Abstract
For the successful treatment of pulmonary tuberculosis, drugs need to penetrate complex lung lesions and permeate the mycobacterial cell wall in order to reach their intracellular targets. However, most currently used anti-tuberculosis drugs were introduced into clinical use without considering the pharmacokinetic and pharmacodynamic properties that influence drug distribution, and this has contributed to the long duration and limited success of current therapies. In this Progress article, I describe new methods to quantify and image drug distribution in infected lung tissue and in mycobacterial cells, and I explore how this technology could be used to design optimized multidrug regimens.
Tuberculosis (TB) is caused by infection with the bacterium Mycobacterium tuberculosis and predominantly affects the lungs, resulting in extensive tissue pathology. The disease is particularly widespread in the developing world and kills one person every 20 seconds, which places it second only to HIV/AIDS among lethal infectious diseases. In 2011, there were an estimated 8.7 million new cases worldwide (13% of which presented as co infections with HIV) and 1.4 million people died from TB, despite the existence of curative chemotherapy1.
It takes at least 6 months to successfully treat uncomplicated drug-sensitive pulmonary TB using multiple antibiotics, whereas most bacterial infections are cured within a week or two of monotherapy. The same combination of four drugs (isoniazid, rifampicin, pyrazinamide and ethambutol) has remained the first-line treatment since 1994 (REFS 2,3). The first three of these drugs were discovered 50–60 years ago and have been part of WHO-recommended tuberculosis treatment regimens since the 1980s. After 2 months of this four-drug combination, treatment is continued with both isoniazid and rifampicin for an additional 4 months2. This lengthy multidrug therapy dramatically affects compliance and adherence to medication; patients are simply overwhelmed by the pill burden or suffer toxic side effects over time and withdraw from treatment before their lungs have been fully sterilized. This can lead to treatment failure, relapse of infection and the emergence of genetic drug resistance, resulting in even longer treatment with less effective and more toxic second-line and third-line drugs4. The fluoroquinolones and aminoglycosides are the mainstay of treatment for multidrug-resistant tuberculosis (MDR TB) owing to resistance to both isoniazid and rifampicin. To cure MDR TB, at least 18–24 months of therapy with four–six drugs, including a fluoroquinolone and one injectable agent (for example, an aminoglycoside or the peptide antibiotic capreomycin), is required5. However, as there are more than a dozen drug candidates in various phases of clinical development — including novel and repurposed traditional antibiotics that have not previously been used against tuberculosis — treatment of MDR TB and extensively drug-resistant TB (XDR TB) is in a transition phase and will certainly evolve in the coming years.
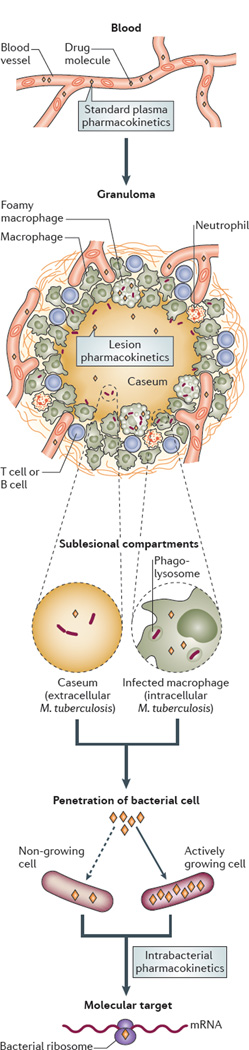
Although tuberculosis is not exclusively a disease of the lungs and can affect many organs and tissues6, this article focuses on the fate and tissue distribution of drugs that are used in the treatment of active pulmonary tuberculosis. To reach their intended target, anti-TB drugs must be transported from the blood compartment to non-vascularized pulmonary lesions, diffuse into necrotic foci and the caseum, permeate the lipid-rich cell envelope of M. tuberculosis and finally reach their molecular target at adequate concentrations and for the required time (FIG. 1). Most first-line drugs that are in use today were launched in the 1950s and 1960s, before pharmacokinetics and pharmacodynamics (PK–PD) constituted an established field in drug discovery. Consequently, correlations between drug concentration in plasma and drug penetration of the infected site were not considered during the drug development process, which has led to suboptimized dosage — a problem that is now widely recognized for rifampicin7, 8. The integration of PK–PD modelling in the development of antibacterial drugs is becoming more widespread9 and, although the tuberculosis field is still lagging behind other infectious and non-communicable disease research areas, plasma-based PK–PD parameters are now increasingly considered in the design of preclinical studies and clinical trials10. However, PK–PD correlations that are made on the basis of drug exposure in plasma might be insufficient to inform drug distribution in TB, owing to the complexity and diversity of TB pathology. For example, although the fluoroquinolone moxifloxacin (MXF) accumulates in cellular granulomas, the concentrations of rifampicin in this niche are only a fraction of those that are achieved in plasma11. Thus, plasma concentrations of anti TB drugs are not generally predictive of concentrations at the infected sites. Little is known about the factors that influence drug distribution from plasma into the range of tissues, nodules and cavities that are inhabited by the pathogen. The striking diversity of cell types, tissue structure and vascular architecture that is present in pulmonary lesions strongly suggests that standard rules of thumb — such as passive equilibration of the free drug fraction between plasma and tissue — cannot be meaningfully and successfully applied to M. tuberculosis-infected sites. To further complicate matters, subpopulations of bacilli that persist in a slowly-growing or non-growing state are recalcitrant to killing by most anti TB drugs — a phenomenon known as phenotypic tolerance (BOX 1).
Figure 1. The path of anti TB drugs from the central blood compartment to their molecular target.
To reach their targets in intracellular bacilli, anti tuberculosis drugs travel from blood vessels and distribute into various types of lesions, and they must overcome several barriers, including variations in lesion architecture and the cellular and chemical composition of tissues, as well as reduced vascularization. From the blood compartment, drugs enter the interstitial space of granulomas and then penetrate and accumulate in immune cells, including within subcellular organelles, such as the phagolysosome, where intracellular bacilli can reside; finally, the drugs permeate the pathogen to reach their molecular target. In necrotic granulomas and cavities, drugs must diffuse through caseum in the absence of vascularization and active transport systems to reach extracellular bacilli that are present in the necrotic centre. Different lesion compartments can harbour bacterial populations in different metabolic and physiological states (for example, slowly-replicating or non-replicating bacilli), which can result in alterations in bacterial cell wall structure and transport mechanisms, which also influence the permeability of the pathogen to small drug molecules. Some drugs show reduced penetration of non-growing cells (dashed arrow) compared to growing cells, whereas others effectively penetrate both cell types (not shown).
Box 1 | Mycobacterial drug resistance.
Mycobacterium tuberculosis exhibits two forms of drug resistance: genetic drug resistance and phenotypic drug resistance. Genetic drug resistance refers to the classical form of heritable bacterial resistance, whereby genetic alterations, such as mutations in target genes, confer drug resistance and are transmitted from mother cells to daughter cells during replication. When a resistance mutation emerges, it is rapidly amplified under antibiotic pressure, provided that the mutation does not confer a high fitness cost93. Resistance as a result of horizontal gene transfer (HGT) is widespread in many bacterial pathogens, but HGT seems to be absent in M. tuberculosis as this species does not contain plasmids and the transfer of genomic DNA has not been shown94. For most anti tuberculosis drugs, the majority of resistance mutations occur in a limited number of genes or genetic loci, allowing for targeted molecular susceptibility testing95. Multidrug-resistant tuberculosis (MDR TB) is defined as being genetically resistant to isoniazid and rifampicin96, which are the two ‘pillars’ of first-line therapy. M. tuberculosis isolates that are also resistant to fluoroquinolones (for example, moxifloxacin) and one injectable drug (for example, an aminoglycoside or the peptide antibiotic capreomycin) are known as extensively drug-resistant tuberculosis (XDR TB)97. The therapeutic options for patients who are infected with MDR-TB and XDR TB are limited and consist of at least 18–24 months of treatment with four or more antibiotics that are less effective and more toxic than first-line drugs5.
Phenotypic drug resistance or drug tolerance arises when genetically susceptible cells become refractory to antibiotic treatment. There are many pathways towards the emergence of phenotypic resistance, including metabolic and physiological adaptations in response to drug exposure and other environmental cues73, 98. This type of resistance is non-heritable and reversible and is often associated with reduced or arrested growth99; for example, it has been shown that non-replicating M. tuberculosis is resistant to the cell wall inhibitor isoniazid, whereas replicating bacilli are susceptible to isoniazid100. Phenotypically resistant populations that survive drug treatment in the absence of genetic resistance are often referred to as persisters. These drug-tolerant cells are suspected to be responsible for the long duration of antibiotic therapy101, although this remains to be formally demonstrated for TB. Although the mechanisms of persistence have been extensively studied in vitro, quantifying the extent of this phenomenon in lesions and elucidating its effect on drug-mediated killing in specific niches is challenging102.
Why are anti-TB drug dynamics important? Understanding the factors that drive drug distribution at the site of infection and into the pathogen could enable more effective use (that is, dosing) of current anti TB drugs and could also facilitate the design of complementary drug regimens to prevent the development of drug resistance and thereby accelerate resolution of the infection. Reducing the treatment period to less than 2 months would revolutionize TB chemotherapy and greatly contribute to global disease control. Thus, methods that are capable of informing the rational combination of drugs (that is, those combinations that have the best potential to substantially reduce the duration of treatment) are needed, particularly as clinical trials for TB drugs are long and complex. Control of the TB epidemic will come from a combination of drugs that complement each other in their ability to penetrate the range of lesions that are found in TB disease, resulting in complementary activity against all bacterial subpopulations that are present in these lesions. In this Progress article, I describe the diversity and complexity of TB lesions in the lung, recent advances in the methods that are available to quantify and image small molecules in these niches and the various factors that influence drug distribution from plasma to lesions to mycobacterial cells. As many bacterial infections can cause similarly complex lesions (such as biofilms and abscesses, among others), the principles that are outlined here might be relevant for other infectious diseases.
Diversity of TB pathology
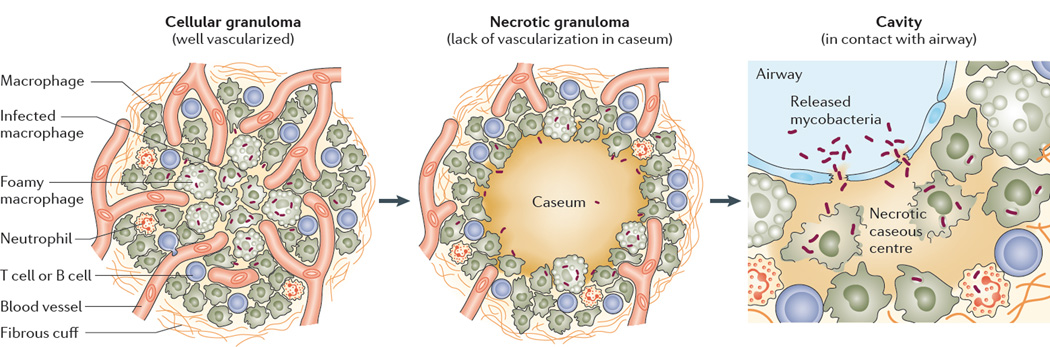
M. tuberculosis infection is initiated by the inhalation of viable bacilli that are present in droplets exhaled into the atmosphere by an individual with active disease. M. tuberculosis is taken up by phagocytic cells and transported across the alveolar epithelium into the lung. Infected macrophages recruit additional macrophages and other immune cells from neighbouring blood vessels to form dynamic, organized structures called granulomas, which are a pathological hallmark of TB12 (FIG. 2). Although granuloma formation is traditionally regarded as an attempt to ‘wall off ’ the infecting pathogen, recent studies have shown that pathogenic mycobacteria use granuloma cells to expand, disseminate and colonize nascent granulomas13, 14. Thus, the pathology of TB is primarily driven by the immune response rather than by damage that is inflicted by the bacterium itself. Pulmonary lesions evolve from cellular granulomas — which are composed of lymphocytes, macrophages, foamy macrophages and neutrophils — to necrotizing granulomas. The necrotic core, which is also known as the caseum, owing to its cheese-like appearance, is the result of host cell and bacterial lysis. The caseum initially forms in the centre of the granuloma and spreads outwards, eventually compressing the surrounding lung tissue and destroying the vasculature15. As the granuloma matures, it often develops several layers of fibroblasts (known as a fibrous cuff), which function to wall off the infection. A cavity forms when an expanding granuloma comes into contact with an airway, which involves transformation of the granuloma into a cavity made of a fibrotic wall filled with liquefied caseum that is directly connected to the luminal surface of the respiratory tract (FIG. 2).
Figure 2. Maturation of pulmonary TB lesions.
The granuloma is initially rich in immune cells and is highly vascularized (it is referred to as a cellular granuloma), which facilitates drug distribution into the core of the lesion. Bacilli reside extracellularly and in activated and foamy macrophages. As the granuloma matures, it begins to necrotize from the centre outwards, and vascularization is gradually destroyed, although the fibrotic rim and cellular layer remain densely vascularized. Bacilli can be found extracellularly in the necrotic caseum and in immune cells. When an expanding granuloma meets an airway, the necrotic centre fuses with the airway structure to form a cavity. Mycobacteria are found extracellularly in the cavity caseum and intracellularly in macrophages, foamy macrophages and neutrophils. Both intracellular and extracellular bacteria are released at the luminal side of the cavity and later appear in sputum23. Image is modified, with permission, from REF. 25 © (2013) Elsevier.
Importantly, bacilli reside in all types of lesions and lesion compartments. In cellular granulomas, bacteria are predominantly, but not exclusively, found inside macrophages, where they are able to evade immune defences and interfere with phagolysosome biogenesis and integrity16, 17. They are also found extracellularly in interstitial tissue. In necrotic granulomas, the caseous centre often contains large numbers of extracellular bacilli, although they frequently go undetected by commonly used staining techniques (such as Ziehl–Neelsen staining) owing to cell wall alterations in slowly-replicating or non-replicating mycobacteria18–22 (BOX 1). This slowly-growing or non-growing population is particularly important as it may constitute the reservoir from which large bacterial numbers emerge when a closed nodule encounters an airway and develops into an aerated cavity (FIG. 2). At the luminal surface of cavities, bacilli are either intracellular (for example, within cell types such as neutrophils and macrophages23) or extracellular. Thus, the granuloma constitutes a friend–foe structure as it functions to contain the pathogen, but it also makes chemotherapeutic eradication extremely difficult, owing to the sequestration of bacilli within remote and shielded lesion compartments24–26. Furthermore, M. tuberculosis can also reside in lung tissue that might seem to be uninvolved macroscopically, but that has a complex spectrum of inflammation and repair, a niche that is often ignored.
In addition to the complexity of the diseased site, the heterogeneous vascular supply within the granuloma impedes drug delivery. Reduced vasculature is widely recognized as one of the main reasons for the resistance of solid cancer tumours to drug treatment27. When concentrations of anticancer drugs are mapped in relation to vasculature density at the disease site, a steep gradient of decreasing drug levels is observed as the distance from blood vessels increases28. Interestingly, reduced vasculature also impairs the supply of oxygen and nutrients, which results in a decrease in cell proliferation and an increase in drug tolerance27. As the vascular architecture is destroyed in the caseous centre of necrotic lesions and cavities, this leads not only to reduced drug supply but also to metabolic quiescence of bacterial cells, as a consequence of reduced oxygen and nutrient supplies, and failed immunity owing to poor access by circulating T lymphocytes29. To reach the centre of necrotic granulomas, where quiescent extracellular bacilli are found in large numbers21, drugs must diffuse from the cellular rim that borders the necrotic centre and penetrate the entire caseous region without the assistance of active or facilitated transport mechanisms (FIG. 2). The lack of blood supply to compartments where large numbers of non-proliferating bacteria reside highlights the importance of assessing drug penetration at these sites.
From blood to lesions
Investigations that were conducted between the 1950s and 1980s using resected lung tissue from patients with TB showed that the levels of isoniazid and rifampicin vary in the lung. These early studies also indicated that the measured levels were dependent on the method of detection that was used as well as on the study design. The methods that were used included the quantification of radioactively labelled drug30, bioassays that quantified drug activity in fluids and tissues31 and an agar diffusion method32, 33. Although these methods were useful, the approach had several drawbacks; for example, drug levels were often measured in resected lung tissue that was collected within a window of several hours after drug dosing rather than at a precise time after drug administration. This introduces a confounding factor in data interpretation as tissue-to plasma drug ratios change over time. Tissue pharmacokinetics of prodrugs, such as isoniazid, which is metabolized by both the host and the pathogen, are challenging and can potentially generate inconsistent data, depending on the methodology that is used for drug quantitation11, 30. Furthermore, although radiolabelled isotopes are highly sensitive, it is not always possible to distinguish between the active parental drug and its active and inactive metabolites30. However, despite such shortcomings, these early data indicated that drug distribution in infected lungs is mostly drug-specific and depends on the lesion type31, 32, 34. They also highlighted the need to refine and expand drug penetration studies in TB lesions using innovative and more robust technologies.
Quantifying drug distribution
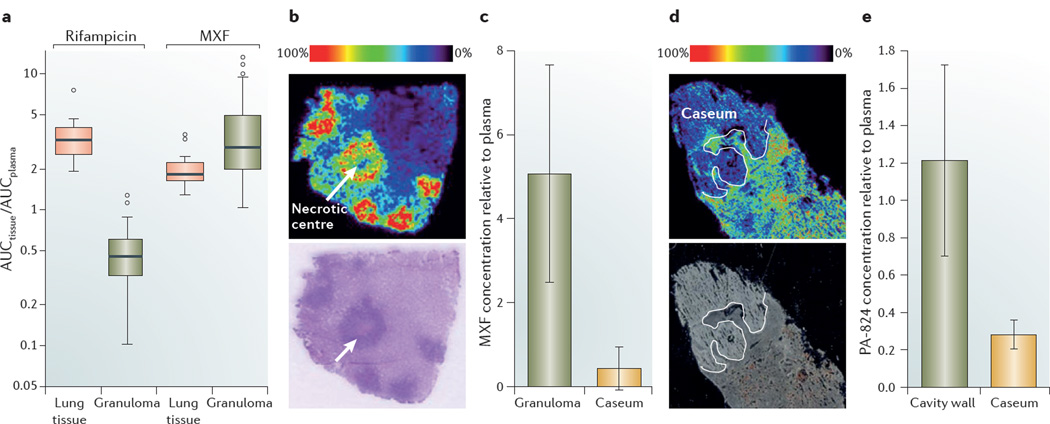
The advent of chromatographic and mass spectrometry instrumentation has made it possible to reliably quantify small molecules in pulmonary lesions. Measuring drug levels by HPLC coupled to tandem mass spectrometry (LC–MS/MS) is invasive and therefore requires the use of animal models to mimic the pathology of human TB and the ability to generate lesions that are large enough to enable accurate dissection and measurements. The rabbit model meets these criteria35, 36 and has therefore been increasingly used in pharmacological studies11, 37, 38. Following the administration of single drugs or drug combinations, population pharmacokinetic modelling enables the rate and extent of drug distribution to be calculated. This has been applied to the measurement of the first-line drugs isoniazid, rifampicin and pyrazinamide, and the second-line drug MXF in rabbit lung tissue and granulomas11. The extent of penetration in lung tissue and lesions follows different trends for each drug; for example, MXF reaches higher levels in lung tissue and granulomas than in plasma (FIG. 3a). Although the mechanism of MXF accumulation in cellular granulomas is unknown, in vitro studies in resting and activated macrophages have revealed that it has high intracellular-to extracellular ratios39, which is consistent with its accumulation in TB lesions (FIG. 3a). By contrast, the concentrations of isoniazid, rifampicin and pyrazinamide in cellular granulomas are lower than in plasma, although rifampicin seems to accumulate in uninvolved lung tissue11, 32 (FIG. 3a). The rate of tissue penetration is rapid for all four drugs; pyrazinamide has the longest equilibration half-life of 1 hour. The four drugs are thus expected to quickly equilibrate between plasma and tissue, although the dynamics of distribution at equilibrium might be more complex than has been suggested by modelling. For example, the prodrugs isoniazid and pyrazinamide are rapidly bioactivated by M. tuberculosis41, 42. This complicates the interpretation of results, as the kinetics of bioactivation and the levels of active metabolites in tissue are crucial to establish PK–PD correlations at the infected site. Bioactivation might also explain the observed discrepancies between the levels of 14C–isoniazid that were measured in lesions by radioactive methods30 and the isoniazid concentrations that were determined in lesion homogenates using either an activity-based assay34 or LC–MS/MS. Transiently active metabolites and reactive species that are generated by these prodrugs are not sufficiently stable to be captured by standard analytical methods. By contrast, the distribution of rifampicin (which is a drug that does not require bioactivation) in pulmonary lesions is consistent, irrespective of the study design and the method that is used — this drug accumulates in uninvolved lung tissue but its levels are reduced in cellular granulomas11, 32 (FIG. 3a).
Figure 3. Drug distribution and imaging in lung tissue and lesions.
a | Boxplots of rifampicin and moxifloxacin (MXF) concentrations in lung tissue and cellular granulomas, relative to plasma concentrations, which show that both drugs are efficiently distributed in healthy lung tissue. Rifampicin concentrations are significantly lower in granulomas than in healthy lung tissue, whereas the opposite is observed for MXF. The box width is the interquartile range and the horizontal line marks the median value. The T-bars indicate the maximum and minimum of ratios within 1.5x the upper and lower quartiles, respectively. Observed datapoints that fall outside this range are represented by open circles. b | A mass spectrometry image that shows the distribution of MXF in necrotizing rabbit granulomas43. The panel contains an ion map (upper panel) and the corresponding hematoxylin and eosin-stained section (lower panel). A signal intensity of 0% corresponds to minimum signal, whereas 100% is assigned to the maximum signal, which, in the case of MXF, corresponds to the cellular cuff of granulomas. The signal responds linearly up to 1 nmol, which is several orders of magnitude higher than the therapeutically relevant drug concentrations that are achieved in tissues. The ion map clearly shows lower drug levels in caseum compared to the surrounding cellular region, which highlights the poor diffusion capacity of MXF in the necrotic core, where extracellular bacilli reside. c | Absolute concentrations of MXF were measured by HPLC coupled to tandem mass spectronomy (LC–MS/MS) in tissue homogenates of caseum and the cellular fraction of granulomas, and these are shown relative to the absolute drug concentration measured in plasma at the time of rabbit necropsy. The bar graph shows the MXF concentration in granuloma and caseum relative to plasma at 2 hours post-dose, which confirms that the drug distributes more efficiently in the cellular fraction of the granuloma than in caseum (V.D., unpublished observations). d | Distribution of the nitroimidazole PA 824 in a large rabbit necrotic granuloma. Similarly to MXF, the ion map (upper panel) shows that PA 824 has limited penetration in caseum. The lower panel is an optical image of the same tissue section prior to matrix coating and imaging44. e | Absolute concentrations of PA 824 were measured by LC–MS/MS in the caseum and cellular wall of three cavities that were found in the same animal at the time of necropsy, and these are shown relative to the absolute drug concentration measured in plasma. The concentration of PA 824 in the cavity wall is higher than in caseum (V.D., unpublished observations), which confirms the results of MALDI–MS (part d). Graph in part a is reproduced, with permission, from REF. 11 © (2012) American Society for Microbiology. The images in part b are reproduced, with permission, from REF. 43 © (2011) American Cell Society. Images in part d are reproduced, with permission, from REF. 44 © (2012) Science Direct.
Imaging drug distribution
Although LC–MS/MS is capable of generating lesion-specific PK–PD indices for use in preclinical studies and clinical trial simulations, drug quantitation in granuloma and cavity homogenates by LC–MS/MS has one major shortcoming: the lack of sublesional spatial resolution for the visualization of drug concentration gradients across lesions and lesion compartments. This is crucial given the varied and complex structure of pulmonary lesions (FIG. 2). MALDI mass spectrometry imaging (MALD–MSI) is ideally suited to study the distribution of small molecules in the various compartments of pulmonary lesions43. Flash-frozen tissues are sectioned and coated with a chemical matrix to enable ionization of the molecules of interest. Laser-assisted desorption of ionized molecules, coupled to detection by mass spectrometry, enables the quantification of small molecules and metabolites across the tissue section, with a spatial resolution of approximately 25–100 mm. A map of relative ion density is computer-generated and displayed as a high-resolution image that can be superimposed onto a stained tissue section to correlate relative drug concentrations with histological features44, 45.
Following up on the favourable accumulation of MXF in lung and pulmonary lesions11 (FIG. 3a), MALDI–MSI was used to generate two-dimensional (2D) ion maps of MXF in necrotic granulomas that were obtained from treated and untreated infected rabbits43 and from patients with TB who were undergoing lung resection (Clinicaltrials.gov identifier: NCT00816426; V.D., unpublished observations). Mapping of MXF molecules in rabbit necrotic granuloma sections shows that a heterogeneous pattern of drug accumulation occurs: the drug accumulates at high levels in macrophages and lymphocytes, but there is little or no penetration of the caseous centre (FIG. 3b). This result is not surprising considering the lack of blood vessels and active transport in this acellular niche. To confirm these findings, absolute drug concentrations were separately measured in the cellular compartment, the cavity wall and in the caseum of dissected rabbit granulomas by LC–MS/MS. These data showed that MXF concentrations are markedly lower in the caseum than in the cellular fraction of granulomas (FIG. 3c), which is consistent with the MALDI ion maps (FIG. 3b). This observation is of particular interest as phenotypically tolerant cells (known as persister cells) typically reside in the caseous foci of necrotic granulomas46, 47. Subinhibitory concentrations of fluoroquinolones have been shown to increase bacterial mutation rates by triggering the SOS response, which accelerates the emergence of antibiotic resistance mutations in Mycobacterium fortuitum48, although this remains to be shown for M. tuberculosis. Thus, the presence of subtherapeutic levels of MXF in caseum may have important clinical implications, such as the enrichment of drug-resistant populations. Similarly to MXF, the nitroimidazole PA 824 (which is a new anti TB drug candidate that is currently in clinical development) distributes more effectively in lung tissue and cellular granulomas than in caseum44 (FIG. 3d,e). Among the anti TB drugs that have so far been investigated by MALDI–MSI, pyrazinamide seems to be the only one that diffuses effectively through caseum (V. D., unpublished observations) and is active against slowly replicating persister cells49. Although there is evidence to suggest that the removal of pyrazinamide from anti TB drug regimens significantly increases the duration of therapy and negatively affects the clinical outcome50, 51, the reasons for these observations remain unknown. Accumulation of pyrazinamide in the necrotic foci of closed nodules, where phenotypically tolerant bacilli persist and where other drugs diffuse poorly, probably contributes to its unique sterilizing activity.
Thus, with MALDI–MSI, it is possible to determine the penetrative power of small molecules in infected tissues. This technology also has the potential to reveal local monotherapies — for example, the presence of only one of several co administered agents in a particular niche, such as the necrotic foci of caseous lesions. Earlier studies suggest that local and temporal windows of monotherapy can develop in specific lesion compartments, which increases the risk of the emergence of resistance52, 53. Such discrepancies in drug distribution are not detectable by conventional LC–MS/MS analysis of lesion homogenates or by whole-body imaging modalities, as these technologies lack sufficient spatial resolution. As we gain further insights into the pulmonary and sublesional distribution of drugs and drug candidates, the rational combination of drugs — each with the ability to preferentially penetrate distinct compartments of the granuloma — is closer to becoming a reality.
One drawback of MALDI–MSI is that it is invasive and relies on accurate tissue resection, which limits the ability to generate large clinical data sets. Non-invasive imaging modalities are emerging to study drug penetration in M. tuberculosis-infected lung tissue and lesions in mice and non-human primates; these include positron emission tomography (PET) using 11C-or 18F-labelled drugs54, 55. As PET is routinely used in humans, its application to imaging anti TB drugs has the potential for rapid bench to bedside translation. One major limitation of PET is finding a label with a sufficiently long half-life that does not alter the chemical structure of the drug. Furthermore, with a spatial resolution of >1 mm, PET does not enable the visualization of sublesional small-molecule distribution in most specimens. However, PET and MALDI–MSI complement each other, with each method compensating for the limitations of the other. Used together, they have the potential to deliver a comprehensive picture of drug pharmacokinetics in multiple organs, including infected lung tissue and granulomas or cavities.
Drug uptake into infected immune cells
M. tuberculosis is a facultative intracellular pathogen of macrophages and other immune cells. In silico modelling and clinical data indicate that intracellular bacterial growth inhibition and killing is important to ensure the complete sterilization of infected lungs56, 57. The presence of intracellular bacteria creates an additional hurdle in the path of anti-TB drugs from the central blood compartment to their bacterial targets, as they must permeate the cell types in which the bacterium resides (FIG. 1). As such, many new drug-formulation approaches rely on liposomes and nanoparticles to achieve targeted drug delivery to macrophages in order to reduce systemic drug levels and toxicities. As particulate carriers, liposomes and nanoparticles naturally target macrophages by taking advantage of their phagocytic properties, which is a feature that can be further enhanced by the addition of ligands for macrophage receptors to their surface58, 59. The potential of these alternative drug delivery systems for the treatment of TB has been comprehensively reviewed elsewhere60, 61.
The requirement for anti-TB drugs to reach intracellular bacilli raises two key questions. First, how do different anti-TB drugs vary in their ability to penetrate macrophages that are grown in vitro and, second, how representative is this data for the penetration of alveolar macrophages and inflammatory cells in vivo? Standard first-line and second-line anti TB drugs have varying intracellular-uptake properties in vitro, with intracellular-to extracellular (I/E) ratios ranging from 0.1 to >20. The β lactams (antibiotics that were developed for non TB infections but which are now increasingly used to treat XDR TB62, 63) and aminoglycosides have I/E ratios that are lower than 1, isoniazid has an I/E ratio of around 1, rifampicin has an I/E ratio of between 2 and 5, and ethambutol and the macrolides have I/E ratios ranging from 10 to >20 (REFS 64,65). These numbers were primarily obtained from in vitro studies that used alveolar macrophages, but the trend remains the same in in vivo clinical studies of pulmonary alveolar cells and epithelium lining fluid (ELF)66, 67. Although rifampicin shows limited penetration of ELF (with an ELF/plasma ratio of around 0.3), it accumulates in alveolar macrophages to levels that are tenfold to 20 fold higher than those measured in plasma67, 68.
The fluoroquinolones show surprisingly variable intracellular penetration properties: MXF concentrations are 20–70 fold higher in pulmonary alveolar macrophages compared with the concentrations that are measured in plasma69, whereas levofloxacin only achieves marginally higher concentrations in alveolar cells compared to plasma70, and ciprofloxacin exhibits intermediate penetration with an alveolar macrophage-to-plasma ratio of 5–10 (REF. 71). The high levels of MXF that are observed by both MALDI–MSI and conventional mass spectrometry in solid granulomas11 (FIG. 3) corroborate these results. However, it is unlikely that in vitro assays of macrophage uptake or in vivo measurements of drug concentrations in alveolar cells are always predictive of drug accumulation in granulomas, as immune cells differ in their uptake ability. Macrophage activation — which occurs after an encounter with an appropriate stimulus, such as a bacterial infection — can induce active transport and efflux systems that contribute to the intracellular accumulation of some drug classes. For example, intracellular accumulation of MXF is markedly increased in activated macrophages, whereas levofloxacin uptake remains unaffected72. Intracellular accumulation of fluoroquinolones is dependent on a dynamic balance between influx, binding of the drug to undefined intracellular constituents and efflux, all of which vary according to the different types of fluoroquinolone that are used39. Differential drug distribution in the cytosol and in membrane-enclosed organelles (such as the phagolysosome) where M. tuberculosis is known to reside also needs to be considered. In order to maximize their efficiency, anti TB drugs must reach, and preferably be retained in, the infected subcellular compartments.
These data clearly show that extensive permeability profiling should be included early during drug discovery in order to design strategies for optimizing drug distribution at the sites of infection. The complexities that are described above highlight the need for in vitro assays that can predict the distribution of drug candidates in the blood, in the cellular and necrotic compartments of granulomas, in multiple immune cell types and, finally, in the subcellular organelles in which the bacterium resides (FIG. 1). In addition, different anti TB compound classes target physiologically distinct mycobacterial populations (BOX 1), and this must also be considered. Ideally, each of the drugs that makes up an effective regimen should preferentially distribute to the site where its most vulnerable target population resides, as seems to be the case for pyrazinamide.
Drug uptake into M. tuberculosis
The inefficiency and extended time period of current TB-treatment strategies is thought to be caused by both pharmacokinetic and pharmacodynamic factors25. The pharmacokinetic factors include differential and suboptimal drug distribution at the site of infection, and the pharmacodynamic factors pertain to subpopulations of phenotypically resistant bacilli (as opposed to genetically resistant bacteria)73. In combination, these PK–PD parameters drive the development of genetic drug resistance.
Limited drug distribution at the infected site and phenotypic drug resistance are not as independent as they might seem to be. Penetration and accumulation of the drug in bacilli is the last step of the long journey to the site of action (FIG. 1), and it has been shown that reduced permeability of M. tuberculosis to small-molecule drugs contributes to the drug tolerance that is associated with the quiescent bacterial population74. Conventional mass spectrometry methods have been optimized to measure the concentrations of unlabelled drugs in the cytosolic fraction of M. tuberculosis cells grown in vitro. Intracellular accumulation of the fluoroquinolones is markedly reduced in nutrient-starved non-replicating M. tuberculosis, and this probably contributes to the loss of fluoroquinolone activity against quiescent populations74. Although efflux seems to have a role in the extrusion of fluoroquinolones in genetically drug-resistant strains75, 76, the intracellular levels of these drugs are unaffected by efflux-pump inhibitors in wild-type (that is, drug-susceptible) M. tuberculosis, at least in vitro77.
By contrast, polyamines do inhibit the uptake of fluoroquinolones in mycobacteria77, which has interesting clinical implications. Increased polyamine synthesis is associated with inflammation78; thus, polyamines accumulate in infected tissues79. More than 60 years ago, it was reported that spermine levels increase at sites of mycobacterial infection and that spermine possesses tuberculostatic activity80. Human monocytes express a non-selective polyamine transporter and increase spermidine (which is a polyamine that is distinct from, but chemically related to, spermine) uptake when they are activated. As polyamines reduce the ability of drugs to permeate M. tuberculosis, it seems that the endogenous production of polyamines in macrophages might contribute to the development of dormancy and phenotypic drug resistance of intracellular bacteria. Interestingly, reduced intrabacterial drug concentrations are associated with enhanced efflux, as growth within macrophages induces M. tuberculosis efflux pumps81. Collectively, these observations establish a multifactorial link between intrabacterial pharmacokinetics and pharmacodynamics and indicate that M. tuberculosis has evolved several mechanisms to limit drug entry during quiescence, although it is not possible to detect all of these in vitro.
Bacterial metabolism of drugs
The metabolism of xenobiotics in the human body is the result of collective biotransformation by all organs and tissues, and the liver is usually the primary site. In the case of infectious diseases, there is another crucial — and often overlooked — player in drug metabolism: the pathogen. Several anti-TB agents are prodrugs, which means that they must be metabolically converted into an active form to be functional. The large number of clinically used prodrugs has long stimulated research efforts in this field and there are several mechanisms of prodrug activation in TB; for example, pyrazinamide is converted by the bacterial enzyme pyrazinamidase into pyrazinoic acid, which has pleiotropic effects on M. tuberculosis82–84. Other prodrugs are converted into reactive species that can form adducts with NAD+ or NADH and potentially other molecules to inhibit the inhA-encoded enoyl-coA reductase and other NAD-dependent targets85, 86. Isoniazid and the thioamides are prototypes of this mechanism; they inactivate enzymes that are involved in the biosynthesis of cell wall constituents and potentially many other cellular processes that require NAD-dependent enzymes85. Interestingly, stochastic variations in the expression of catalase-peroxidase-peroxynitritase katG (which encodes the enzyme responsible for isoniazid activation) have been observed in Mycobacterium smegmatis during isoniazid treatment87, leading to the emergence of persister cells, which highlights the importance of bioactivation for bacterial survival. Some prodrugs are converted into pleiotropic reactive species that interact with, and derail, multiple cellular pathways; for example, intrabacterial redox activation of PA 824 releases several reactive nitrogen species that have broad toxic effects88. Finally, an elegant recent study has shown that para-aminosalicylate (PAS) is a prodrug that poisons folate biosynthesis by substituting for the natural substrate (which is para-aminobenzoic acid) of dihydropteroate synthase, causing early termination of folate biosynthesis by generating inhibitors of subsequent steps in the pathway89. Notably, transiently active metabolites and reactive species that are generated by prodrugs are not sufficiently stable to be captured by the analytical and imaging methods that are described here. However, these examples emphasize the clinical relevance of bioactivation by M. tuberculosis. Similar studies with all major anti-TB drug classes have the potential to reveal not only new modes of action of anti TB drugs and new targets for drug discovery but also new unsuspected prodrugs and bioactivation pathways, opening an entire new field of intrabacterial PK–PD investigations. Computational frameworks for predicting biodegradation of foreign small molecules have been established for some bacterial species, such as Gram-negative environmental bacteria90, but similar systems biology investigations are still lacking in mycobacteria. Much remains to be discovered about the ‘prodrugome’ and the ‘degradome’ of mycobacteria, including the networks that are responsible for activating and inactivating xenobiotics.
Outlook
There is growing evidence that TB pathology encompasses a broad range of lesion types with heterogeneous architectures, which limit the ability of drugs to access the infected site. Moreover, each distinct compartment hosts bacteria that can differ in their susceptibility to drugs. Thus, it is clear that no single drug in our current antibiotic arsenal can reach and kill all mycobacterial subpopulations, which highlights the need for combination therapies. However, despite the use of up to four drugs for 6–9 months against uncomplicated TB, resistance is increasing and threatens our ability to control the TB epidemic. How can we curb this trend? Optimization of treatment duration with the current four-drug regimen was a mostly empirical process, which was developed after 40 years of controlled trials by the British Medical Research Council Tuberculosis Units91. The pharmacokinetic and imaging methodologies that are described in this Progress article have the potential to inform rationally designed first-line and second-line treatment regimens that are based on the differential abilities of each companion drug to distribute into multiple types of lesions, and to ultimately combine drugs that have complementary distribution properties. To achieve this, the optimization of drug doses and regimens should rely on tissue-specific PK–PD parameters in addition to the conventional plasma-based indices.
Clearly, drug distribution is only ‘one side of the coin’ and needs to be considered in conjunction with the ability of each drug to kill the bacterial population or populations that are present in the corresponding lesion compartment. Animal studies that integrate both lesion-centric pharmacokinetic and efficacy readouts are needed to understand the effect of lesion diversity on bacterial physiology and phenotypic susceptibility to anti TB drugs (BOX 1) and to determine whether local and temporal monotherapies in specific lesion compartments could lead to the emergence of drug resistance. PK–PD indices that integrate ‘lesional’ pharmacokinetic and drug-susceptibility values can be used to determine how much of a drug is required and for how long in each lesion type, thus informing the rational design of new drug regimens. Although the rabbit model of active cavitary TB clearly recapitulates the major pathological features of human disease35, 36, 92, confirming the findings in other validated animal models (such as non-human primates) before embarking on long clinical trials is important. Lesion pharmacokinetic studies in patients with TB who are undergoing resection lung surgery are currently being carried out to identify the respective strengths and limitations of each animal model. Finally, the ability of drugs to penetrate lesions, necrotic tissue, immune cells and both active and quiescent bacilli should be considered early during the drug discovery process to guide medicinal chemistry efforts. This will require the development of medium-throughput in vitro assays that can be easily integrated during lead-compound discovery and lead-compound optimization campaigns. Knowledge of the mechanisms that drive the differential distribution of each antibiotic might also be useful for informing the rational design of drugs on the chemical level. The next major step towards curing TB and preventing the development of resistance will come from a combination of complementary drugs, each of which preferentially distributes in the lesion or lesion compartment where its most vulnerable target bacterial population resides.
Acknowledgements
The author thanks T. Dick, J. Sarathy and B. Prideaux for many stimulating discussions. V.D. is funded by the Tuberculosis Drug Accelerator program of the Bill and Melinda Gates Foundation and grant R01AI106398-01 from the US National Institutes of Health-National Institute of Allergy and Infectious Diseases (NIH-NIAID).
Glossary
- Equilibration half-life
A measure of the time that is required to reach a steady-state drug concentration at the site of drug action, assuming that the concentration remains constant in plasma.
- Foamy macrophages
Lipid-loaded macrophages that are found in the inner layers of pulmonary granulomas.
- Free drug fraction
The percentage of drug molecules that are not bound to proteins such as albumin, globulins, glycoproteins and lipoproteins; typically calculated in the plasma. It is generally accepted that only this fraction is capable of passively diffusing between body compartments and it is therefore the drug fraction that can exert activity at the site of infection.
- HPLC coupled to tandem mass spectrometry
(LC–MS/MS). An analytical method that combines high performance chromatographic separation of analytes with mass-based quantification of molecular ions. Tandem mass spectrometry (MS/MS) enables the quantitation of small-molecule drugs in the complex biological matrices that are typical of biological fluids and tissues.
- Intrabacterial pharmacokinetics
The change in drug concentrations over time in individual bacterial cells grown in vitro, in which intracellular drug concentrations change as a result of passive or active uptake, pathogen-mediated metabolism and efflux of the drug.
- Liposomes
Artificially prepared vesicles that are composed of a lipid bilayer and are used as vehicles for the administration and slow release of nutrients and pharmaceutical drugs. Liposomes can include surface ligands that enable targeting of specific tissues and cell types.
- MALDI mass spectrometry imaging
(MALDI–MSI). A label-free semiquantitative imaging technology that generates two-dimensional ion maps of molecules and their metabolites in biological tissue sections using mass-based detection. MSI preserves the spatial profile and tissue architecture, which enables the high-resolution localization of drugs, lipids and peptides of interest, relative to the underlying tissue structure.
- Phagolysosome
A cytoplasmic body that is formed from the fusion of a phagosome (which is a vesicle formed around a particle by phagocytosis) with a lysosome (which contains hydrolytic enzymes).
- Pharmacodynamics
The effects that a drug has on an organism (that is, ‘how the drug affects the body’) or on bacterial cultures in vitro. In the case of antibiotics, this is often determined in animal models of disease and is typically quantified as the difference in bacterial load (or colony forming units (CFU)) over time in selected tissues.
- Pharmacokinetics
The change in drug concentrations over time in blood or tissues, which is determined by absorption through the gastrointestinal tract, distribution from one compartment to another, metabolism and elimination from the body. Pharmacokinetics is frequently referred to as ‘how the body handles the drug’.
- PK–PD parameters
(Pharmacokinetic–pharmacodynamic parameters). The ratios between certain drug-exposure variables (such as peak plasma concentration (Cmax) or area under the concentration–time curve (AUC)) at a given dose and the antibacterial activity (minimum inhibitory concentration) of the drug in vitro. These ratios provide an estimate of in vivo drug exposure relative to in vitro potency, and rules-of-thumb have been established for all of the major antibiotic classes (for example, the efficacy of aminoglycosides is mostly driven by Cmax/MIC). By correlating these parameters with the observed efficacy of the drug in animal models at the corresponding dose, it is possible to predict the dose and dosing frequency that are required to achieve a desired pharmacological effect in patients.
- Polyamines
Organic compounds that contain two or more primary amino groups, which are found at reasonably high concentrations in both prokaryotic and eukaryotic cells.
- Positron emission tomography
(PET). A nuclear medical and preclinical whole-body imaging modality that produces a three-dimensional image of functional processes or drugs in the body.
- Prodrugs
Drugs that are delivered as inactive precursors and that require enzymatic conversion to one or more active derivatives either by the host or by the pathogen. A number of anti-tuberculosis agents are prodrugs, such as isoniazid, pyrazinamide, the thioamides and nitroimidazoles.
- SOS response
A global response to DNA damage in bacteria.
Footnotes
Competing interests statement
The author declares no competing interests.
References
- 1.World Health Organization. Global Tuberculosis Report. WHO; 2012. [Google Scholar]
- 2.Bass JB, Jr, et al. Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and the Centers for Disease Control and Prevention. Am. J. Respir. Crit. Care Med. 1994;149:1359–1374. doi: 10.1164/ajrccm.149.5.8173779. [DOI] [PubMed] [Google Scholar]
- 3.Bates JH, Nardell E. Institutional control measures for tuberculosis in the era of multiple drug resistance: ACCP/ATS Consensus Conference. Chest. 1995;108:1690–1710. doi: 10.1378/chest.108.6.1690. [DOI] [PubMed] [Google Scholar]
- 4.Monedero I, Caminero JA. MDR-/XDR TB management: what it was, current standards and what is ahead. Expert Rev. Respir. Med. 2009;3:133–145. doi: 10.1586/ers.09.6. [DOI] [PubMed] [Google Scholar]
- 5.Caminero JA, Sotgiu G, Zumla A, Migliori GB. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect. Dis. 2010;10:621–629. doi: 10.1016/S1473-3099(10)70139-0. [DOI] [PubMed] [Google Scholar]
- 6.Golden MP, Vikram HR. Extrapulmonary tuberculosis. Am. Family Physician. 2005;72:1761–1768. [PubMed] [Google Scholar]
- 7.Diacon AH, et al. Early bactericidal activity of high-dose rifampin in patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob. Agents Chemother. 2007;51:2994–2996. doi: 10.1128/AAC.01474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Ingen J, et al. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin. Infect. Dis. 2011;52:e194–e199. doi: 10.1093/cid/cir184. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen EI, Friberg LE. Pharmacokinetic–pharmacodynamic modeling of antibacterial drugs. Pharmacol. Rev. 2013;65:1053–1090. doi: 10.1124/pr.111.005769. [DOI] [PubMed] [Google Scholar]
- 10.Egelund EF, Barth AB, Peloquin CA. Population pharmacokinetics and its role in anti-tuberculosis drug development and optimization of treatment. Curr. Pharm. Des. 2011;17:2889–2899. doi: 10.2174/138161211797470246. [DOI] [PubMed] [Google Scholar]
- 11.Kjellsson MC, et al. Pharmacokinetic evaluation of the penetration of antituberculosis agents in rabbit pulmonary lesions. Antimicrob. Agents Chemother. 2012;56:446–457. doi: 10.1128/AAC.05208-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nature Immunol. 2009;10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva Miranda M, Breiman A, Allain S, Deknuydt F, Altare F. The tuberculous granuloma: an unsuccessful host defence mechanism providing a safety shelter for the bacteria? Clin. Dev. Immunol. 2012;2012:139127. doi: 10.1155/2012/139127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leong FJ, Eum SY, Via LE, Barry CE., 3rd . In: A Color Atlas of Comparative Pathology of Pulmonary Tuberculosis. Leong FJ, Dartois V, Dick T, editors. CRC Press; 2011. pp. 53–81. [Google Scholar]
- 16.Vergne I, et al. Mechanism of phagolysosome biogenesis block by viable. Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA. 2005;102:4033–4038. doi: 10.1073/pnas.0409716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simeone R, et al. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog. 2012;8:e1002507. doi: 10.1371/journal.ppat.1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seiler P, et al. Cell-wall alterations as an attribute of Mycobacterium tuberculosis in latent infection. J. Infect. Dis. 2003;188:1326–1331. doi: 10.1086/378563. [DOI] [PubMed] [Google Scholar]
- 19.Fenhalls G, et al. In situ detection of Mycobacterium tuberculosis transcripts in human lung granulomas reveals differential gene expression in necrotic lesions. Infect. Immun. 2002;70:6330–6338. doi: 10.1128/IAI.70.11.6330-6338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan GJ, et al. Multiple M. tuberculosis phenotypes in mouse and guinea pig lung tissue revealed by a dual-staining approach. PLoS ONE. 2010;5:e11108. doi: 10.1371/journal.pone.0011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Driver ER, et al. Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against. Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2012;56:3181–3195. doi: 10.1128/AAC.00217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoff DR, et al. Location of intra- and extracellular M. tuberculosis populations in lungs of mice and guinea pigs during disease progression and after drug treatment. PLoS ONE. 2010;6:e17550. doi: 10.1371/journal.pone.0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eum SY, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137:122–128. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin EJ. The granuloma in tuberculosis — friend or foe? N. Engl. J. Med. 2009;360:2471–2473. doi: 10.1056/NEJMcibr0902539. [DOI] [PubMed] [Google Scholar]
- 25.Dartois V, Barry CE., 3rd A medicinal chemists’ guide to the unique difficulties of lead optimization for tuberculosis. Bioorg. Med. Chem. Lett. 2013;23:4741–4750. doi: 10.1016/j.bmcl.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dartois V, Barry CE. Clinical pharmacology and lesion penetrating properties of second- and third-line antituberculous agents used in the management of multidrug-resistant (MDR) and extensively-drug resistant (XDR) tuberculosis. Curr. Clin. Pharmacol. 2010;5:96–114. doi: 10.2174/157488410791110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nature Rev. Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 28.Kyle AH, Huxham LA, Yeoman DM, Minchinton AI. Limited tissue penetration of taxanes: a mechanism for resistance in solid tumors. Clin. Cancer Res. 2007;13:2804–2810. doi: 10.1158/1078-0432.CCR-06-1941. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan G, et al. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect. Immun. 2003;71:7099–7108. doi: 10.1128/IAI.71.12.7099-7108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barclay WR, Ebert RH, Le Roy GV, Manthei RW, Roth LJ. Distribution and excretion of radioactive isoniazid in tuberculous patients. J. Am. Med. Assoc. 1953;151:1384–1388. [PubMed] [Google Scholar]
- 31.Kislitsyna NA, Kotova NI. Rifampicin and isoniazid concentration in the blood and resected lungs in tuberculosis with combined use of the preparations. Probl Tuberk. 1980;8:63–65. (in Russian) [PubMed] [Google Scholar]
- 32.Canetti G, Parrot R, Porven G, Le Lirzin M. Rifomycin levels in the lung and tuberculous lesions in man. Acta Tuberc Pneumol Belg. 1969;60:315–322. (in French) [PubMed] [Google Scholar]
- 33.Kiss IJ, Farago E, Juhaz I, Bacsa S, Fabian E. Investigation on the serum and lung tissue level of rifampicin in man. Int. J. Clin. Pharmacol. Biopharm. 1976;13:42–47. [PubMed] [Google Scholar]
- 34.Kislitsyna NA. Comparative evaluation of rifampicin and isoniazid penetration into the pathological foci of the lungs in tuberculosis patients. Probl Tuberk. 1985;4:55–57. (in Russian) [PubMed] [Google Scholar]
- 35.Dannenberg AM., Jr . Pathogenesis of Human Pulmonary Tuberculosis. ASM Press; 2006. pp. 22–33. [Google Scholar]
- 36.Kaplan G, Tsenova L. In: A Color Atlas of Comparative Pathology of Pulmonary Tuberculosis. Leong FJ, Dartois V, Dick T, editors. CRC Press; 2011. pp. 107–128. [Google Scholar]
- 37.Subbian S, et al. Phosphodiesterase-4 inhibition combined with isoniazid treatment of rabbits with pulmonary tuberculosis reduces macrophage activation and lung pathology. Am. J. Pathol. 2011;179:289–301. doi: 10.1016/j.ajpath.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Via LE, et al. Infection dynamics and response to chemotherapy in a rabbit model of tuberculosis using [18F]2 fluoro-deoxy d glucose positron emission tomography and computed tomography. Antimicrob. Agents Chemother. 2012;56:4391–4402. doi: 10.1128/AAC.00531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michot JM, Seral C, Van Bambeke F, Mingeot- Leclercq MP, Tulkens PM. Influence of efflux transporters on the accumulation and efflux of four quinolones (ciprofloxacin, levofloxacin, garenoxacin, and moxifloxacin) in J774 macrophages. Antimicrob. Agents Chemother. 2005;49:2429–2437. doi: 10.1128/AAC.49.6.2429-2437.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dutta S, et al. Steady-state propofol brain:plasma and brain:blood partition coefficients and the effect-site equilibration paradox. Br. J. Anaesth. 1998;81:422–424. doi: 10.1093/bja/81.3.422. [DOI] [PubMed] [Google Scholar]
- 41.Mackaness GB. The intracellular activation of pyrazinamide and nicotinamide. Am. Rev. Tuberc. 1956;74:718–728. doi: 10.1164/artpd.1956.74.5.718. [DOI] [PubMed] [Google Scholar]
- 42.Marcinkeviciene JA, Magliozzo RS, Blanchard JS. Purification and characterization of the Mycobacterium smegmatis catalase-peroxidase involved in isoniazid activation. J. Biol. Chem. 1995;270:22290–22295. doi: 10.1074/jbc.270.38.22290. [DOI] [PubMed] [Google Scholar]
- 43.Prideaux B, et al. High-sensitivity MALDI–MRM–MS imaging of moxifloxacin distribution in tuberculosis-infected rabbit lungs and granulomatous lesions. Anal. Chem. 2011;83:2112–2118. doi: 10.1021/ac1029049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prideaux B, Stoeckli M. Mass spectrometry imaging for drug distribution studies. J. Prot. 2012;75:4999–5013. doi: 10.1016/j.jprot.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 45.Seeley EH, Caprioli RM. 3D imaging by mass spectrometry: a new frontier. Anal. Chem. 2012;84:2105–2110. doi: 10.1021/ac2032707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenaerts AJ, et al. Location of persisting mycobacteria in a Guinea pig model of tuberculosis revealed by r207910. Antimicrob. Agents Chemother. 2007;51:3338–3345. doi: 10.1128/AAC.00276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin PL, et al. Metronidazole prevents reactivation of latent Mycobacterium tuberculosis infection in macaques. Proc. Natl Acad. Sci. USA. 2012;109:14188–14193. doi: 10.1073/pnas.1121497109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gillespie SH, Basu S, Dickens AL, O’Sullivan DM, McHugh TD. Effect of subinhibitory concentrations of ciprofloxacin on Mycobacterium fortuitum mutation rates. J. Antimicrob. Chemother. 2005;56:344–348. doi: 10.1093/jac/dki191. [DOI] [PubMed] [Google Scholar]
- 49.Mitchison DA. The search for new sterilizing anti-tuberculosis drugs. Front. Biosci. 2004;9:1059–1072. doi: 10.2741/1293. [DOI] [PubMed] [Google Scholar]
- 50.Mitchison D, Davies G. The chemotherapy of tuberculosis: past, present and future. Int. J. Tuberc Lung Dis. 2012;16:724–732. doi: 10.5588/ijtld.12.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joint Tuberculosis Committee of the British Thoracic Society. Chemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998. Thorax. 1998;53:536–548. [PMC free article] [PubMed] [Google Scholar]
- 52.Ginsburg AS, et al. Emergence of fluoroquinolone resistance in Mycobacterium tuberculosis during continuously dosed moxifloxacin monotherapy in a mouse model. Antimicrob. Agents Chemother. 2005;49:3977–3979. doi: 10.1128/AAC.49.9.3977-3979.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colijn C, Cohen T, Ganesh A, Murray M. Spontaneous emergence of multiple drug resistance in tuberculosis before and during therapy. PLoS ONE. 2011;6:e18327. doi: 10.1371/journal.pone.0018327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinstein EA, et al. Noninvasive determination of 2-[18F]-fluoroisonicotinic acid hydrazide pharmacokinetics by positron emission tomography in Mycobacterium tuberculosis-infected mice. Antimicrob. Agents Chemother. 2012;56:6284–6290. doi: 10.1128/AAC.01644-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu L, et al. Radiosynthesis and bioimaging of the tuberculosis chemotherapeutics isoniazid, rifampicin and pyrazinamide in baboons. J. Med. Chem. 2010;53:2882–2891. doi: 10.1021/jm901858n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goutelle S, Bourguignon L, Jelliffe RW, Conte JE, Jr, Maire P. Mathematical modeling of pulmonary tuberculosis therapy: insights from a prototype model with rifampin. J. Theor. Biol. 2011;282:80–92. doi: 10.1016/j.jtbi.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 57.Mitchison DA. The action of antituberculosis drugs in short-course chemotherapy. Tubercle. 1985;66:219–225. doi: 10.1016/0041-3879(85)90040-6. [DOI] [PubMed] [Google Scholar]
- 58.Kelly C, Jefferies C, Cryan SA. Targeted liposomal drug delivery to monocytes and macrophages. J. Drug Deliv. 2011;2011:727241. doi: 10.1155/2011/727241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clemens DL, et al. Targeted intracellular delivery of antituberculosis drugs to Mycobacterium tuberculosis-infected macrophages via functionalized mesoporous silica nanoparticles. Antimicrob. Agents Chemother. 2012;56:2535–2545. doi: 10.1128/AAC.06049-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Griffiths G, Nystrom B, Sable SB, Khuller GK. Nanobead-based interventions for the treatment and prevention of tuberculosis. Nature Rev. Microbiol. 2010;8:827–834. doi: 10.1038/nrmicro2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pinheiro M, Lucio M, Lima JL, Reis S. Liposomes as drug delivery systems for the treatment of TB. Nanomed. (Lond.) 2011;6:1413–1428. doi: 10.2217/nnm.11.122. [DOI] [PubMed] [Google Scholar]
- 62.De Lorenzo S, et al. Efficacy and safety of meropenem–clavulanate added to linezolid-containing regimens in the treatment of MDR-/XDR TB. Eur. Respir. J. 2013;41:1386–1392. doi: 10.1183/09031936.00124312. [DOI] [PubMed] [Google Scholar]
- 63.Payen MC, et al. Clinical use of the meropenem–clavulanate combination for extensively drug-resistant tuberculosis. Int. J. Tuberc Lung Dis. 2012;16:558–560. doi: 10.5588/ijtld.11.0414. [DOI] [PubMed] [Google Scholar]
- 64.Hand WL, Corwin RW, Steinberg TH, Grossman GD. Uptake of antibiotics by human alveolar macrophages. Am. Rev. Respir. Dis. 1984;129:933–937. doi: 10.1164/arrd.1984.129.6.933. [DOI] [PubMed] [Google Scholar]
- 65.Johnson JD, Hand WL, Francis JB, King- Thompson N, Corwin RW. Antibiotic uptake by alveolar macrophages. J. Lab Clin. Med. 1980;95:429–439. [PubMed] [Google Scholar]
- 66.Conte JE, et al. Effects of AIDS and gender on steady-state plasma and intrapulmonary ethambutol concentrations. Antimicrob. Agents Chemother. 2001;45:2891–2896. doi: 10.1128/AAC.45.10.2891-2896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodvold KA, Yoo L, George JM. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antifungal, antitubercular and miscellaneous anti-infective agents. Clin. Pharmacokinet. 2011;50:689–704. doi: 10.2165/11592900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 68.Ziglam HM, Baldwin DR, Daniels I, Andrew JM, Finch RG. Rifampicin concentrations in bronchial mucosa, epithelial lining fluid, alveolar macrophages and serum following a single 600 mg oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 2002;50:1011–1015. doi: 10.1093/jac/dkf214. [DOI] [PubMed] [Google Scholar]
- 69.Soman A, Honeybourne D, Andrews J, Jevons G, Wise R. Concentrations of moxifloxacin in serum and pulmonary compartments following a single 400 mg oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 1999;44:835–838. doi: 10.1093/jac/44.6.835. [DOI] [PubMed] [Google Scholar]
- 70.Andrews JM, et al. Concentrations of levofloxacin (HR 355) in the respiratory tract following a single oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 1997;40:573–577. doi: 10.1093/jac/40.4.573. [DOI] [PubMed] [Google Scholar]
- 71.Schuler P, et al. Penetration of sparfloxacin and ciprofloxacin into alveolar macrophages, epithelial lining fluid, and polymorphonuclear leucocytes. Eur. Respir. J. 1997;10:1130–1136. doi: 10.1183/09031936.97.10051130. [DOI] [PubMed] [Google Scholar]
- 72.Van de Velde S, et al. Contrasting effects of human THP-1 cell differentiation on levofloxacin and moxifloxacin intracellular accumulation and activity against Staphylococcus aureus and Listeria monocytogenes. J. Antimicrob. Chemother. 2008;62:518–521. doi: 10.1093/jac/dkn232. [DOI] [PubMed] [Google Scholar]
- 73.Sacchettini JC, Rubin EJ, Freundlich JS. Drugs versus bugs: in pursuit of the persistent predator. Mycobacterium tuberculosis. Nature Rev. Microbiol. 2008;6:41–52. doi: 10.1038/nrmicro1816. [DOI] [PubMed] [Google Scholar]
- 74.Sarathy J, Dartois V, Dick T, Gengenbacher M. Reduced drug uptake in phenotypically resistant nutrient-starved non-replicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2013;57:1648–1653. doi: 10.1128/AAC.02202-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Escribano I, et al. Importance of the efflux pump systems in the resistance of Mycobacterium tuberculosis to fluoroquinolones and linezolid. Chemotherapy. 2007;53:397–401. doi: 10.1159/000109769. [DOI] [PubMed] [Google Scholar]
- 76.Louw GE, et al. Rifampicin reduces susceptibility to ofloxacin in rifampicin-resistant Mycobacterium tuberculosis through efflux. Am. J. Respir. Crit. Care Med. 2011;184:269–276. doi: 10.1164/rccm.201011-1924OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarathy JP, Lee E, Dartois V. Polyamines inhibit porin-mediated fluoroquinolone uptake in mycobacteria. PLoS ONE. 2013;8:e65806. doi: 10.1371/journal.pone.0065806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Babbar N, Gerner EW. Targeting polyamines and inflammation for cancer prevention. Recent Results Cancer Res. 2011;188:49–64. doi: 10.1007/978-3-642-10858-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clarke JR, Tyms AS. Polyamine biosynthesis in cells infected with different clinical isolates of human cytomegalovirus. J. Med. Virol. 1991;34:212–216. doi: 10.1002/jmv.1890340403. [DOI] [PubMed] [Google Scholar]
- 80.Hirsch JG, Dubos RJ. The effect of spermine on tubercle bacilli. J. Exp. Med. 1952;95:191–208. doi: 10.1084/jem.95.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adams KN, et al. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell. 2011;145:39–53. doi: 10.1016/j.cell.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y, Mitchison D. The curious characteristics of pyrazinamide: a review. Int. J. Tuberc Lung Dis. 2003;7:6–21. [PubMed] [Google Scholar]
- 83.Shi W, et al. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science. 2011;333:1630–1632. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zimhony O, Cox JS, Welch JT, Vilcheze C, Jacobs WR., Jr Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of. Mycobacterium tuberculosis. Nature Med. 2000;6:1043–1047. doi: 10.1038/79558. [DOI] [PubMed] [Google Scholar]
- 85.Argyrou A, Jin L, Siconilfi-Baez L, Angeletti RH, Blanchard JS. Proteome-wide profiling of isoniazid targets in. Mycobacterium tuberculosis. Biochemistry. 2006;45:13947–13953. doi: 10.1021/bi061874m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nishida CR, Ortiz de Montellano PR. Bioactivation of antituberculosis thioamide and thiourea prodrugs by bacterial and mammalian flavin monooxygenases. Chem. Biol. Interact. 2011;192:21–25. doi: 10.1016/j.cbi.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wakamoto Y, et al. Dynamic persistence of antibiotic-stressed mycobacteria. Science. 2013;339:91–95. doi: 10.1126/science.1229858. [DOI] [PubMed] [Google Scholar]
- 88.Singh R, et al. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science. 2008;322:1392–1395. doi: 10.1126/science.1164571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chakraborty S, Gruber T, Barry CE, 3rd, Boshoff HI, Rhee KY. Para-aminosalicylic acid acts as an alternative substrate of folate metabolism in Mycobacterium tuberculosis. Science. 2013;339:88–91. doi: 10.1126/science.1228980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Finley SD, Broadbelt LJ, Hatzimanikatis V. Computational framework for predictive biodegradation. Biotechnol. Bioeng. 2009;104:1086–1097. doi: 10.1002/bit.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int. J. Tuberc Lung Dis. 1999;3:S231–S279. [PubMed] [Google Scholar]
- 92.Subbian S, et al. Spontaneous latency in a rabbit model of pulmonary tuberculosis. Am. J. Pathol. 2012;181:1711–1724. doi: 10.1016/j.ajpath.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cohen T, Murray M. Modeling epidemics of multidrug-resistant M. tuberculosis of heterogeneous fitness. Nature Med. 2004;10:1117–1121. doi: 10.1038/nm1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zainuddin ZF, Dale JW. Does Mycobacterium tuberculosis have plasmids? Tubercle. 1990;71:43–49. doi: 10.1016/0041-3879(90)90060-l. [DOI] [PubMed] [Google Scholar]
- 95.Ramaswamy S, Musser JM. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber Lung Dis. 1998;79:3–29. doi: 10.1054/tuld.1998.0002. [DOI] [PubMed] [Google Scholar]
- 96.Gupta R, Espinal M. A prioritised research agenda for DOTS-Plus for multidrug-resistant tuberculosis (MDR TB) Int. J. Tuberc Lung Dis. 2003;7:410–414. [PubMed] [Google Scholar]
- 97.Migliori GB, et al. Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur. Respir. J. 2007;30:623–626. doi: 10.1183/09031936.00077307. [DOI] [PubMed] [Google Scholar]
- 98.Nathan C. Fresh approaches to anti-infective therapies. Sci Transl Med. 2012;4:140sr2. doi: 10.1126/scitranslmed.3003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinb.) 2004;84:29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 100.Mitchison DA, Jindani A, Davies GR, Sirgel F. Isoniazid activity is terminated by bacterial persistence. J. Infect. Dis. 2007;195:1871–1873. doi: 10.1086/518046. [DOI] [PubMed] [Google Scholar]
- 101.Levin BR, Rozen DE. Non-inherited antibiotic resistance. Nature Rev. Microbiol. 2006;4:556–562. doi: 10.1038/nrmicro1445. [DOI] [PubMed] [Google Scholar]
- 102.Dartois V, Leong FJ, Dick T. In: Drug Discovery in Infectious Diseases. Seltzer P, editor. Wiley-VCH; 2009. pp. 415–440. [Google Scholar]