Abstract
Background
Rapid desensitization,a procedure in which individuals allergic to an antigen are treated at short intervals with increasing doses of that antigen until they tolerate a large dose, is an effective, but risky way to induce temporary tolerance.
Objective
To determine whether this approach can be adapted to suppress all IgE-mediated in mice by injecting serially increasing doses of monoclonal antibodies (mAbs) to IgE or FcεRIα.
Methods
Active and passive models of antigen- and anti-IgE mAb-induced IgE-mediated anaphylaxis were used. Mice were desensitized with serially increasing doses of anti-IgE mAb, anti-FcεRIα mAb or antigen. Development of shock (hypothermia), histamine and mast cell protease release, cytokine secretion, calcium flux and changes in cell number and FcεRI and IgE expression were evaluated.
Results
Rapid desensitization with anti-IgE mAb suppressed IgE-mediated immediate hypersensitivity; however, some mice developed mild anaphylaxis during desensitization. Rapid desensitization with anti-FcεRIα mAb that only binds FcεRI that is not occupied by IgE suppressed both active and passive IgE-mediated anaphylaxis without inducing disease. It quickly, but temporarily, suppressed IgE-mediated anaphylaxis by decreasing mast cell signaling through FcεRI, then slowly slowlyinduced longer lasting mast cell unresponsiveness by removing membrane FcεRI. Rapid desensitization with anti-FcεRIα mAb was safer and longer-lasting than rapid desensitization with antigen.
Conclusion
A rapid desensitization approach with anti-FcεRIα mAb safely desensitizes mice to IgE-mediated anaphylaxis by inducing mast cell anergy and later, removing all mast cell IgE. Rapid desensitization with an anti-human FcεRIα mAb may be able to prevent human IgE-mediated anaphylaxis.
Keywords: anaphylaxis, basophils, IgE, FcεRI, mast cells, mouse, rapid desensitization
Introduction
Allergic disorders, including allergic rhinitis, asthma, atopic dermatitis, food allergy and anaphylaxis are an increasingly common cause of morbidity in developed countries and, in the case of asthma and anaphylaxis, a not infrequent cause of death1–4. All of these disorders are mediated, to some extent, by immediate hypersensitivity reactions in which the activation of inflammatory cells by the crosslinking of immunoglobulin (Ig) Fc receptors (R) leads rapidly to the release of vasoactive mediators, such as histamine and platelet activating factor (PAF), cytokines and proteolytic enzymes5. Such immediate hypersensitivity reactions are the critical pathogenic mechanism in anaphylaxis and IgE-mediated food allergy and an important contributing mechanism in asthma, atopic dermatitis and allergic rhinitis5. In both humans and mice, immediate hypersensitivity reactions can be mediated by antigen crosslinking of antigen-specific IgE bound to the high affinity IgE receptor, FcεRI, on mast cells and basophils6,7.
Although some of these allergic disorders can be treated pharmacologically, manipulation of the immune system by administering increasing doses of allergen over time can also be an efficacious, albeit sometimes risky, way to suppress disease8. Two different general strategies of allergen immunotherapy have been widely used. Standard immune desensitization involves administration of increasing doses of allergen through a subcutaneous, oral, rectal or sublingual route over a period of weeks to months. This procedure has been shown in mice to suppress IgE-mediated disease through at least two mechanisms: 1) increased production of IgG antibodies that can activate an inhibitory Ig receptor, FcγRIIb and intercept antigen before it can access mast cell and basophil IgE; and 2) induction of regulatory T cells, that can suppress production of IgE9,10. Rapid desensitization procedures, in contrast, administer increasing concentrations of allergen over a period of hours or days. This time period is too short to work by altering Ig production; however, the precise mechanisms are not established11. Unlike conventional desensitization, the suppressive effects of rapid desensitization can be quickly lost when allergen administration is discontinued8,11.
To date, rapid desensitization techniques have involved the administration of allergen. Although effective, this can be of limited utility in individuals who are allergic to multiple antigens. In addition, the presence of serum antibodies, including IgG, that can bind inoculated allergens may make rapid desensitization more risky if the initial, small allergen doses are neutralized before they can access mast cell- or basophil-bound IgE, so that the first dose of allergen that interacts with cell-bound IgE is sufficiently large to induce a severe reaction. The presence of antigen-specific antibodies probably also contributes to the rapid clearance of desensitizing antigens and consequently, to the limited duration of the suppressive effects of rapid desensitization. These limitations of rapid desensitization suggested the possibility of attempting a similar procedure with antibodies to IgE or to FcεRI, the receptor involved in IgE-mediated anaphylaxis. We hypothesized that although administration of a single large dose of the IgG anti-IgE mAb, EM-95, or the IgG anti-FcεRIα mAb, MAR-1, can induce an anaphylactic response, administration of sequentially increasing doses of either of these mAbs, starting with a dose too small to induce detectable disease, might inhibit all IgE-mediated immediate hypersensitivity. Furthermore, the long in vivo half-life of IgG and the absence of preexisting antibodies to the administered IgGs might allow safer and more persistent desensitization. The results of these studies in a mouse model demonstrate the feasibility of this approach and shed light on the mechanisms that are involved.
Results
Rapid desensitization with an activating anti-IgE mAb
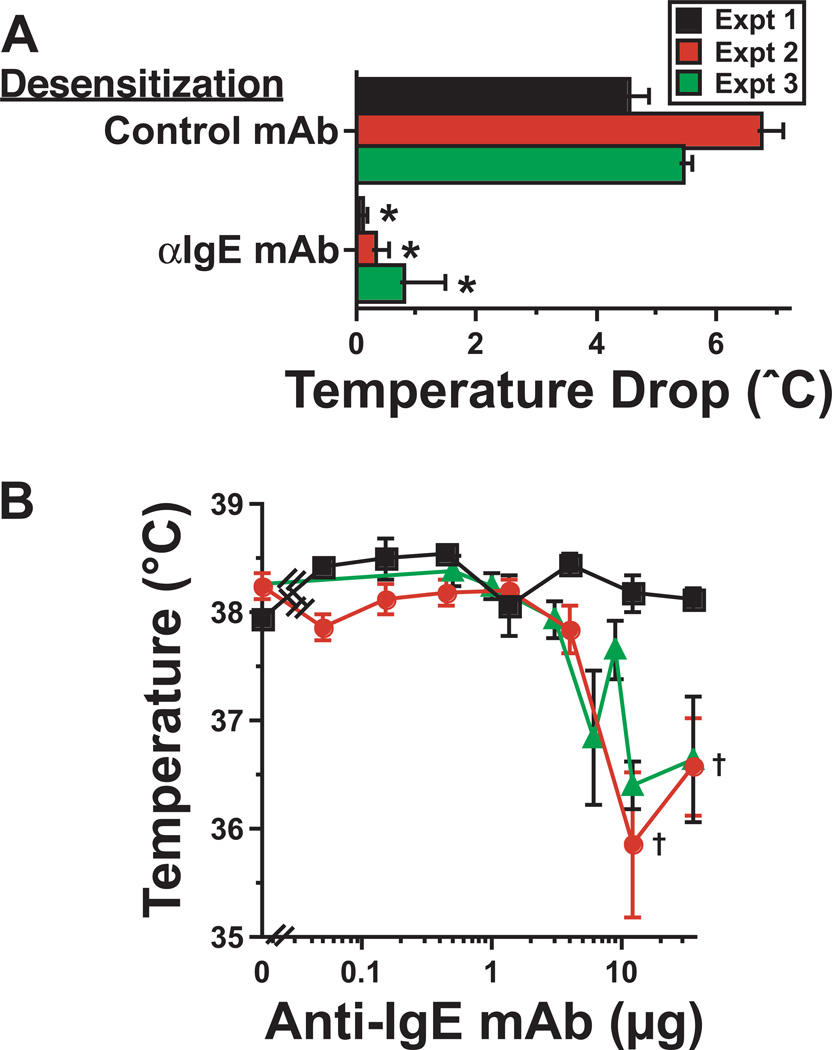
Injection of BALB/c mice with a single >10 µg dose of the activating rat IgG2a mAb to mouse IgE, EM-95, has been shown to induce anaphylaxis characterized by decreased mobility and hypothermia12. To determine whether this mAb could be used to rapidly desensitize mice to IgE-mediated responses, mice were first injected i.v. with 50 ng of EM-95, a dose too small to induce hypothermia, then subsequently injected every 90 min with 2–3 times the previous dose, until a dose that would normally induce severe shock (36 µg) was reached. This protocol prevented anaphylaxis induction by 36 µg of EM-95 (Fig. 1A) and, in most mice, was performed without a noticeable reaction (Fig. 1B). Some mice, however, did develop relatively mild hypothermia during the desensitization procedure (Fig. 1B). This developed in different individual mice at different doses of EM-95 and may have resulted from neutralization of the initial EM-95 doses by serum IgE, so that the first dose of EM-95 that interacted with mast cell and basophil IgE was sufficient to induce disease. The unpredictability of this problem and its potential severity mitigated against its clinical use for polyclonal desensitization and led us to try the alternative approach of desensitizing mice with an antibody to FcεRIα, the IgE-binding chain of the high affinity IgER13, which is expressed solely on cells.
Figure 1. Rapid desensitization with an activating anti-IgE mAb.
A. In 3 separate experiments, BALB/c mice (4/group) were injected i.v. every 90 min with doubling or tripling doses of anti-IgE mAb (EM-95) or an isotype-matched control mAb for a total of 8 doses, starting with 0.05 µg. Mice were then challenged the next day i.v. with 36 µg of anti-IgE mAb. Rectal temperatures were determined during the 90 min after challenge. Means of maximum decreases in temperature ± SEs are shown. B. The lowest temperature after each i.p. dose of anti-IgE mAb is shown for each of the 3 experiments. Additional experiments that varied dose number and dose increment did not reliably avoid temperature drops >0.5°C. * signifies p < 0.05 as compared to mice desensitized with control mAb. † signifies p < 0.05 compared to temperature of unchallenged mice.
MAR-1 anti-FcεRIα mAb binds to and activates mast cells and basophils
Initial experiments with MAR-1, a hamster IgG mAb to mouse FcεRIα, confirmed that this mAb stains mast cells, basophils, some monocytes (to a slight extent) and a very small population of dendritic cells, but not neutrophils, NK cells, T cells or B cells (see Fig. E1 in the Online Respository). Our experiments also confirmed previous observations that this mAb can activate both mast cells and basophils14, causing hypothermia (Fig. E2A), increased serum levels of MMCP1 (Fig. E2B) and histamine (Fig. E2C) and secretion of a large quantity of IL-4 (Supplemental Fig. E2D). A single 0.2–0.8 µg dose of MAR-1, injected i.v., was sufficient to induce considerable hypothermia (Fig. E2E), which is mast cell-dependent12, while 5–50 ng of MAR-1 was sufficient to induce a large increase in IL-4 production (Fig. E2D), which is basophil-dependent15.
Rapid desensitization with MAR-1 can prevent anaphylaxis
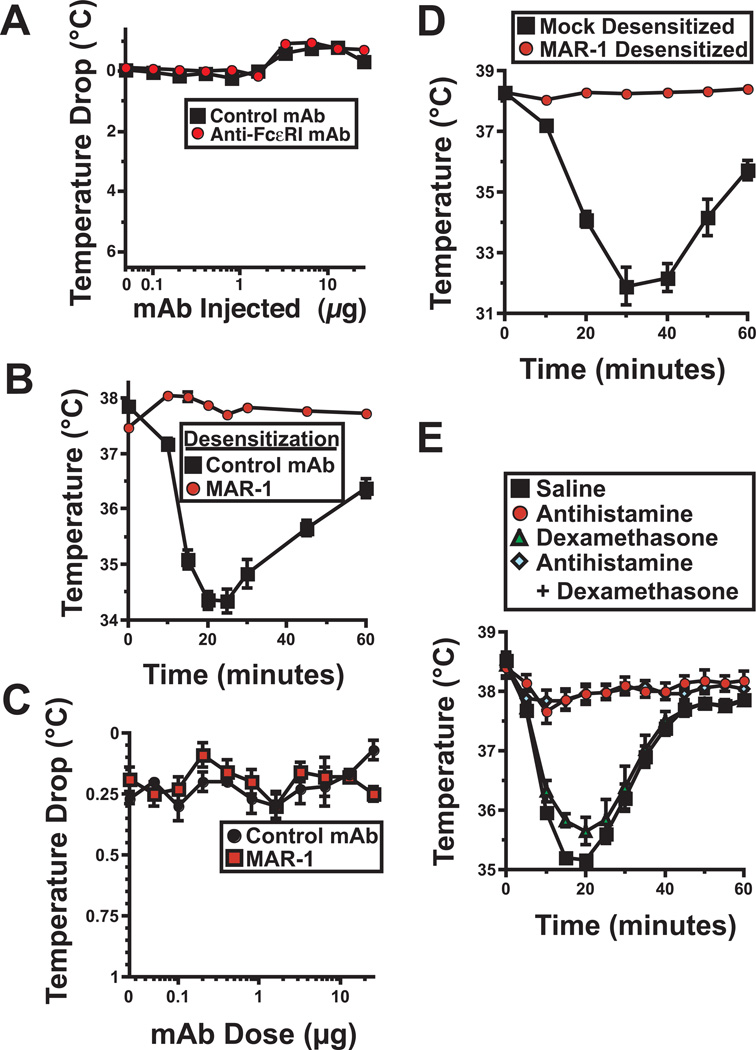
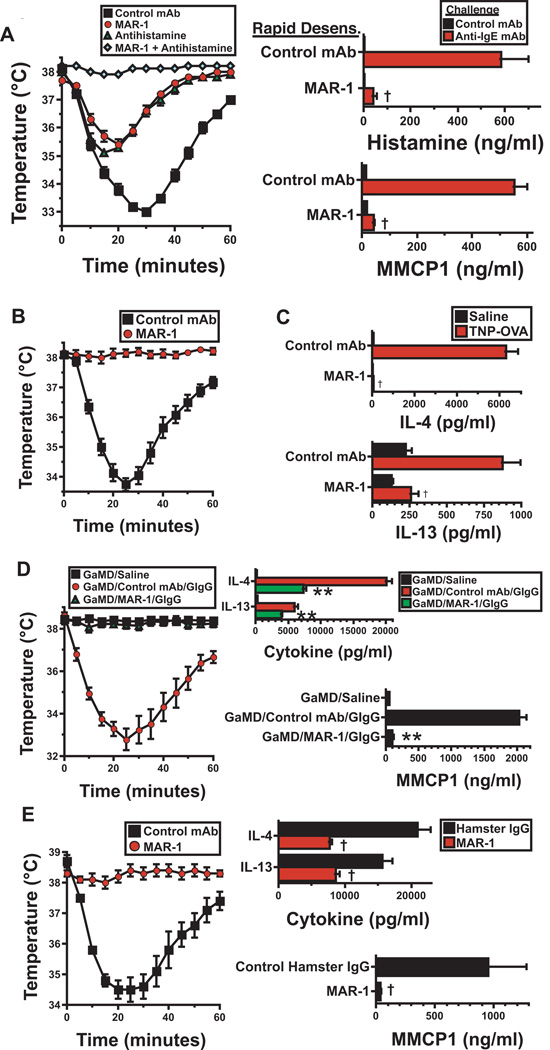
Hypothermia could be prevented by administering MAR-1 through a rapid desensitization approach (Fig. 2A). Rapid desensitization with MAR-1 also prevented the induction of hypothermia by i.v. injection of 40 µg of MAR-1, a dose ~100-fold greater than that required to induce hypothermia in naïve mice (compare Fig. E2E and Figure 2B). Rapid desensitization with i.p. MAR-1 failed to induce hypothermia and prevented the hypothermia response to challenge with a full dose of MAR-1 even when mice were pretreated with a long-acting formulation of IL-4 to make them more sensitive to vasoactive mediators16 (Fig. 2C and D). In the absence of rapid desensitization, MAR-1 induction of anaphylaxis could also be largely suppressed by pretreating mice with an antihistamine prior to MAR-1 injection, while corticosteroid pretreatment had little effect (Fig. 2E).
Figure 2. Anti-Fcε RIα mAb induces anaphylaxis that can be blocked by rapid desensitization or antihistamine.
A. Mice were serially injected i.p. every 60 – 90 min with the doses of MAR-1 or control mAb shown, starting with 0.05 µg. The mean maximum decrease in temperature ± SE during the 60 min after each injection is shown. 4 mice/group; results representative of 2 experiments. B. Mice were mock-desensitized with control mAb or desensitized with MAR-1 as in A, then challenged i.v. with 40 µg of MAR-1. Rectal temperatures were followed for the next 60 min. 4 mice/group; results representative of 2 experiments. C. Mice were injected i.p. with IL-4C containing 1 µg of IL-4 then the next day serially injected i.p. every 60 – 90 min with the doses of MAR-1 or control mAb shown, starting with 0.05 µg. The mean maximum decrease in temperature ± SE during the 60 min after each injection is shown. Results pooled from 2 experiments; total 8 mice/group for MAR-1-treated mice; 6/group for control mAb-treated mice. D. In a separate experiment, mice that had been IL-4C–treated and desensitized with MAR-1 or mock-desensitized with control mAb, as in Fig. 4C, were challenged i.v. with 40 µg of MAR-1. E. Mice were injected i.v. with a single 40 µg dose of MAR-1 45 min after pretreatment with saline, antihistamine (triprolidine), dexamethasone, or antihistamine plus dexamethasone. Rectal temperatures were determined during the subsequent 60 min. Results pooled from 2 experiments; total 8 mice/group.
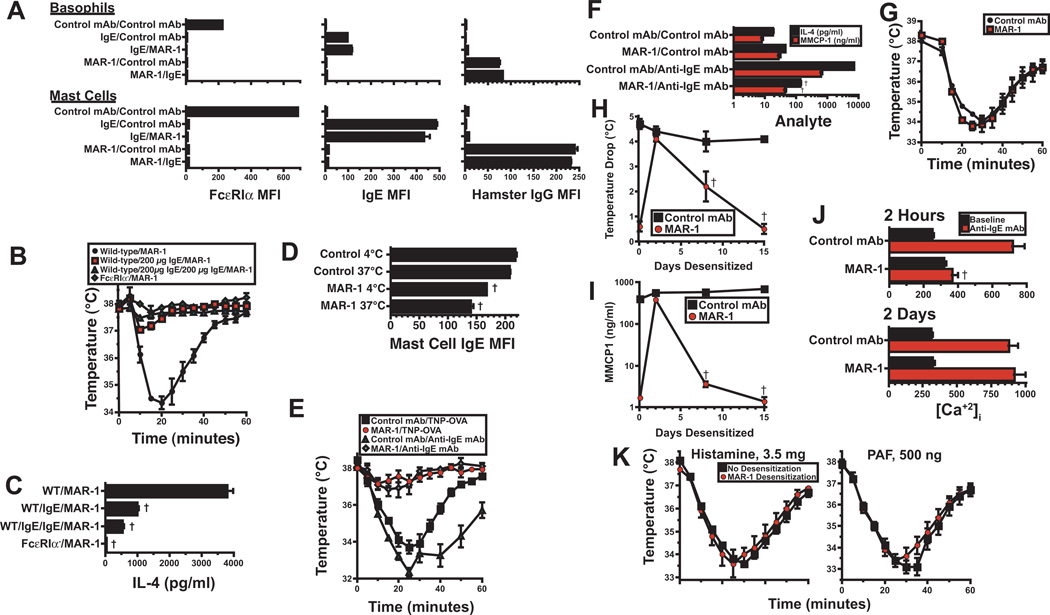
MAR-1 and IgE compete for binding to FcεRI
The interaction of mast cells and basophils with MAR-1 was more complicated, however, than the interaction of these cells with an anti-IgE mAb, because MAR-1aFcεRIα mAb only bound FcεRI that was not already occupied by IgE. That is, pre-treatment of these cells in vitro with IgE under non-capping conditions blocked the binding of MAR-1 (Fig. 3A and Fig. E3) and pretreatment with MAR-1 blocked the binding of IgE (Fig. 3A). For this reason, MAR-1 failed to induce anaphylaxis in mice that had been pretreated with sufficient exogenous IgE to saturate FcεRI (Fig. 3B) and induced relatively little IL-4 secretion in these mice (Fig. 3C). In addition, MAR-1 anti-FcεRIα mAb treatment failed to remove most IgE from mast cells during a 1 hr incubation at either 4°C or 37°C (Fig. 3D). Thus, MAR-1 antiFcεRIα mAb, unlike anti-IgE mAb, has little ability to interact with and modulate FcεRI that has already bound IgE.
Figure 3. Mechanism of MAR-1 rapid desensitization.
A. Peritoneal mast cells and blood basophils from IgE-deficient mice were incubated on ice with 20 µg of IgE anti-TNP mAb, MAR-1, or control mAb, then washed and incubated with one of the 3 same mAbs. Cells were washed again and stained for FcεRIα (left panels), IgE (middle panels), or hamster IgG (right panels) and analyzed for surface fluorescence after gating on basophil or mast cell markers. 4 mice/group; representative results from 1 of 2 experiments. B. WT and FcεRIα mice were injected i.v. once or twice with saline or 200 µg of mouse IgE at 2 hr intervals, then challenged i.v. 1 hr later with 40 µg of MAR-1. Rectal temperatures were determined. 4 mice/group; representative results from 1 of 2 experiments. C. IL-4 secretion was determined by IVCCA for 4 hr following challenge with MAR-1 in the same experiment shown in B. Representative results from 1 of 2 experiments. D. Peritoneal mast cells from wild-type BALB/c mice were cultured for 1 hr at 4°C or 37°C with 20 µg/ml of MAR-1 or control mAb, then stained for IgE and analyzed by flow cytometry. 4 mice/group; representative results from 1 of 2 experiments. E. BALB/c mice were injected i.v. with 10 µg of IgE anti-TNP mAb, then rapidly desensitized with MAR-1 or mock-desensitized with control mAb. Next, mice were injected i.v. with 50 µg of TNP-OVA or 100 µg of EM-95 2 hr after the last MAR-1 or control mAb dose. Rectal temperatures were determined. 4 mice/group; representative results from 1 of 2 experiments. F. BALB/c mice were left untreated or were rapidly desensitized with MAR-1. Some mice were injected i.v. 2 hr after the last MAR-1 dose with 100 µg of anti-IgE mAb. Serum MMCP1 and IL-4 production were evaluated. 4 mice/group; representative results from 1 of 2 experiments. G. BALB/c mice were rapidly desensitized with MAR-1 or mock-desensitized with control mAb, then injected i.v. 2 d later with 100 µg of anti-IgE mAb. Rectal temperatures were determined. 4 mice/group; representative results from 1 of 2 experiments. H and I. BALB/c mice were injected i.p. with 10 µg of IgE anti-TNP mAb. Mice were rapidly desensitized 24 hr later with MAR-1 or treated with equal doses of normal hamster IgG. Mice were re-injected with 10 µg of IgE anti-TNP mAb 2, 5 and 11 d after the initial IgE anti-TNP mAb treatment and with 40 µg of MAR-1 or control mAb 2, 5, 8 and 11 d after the initial MAR-1 treatment. Mice were challenged i.v. with 50 µg of TNP-OVA on the days shown. The mean maximum decrease in rectal temperature (H) and the mean serum MMCP1 level (I) 4 hr following challenge are shown for mice challenged 2 hr or 2, 4, 8, or 14 d after desensitization. 4 mice/group; similar results were obtained in a second experiment in which MAR-1 or control mAb was injected without rapid desensitization. J. Peritoneal lavage cells from mice rapidly desensitized with MAR-1 or control mAb 2 hr or 2 d prior to cell collection were loaded with Fluo-4. Relative levels of intracellular Ca++ ([Ca+2]i) were determined at baseline and immediately after in vitro challenge with anti-IgE mAb. Results pooled from 2 experiments; total of 8 mice/group. †p < 0.05 for decrease as compared to control. K. BALB/c mice were left untreated or rapidly desensitized with MAR-1, then injected i.v. with 3.5 mg of histamine or 500 ng of PAF. Rectal temperatures were determined. Results pooled from 2 experiments; total of 8 mice/group.
Mechanisms of rapid desensitization with MAR-1
The failure of MAR-1 to bind well to IgE-associated FcεRI allowed us to investigate whether rapid desensitization with this mAb prevents anaphylaxis by (a) removing IgE from mast cells; (b) decreasing mast cell responsiveness to IgE crosslinking; (c) by decreasing responses to mast cell-secreted mediators; or (d) by eliminating mast cells. Initial experiments, in which mice were injected with IgE anti-TNP mAb prior to treatment with MAR-1, showed that MAR-1 rapid desensitization induced a considerable decrease in the degree of hypothermia induced by either anti-IgE mAb or antigen challenge in MAR-1-pretreated mice (Fig. 3E), as well as decreases in IL-4 and MMCP1 responses to anti-IgE mAb by factors of ~100 and ~30, respectively (Fig. 3F). Decreased severity of hypothermia appears to result from temporary mast cell anergy, because EM-95 anti-IgE mAb challenge 2 days later, when MAR-1 was still present in blood, induced nearly the same degree of hypothermia as was seen in mice that had been pretreated with a control mAb instead of MAR-1 (Fig. 3G). This interpretation was confirmed by a second experiment, performed as part of a larger kinetic study of the in vivo effects of MAR-1, that demonstrated MAR-1 suppression of IgE-mediated, antigen-triggered anaphylaxis and MMCP1 secretion 2 hours, but not 2 days after completing administration of serially increasing doses of MAR-1 (Fig. 3H and 3I). Consistent with the mast cell anergy hypothesis, ex vivo FcεRI crosslinking failed to increase mast cell intracellular Ca++ 2 hr after the completion of rapid desensitization with MAR-1, although the Ca++ response was again completely intact 2 days after rapid desensitization (Fig. 3J).
In contrast, the short lived suppression of IgE-mediated anaphylaxis by rapid desensitization with MAR-1 did not involve decreased sensitivity of target organs to mediators released by activated mast cells, such as histamine and PAF, because histamine or PAF injection of mice that had just undergone rapid desensitization induced the same degree of hypothermia as injection of naïve mice with the same dose of the same vasoactive mediator (Fig. 3K). Thus, acute crosslinking of unoccupied FcεRI on mast cells temporarily decreases susceptibility to IgE-mediated anaphylaxis by inducing a relatively short-lived decrease in mast cell responsiveness to signaling through IgE-occupied FcεRI, rather than by decreasing sensitivity to mast cell-secreted mediators.
Rapid desensitization with MAR-1 eliminates basophils, but not mast cells
As has previously been reported for mice injected with full doses of MAR-117, rapid desensitization with this mAb eliminated >95% of basophils (Fig. E4A and B). Basophil elimination, however, seems to require more than a single round of FcεRI crosslinking, because rapid desensitization with EM-95 anti-IgE mAb had no effect on basophil number (Fig. E4C). Rapid desensitization with MAR-1, followed by MAR-1 treatment every 2–3 d, had a smaller effect on peritoneal and tongue mast cells, decreasing their number by up to 40% after 3 days, while having no significant effect on peritoneal mast cells after 14 days, but decreasing tongue mast cells at this time by ~50% (Fig. E4A and B). Importantly, decreases in mast cell number had little, if any effect on the severity of anaphylaxis or MMCP1 secretion (Fig. 3G and I), which are not decreased 2 d after MAR-1 injection.
MAR-1 treatment did not substantially alter the number of other cell types, including most dendritic cells (Fig. E4A and B), but did eliminate the small population of FcεRI+ “inflammatory” dendritic cells, as has been reported18 (Fig. E5).
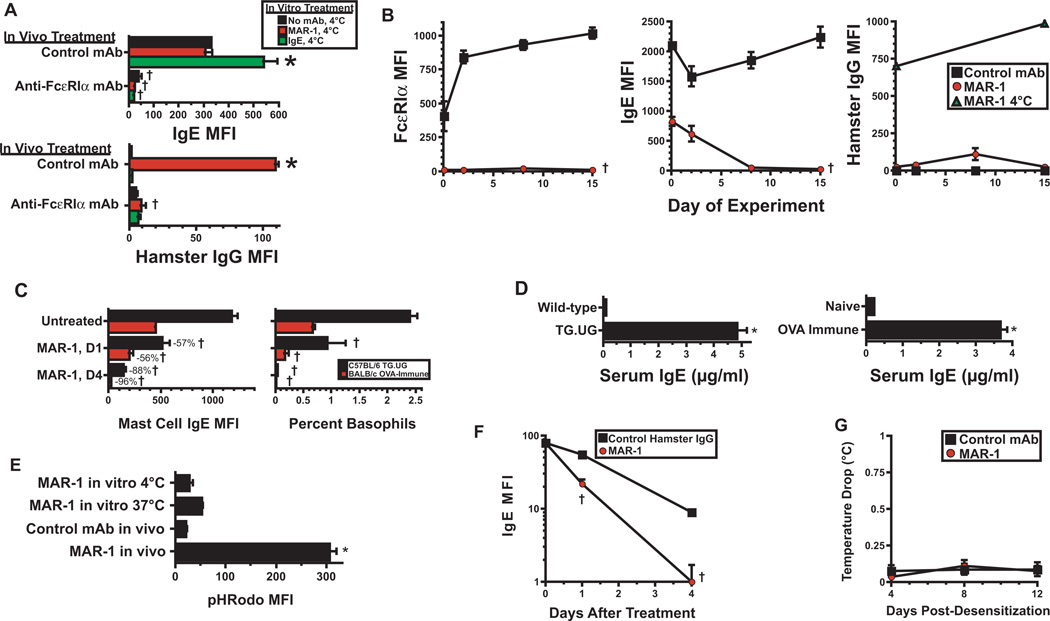
MAR-1 treatment causes progressive internalization and loss of mast cell FcεRI and IgE
Although MAR-1 does not quickly remove pre-existing IgE from mast cells and basophils, it progressively causes the loss of nearly all mast cell FcεRI and IgE expression over time (Fig. 4A and B and Fig. E6). Loss of most mast cell IgE over a 4 day period was seen in rapidly desensitized wild-type mice that were injected only with MAR-1 (Fig. E6), wild-type mice that were also injected every 2–3 days with a large dose of IgE anti-TNP mAb (Figure 4B) and in IL-4-overproducing transgenic C57BL/6 TG.UG mice and OVA-immunized BALB/c mice (Fig. 4C), which have high endogenous serum IgE levels (Fig. 4D). The rates of loss of mast cell IgE in the mice that had high serum IgE levels (Fig. 4C) were similar to those observed in naïve mice that had much lower levels of serum IgE (46% loss in 1 day, 87% loss in 4 days; not shown in figure). The reduction in mast cell IgE appears to be a consequence of two effects of MAR-1 binding to FcεRI: 1) removal of FcεRI from the mast cell surface (Fig. 4B) that results from internalization of the MAR-1/FcεRI complex (as shown by in vivo staining with MAR-1αFcεRIα mAb labeled with the fluorochrome pHRodo, which only exhibits detectable fluorescence after internalization and acidification (Fig. 4E)), which prevents newly expressed FcεRI from binding IgE; and 2) an increased rate of turnover of IgE-occupied FcεRI or dissociation of IgE from FcεRI (Fig. 4F). Importantly, the repeated injections of MAR-1 that are necessary to remove nearly all IgE from mast cells did not cause anaphylaxis (Fig. 4G), presumably because residual MAR-1 from the previous injection kept mast cell levels of free FcεRI too low to trigger anaphylaxis (Fig. 4B, left panel).
Figure 4. Suppression of FcεRI expression by anti-FcεRIα mAb.
A. WT BALB/c mice were rapidly desensitized with with MAR-1 or mock-desensitized with control mAb (maximum dose 40 µg), then treated every 2 d for 8 d with 40 µg of MAR-1 or control mAb. Peritoneal nucleated cells were then incubated for 30 min on ice with no mAb, 12.5 µg/ml of MAR-1, or 12.5 µg/ml of IgE anti-TNP mAb, then stained for IgE or hamster IgG. Average mean fluorescence is shown. 1 experiment, 4 mice/group. B. WT BALB/c mice were treated with IgE anti-TNP mAb (10 µg on d 0, 4, 6, and 9), and rapidly desensitized with MAR-1 or isotype control mAb on d 1. Mice were re-injected with 40 µg of MAR-1 or isotype control mAb on days 5, 7 and 10. Peritoneal mast cells obtained on the days shown were stained for FcεRIα, IgE, or hamster IgG. In addition, peritoneal mast cells obtained on days 0 and 16 from control mAb-treated mice were incubated with MAR-1 at 4°C, then stained for hamster IgG (right panel). Average mean fluorescence is shown. Representative of 2 experiments, 4 mice/group. C. Naïve WT, naïve IL-4 transgenic (TG.UG) and OVA-immune WT mice were left untreated or rapidly desensitized with MAR-1 or control mAb. Peritoneal mast cells obtained prior to or 1 or 4 days after MAR-1 injection were stained for IgE and analyzed by flow cytometry. Peripheral blood was analyzed for percent basophils. 4 mice/group. Similar results were obtained in a second experiment in which mice were injected i.p. with a single 40 µg dose of MAR-1 or control mAb. D. Serum IgE levels were determined by ELISA for naïve WT and TG.UG C57BL/6 mice (left) and for naïve and OVA-immune BALB/c mice (right). One experiment, 4 mice/group. E. BALB/c peritoneal mast cells were incubated on ice with pHRhodo-labeled MAR-1 or control mAb. BALB/c mice were rapidly desensitized with the same mAbs and peritoneal mast cells were obtained 3 hr later. Cells were analyzed for pHRodo fluorescence by flow cytometry. Representative of 2 experiments, 4 mice/group. F. IgE-deficient mice were injected i.v. with 500 µg of anti-CD23 mAb to block FcεRII, then with 10 µg of IgE anti-TNP mAb. The next day, mice were injected with 50 µg of MAR-1 or control mAb. Peritoneal mast cells obtained prior to or 1 or 4 days after MAR-1 or control mAb injection were analyzed for membrane IgE. Representative of 2 experiments, 4 mice/group. G. WT BALB/c mice were rapidly desensitized with MAR-1 or control mAb, then injected i.p. with 50 µg of the same mAb on d 4, 8 and 12. Rectal temperatures were obtained for 1 hr after each repeated mAb dose. Means and SEs of temperatures after each dose are shown. Data pooled from 2 experiments, total 7–8 mice/group. *p < 0.05 for increase as compared to control; †p < 0.05 for decrease as compared to control.
Chronic MAR-1 treatment suppresses antigen-induced IgE-mediated anaphylaxis by removing mast cell FcεRI and IgE
Although mast cell IgE levels were greatly reduced after 22 days of MAR-1 anti-FcεRIα mAb treatment, they were still sufficient for i.v. challenge with EM-95 αIgE mAb to induce mild, histamine-dependent shock (Fig. 5A). However, because this mAb reacts with all mast cell-associated IgE molecules, while an antigen reacts only with the subset of mast cell-associated IgE molecules that is specific for that antigen, an anti-IgE mAb that is capable of crosslinking IgE, such as EM-95, is likely to be a stronger stimulus of mast cell degranulation than a conventional antigen, especially once anti-FcεRIα mAb treatment has greatly decreased the quantity of mast cell-associated IgE. Because of this, antigen challenge may be more appropriate than anti-IgE mAb challenge to evaluate the ability of desensitization with anti-FcεRIα mAb to suppress IgE-mediated anaphylaxis. Consequently, we evaluated the effects of pretreating mice with IgE anti-TNP mAb and continuing treatment with this mAb while administering MAR-1 anti-FcεRIα or a control mAb every 7 d for 21 d, on their response to challenge with TNP-OVA. Results demonstrated the development of hypothermia in the control mAb-treated, but not in the MAR-1-treated, Ag-challenged mice (Fig. 5B). MAR-1 treatment also totally prevented the basophil-dependent IL-4 response and the mast cell- and basophil-dependent IL-13 response to TNP-OVA challenge (Fig. 5C). Furthermore, MAR-1 treatment for even 7 days totally blocked hypothermia and the MMCP1 response and significantly inhibited IL-4 and IL-13 responses in mice actively immunized with goat anti-mouse IgD antibody and challenged i.v. with goat IgG10 (Fig.5D) (note that anti-FcγRII/RIII mAb (2.4G2) was used in these studies to block IgG-mediated anaphylaxis12 and that IL-4 and IL-13 responses in this system are made by CD4+ T cells as well as basophils). Similar suppression of IgE-mediated anaphylaxis by MAR-1 treatment was observed in mice that were immunized with OVA/alum and challenged i.v. with the same antigen (Fig. 5E). Thus, rapid desensitization with MAR-1, followed by repeated treatment with this mAb, prevents antigen-specific IgE-mediated passive anaphylaxis when exposure to antigen-specific IgE precedes MAR-1 treatment and is continued during the course of this treatment, as well as 2 models of active IgE-mediated anaphylaxis that are associated with high serum IgE levels.
Figure 5. Suppression of IgE-mediated anaphylaxis with MAR-1.
A. BALB/c mice were rapidly desensitized with MAR-1 or control mAb, then treated with 40 µg of MAR-1 or control mAb on days 5, 12, and 18 and injected on day 22 with antihistamine or saline and challenged with anti-IgE mAb. Rectal temperature was determined. Sera obtained 5 min and 4 hr later were assayed for histamine and MMCP1, respectively. Data pooled from 2 experiments, total of 8 mice/group. B. BALB/c mice were initially injected i.v. with 10 µg of IgE anti-TNP mAb, followed by MAR-1 or control mAb, given initially as a single 40 µg i.p. injection or by rapid desensitization. All mice then were injected every 7 days i.p. for 21 days with 40 µg doses of MAR-1 or control mAb. All mice received a second 10 µg dose of IgE anti-TNP mAb on day 23 and were challenged on day 24 with TNP-OVA. Rectal temperatures were determined. 8 mice/group pooled from 2 experiments; similar results were obtained when mice were initially injected once or rapidly desensitized with MAR-1. C. In the same experiments shown in “B,” IL-4 and IL-13 production were determined by IVCCA for the 4 hr after TNP-OVA challenge. pooled from 2 experiments, total 8 mice/group. D. Mice immunized i.p. with goat anti-mouse IgD antiserum on d 0 were rapidly desensitized with MAR-1 or control mAb i.p. on d 8 and re-injected with the same mAb on d 12. All mice were injected i.p. on d 14 with 500 µg of anti-FcγRIIb/RIII mAb to block IgG-mediated anaphylaxis (2.4G2 has no effect on IgE-mediated histamine responses (data not shown)). Mice were challenged i.v. with saline or with 5 mg of goat IgG on d 15. Rectal temperatures and IL-4, IL-13 and MMCP1 secretion were determined. Data pooled from 2 experiments, total of 8 mice/group. E. BALB/c mice were immunized i.p. with OVA/alum on days 0 and 12, then rapidly desensitized i.p. with MAR-1 on d 14 and injected i.p. on d 16 and 18 with 40 µg of MAR-1 or control mAb. Mice were injected i.p. with 500 µg of 2.4G2 on d 19 to block IgG-mediated anaphylaxis and challenged i.v. with 200 µg of OVA the next day. Changes in rectal temperature and IL-4, IL-13 and MMCP1 secretion were determined. Data pooled from 2 experiments, total of 8 mice/group. †p < 0.05 for decrease as compared to control. **p < 0.05 for decrease as compared to control mAb-treated mice.
Comparison of antigen vs. anti-FcεRIα-mediated rapid desensitization
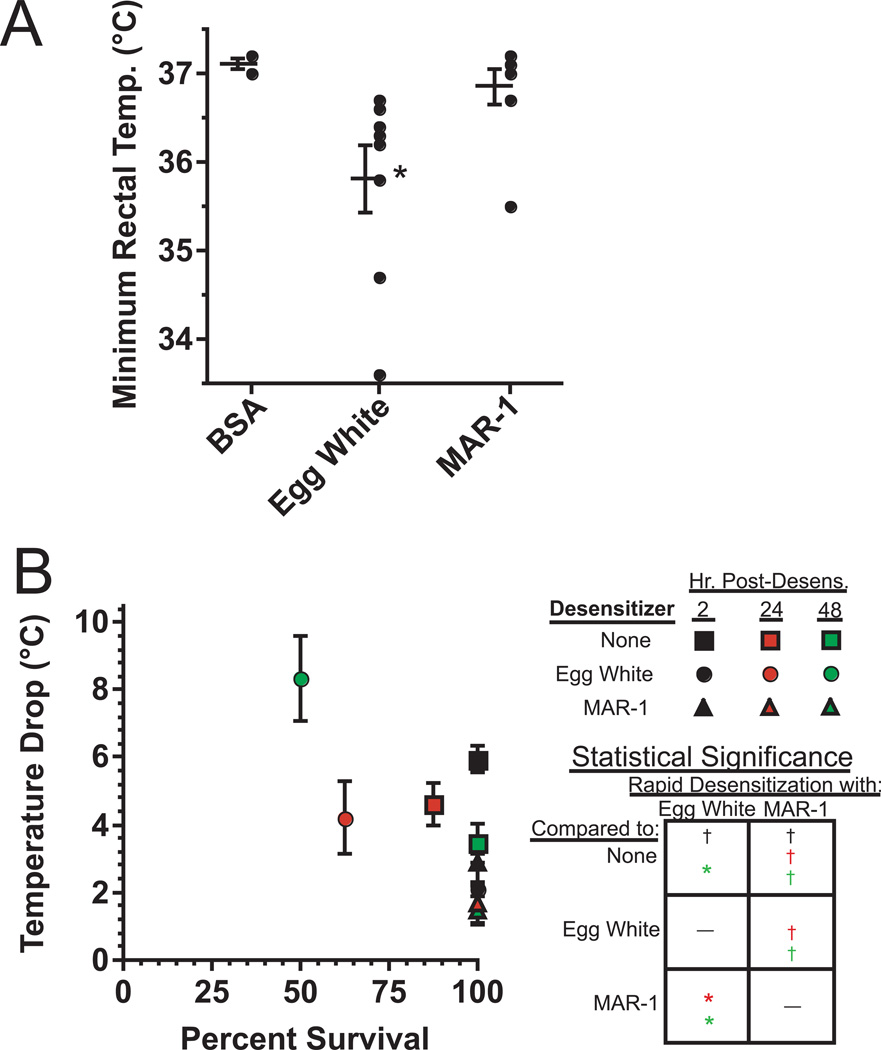
To compare the safety and efficacy of rapid desensitization with antigen vs. anti-FcεRα mAb in mice that had been sensitized to antigen by active immunization, we immunized mice with egg white (EW)/alum, then attempted to rapidly desensitize them with serially increasing i.p. injections of EW or MAR-1. Upon finding that none of these rapid desensitization treatments caused a significant drop in rectal temperature (data not shown), we repeated the study, but increased sensitivity to anaphylaxis by injecting mice with IL-4/anti-IL-4 complexes 1 day prior to desensitization, to mimic possible conditions in allergic people who produce high concentrations of Th2 cytokines. With this modification, we found that most mice that were rapidly desensitized with EW developed hypothermia, while this rarely occurred in mice that were rapidly desensitized with MAR-1 (Fig. 6A). Thus, rapid desensitization with an anti-FcεRIα mAb appears to be safer than rapid desensitization with Ag.
Figure 6. Suppression of active anaphylaxis with anti-FcεRIα mAb.
A. BALB/c mice were immunized twice i.p. with EW/alum, then treated i.p. with IL-4C that contained 1 µg of IL-4. The next day, mice were rapidly desensitized with EW (6 doubling doses, starting at 6 µg) or MAR-1 (8 doubling doses, starting at 0.4 µg), or mock-desensitized with BSA (negative control, 6 doubling doses, starting at 6 µg). The minimum rectal temperature for each mouse during rapid desensitization is shown, along with the mean ± SEM of minimum rectal temperature for the group. Data pooled from 2 experiments except 1 experiment for the BSA (negative control) group; 4 mice/group. Total of 8 mice/group, except 4 mice for the BSA group. * indicates significant lowering in rectal temperature as compared to BSA group. B. BALB/c mice were immunized with EW as in A, then injected i.p. 17 days after the initial immunization with 500 µg of 2.4G2 to block IgG-mediated anaphylaxis. Mice were then rapidly desensitized with EW or MAR-1 or mock-rapidly desensitized and challenged i.v. with 200 µg of EW 2, 24, or 48 hr after rapid desensitization. All mice were challenged with EW 1–2 d after 2.4G2 injection. 2 experiments, total of 8 mice/group. Mean maximum temperature drop and percent survival for >2 hr is shown for each group. Statistical significance box: † indicates a significantly lower decrease in temperature drop and/or mortality for a group above the box as compared to a group to the right of the box. * indicates a significantly greater decrease in temperature drop and/or mortality for a group above the box as compared to a group to the right of the box. Colors code for comparisons for groups challenged 2 hours (black), 24 hours (red) or 48 hours (green) after rapid desensitization.
We next evaluated the abilities of rapid desensitization with EW or MAR-1 to suppress IgE -mediated anaphylaxis. Because Ag challenge can cause both IgE- and IgG-mediated anaphylaxis in EW-immunized mice, we treated EW-sensitized mice with a single 500 µg dose of 2.4G2 to suppress IgG-mediated anaphylaxis26 prior to rapid desensitization with EW or MAR-1. The severity of IgE-mediated anaphylaxis induced by EW challenge was substantially reduced in mice that had completed rapid desensitization 2 hr earlier with EW or MAR-1 (Fig. 6B). However, anaphylaxis was actually exacerbated in these mice 1–2 days after rapid desensitization with EW, while the protective effects of rapid desensitization with MAR-1 increased during this time period (Fig. 6B).
Discussion
Our observations demonstrate that activating mAbs specific for IgE or FcεRIα, delivered by a rapid desensitization approach, can be used to block IgE-mediated anaphylaxis. This approach takes advantage of the abilities of a mAb specific for IgE to neutralize serum IgE and remove cell membrane IgE from mast cells and the ability of anti-FcεRIα mAb to remove membrane FcεRI from cells that express it. As a result of the latter effect, thanti-RmAbs acts more like a non-competitive than a competitive inhibitor and allow a relatively small quantity of anti-receptor mAb to block the binding of a large quantity of IgE. In contrast, the non-activating anti-human IgE mAb, omalizumab, which does not interact directly with FcεRI-bound IgE, but blocks the ability of serum IgE to bind to unoccupied FcεRI, acts more like a competitive inhibitor of IgE-mediated disease and consequently, lacks effectiveness in individuals who have highly elevated IgE levels.
The two techniques that we have evaluated for rapid desensitization of IgE-mediated disease, injection of an activating anti-IgE mAb and injection of an activating anti-FcεRIα mAb, each has advantages and disadvantages. The main advantage of anti-IgE mAb is the rapidity of its suppressive effects. Unlike anti-FcεRIα mAb or omalizumab, which do not directly perturb IgE/FcεRI complexes, EM-95 anti-mouse IgE mAb neutralizes cell-associated IgE as well as IgE in plasma and lymph. This advantage, however, comes at a price: 1) the therapeutic effects of an activating anti-IgE mAb is likely to be limited by IgE concentration, as has been seen with omalizumab; consequently, more anti-IgE mAb would be needed to treat individuals who have high serum IgE than those with lower amounts of this isotype; 2) the process of rapid desensitization with anti-IgE mAb is less predictable, and hence, more risky, because most of the small doses of mAb that are initially injected will probably be adsorbed by serum IgE without influencing mast cells or basophils. Consequently, it is difficult to gauge how much anti-IgE mAb needs to be administered in small doses before it can safely be given in ascending doses. Our inability to avoid mild hypothermia in some mice during rapid desensitization with EM-95 is probably a consequence of this difficulty. Antigen-based rapid desensitization, as it is currently performed by allergists, theoretically shares this second problem with an activating anti-IgE mAb, because injected Ag will most likely be neutralized by Ag-specific Ab of all isotypes before it can access IgE on mast cells or basophils. This may account for the fairly common induction of allergic responses with this technique, especially in patients with IgE-mediated food allergy8 and the greater propensity of EW, than anti-FcεRIα mAb to cause shock during the desensitization process in our mouse models.
In contrast, neither of these problems is likely with anti-FcεRIα mAb, which has a much smaller and more predictable target than anti-IgE mAb and which is entirely directed against cell-associated FcεRI. Administration of sequentially increasing doses of this mAb is unlikely to induce hypothermia, even when mice were made particularly sensitive to vasoactive mediators by pretreating them with IL-4. However, while rapid desensitization with anti-FcεRIα mAb can quickly suppress the ability of this mAb to induce anaphylaxis, its inability to rapidly remove FcεRI-bound IgE from mast cells limits its ability to rapidly prevent disease mediated by IgE that was already bound by FcεRI prior to the initiation of rapid desensitization. Consequently, desensitization with an anti-FcεRIα mAb similar to MAR-1 would not be useful in situations in which it is necessary to rapidly suppress an established IgE-mediated allergic response, unless a way can be developed to maintain the short-lived mast cell anergy that is rapidly induced by desensitization with MAR-1. Although this limitation might potentially be overcome by engineering a mAb that binds to a site on FcεRIα that is not blocked by bound IgE, no such mAb to mouse FcεRIα is currently available. In contrast, treatment with increasing doses of anti-FcεRIα mAb over a one day period, followed by additional treatment with this mAb for 1–3 weeks, appears to be a safe and effective, albeit less rapid way to suppress antigen-specific IgE-mediated allergic reactions, as shown by our studies in both passive and active anaphylaxis models.
Although our studies with approaches to rapid polyclonal desensitization were primarily carried out to test the feasibility, rather than the mechanism of this approach, they provide insights into the mechanisms involved in antigen-based rapid desensitization. The ability of rapid desensitization with anti-FcεRIα mAb to initially partially suppress IgE-mediated anaphylaxis before substantially decreasing mast cell-associated IgE suggests that the slow, persistent mast cell activation decreases mast cell responsiveness. This state of partial anergy is short-lived, however, inasmuch as the decrease in the severity of IgE-mediated anaphylaxis is lost within 2 days, despite the continued presence of anti-FcεRIα mAb (which has very limited ability to activate mast cells at this point because it now can bind only to FcεRIα that is newly inserted into the mast cell membrane). Full suppression of IgE-mediated anaphylaxis by anti-FcεRIα mAb occurs only after several days, when FcεRI turnover, coupled with anti-FcεRIα mAb modulation of its target, has reduced mast cell membrane IgE to a level incapable of mediating the amount of mast cell degranulation that is required to induce anaphylaxis. It seems likely that antigen-induced rapid desensitization works in the same ways: limited induction of partial anergy, but more importantly, depletion of antigen-specific IgE. In vitro studies that demonstrate that mast cells can be desensitized by Ag removal of their membrane IgE without making them unresponsive to repeated IgE priming and Ag challenge19, as well as in vivo studies that demonstrate that rapid desensitization with antigen is usually short-lived in the absence of continuing antigen administration8 are consistent with this conclusion.
Anti-FcεRIα mAb may also decrease the severity of anaphylaxis by eliminating basophils and IgE+ inflammatory dendritic cells, which could decrease IL-4 production and antigen presentation, respectively. However, these effects appear to be less important than elimination of mast cell FcεRI, because anaphylaxis continues to be severe after basophils and FcεRIα+ DCs have been eliminated and until mast cell FcεRI expression is almost totally suppressed (Fig. 3H and 3I).
In sum, our data provide evidence that polyclonal rapid desensitization with anti-FcεRIα mAb can be a feasible way to suppress IgE-mediated immediate hypersensitivity reactions. Although the antibody used can induce anaphylaxis if administered initially in full doses, this is also true for rapid desensitization protocols that administer an allergen rather than an antibody. An additional layer of protection for anti-FcεRIα-mediated rapid desensitization can be provided by pretreatment with an antihistamine. Furthermore, while rapid desensitization with an allergen may last only a short time, because most allergens have a short in vivo half-life and allergen-specific IgE is likely to reaccumulate once the allergen is no longer present, the long in vivo half-life of IgG antibodies prolonged protection. This should be particularly true for humans, in whom IgG has a much longer half-life than it has in mice20. Compared to treatment with a non-activating anti-IgE mAb, such as omalizumab, rapid desensitization with anti-FcεRIα mAbs would be expected to be more effective, particularly in patients who have high levels of serum IgE, but also more risky. Even this disadvantage is not totally clear, because omalizumab has also induced anaphylactic reactions21, although the mechanisms involved are not fully understood. All in all, rapid desensitization with anti-FcεRIα mAb appears to provide a safe and effective way to suppress IgE-mediated immediate hypersensitivity reactions in mice, regardless of the allergen involved.
Although it is impossible to fully extrapolate rodent data to humans, these observations suggest that it would be reasonable to generate anti-FcεRIα mAb appropriate for human use and evaluate whether they have the same advantages and safety in humans that we have found in mice. If so, we would expect an anti-human FcεRIα mAb to be useful for treating food allergy, atopic dermatitis, asthma and other disorders that are primarily IgE-mediated or have an important IgE-dependent component. Anti-FcεRIα mAbs that can bind to FcεRI regardless of whether it is associated with IgE would be expected to be particularly useful, in that they should be able to rapidly prevent IgE-mediated anaphylaxis by removing both free FcεRI and FcεRI/IgE complexes from cells.
Key messages.
Treatment of mice with an anti-FcεRIα mAb suppresses IgE-mediated anaphylaxis by 2 separate mechanisms: rapid, temporary induction of mast cell anergy and slow removal of FcεRI and IgE from the mast cell surface.
Application of this process to humans could provide a safe, effective way to suppress all IgE-mediated allergy.
Acknowledgements
We thank Carolyn Cuff and Andrew Long of Abbott for their gift of anti-IL-13 mAbs and Christopher Karp for his helpful suggestions.
Research presented here was funded by a Merit Award to FDF from the United States Department of Veterans Affairs.
Abbreviations
- BSA
bovine serum albumin
- d
day
- EM95
a rat IgG monoclonal antibody to mouse IgE
- EW
egg white
- FcεRIα
α chain for the high affinity IgE receptor
- HN
Hank’s balanced salt solution plus 5% fetal bovine serum
- HNA
Hank’s balanced salt solution plus 5% fetal bovine serum and 0.2%sodium azide hr hour
- IL-4C
a long acting formulation of IL-4 produced by mixing IL-4 and anti-IL-4 monoclonal antibody at a 2:1 molar ratio
- i.p.
intraperitoneal
- i.v.
intravenous
- IVCCA
in vivo cytokine capture assay
- mAb
monoclonal antibody
- MAR-1
a hamster IgG anti-mouse FcεRIα monoclonal antibody
- MMCP1
mouse mast cell protease 1
- OVA
chicken ovalbumin
- PAF
platelet activating factor
- R
receptor
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Materials and Methods: Please see online supplement.
References
- 1.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R, Sheikh A, Strachan DP, Anderson HR. Time trends in allergic disorders in the UK. Thorax. 2007;62:91–96. doi: 10.1136/thx.2004.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2010;127:594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 4.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Suppl 3):S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 5.Galli SJ, Lantz CS. Allergy. In: Paul WE, editor. Fundamental Immunology. 4 ed. Philadelphia: Lippincott-Raven; 1999. [Google Scholar]
- 6.Boyce JA, Austen KF. Biology of the mast cell. In: Austen KF, Frank MM, Atkinson JP, Cantor H, editors. Samter's Immunolgic Diseases. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 253–269. [Google Scholar]
- 7.Bochner BS, Schroder JM, Basophils . In: Samter's Immunologic Diseases. 6th ed. Austen KF, Frank MM, Atkinson JP, Cantor H, editors. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 244–252. [Google Scholar]
- 8.Nowak-Wegrzyn A, Sampson HA. Future therapies for food allergies. J Allergy Clin Immunol. 2011;127:558–573. doi: 10.1016/j.jaci.2010.12.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Till SJ, Francis JN, Nouri-Aria K, Durham SR. Mechanisms of immunotherapy. J Allergy Clin Immunol. 2004;113:1025–1034. doi: 10.1016/j.jaci.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and FcyRIIb cross-linking. J Clin Invest. 2006;116:833–841. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouza TR, Palis RI, Legere HJ, III, Castells MC. Rapid desensitization for antibiotic-induced hypersensitivity reactions and anaphylaxis. In: Castells MC, editor. Anaphylaxis and Hypersensitivity Reactions. New York: Humana Press; 2011. pp. 313–332. [Google Scholar]
- 12.Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol. 2002;109:658–668. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 13.Metzger H, Alcaraz G, Hohman R, Kinet JP, Pribluda V, Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- 14.Hubner MP, Larson D, Torrero MN, Mueller E, Shi Y, Killoran KE, et al. Anti-FcepsilonR1 antibody injections activate basophils and mast cells and delay Type 1 diabetes onset in NOD mice. Clin Immunol. 2011;141:205–217. doi: 10.1016/j.clim.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khodoun MV, Orekhova T, Potter C, Morris S, Finkelman FD. Basophils initiate IL-4 production during a memory T-dependent response. J Exp Med. 2004;200:857–870. doi: 10.1084/jem.20040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strait RT, Morris SC, Smiley K, Urban JF, Jr., Finkelman FD. IL-4 exacerbates anaphylaxis. J Immunol. 2003;170:3835–3842. doi: 10.4049/jimmunol.170.7.3835. [DOI] [PubMed] [Google Scholar]
- 17.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, et al. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubinchik E, Shalit M, Levi-Schaffer F. Responsiveness of human skin mast cells to repeated activation: an in vitro study. Allergy. 1998;53:14–19. doi: 10.1111/j.1398-9995.1998.tb03768.x. [DOI] [PubMed] [Google Scholar]
- 20.Morell A, Terry WD, Waldmann TA. Metabolic properties of IgG subclasses in man. J Clin Invest. 1970;49:673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dreyfus DH, Randolph CC. Characterization of an anaphylactoid reaction to omalizumab. Ann Allergy Asthma Immunol. 2006;96:624–627. doi: 10.1016/S1081-1206(10)63560-0. [DOI] [PubMed] [Google Scholar]