Abstract
Background
An assessment of historical trends in patient survival is important to determine the progress towards patient outcomes and to reveal where advancements must be made. The goal of this study was to assess changes in demographics and overall survival of non-small cell lung cancer (NSCLC) patients who were seen at the H. Lee Moffitt Cancer Center (MCC) and Research Institute spanning over 22 years.
Methods
This analysis included 4,997 NSCLC patients who were treated at the MCC over five time periods: 1986 to 1988, 1991 to 1993, 1996 to 1998, 2001 to 2003, and 2006 to 2008. Kaplan-Meier survival curves and the log-rank statistic were used to assess changes in 5-year survival rates over the five time periods and multivariable hazard ratios were estimated from Cox proportional hazards models.
Results
From 1986 to 2008 we observed statistically significant increases in the percentage of patients over the age of 70 years, women, never- and former smokers, and patients with stage I tumors. Over the same time period the median survival time statistically significantly increased from 1.09 years (95% CI 0.95 – 1.34, P < 0.001) to 2.27 years (2.07 – 2.46, P < 0.001) and the overall 5-year survival rate for all patients significantly increased from 14.7% to 31.1% (< 0.001). Among stage I patients the 5-year survival rate increased from 31.7% to 54.0% (P < 0.001), 13.3% to 36.0% for Stage II (P < 0.001), 10.5% to 21.7% for Stage III (P < 0.001), and 3.4% to 9.6% for Stage IV (P < 0.001).
Conclusions
This analysis demonstrated important temporal changes in the demographics and improvements in overall survival of NSCLC patients treated at the MCC from 1986 to 2008. The 5-year survival rates and median survival time of patients diagnosed with NSCLC has significantly improved across all stages including late stage patients.
Keywords: Lung cancer, overall survival, non-small cell lung cancer, epidemiology, cancer registry
Introduction
In the US lung cancer is the second most common cancer in men after prostate cancer and the second most common cancer in women after breast cancer. In 2013 an estimated 228,190 new cases of lung cancer are expected in the US, accounting for about 14% of all cancer diagnoses.1 The incidence rate has been declining in men over the past two decades, but in women the incidence rate has just recently started to decrease. Lung cancer is the leading cause of cancer-related death among both men and women in the US and accounts for more deaths than any other cancer in both sexes. In 2013 an estimated 159,480 deaths, or about 27% of all cancer deaths, are expected to occur. The number of annual lung cancer deaths accounts for more deaths than prostate, breast, colon, and pancreatic cancer combined.1 Non-small cell lung cancer represents more than 80% of lung cancer diagnoses and has an overall 5-year survival rate of ~16% that decreases precipitously among patients diagnosed with late stage disease.2,3
An assessment of historical trends in patient survival is an important evaluation to determine the progress towards patient outcomes and to reveal where advancements must be made. To date, few studies have evaluated survival trends over time in patients with lung cancer. The goal of this study was to assess changes in demographics and overall survival of non-small cell lung cancer patients across five time period spanning over 22 years who were seen at the H. Lee Moffitt Cancer Center and Research Institute.
Material and Methods
Study population
This analysis included 4,997 non-small cell lung cancer patients who were treated at the Moffitt Cancer Center between 1986 and 2008. The five periods that were selected for analysis were 1986 to 1988, 1991 to 1993, 1996 to 1998, 2001 to 2003, and 2006 to 2008. These time period ranges were selected to compare changes in demographics and overall survival within and across decades. Each study period included 3 years of patient data with a 2 to 3 year interval in between. This research was approved by the University of South Florida Institutional Review Board.
Cancer registry data
The primary source of data for this analysis was Moffitt’s Cancer Registry which abstracts information from patient electronic medical records on demographics, history of smoking, stage, histology, and treatment. Patients seen for second opinions are not included in the Cancer Registry database because they do not fall under current reportable state and/or federal guidelines. Follow-up for survival and vital status information occurs annually through passive and active methods. The Cancer Registry defines “first course of treatment” as all methods of treatment recorded in the treatment plan and administered to the patient before disease progression or recurrence or death. For this analysis smoking status was categorized as self-reported current-, former-, or never smoker. Where available pathological TNM staging was utilized and if these data were missing we utilized clinical stage information.
Statistical Analysis
Pearson’s chi-square was used to test for differences in the patient characteristics across the five time groups. Overall survival was right-censored at five years and survival analyses were performed using Kaplan-Meier survival curves and the log-rank statistic. Multivariable Cox proportional hazard regression was utilized to generate hazard ratios (HR) and 95% confidence intervals (CI) for each time period. All statistical analyses were performed using R version 2.14 (R Project for Statistical Computing, http://www.r-project.org).
Results
For this analysis 4,997 non-small cell lung cancer patients were available across the five time periods: 1986 to 1988 (No. = 207), 1991 to 1993 (No. = 379), 1996 to 1998 (No. = 792), 2001 to 2003 (No. = 1669), and 2006 to 2008 (No. = 1950). The demographics and 5-year survival data across the five time periods are presented in Table 1. From 1986 to 2008, the percentage of patients who were diagnosed over the age of 70 years of age increased from 19.8% to 38.4% (P < 0.001), the percentage of women increased from 35.7% to 50.9% (P < 0.001), the percentage of current smokers decreased from 47.8% to 28.3% P < 0.001), the percentage of squamous cell carcinoma decreased from 36.7% to 19.9% (P < 0.001), and the percentage of stage I patients increased 19.8% to 27.7% (P < 0.001). Although the percentage of surgeries remained relatively constant for first course of treatment (26.6% to 23.6%), the actual number of surgical patients increased from 55 in the 1986 to 1998 time period to 461 in the 2006 to 2008 time period. Furthermore, combination first course of treatment, which would include surgical patients who receive adjuvant chemotherapy, increased significantly (27.1% to 35.4%, P < 0.001).
Table 1.
Demographic and clinical characteristics of the non-small cell lung cancer patients for each time period
| Characteristic | 1986 to 1988 (N = 207) |
1991 to 1993 (N = 379) |
1996 to 1998 (N = 792) |
2001 to 2003 (N = 1669) |
2006 to 2008 (N = 1950) |
P-value2 |
|---|---|---|---|---|---|---|
| Age, N (%) | < 0.001 | |||||
| < 50 | 24 (11.6) | 42 (11.1) | 74 (9.3) | 165 (9.9) | 165 (8.5) | |
| ≥ 50 to ≤ 59 | 52 (25.1) | 66 (17.4) | 133 (16.8) | 361 (21.6) | 376 (19.3) | |
| ≥ 60 to ≤ 69 | 90 (43.5) | 166 (43.8) | 281 (35.5) | 556 (33.3) | 660 (33.9) | |
| ≥ 70 | 41 (19.8) | 105 (27.7) | 304 (38.4) | 587 (35.2) | 748 (38.4) | |
| Gender, N (%) | < 0.001 | |||||
| Male | 133 (64.3) | 212 (55.9) | 422 (53.3) | 896 (53.7) | 957 (49.1) | |
| Female | 74 (35.7) | 167 (44.1) | 370 (46.7) | 773 (46.3) | 993 (50.9) | |
| Smoking status, N (%) | < 0.001 | |||||
| Never | 11 (5.3) | 31 (8.2) | 51 (6.4) | 111 (6.7) | 140 (7.2) | |
| Former | 84 (40.6) | 167 (44.1) | 439 (55.4) | 856 (51.3) | 1006 (51.6) | |
| Current | 99 (47.8) | 172 (45.4) | 292 (36.9) | 586 (35.1) | 551 (28.3) | |
| Race, N (%) | 0.0083 | |||||
| White | 203 (98.1) | 367 (96.8) | 771 (97.3) | 1605 (96.2) | 1817 (93.2) | |
| Black | 4 (1.9) | 10 (2.6) | 14 (1.8) | 49 (2.9) | 81 (4.2) | |
| Other or unknown | 0 (0) | 2 (0.5) | 7 (0.9) | 15 (0.9) | 52 (2.7) | |
| Ethnicity, N (%) | 0.0254 | |||||
| Non-Spanish | 203 (98.1) | 375 (98.9) | 776 (98) | 1612 (96.6) | 1856 (95.2) | |
| Spanish | 4 (1.9) | 4 (1.1) | 15 (1.9) | 50 (3) | 68 (3.5) | |
| Unknown | 0 (0) | 0 (0) | 1 (0.1) | 7 (0.4) | 26 (1.3) | |
| Histology, N (%) | < 0.001 | |||||
| Adenocarcinoma | 89 (43.0) | 167 (44.1) | 382 (48.2) | 697 (41.8) | 751 (38.5) | |
| Squamous Cell Carcinoma | 76 (36.7) | 139 (36.7) | 225 (28.4) | 431 (25.8) | 389 (19.9) | |
| Other NSCLC | 42 (20.3) | 73 (19.3) | 185 (23.4) | 541 (32.4) | 810 (41.5) | |
| Stage, N (%) | < 0.0015 | |||||
| I | 41 (19.8) | 79 (20.8) | 223 (28.2) | 419 (25.1) | 532 (27.3) | |
| II | 15 (7.2) | 40 (10.6) | 56 (7.1) | 161 (9.6) | 135 (6.9) | |
| III | 77 (37.2) | 136 (35.9) | 268 (33.8) | 450 (27) | 446 (22.9) | |
| IV | 61 (29.5) | 100 (26.4) | 211 (26.6) | 495 (29.7) | 521 (26.7) | |
| NA | 13 (6.3) | 24 (6.3) | 34 (4.3) | 144 (8.6) | 316 (16.2) | |
| First Course of Treatment1, N (%) | < 0.0015 | |||||
| Combination | 56 (27.1) | 102 (26.9) | 324 (40.9) | 706 (42.3) | 691 (35.4) | |
| Surgery | 55 (26.6) | 112 (29.6) | 241 (30.4) | 485 (29.1) | 461 (23.6) | |
| Chemotherapy | 12 (5.8) | 30 (7.9) | 117 (14.8) | 207 (12.4) | 228 (11.7) | |
| Radiation | 62 (30) | 115 (30.3) | 67 (8.5) | 110 (6.6) | 129 (6.6) | |
| None | 22 (10.6) | 20 (5.3) | 42 (5.3) | 102 (6.1) | 105 (5.4) | |
| NA | 0 (0) | 0 (0) | 1 (0.1) | 59 (3.5) | 336 (17.2) |
Abbreviations: NSCLC, non-small cell lung cancer; NA, not available
Bold font p-values indicate a statistically significant difference across the five time periods
First course of treatment includes all methods of treatment recorded in the treatment plan and administered to the patient before disease progression or recurrence. Combination is treatment with two or more of surgery, chemotherapy, of radiation.
P-values calculated from Pearson’s chi-square to test for differences across the five time periods.
Excludes ‘Other or unknown’ group
Excludes ‘Unknown’ group
Excludes ‘NA’ group
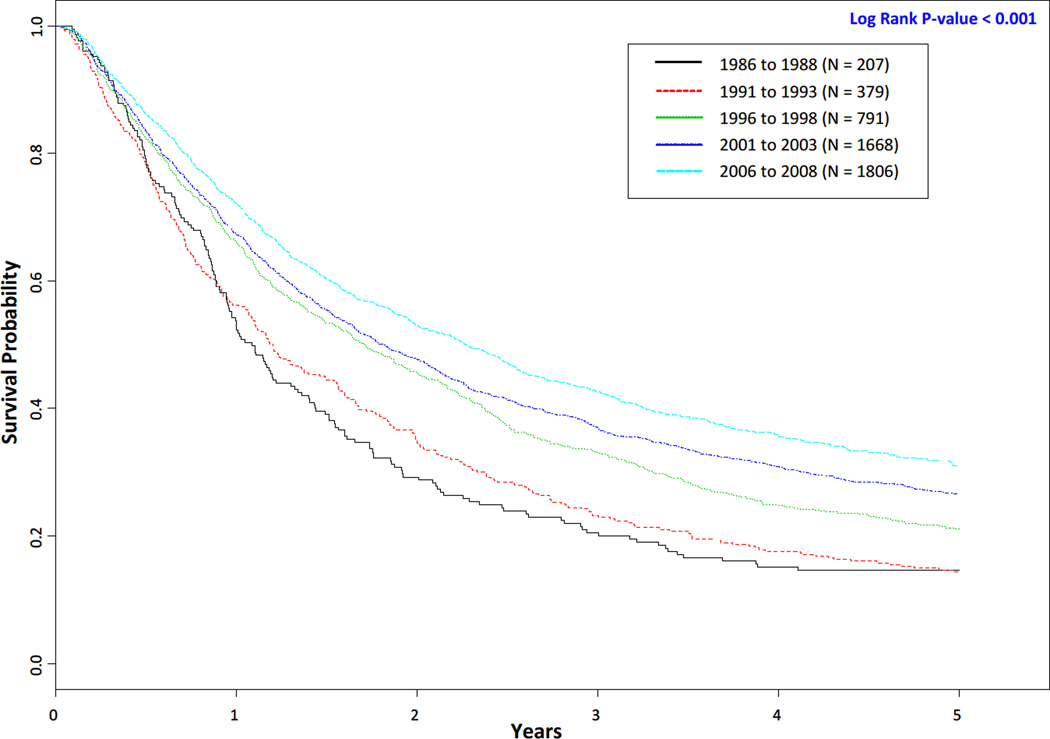
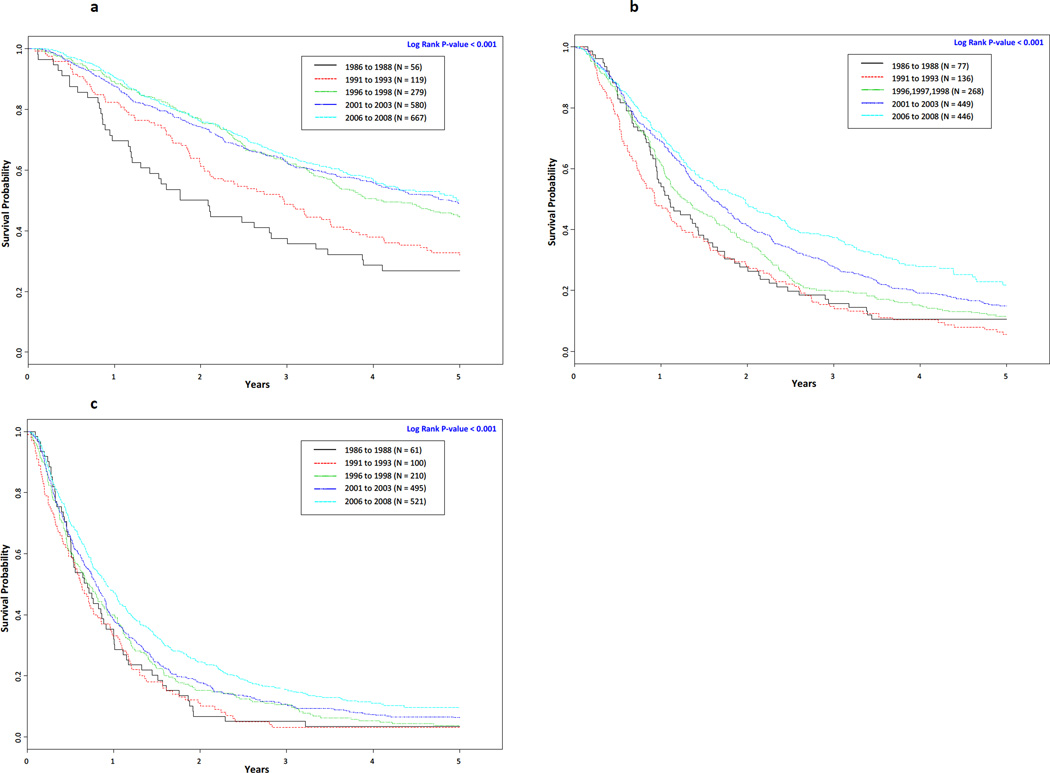
Complete follow-up data were not available on all patients. The number of patients with complete follow-up data per time period is noted in Table 2 and Figure 1. From 1986 to 2008 among all non-small cell lung cancer patients the median survival time statistically significantly increased (< 0.001) from 1.09 years (95% CI 0.95 – 1.34) to 2.27 years (95% CI 2.07 – 2.46) and the overall 5-year survival rate significantly increased (< 0.001) from 14.7% to 31.1% (Table 2). Although there was no substantial difference in the overall 5-year rate for the first two time periods (14.7% for 1986 to 1988 vs. 14.2% for 1991 to 1993), the 5-year Kaplan–Meier survival curves (Figure 1) demonstrated statistically significantly improved survival across the five time periods (P < 0.001). When the data were stratified by stage, we observed increases in the 5-year survival rates for each stage. Among stage I patients the 5-year survival rate increased from 31.7% to 54.0% (P < 0.001), 13.3% to 36.0% for stage II (P < 0.001), 10.5% to 21.7% for stage III (P < 0.001), and 3.4% to 9.6% for stage IV (P < 0.001). The 5-year survival rates for the two most recent time periods were nearly the same for stage (54.3% for 2001 to 2003 vs. 54.0% for 2006 to 2008) and stage II patients (35.3% for 2001 to 2003 vs. 36.0% for 2006 to 2008). As noted in Figure 2A, when stage I and II patients were combined the 5-year survival curves demonstrated statistically significantly improved survival across the five time periods (P < 0.001). Similarly, 5-year survival curves demonstrated statistically significantly improved survival across the five time periods for Stage III (Figure 2B, P < 0.001) and Stage IV (Figure 2C, P < 0.001) patients as well.
Table 2.
Median survival time and five-year survival rate for each time period
| 1986 to 1988 (N = 207) |
1991 to 1993 (N = 379) |
1996 to 1998 (N = 791) |
2001 to 2003 (N = 1668) |
2006 to 2008 (N= 1803) |
P-value2 | |
|---|---|---|---|---|---|---|
| Median survival time, years (95% CI)1 | 1.09 (0.95 – 1.34) | 1.20 (1.07 – 1.49) | 1.71 (1.53 – 1.93) | 1.82 (1.66 – 2.01) | 2.27 (2.07 – 2.46) | < 0.001 |
| 5-year survival rate, % | ||||||
| Overall | 14.7 | 14.2 | 21.1 | 26.5 | 31.1 | < 0.001 |
| By stage | ||||||
| I | 31.7 | 36.7 | 48.0 | 54.3 | 54.0 | < 0.001 |
| II | 13.3 | 22.5 | 30.4 | 35.3 | 36.0 | < 0.001 |
| III | 10.5 | 5.5 | 11.6 | 15.0 | 21.7 | < 0.001 |
| IV | 3.4 | 3.0 | 3.8 | 6.3 | 9.6 | < 0.001 |
Abbreviations: CI, confidence interval
Bold font p-values indicate a statistically significant difference across the five time periods
Median survival time was calculated from the Kaplan–Meier survival curves
P-values calculated from the log-rank test
Figure 1.
Kaplan–Meier survival curves for overall survival of non-small cell lung cancer patients by time period.
Figure 2.
Stage-specific Kaplan-Meier survival curves for overall survival of non-small cell lung cancer patients by time period: Figure 2A. Kaplan-Meier survival curve for stages I & II lung cancer; Figure 2B. Kaplan-Meier survival curve for stage III lung cancer; and Figure 2C. Kaplan-Meier survival curve for stage IV lung cancer.
Table 3 presents the multivariable hazard ratios (mHRs) models for all covariates for each time period. Across the five time periods an increased risk of death was generally observed for patients over 70 years of age, men, and current smokers. Patients treated with radiation were generally associated with an elevated risk of death across the five time periods and increasing stage for each of the time periods was associated with an incremental increased risk of death. For example, in the 2006 to 2008 time period the hazard ratio for stage II patients was 1.81 (95% CI 1.36 – 2.41), 2.72 (95% CI 2.72 – 3.38) for stage III patients, and 5.17 (95% CI 4.15 – 6.45) for stage IV patients.
Table 3.
Multivariable Cox proportional hazard models for each time period
| 1986 to 1988 mHR (95% CI) |
1991 to 1993 mHR (95% CI) |
1996 to 1998 mHR (95% CI) |
2001 to 2003 mHR (95% CI) |
2006 to 2008 mHR (95% CI) |
|
|---|---|---|---|---|---|
| Age | |||||
| < 50 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| ≥ 50 to ≤ 59 | 0.83 (0.47 – 1.47) | 1.24 (0.75 – 2.03) | 1.29 (0.92 – 1.83) | 1.00 (0.79 – 1.25) | 0.93 (0.72 – 1.20) |
| ≥ 60 to ≤ 69 | 1.06 (0.62 – 1.80) | 1.28 (0.84 – 1.96) | 1.34 (0.97 – 1.84) | 0.95 (0.77 – 1.19) | 1.03 (0.81 – 1.32) |
| ≥ 70 | 1.74 (0.94 – 3.24) | 1.85 (1.17 – 2.91) | 1.73 (1.25 – 2.41) | 1.28 (1.02 – 1.60) | 1.16 (0.90 – 1.48) |
| Gender | |||||
| Female | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Male | 1.21 (0.84 – 1.73) | 1.58 (1.23 – 2.03) | 1.42 (1.19 – 1.68) | 1.39 (1.23 – 1.58) | 1.24 (1.09 – 1.41) |
| Smoking status | |||||
| Never | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Former | 1.04 (0.47 – 2.31) | 1.10 (0.69 – 1.74) | 1.03 (0.72 – 1.47) | 1.14 (0.89 – 1.47) | 1.39 (1.08 – 1.79) |
| Current | 0.96 (0.44 – 2.10) | 1.16 (0.74 – 1.83) | 1.38 (0.96 – 1.98) | 1.34 (1.03 – 1.74) | 1.72 (1.32 – 2.25) |
| Race | |||||
| White | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Black | 2.92 (0.85 – 9.97) | 2.41 (1.20 – 4.88) | 0.60 (0.29 – 1.24) | 1.21 (0.83 – 1.76) | 1.52 (1.14 – 2.02) |
| Other or unknown | NA | 2.87 (0.67 – 12.31) | 1.51 (0.69 – 3.28) | 1.87 (0.99 – 3.53) | 0.76 (0.48 – 1.19) |
| Ethnicity | |||||
| Non-Spanish | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Spanish | 0.33 (0.07 – 1.56) | 1.58 (0.48 – 5.17) | 0.84 (0.45 – 1.59) | 0.90 (0.64 – 1.25) | 1.19 (0.86 – 1.65) |
| Unknown | NA | NA | 0.88 (0.12 – 6.56) | 1.41 (0.58 – 3.43) | 2.14 (1.14 – 4.04) |
| Histology | |||||
| Adenocarcinoma | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Squamous Cell Carcinoma | 0.79 (0.54 – 1.17) | 0.85 (0.63 – 1.13) | 0.93 (0.76 – 1.14) | 0.92 (0.79 – 1.08) | 1.10 (0.92 – 1.31) |
| Other NSCLC | 0.98 (0.62 – 1.57) | 1.01 (0.73 – 1.40) | 0.96 (0.77 – 1.20) | 0.88 (0.77 – 1.02) | 0.92 (0.80 – 1.07) |
| First Course of Treatment1 | |||||
| Combination | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Surgery | 0.37 (0.19 – 0.72) | 0.86 (0.57 – 1.29) | 0.88 (0.67 – 1.17) | 0.70 (0.57 – 0.85) | 0.86 (0.70 – 1.07) |
| Chemotherapy | 0.71 (0.34 – 1.47) | 1.30 (0.82 – 2.05) | 1.41 (1.09 – 1.82) | 1.40 (1.17 – 1.67) | 1.19 (0.99 – 1.42) |
| Radiation | 1.37 (0.90 – 2.09) | 1.51 (1.10 – 2.06) | 2.45 (1.82 – 3.31) | 2.52 (1.98 – 3.20) | 2.59 (2.03 – 3.29) |
| Stage | |||||
| I | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| II | 1.06 (0.48 – 2.34) | 1.40 (0.86 – 2.26) | 1.55 (1.04 – 2.33) | 1.40 (1.08 – 1.82) | 1.81 (1.36 – 2.41) |
| III | 1.01 (0.52 – 1.95) | 2.76 (1.79 – 4.24) | 3.33 (2.49 – 4.46) | 2.38 (1.92 – 2.96) | 2.72 (2.19 – 3.38) |
| IV | 1.87 (0.96 – 3.64) | 5.40 (3.28 – 8.89) | 5.26 (3.83 – 7.23) | 4.30 (3.46 – 5.36) | 5.17 (4.15 – 6.45) |
Abbreviations: mHR, multivariable hazard ratio; CI, confidence interval; NSCLC, non-small cell lung cancer; NA, not available
Bold font indicates a statistically significant hazard ratio.
First course of treatment includes all methods of treatment recorded in the treatment plan and administered to the patient before disease progression or recurrence.
Discussion
This analysis of Cancer Registry data from five time-periods of lung cancer patients at the Moffitt Cancer Center demonstrates statistically significant changes in demographics and survival of non-small cell lung cancer patients over the past 22 years. Most importantly, from 1986 to 2008 the overall median survival time and overall 5-year survival rate have both more than doubled. Additionally, we observed statistically significant increases in stage-specific 5-year survival rates over the five time-periods.
The most recent Surveillance, Epidemiology, and End Results (SEER) monograph, published in 2007, on lung cancer reported overall and stage-specific survival rates based on patients diagnosed between 1998 to 2001 from twelve SEER areas.4 Since none of the five time-periods in our study completely overlapped with the time period in the SEER study, we explored the 1998 to 2001 time-period in our data so we could make comparisons. The overall 5-year survival for SEER was 15.5% vs. 25.0% in our data and the stage-specific rates in the SEER study vs. our study, respectively, were: 56.9% vs. 52.4% for stage I, 33.7% vs. 33.6% for stage II, 9.4% vs. 14.9% for stage III, and 9.4% vs. 3.9% for stage IV. Thus, their results are comparable to the rates found in our analysis especially among stage I, II, and III patients.
Other studies have also analyzed the temporal changes in lung cancer survival, but our study is by far the largest from a single institution. An analysis performed at the The University of Texas M. D. Anderson Cancer Center5 over three study periods (1985 to 1989, 1993 to 1997, and 2000 to 2004) found that the overall median survival duration increased 12.0 months in 1985 to 1989 to 17.5 months in 2000 to 2004. Additionally, their study reported that the probability of survival of patients who were alive at 2 years after diagnosis increased from 26.5% in 1985 to 1989 to 40.8% in 2000 to 2004. A Japanese study5 compared the survival of lung cancer patients who were detected by screening conducted between 1976 and 1984 with patients that were detected by screening between 1989 and 1997 and reported an increase in both median survival time of 27.8 months to 49.8 months and an increase in 5-year survival 34.8% to 47.8% (P < 0.01). There also have been two studies6,7 in Canada that used national databases to assess temporal changes in cancer survival rates. A recent study by Kachuri et al. 6 assessed temporal changes across four time periods (1992 to 1994, 1996 to 1998, 2000 to 2002, and 2005 to 2007) and found only a 2.6% absolute difference in the five-year age-standardized relative survival ratios for all lung cancers combined. An earlier study conducted in Canada7 analyzed survival trends across five time periods (1969 to 1973, 1974 to 1978, 1979 to 1983, 1984 to 1988, and 1989 to 1991) and found no significant differences across the study periods. Unfortunately, neither Canadian study6,7 presented stage-specific results and combined all lung cancers together rather than focusing on specific histological subtypes.
The multivariable models in the present analysis revealed that older patients, men, current smokers, and late stage patients were associated an increased risk of death. The findings for age, gender, and smoking status have been reported in previous studies3,4,8 and tumor stage is a well-documented prognostic and predictive factor for non-small cell lung cancer.9–11 First course of treatment with radiation was also associated with an increased risk of death. Kachroo et al. 8 reported a similar finding for radiation therapy where patients treated with radiation only were associated with a 3.9-fold (95% CI 1.12 – 13.33) increased risk of death. Lung cancer patients who receive radiotherapy typically have more advanced disease (e.g., post-operative stage IIIA, unresectable stage IIIA/B, or stage IV). Additionally, some early-stage lung cancer patients are also referred for radiotherapy if they have poor performance status and are not surgical candidates. Thus, the increased risk of death among patients that receive radiotherapy likely reflects the poor performance status and inherent poor prognosis of the patients rather than the treatment itself. We also observed that surgery only patients were generally associated with a reduced risk of death. This finding is not unexpected since surgery is the typical treatment for early stage lung cancer patients with good performance status.
A few limitations must be acknowledged about this study. First, we acknowledge the possible lack of generalizability of our study population because the lung cancer patients in this analysis were derived from a single cancer care center and were composed of mostly non-Hispanic Whites. Hence, the results may not be generalizable to community practices or even other cancer centers. However, we must consider that lung cancer patients at a tertiary cancer center like Moffitt could represent more complex cases and thus our 5-year survival rates could be conservative. This is just speculation since we do not have demographic and 5-year survival data for regional practices. Additionally, our analyses was limited to Cancer Registry data which does not include a comprehensive and systematic assessment of lung cancer risk factors such a detailed smoking history, family history of cancer, occupational exposures, and medical history. The data abstracted by Cancer Registry are limited to information that is available in patient medical records.
Conclusions
This analysis demonstrated important temporal changes in the demographics and improvements in overall survival of non-small cell lung cancer patients treated at the Moffitt Cancer Center from 1986 to 2008. The 5-year survival rates and median survival time of patients diagnosed with non-small cell lung cancer has significantly improved across all stages including late stage patients. The observed improvement in lung cancer survival over the last 22 years is likely attributed to several factors including advancements in surgery, chemotherapy regimes, management of comorbidities, and a slightly higher percentage of early stage patients which are more amendable to treatment. Further studies will be required to assess potential temporal changes in targeted molecular-based therapies, advancements in surgical procedures, and patients enrolled into chemotherapy trials.
Acknowledgments
This work was supported was by a National Institutes of Health/National Cancer Institutive (NIH/NCI) Specialized Program of Research Excellence (SPORE) Grant (P50 CA119997) and an NIH/NCI American Recovery and Reinvestment Act (ARRA) Grant (5 UC2 CA 148322-02). For contributions in data collection, curation, management, and access the authors thank the Moffitt Cancer Registry (Director: Karen A. Coyne), Edward T. Chwieseni, Research Information Technology (IT), and the Data Management and Integration Technology (DMIT) group.
References
- 1.American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.McErlean A, Ginsberg MS. Epidemiology of lung cancer. Semin Roentgenol. 2011 Jul;46(3):173–177. doi: 10.1053/j.ro.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011 Dec;32(4):605–644. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J, editors. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988–2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub. No. 07-6215; Bethesda, MD: 2007. [Google Scholar]
- 5.Kitajima T, Nishii K, Ueoka H, et al. Recent improvement in lung cancer screening: a comparison of the results carried out in two different time periods. Acta Med Okayama. 2006 Jun;60(3):173–179. doi: 10.18926/AMO/30751. [DOI] [PubMed] [Google Scholar]
- 6.Kachuri L, De P, Ellison LF, Semenciw R. Cancer incidence, mortality and survival trends in Canada, 1970–2007. Chronic Dis Inj Can. 2013 Mar;33(2):69–80. [PubMed] [Google Scholar]
- 7.Ugnat AM, Xie L, Semenciw R, Waters C, Mao Y. Survival patterns for the top four cancers in Canada: the effects of age, region and period. Eur J Cancer Prev. 2005 Apr;14(2):91–100. doi: 10.1097/00008469-200504000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Kachroo S, Tong L, Spitz MR, et al. Trends in prevalence of prognostic factors and survival in lung cancer patients from 1985 to 2004 at a tertiary care center. Cancer Detect Prev. 2008;32(2):101–108. doi: 10.1016/j.cdp.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E., Jr. The 7th lung cancer TNM classification and staging system: Review of the changes and implications. World J Radiol. 2012 Apr 28;4(4):128–134. doi: 10.4329/wjr.v4.i4.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol. 2009 Jul;4(7):792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 11.Bergman P, Brodin D, Lewensohn R, de Petris L. Validation of the 7th TNM classification for non-small cell lung cancer: A retrospective analysis on prognostic implications for operated node-negative cases. Acta Oncol. 2013 Aug;52(6):1189–1194. doi: 10.3109/0284186X.2012.742960. [DOI] [PubMed] [Google Scholar]