RNA levels of 200 copies per milliliter or higher. All sequences were edited manually, and nucleotide positions with multiple peaks that were more than 20% above background were considered to be mixtures. Resistance mutations were identified with the use of the Stanford Calibrated Population Resistance Tool.12
STATISTICAL ANALYSIS
The study was designed to enroll 1000 women per group who would be followed through the end of the study or for a maximum of 36 months, with a minimum follow-up period of 12 months. In the study design, we assumed a background HIV incidence rate of 3% and aimed to analyze 94 HIV seroconversions for each of three pairwise comparisons. The study was designed to have 90% power to detect at least 55% effectiveness while ruling out effectiveness of less than 25%, with false positive error rate of 0.0025.
The primary analysis was performed in a modified intention-to-treat population and included as end points only HIV-1 infections that were deemed to have been acquired after enrollment; participants with detectable HIV-1 RNA in plasma at enrollment were categorized as having acute HIV-1 infection and were excluded. Cox proportional-hazards models stratified according to site were used to assess the time to HIV-1 seroconversion. The cumulative probabilities of infection were estimated by means of the Kaplan– Meier method. Analyses were conducted with the use of SAS software, version 9.2 (SAS Institute), and R software, version 2.15.1 (R Project for Statistical Computing). All P values are two-sided.
For the analysis of plasma TFV concentrations, we used a case–cohort design,13 in which a random subcohort was selected from the active study-product groups (cohort) and was enriched with all remaining participants who had undergone HIV-1 seroconversion (cases). For every participant who underwent seroconversion, we sampled approximately three participants who remained uninfected with HIV-1 throughout participation. Predictors of TFV detection were assessed with the use of generalized estimating equations with a logistic link function and exchangeable working correlation matrix. We conducted multivariate survival analyses to assess the association between the detection of the drug in plasma obtained at follow-up visits and time to HIV-1 infection.14 Generalized-estimating-equation models with a binomial link, exchangeable correlation structure, and robust standard errors were used to determine baseline predictors of plasma TFV detection. A Cox regression was used to determine baseline predictors of HIV acquisition and to analyze the association between TFV detection and HIV acquisition.
RESULTS
PARTICIPANTS
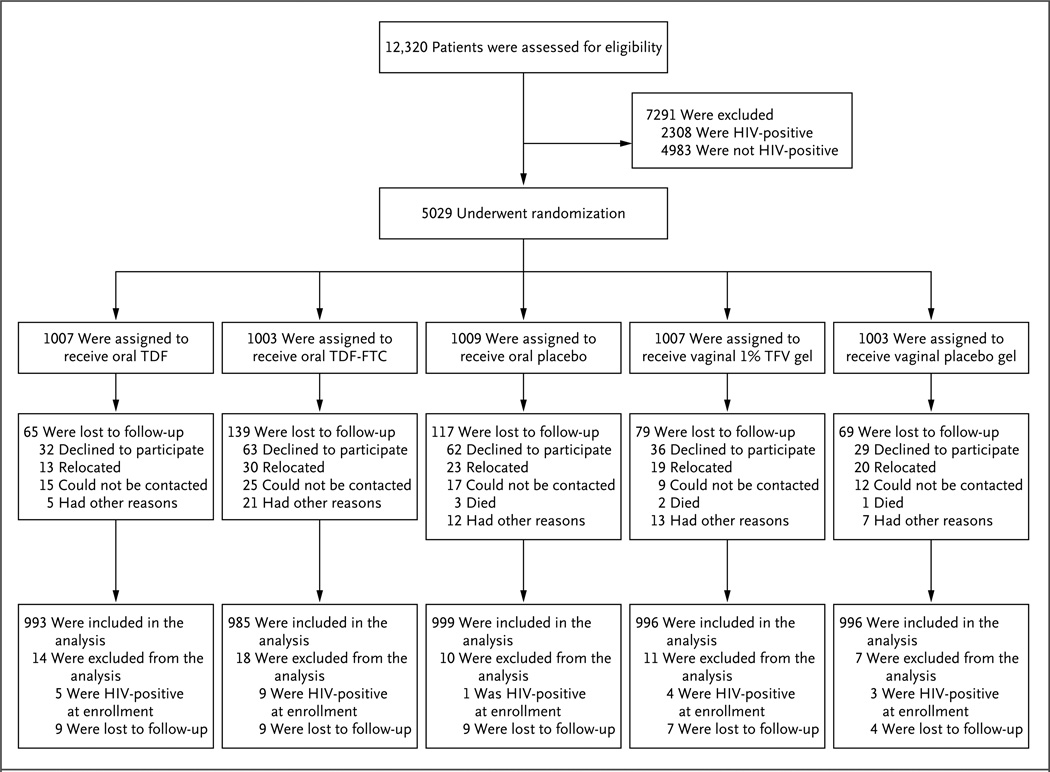
We assessed 12,320 women for eligibility and randomly assigned 5029 women to a study group (Fig. 1). The primary exclusion criterion was HIV-1 infection (32%; range, 3 to 53% according to site). A total of 61.8% of the participants were from Durban, South Africa; the majority of participants from this site were unmarried women who were younger, on average, than other participants. The characteristics of the participants were similar among the study groups (Table 1). The mean age was 25.3 years, 21% of the women were married, and 71% used injectable hormonal contraception. The participants reported a mean number of 2.5 episodes of vaginal intercourse during the 7 days before enrollment.
Figure 1. Study Cohort.
The most common reason for exclusion from participation was prevalent HIV infection (33% of excluded participants). Failure to complete screening and enrollment within the 56-day window resulted in the exclusion of 21% of potential participants. Collectively, abnormal laboratory results accounted for 16% (range, 5 to 23) of all exclusions; the most common results were positivity for the hepatitis B surface antigen (3.4%), anemia (2.9%), abnormal (grade 2 or higher) Papanicolaou smear (2.7%), and a urine-dipstick result of 2+ or higher for protein (2.5%). Conditions related to reproductive outcomes resulted in the exclusion of 9% of the women: 5.9% were pregnant at the time of screening, with the remainder reporting current breast-feeding or the intention to become pregnant in the next 2 years. A total of 22 participants who were found to have HIV-1 RNA, as assessed by means of a polymerase-chain-reaction assay, at the time of enrollment were classified as having acute HIV-1 infection and were excluded from the analysis. FTC denotes emtricitabine, TDF tenofovir disoproxil fumarate, and TFV tenofovir.
Table 1.
Baseline Characteristics of the Study Participants, According to Study Group.*
| Characteristic | Total (N = 5029) |
Oral TDF (N = 1007) |
Oral TDF-FTC (N = 1003) |
Oral Placebo (N = 1009) |
TFV Gel (N = 1007) |
Placebo Gel (N = 1003) |
|---|---|---|---|---|---|---|
| Age — yr | ||||||
| Mean | 25.3±5.2 | 25.5±5.1 | 25.2±5.2 | 25.3±5.2 | 25.3±5.2 | 25.3±5.1 |
| Median (range) | 24 (18–40) | 25 (18–40) | 24 (18–40) | 24 (18–40) | 24 (18–40) | 24 (18–40) |
| Some secondary school education or higher — no. (%) | 4622 (92) | 924 (92) | 929 (93) | 926 (92) | 920 (91) | 923 (92) |
| Earns own income — no. (%) | 2881 (57) | 569 (57) | 569 (57) | 586 (58) | 587 (58) | 570 (57) |
| Live births — no. | 1.5±1.1 | 1.6±1.1 | 1.5±1.1 | 1.5±1.2 | 1.5±1.1 | 1.5±1.2 |
| Currently married — no. (%) | 1052 (21) | 207 (21) | 209 (21) | 211 (21) | 210 (21) | 215 (21) |
| ≥2 male sex partners in the past 3 months — no./total no. (%) | 1104/4991 (22) | 236/1001 (24) | 208/994 (21) | 244/1003 (24) | 217/998 (22) | 199/995 (20) |
| Episodes of vaginal intercourse in the past 7 days — no.† | 2.5±3.1 | 2.5±2.8 | 2.5±3.4 | 2.5±2.6 | 2.6±3.6 | 2.6±2.9 |
| Condom use during last vaginal sex — no./total no. (%) | 3766/4420 (85) | 763/880 (87) | 760/888 (86) | 742/872 (85) | 768/895 (86) | 733/885 (83) |
| Anal sex in the previous 3 mo — no./total no. (%) | 868/4991 (17) | 164/1001 (16) | 175/994 (18) | 174/1003 (17) | 179/998 (18) | 176/995 (18) |
| Contraception method — no. (%) | ||||||
| Injectable | 3565 (71) | 709 (70) | 723 (72) | 700 (69) | 707 (70) | 726 (72) |
| Oral pills | 1140 (23) | 226 (22) | 223 (22) | 238 (24) | 238 (24) | 215 (21) |
| Infection present | ||||||
| Chlamydia trachomatis — no. (%)‡ | 611 (12) | 122 (12) | 117 (12) | 127 (13) | 116 (12) | 129 (13) |
| Neisseria gonorrhoeae — no. (%)‡ | 163 (3) | 42 (4) | 27 (3) | 34 (3) | 24 (2) | 36 (4) |
| Trichomonas vaginalis — no. (%)§ | 301 (6) | 68 (7) | 54 (5) | 66 (7) | 62 (6) | 51 (5) |
| Syphilis — no. (%)¶ | 68 (1) | 12 (1) | 15 (1) | 16 (2) | 14 (1) | 11 (1) |
| HSV-2 — no./total no. (%)‖ | 2289/5005 (46) | 482/1002 (48) | 449/997 (45) | 455/1006 (45) | 438/1004 (44) | 465/996 (47) |
| Bacterial vaginosis — no./ total no. (%)** | 2023/5010 (40) | 422/1000 (42) | 410/1002 (41) | 401/1008 (40) | 397/1003 (40) | 393/997 (39) |
Plus–minus values are means ±SD. FTC denotes emtricitabine, HSV-2 herpes simplex virus type 2, TDF tenofovir disoproxil fumarate, and TFV tenofovir.
Data on the number of sex acts in the past 7 days were available for 4566 participants.
Testing for C. trachomatis and N. gonorrhoeae was performed with the use of a strand-displacement amplification assay (BD ProbeTec, Becton Dickinson).
Testing for T. vaginalis was performed with the use of the OSOM Trichomonas Rapid Test (Genzyme).
Syphilis testing was performed with the use of a rapid plasma reagin screening test followed by a confirmatory microhemagglutinin assay for Treponema pallidum or a T. pallidum hemagglutination assay for reactive samples.
HSV-2 seropositivity was determined with the use of the HerpeSelect 2 enzyme immunoassay (Focus Technologies) at the time of enrollment; an index value of 3.5 or greater was considered to be a positive result.
Bacterial vaginosis was determined by the Nugent score on Gram’s staining of vaginal fluid.
FOLLOW-UP AND ADHERENCE ASSESSMENTS
The primary end point was determined for 4969 participants (99% of enrollees) during 5509 person-years of follow-up, which represented 95% of the anticipated person-years. The rate of retention in the study, defined as completion of end-of-study visit procedures (including physical examination and relevant laboratory testing), was 91% and did not differ significantly among study groups. In September 2011, the data and safety monitoring board recommended that treatment with oral TDF alone be discontinued for futility; in November 2011, a similar determination was made regarding TFV gel. The board recommended that the TDF-FTC and oral placebo groups continue follow-up until the end of the study, in August 2012.
On the basis of returned-product counts, the mean rate of adherence (calculated as the proportion of product not returned divided by the number of days since the previous visit at which study products were dispensed) was 86% (Table 2). On the basis of self-reporting, the mean rate of adherence was 90% as reported in interviews and 88% as assessed with an ACASI. In the ACASIs, 47% of the participants rated their ability to use the product daily, as instructed, during the previous 4 weeks as “very good or excellent.”15 The study product was withheld for 246 person-years, primarily because of pregnancy; the rates for withholding of the study product were similar among the study groups.
Table 2.
Adherence to Study Products.*
| Measure of Adherence | Total (N = 5007) |
Oral TDF (N = 1002) |
Oral TDF-FTC (N = 994) |
Oral Placebo (N = 1008) |
TFV Gel (N = 1003) |
Placebo Gel (N = 1000) |
|---|---|---|---|---|---|---|
| Mean rate of adherence (%) | ||||||
| Assessed by clinic-based product count† | 86 | 84 | 88 | 90 | 83 | 84 |
| Assessed by face-to-face interview‡ | 90 | 91 | 90 | 91 | 90 | 90 |
| Assessed by ACASI§ | 88 | 87 | 87 | 88 | 88 | 89 |
| Mean proportion of quarterly plasma samples with TFV detected (%)¶ | NA | 30 | 29 | NA | 25 | NA |
| Proportion of women with TFV not detected in any quarterly plasma samples (%)¶ | NA | 58 | 50 | NA | 57 | NA |
| Mean proportion of vaginal swab samples with TFV detected (%)‖ | NA | NA | NA | NA | 49 | NA |
| Proportion of women with TFV not detected in any vaginal swab samples (%)‖ | NA | NA | NA | NA | 41 | NA |
ACASI denotes audio computer-assisted self-interview, and NA not applicable.
Participants were asked to return empty pill bottles, unused pills, and unused vaginal gel applicators at each monthly visit. Percentages are the estimated mean proportions of doses not returned and presumed to have been used. The mean proportion in the Total column is based on 4994 participants with follow-up data.
In the interviews, women were asked to report the number of doses of study product they used in the previous week, and these were summed across monthly follow-up visits. The mean proportion in the Total column is based on 4935 participants.
In the ACASI, women were asked to report the number of doses of study product they used in the previous week, and these were summed across quarterly follow-up visits. The mean proportion in the Total column is based on 4739 participants.
Proportions were calculated among the 488 participants in the random cohort of the case–cohort subset who had quarterly plasma samples available (158 in the TDF group, 157 in the TDF-FTC group, and 173 in the TFV gel group); the median number of quarterly samples tested per participant was 3 (range, 1 to 10).
Proportions were calculated among the 170 participants randomly assigned to the TFV gel group who were in the random cohort of the case–cohort subset and provided at least one vaginal swab sample (with samples collected at 6-month intervals). A total of 255 swabs were collected from these participants between the month 5 and month 17 visits and were available for analysis; 85 participants had two swabs tested, and 85 had one swab tested.
EFFECT OF TDF, TDF-FTC, AND TFV ON HIV-1 ACQUISITION
There were 334 seroconversions among study participants, including 22 events that were defined as acute HIV-1 infection with RNA present at enrollment. When these 22 participants were excluded, the incidence of HIV infection was 5.7 per 100 person-years (0.8 to 9.9 per 100 person-years according to site). The incidence was highest among participants who were younger than 25 years of age (8.0 per 100 person-years) and among those who were unmarried (7.2 per 100 person-years).
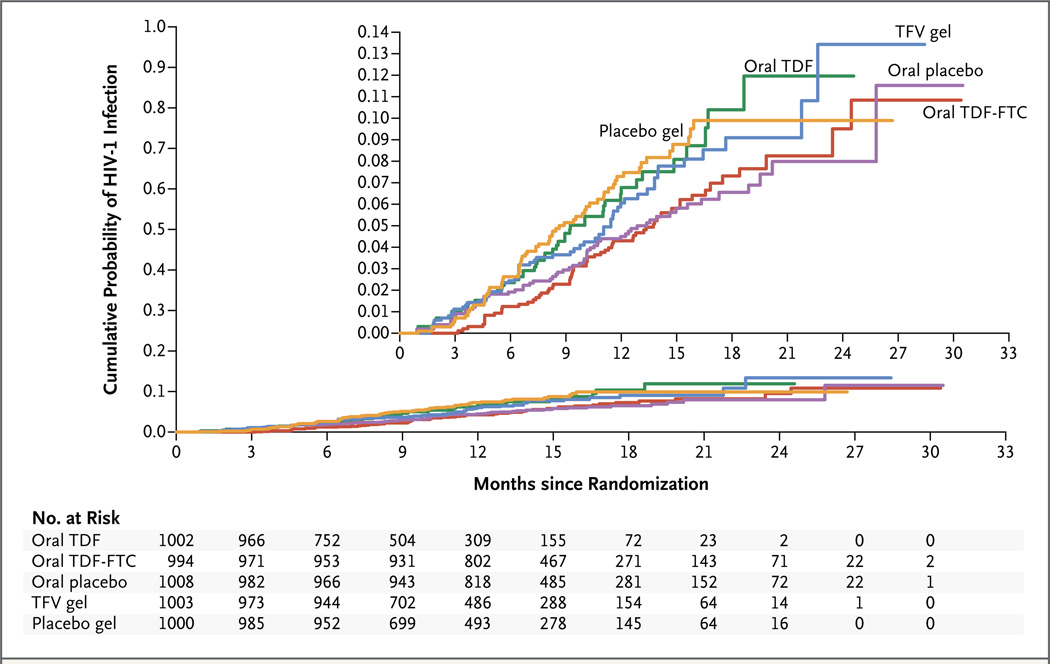
Of the 312 seroconversions included in the primary analysis, 52 occurred in the TDF group, 61 in the TDF-FTC group, 61 in the TFV gel group, 60 in the oral placebo group, and 70 in the placebo gel group (Table 3). The effectiveness was −49.0% with TDF (hazard ratio for infection, 1.49; 95% confidence interval [CI], 0.97 to 2.29), −4.4% with TDF-FTC (hazard ratio, 1.04; 95% CI, 0.73 to 1.49), and 14.5% with TFV gel (hazard ratio, 0.85; 95% CI, 0.61 to 1.21). There was no significant difference in the rate of HIV-1 seroconversion between a group receiving any study product and the group receiving the corresponding placebo, and the incidence of HIV-1 seroconversion did not change significantly during the study (Fig. 2). HIV-1 seroconversion was identified in 34 women after the scheduled completion of the period of product use; the rates did not differ according to study group.
Table 3.
Primary Efficacy Results.
| Result | Oral TDF* | Oral TDF-FTC | Oral Placebo | TFV Gel | Placebo Gel | |
|---|---|---|---|---|---|---|
| Active Agent | Placebo | |||||
| Person-years | 823 | 838 | 1284 | 1308 | 1024 | 1030 |
| Number of HIV-1 infections | 52 | 35 | 61 | 60 | 61 | 70 |
| HIV-1 incidence — cases per 100 person-years (95% CI) | 6.3 (4.7–8.3) | 4.2 (2.9–5.8) | 4.7 (3.6–6.1) | 4.6 (3.5–5.9) | 6.0 (4.6–7.6) | 6.8 (5.3–8.6) |
| Hazard ratio (95% CI) | 1.49 (0.97–2.29) | — | 1.04 (0.73–1.49) | — | 0.85 (0.61–1.21) | — |
| P value | 0.07 | — | 0.81 | — | 0.37 | — |
Data were censored on the date when sites were asked to discontinue treatment in the oral TDF group.
Figure 2. Cumulative Probability of HIV-1 Infection, According to Study Group.
The numbers shown below the graph are the numbers of participants who were at risk at the start of each quarterly interval. The inset shows the same data on an enlarged y axis.
ANTIRETROVIRAL RESISTANCE
Of 334 participants who underwent HIV-1 seroconversion, 322 (96%) had plasma available with sufficient HIV-1 RNA copies for resistance testing. Among the 21 of these participants who were infected at the time of randomization, resistance to FTC (M184I/V mutation) was detected in 2 participants who had been assigned to the TDF-FTC group (Table S5 in the Supplementary Appendix). Among the 301 participants who acquired HIV-1 infection after randomization, resistance to FTC (M184V mutation) developed in 1 participant in the TDF-FTC group. No participants who acquired HIV-1 infection were infected with TDF-resistant strains (K65R mutation). Strains with the M184I/V or K65R mutation were not present in 33 participants who were infected after they stopped using the study product.
DETECTION OF STUDY DRUG IN BIOLOGIC SAMPLES AND ASSOCIATION WITH HIV-1 SEROCONVERSION
The case–cohort sample comprised 647 participants from the active-product study groups, from whom 2987 plasma samples were available for TFV measurement. In the random subcohort, TFV was detected in a mean of 30% of available plasma samples collected quarterly from participants randomly assigned to the TDF group, in 29% of available plasma samples from the TDF-FTC group, and in 25% from the TFV gel group (Table 2). At the first quarterly visit, less than 40% of plasma samples in all three active-product groups had detectable TFV. A total of 58% of the participants in the TDF group, 50% in the TDF-FTC group, and 57% in the TFV gel group had no plasma TFV detected at any quarterly visit (Table S6 in the Supplementary Appendix). Among participants randomly assigned to the TFV gel group, TFV was detected in a mean of 49% of swabs tested (Table 2).
Baseline characteristics associated with plasma TFV detection in the active-product groups, adjusted for study site, included being older than 25 years of age (adjusted odds ratio, 1.62; 95% CI, 1.12 to 2.34), being married (adjusted odds ratio, 2.24; 95% CI, 1.12 to 4.49), having an independent income (adjusted odds ratio, 1.42; 95% CI, 0.98 to 2.07), and being multiparous (adjusted odds ratio, 1.84; 95% CI, 1.26 to 2.69). These characteristics were also associated with a lower risk of HIV-1 acquisition in both placebo groups, which suggests that participants with better adherence had a lower risk of acquiring HIV than did participants who were less likely to adhere (Table S6E in the Supplementary Appendix). We adjusted for these characteristics when assessing the association between TFV detection and HIV-1 acquisition. A Cox regression assessing the time to HIV-1 infection and the detection of TFV in plasma at each follow-up visit did not yield a significant association. However, plasma TFV detection at the first quarterly visit was predictive of TFV detection at later visits. Most women in whom TFV was not detected at the first quarterly visit had none detected at subsequent visits (70% in the FTC-TDF group, 83% in the TDF group, and 72% in the TFV gel group). When participants were evaluated according to whether TFV was detected in plasma at the first quarterly visit, those in the TFV gel group with detectable TFV levels had a significantly lower likelihood of HIV acquisition than did those with no TFV detected (HIV incidence per 100 person-years: 1.9 vs. 6.1, adjusted hazard ratio, 0.34; 95% CI, 0.13 to 0.87; P = 0.02). Similar results were obtained when participants were grouped according to whether the drug was ever detected or was never detected at any time during the follow-up period (Table S6C in the Supplementary Appendix).
SAFETY AND ADVERSE-EVENT PROFILES
Elevated serum creatinine levels were seen more frequently among participants randomly assigned to the oral TDF-FTC group than among those in the oral placebo group (1.3% vs. 0.2%, P = 0.004); all but one of these elevations were mild (Table S7G in the Supplementary Appendix). We observed no other significant differences between the groups with respect to safety outcomes of concern. The incidence of pregnancy was 7.8 per 100 person-years and did not differ by group.
DISCUSSION
In our population of predominantly young, unmarried women in sub-Saharan Africa, daily adherence to study products — oral or vaginal TFV-based formulations — was low, and no regimen significantly reduced the risk of HIV-1 acquisition in a modified intention-to-treat analysis. Lower adherence, as assessed by measurement of TFV levels in plasma, was associated with characteristics that predicted a higher risk of HIV acquisition. The detection of TFV in plasma among participants randomly assigned to the TFV gel group was associated with a lower likelihood of HIV acquisition after adjustment for these characteristics. However, we were not able to measure (and thus control for) the most important potential confounder in this analysis — namely, the likelihood of HIV-1 exposure. On the basis of differences in characteristics between participants who used the study products and those who did not use the study products, the likelihood of HIV-1 exposure may also have differed between these two groups, beyond our ability to control for it. Moreover, as observed in other trials of preexposure prophylaxis, participants with detectable drug levels did acquire HIV-1 infection.
Our results are consistent with those of the FEM-PrEP trial, in which daily TDF-FTC use did not reduce HIV-1 acquisition among women and in which study-drug adherence was also low.6 These findings have implications for biomedical prevention research in these populations, as well as for the implementation of interventions with proven efficacy. First, despite increasing coverage with antiretroviral therapy for the treatment of HIV-infected persons and the potential for associated declines in community-wide incidence,16 we observed HIV-1 incidence rates exceeding those we anticipated, particularly among young women in Durban — up to 9.9 per 100 person-years.
Second, most participants did not use the study products daily, a finding that is not consistent with prestudy assessments of the willingness of the target populations to use such products,17 adherence assessments based on clinic-based product counts and self-reporting, and the high rates of retention. Despite using multiple measures to assess adherence, including ACASIs, we found that fewer than half the participants disclosed nonadherence or barriers to use, and in fact products were returned in a manner consistent with high adherence. This highlights the need for measures of adherence that do not rely solely on self-reporting and that are not easily manipulated by participants, such as real-time biologic monitoring of drug levels. In a qualitative ancillary study conducted concurrently among randomly selected participants in the VOICE trial in Johannesburg, consistent themes emerged: the unknown efficacy of products, their identification with HIV infection, and the lack of social support for study participation.11 These may be some of the factors that discouraged consistent use of the study products.
Third, our findings contrast with those from other trials. In the Partners PrEP trial, the reductions in the risk of HIV-1 acquisition among HIV-1–uninfected women were 71% with TDF and 66% with TDF-FTC.2 Adherence was good; this was probably facilitated by the enrollment of serodiscordant couples, the disclosure of HIV infection status within couples, and the partners’ mutual engagement in product use.18 The VOICE participants who were most likely to adhere were similar, in terms of age and marital status, to women in the Partners PrEP trial. In the CAPRISA 004 trial, pericoital administration of 1% TFV gel was associated with a reduction in the risk of HIV-1 acquisition of 39% (95% CI, 6 to 60).9 Effectiveness was associated with adherence, as assessed through self-reporting, applicator counts, and TFV concentration in the cervicovaginal fluid. 19 Pericoital administration of 1% TFV gel is currently being studied in the Follow-on African Consortium for Tenofovir Studies (FACTS) 001 Trial in South Africa.
We observed one case of resistance (M184V mutation) in a participant in an active-product group who underwent seroconversion 11 months after enrollment. Five cases of resistance have been reported in participants in active-product groups in trials of preexposure prophylaxis; all five participants were enrolled while they had acute HIV-1 infection.1–3 In the FEM-PrEP trial, three cases of M184I/V resistance occurred within 12 weeks after enrollment in the TDF-FTC group; the investigators could not exclude the possibility that the participants were infected at the time of enrollment.6 Acquired resistance was rare in the VOICE trial, a finding that could be explained by consistently low, rather than intermittent, adherence to the study product.
Our results reaffirm the need for effective and acceptable prevention interventions for women at high risk for sexual acquisition of HIV-1 and suggest that more accurate measures are critical for the estimation of product use during biomedical HIV-1–prevention trials.7 Real-time monitoring of biomarkers of product adherence may facilitate the efficiency of such trials. Products that do not require daily use, including sustained delivery of antiretroviral agents from vaginal rings or injections, may be more suitable for some women. Successful adoption of daily preexposure prophylaxis may require different social and operational strategies to support daily use. Indeed, our retention and qualitative findings suggest that participants valued the study and its contribution to their health.11 It is critical that investigators understand the participants’ motivation for enrolling in a study, as well as their perceptions of the investigational products, and that they leverage this insight when conducting biomedical research on HIV-1 prevention.
Supplementary Material
Acknowledgments
Funded by the National Institutes of Health
Dr. Hendrix reports receiving grant support from Gilead Sciences, and Dr. Rooney, being an employee of and holding stock options in Gilead Sciences. Dr. Rooney also reports pending patents related to the use of tenofovir for prevention of genital herpes simplex virus infection (US 13/700,710 and WO 2011/156416).
We thank the study participants.
APPENDIX
The authors’ affiliations are as follows: the University of Washington (J.M.M.) and the Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center (B.A.R., J.Y.D., B. Mâsse) — both in Seattle; the HIV Prevention Research Unit, Medical Research Council (G.R., S.G.), and the Centre for AIDS Programme of Research in South Africa (CAPRISA), Durban (G.N.), Witwatersrand Reproductive Health and HIV Research Institute (T.P.) and Perinatal HIV Research Unit (B. Mkhize), Johannesburg, and the AURUM Institute, Klerksdorp (M.T.) — all in South Africa; FHI 360, Durham, NC (K.G.); University of Zimbabwe–University of California San Francisco Research Programme, Harare, Zimbabwe (N.M., Z.M.C.); Makerere University–Johns Hopkins University Research Collaboration, Kampala, Uganda (C.N.); Women’s Global Health Imperative, Research Triangle Institute (RTI) International, San Francisco (A.S.); Magee–Womens Research Institute, University of Pittsburgh Medical Center, Pittsburgh (L.N., U.M.P., S.L.H., I.M.M.); Johns Hopkins University School of Medicine, Baltimore (C.W.H., M.A.M.); Division of AIDS, National Institute of Allergy and Infectious Diseases (J.P.), National Institutes of Mental Health (C.G.), and the Eunice Shriver Kennedy National Institute of Child Health and Human Development (H.W.), National Institutes of Health — all in Bethesda, MD; Gilead Sciences, Foster City, CA (J.R.); CONRAD, Arlington, VA (J.L.S.); and Centre Hospitalier Universitaire Sainte-Justine, University of Montreal, Montreal (B. Mâsse).
Footnotes
A complete list of members of the Vaginal and Oral Interventions to Control the Epidemic (VOICE) Study Team is provided in the Supplementary Appendix, available at NEJM.org.
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States — 2014. U.S. Public Health Service. 2014 ( http://www.cdc.gov/hiv/pdf/prepprovidersupplement2014.pdf)
- 6.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cranage M, Sharpe S, Herrera C, et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med. 2008;5(8):e157. doi: 10.1371/journal.pmed.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh UM, Dobard C, Sharma S, et al. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol. 2009;83:10358–10365. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson BA, Kelly C, Ramjee G, et al. Appropriateness of hydroxyethylcellulose gel as a placebo control in vaginal microbicide trials: a comparison of the two control arms of HPTN 035. J Acquir Immune Defic Syndr. 2013;63:120–125. doi: 10.1097/QAI.0b013e31828607c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Straten A, Stadler J, Montgomery E, et al. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PLoS One. 2014;9(2):e89118. doi: 10.1371/journal.pone.0089118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis. 2006;42:1608–1618. doi: 10.1086/503914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prentice RL. A case-cohort design for epidemiological cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 14.Lumley T. Complex surveys: a guide to analysis using R. Hoboken, NJ: John Wiley; 2010. [Google Scholar]
- 15.Lu M, Safren SA, Skolnik PR, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav. 2008;12:86–94. doi: 10.1007/s10461-007-9261-4. [DOI] [PubMed] [Google Scholar]
- 16.Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mensch BS, van der Straten A, Katzen LL. Acceptability in microbicide and PrEP trials: current status and a reconceptualization. Curr Opin HIV AIDS. 2012;7:534–541. doi: 10.1097/COH.0b013e3283590632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware NC, Wyatt MA, Haberer JE, et al. What’s love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. J Acquir Immune Defic Syndr. 2012;59:463–468. doi: 10.1097/QAI.0b013e31824a060b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karim SS, Kashuba AD, Werner L, Karim QA. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011;378:279–281. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.