Abstract
Development of new drugs is one of the strategies for malaria control. The biosynthesis of several isoprenoids in Plasmodium falciparum was recently described. Interestingly, some intermediates and final products biosynthesized by this pathway in mammals differ from those biosynthesized in P. falciparum. These facts prompted us to evaluate various terpenes, molecules with a similar chemical structure to the intermediates of the isoprenoids pathway, as potential antimalarial drugs. Different terpenes and S-farnesylthiosalicylic acid were tested on cultures of the intraerythrocytic stages of P. falciparum, and the 50% inhibitory concentrations for each one were found: farnesol, 64 μM; nerolidol, 760 nM; limonene, 1.22 mM; linalool, 0.28 mM; and S-farnesylthiosalicylic acid, 14 μM. All the terpenes tested inhibited dolichol biosynthesis in the trophozoite and schizont stages when [1-(n)-3H]farnesyl pyrophosphate triammonium salt ([3H]FPP) was used as precursor. Farnesol, nerolidol, and linalool showed stronger inhibitory activity on the biosynthesis of the isoprenic side chain of the benzoquinone ring of ubiquinones in the schizont stage. Treatment of schizont stages with S-farnesylthiosalicylic acid led to a decrease in intensity of the band corresponding a p21ras protein. The inhibitory effect of terpenes and S-farnesylthiosalicylic acid on the biosynthesis of both dolichol and the isoprenic side chain of ubiquinones and the isoprenylation of proteins in the intraerythrocytic stages of P. falciparum appears to be specific, because overall protein biosynthesis was not affected. Combinations of some terpenes or S-farnesylthiosalicylic acid tested in this work with other antimalarial drugs, like fosmidomycin, could be a new strategy for the treatment of malaria.
Despite attempts at eradication made since the 1950s, malaria is still the most important parasitic disease of humans. As a consequence of increasing parasite resistance to virtually all reagents used for malaria therapy, new approaches to drug design are urgently needed (38).
Isoprenoids play important roles as components of structural cholesterol, steroid hormones in mammals, carotenoids in plants, and ubiquinones in all living organisms (34, 35).
Recently, the identification of two genes encoding the enzymes 1-deoxy-d-xylulose-5-P (DOXP) synthase and DOXP reductoisomerase suggested that isoprenoid biosynthesis in Plasmodium falciparum depends on the DOXP/2-C-methyl-d-erythritol-4-phosphate (MEP) pathway (21). In contrast, in humans, isoprenoids are synthesized via the mevalonate pathway (18). Fosmidomycin, an inhibitor of DOXP reductoisomerase, showed antimalarial activity in vitro and in vivo (21); however, 10% of patients failed to show cure with this treatment (42). To avoid resistance or the rapid emergence of resistance, combinations with antimalarial inhibitors operating on the different points in the same metabolic pathway could be synergistic (39). In P. falciparum all the metabolic pathways and associated enzymes downstream of isopentenyl pyrophosphate (IPP) need to be identified and characterized (39).
In a previous work, we showed the presence of an active isoprenoid pathway for the biosynthesis of dolichol of 11 and 12 isoprenic units (4) and side chain of the 8 and 9 isoprenic units attached to benzoquinone rings of ubiquinones in P. falciparum (7). In mammalian cells, the isoprenic chains of dolichol and ubiquinones comprise 20 to 22 and 10 isoprenic units, respectively (2, 23). In plants, the isoprenoid pathway biosynthesizes terpenes whose antibacterial, antifungal, and antiparasitic activities have been previously reported (5, 34). The chemical structure of some terpenes resembles that of some intermediates of the isoprenoid pathway (10) and may interfere with the biosynthesis of the latter in other organisms. In our laboratory, we demonstrated the inhibition of P. falciparum protein isoprenylation by the monoterpene limonene (29). One of the oldest antimalarial drugs is a sesquiterpene lactone, artemisinin, whose mechanism of action has been recently described (13).
Therefore, the possibility of developing new antimalarial drugs that could interfere with the biosynthesis of the dolichols, with the isoprenic chain of ubiquinones, and with protein isoprenylation led us to study the effects of different terpenes purified from essential oils (6, 27).
In the present study we investigated the effects of various terpenes (farnesol, nerolidol, limonene, and linalool) and the S-farnesylthiosalicylic acid (FTS) on the biosynthesis of dolichol and the isoprenic side chain of ubiquinones as well as on protein isoprenylation in the intraerythrocytic stages of P. falciparum.
MATERIALS AND METHODS
Parasite culture.
The experiments were performed with isolate NF54, clone 3D7 of P. falciparum. Parasites were cultured according to the method of Trager and Jensen (37) as modified by Kimura et al. (25). The gas mixture of the tissue culture flasks (75 cm2) contained 5.05% CO2, 4.93% O2, and 90.2% N2.
Parasite development and multiplication was monitored by microscopic evaluation of Giemsa-stained thin smears. Synchronization was obtained by two treatments with 6% (wt/vol) Plasmagel (Laboratoire Roger Bellon, Neuilly sur Seine, France) solution in physiological saline (31). Starting with asynchronous cultures, schizont stages were concentrated by flotation in Plasmagel and subcultured with fresh erythrocytes at 48-h intervals. Ring (1 to 20 h after reinvasion), trophozoite (20 to 30 h after reinvasion), and schizont (30 to 45 h after reinvasion) forms were purified on a 40%/70%/80% discontinuous Percoll (Pharmacia LKB, Uppsala, Sweden) gradient (1).
Inhibition tests.
Terpenes and FTS were purchased from Sigma (St. Louis, Mo.), diluted in methanol, and used at the following concentrations: farnesol, 1 to 200 μM; nerolidol, 5 to 2,500 nM; limonene, 0.05 to 5.0 mM; linalool, 0.01 to 1 mM. Controls with methanol were performed in parallel. FTS was diluted in chloroform at 0.1 M stock solution. Twenty-five microliters of 0.1 M FTS in chloroform was evaporated to dryness and then dissolved in 4 μl of ethanol plus 7 μl of 1 M NaOH to which 900 μl of 0.007 M Na2HPO4-0.01 M NaH2PO4 (pH 7.4)-0.15 M NaCl (PBS) was subsequently added (28). The final concentrations of FTS used were between 0.1 and 500 μM.
The method of Desjardins et al. (8) was used to determine the 50% inhibitory concentrations (IC50s) and the IC90s of the various terpenes and FTS. Briefly, ring stage parasite cultures (5% hematocrit, 2% parasitemia) were exposed to increasing drug concentrations. After 24 h in culture, [G-3H]hypoxanthine (270 GBq/mmol; Amersham Life Sciences, Little Chalfont, Buckinghamshire, United Kingdom) was added (final concentration, 5 μCi/ml), and cells were harvested after an additional 24-h incubation period. All tests were done in triplicate. Suspensions of similarly treated uninfected erythrocytes were used for background subtraction. Parasitemia and parasite morphology were determined by Giemsa-stained smears immediately before the start and at the end of assay. The IC50s and IC90s of growth inhibition were calculated by Probit analysis (Statistical Software 13.30; Minitab Inc.) (29).
Inhibition tests with each of the terpenes and FTS were carried out in flat-bottom microtiter plates (Falcon). Freshly synchronized cultures of 5% hematocrit and 1% parasitemia (ring stage parasites) were exposed to several dilutions of the compound to be tested in normal culture medium. After 24, 48, 72, and 96 h (if not otherwise stated), the percentage comprised by each form was determined. After counting, values for each form were expressed as percentages of total number of parasites (counting multinuclear schizont-infected red blood cells as one cell) (29). Student's t test was applied to the results of three independent experiments to check for significant discrepancies between each form per time point in treated versus untreated parasites.
Metabolic labeling.
Mixed cultures of P. falciparum with parasitemias around 10% were treated with 25 μM farnesol, 500 nM nerolidol, 0.5 mM limonene, 0.1 mM linalool, or 5.5 μM FTS for 30 h and labeled for 18 h, in the presence of each drug with [1-(n)-3H]geranylgeranyl pyrophosphate triammonium salt ([3H]GGPP) (16.5 Ci/mmol, 3.125 μCi/ml; Amersham), [1-(n)-3H]farnesyl pyrophosphate triammonium salt ([3H]FPP) (16.5 Ci/mmol, 3.125 μCi/ml; Amersham), or [1-14C]isopentenyl pyrophosphate triammonium salt ([14C]IPP) (55.0 Ci/mmol; 3.125 μCi/ml Amersham) in RPMI 1640 normal medium. The same protocol was used when parasites were labeled with l-[35S]methionine (25 μCi/ml; >1,000 Ci/mmol; Amersham) in 10 mM methionine-deficient RPMI medium. Untreated parasites were developed under identical experimental conditions and used as controls (4).
Each stage (ring, trophozoite, and schizont forms) was then purified as described above, followed by lysis of cells in twice their volume of a solution of ice-cold 10 mM Tris-HCl (pH 7.2), 150 mM NaCl, 2% (vol/vol) Triton X-100, 0.2 mM phenylmethylsulfonyl fluoride, 5 mM iodoacetamide, 1 mM N-(p-tosyl-lysine) chloromethyl ketone, and leupeptin (1 μg/ml). After incubation for 15 min at 4°C, lysates were centrifuged at 10,000 × g for 30 min and supernatants were stored in liquid N2 for subsequent sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. For the analysis of isoprenoids each purified parasite stage was freeze-dried prior to lipid extraction (4, 25).
Another protocol was used to evaluate protein biosynthesis. Synchronous P. falciparum ring stage cultures, untreated or treated with terpenes or FTS for 48 h, were labeled with l-[35S]methionine (25 μCi/ml) in 10 μM methionine-deficient RPMI medium, at the beginning or after 24 h of treatment. Aliquots were collected at different times (0 to 48 h) and precipitated with 12% (wt/vol) trichloroacetic acid, and radioactivity was measured with a Beckman 5000 β-counter (4).
Lipid extraction.
Each purified and freeze-dried stage was extracted with hexane (three times; 0.5 ml each). The pooled extracts were dried under a nitrogen stream and resuspended in 500 μl of hexane. Aliquots of each extract were monitored for radioactivity (4).
HPTLC.
Hexane extracts obtained from similar amounts of parasites treated with terpene and FTS or untreated parasites or uninfected erythrocytes were analyzed by high-performance thin-layer chromatography (HPTLC). Plates were developed with hexane-diethylether-acetic acid (80:20:1, vol/vol/vol) at 4°C (30). Authentic standards of coenzyme Q7-10, farnesol, geraniol, and dolichol 11 were run on the same plate. Plates were sprayed with En3Hance (DuPont NEN) and subjected to autoradiography for several days at −70°C. Standards were visualized with iodine vapor.
Gel electrophoresis.
SDS-PAGE was performed in 12.5% gels as described elsewhere (26). The same number of drug-treated or untreated parasites as mentioned above were solubilized in SDS sample buffer and applied to each well for analysis. All gels were treated with Amplify (Amersham), dried, and exposed to Kodak X-Omat film with intensifying screen sets at −70°C.
Immunoprecipitation assays.
Samples stored in liquid N2 were resuspended in immunoprecipitation buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% [vol/vol] Triton X-100, 0.5% [wt/vol] sodium deoxycholate, 0.1% [wt/vol] SDS, and a 5-μg/ml concentration of a protease inhibitor cocktail [0.2 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 2 mM β-mercaptoethanol, chymostatin {5 mg/ml}, and a 1-μg/ml concentration each of leupeptin, antipain, and pepstatin A]) and then precleared with protein A-Sepharose beads (Pharmacia) (29). Each stage (ring, trophozoite, and schizont forms) was then incubated with anti-human-Ras or anti-Rap 1/Krev-1 monoclonal immunoglobulins (1:50 dilution; Santa Cruz Biotechnology, Inc., Calif.) for 2 h at 4°C. The antigen-antibody complex was precipitated using 100 μl of a 10% protein A-Sepharose slurry. After five washes with PBS, the bound antigen was released in SDS sample buffer and analyzed by SDS-PAGE and autoradiography (24).
Reverse-phase high-performance liquid chromatography (HPLC).
Each purified parasite (ring, trophozoite, and schizont forms) stage was freeze-dried and successively extracted with hexane (three times; 0.5 ml each) (extract I), chloroform-methanol (1:1; vol/vol; three times; 0.5 ml each) (extract II), and chloroform-methanol-water (10:10:3; vol/vol/vol; three times; 0.5 ml each) (extract III) (4). For the analysis of dolichyl-phosphate (dolichyl-P) and dolichyl-pyrophosphate (dolichyl-PP), extract II or extract III was subjected to acid hydrolysis. Samples were hydrolyzed with 100 μl of a solution of 50% HCl in a screw-cap test tube for 3 h at room temperature. After incubation, the sample was neutralized with a saturated solution of LiOH and then extracted with hexane as described above (33).
Aliquots of each extract were monitored for radioactivity. Samples were analyzed on an Ultrasphere ODS Beckman column (4.6 mm by 25 cm) with a Gilson HPLC apparatus equipped with UV/VIS 151 and 152 detectors. For dolichol analysis, the eluent was monitored at 210 nm. Fractions of 0.5 min were collected, and aliquots were subjected to liquid scintillation counting. A gradient elution system was used, with methanol-water (9:1, vol/vol) as solvent A and hexane-propan-2-ol-methanol (1:1:2, vol/vol/vol) as solvent B. A linear gradient from 5 to 100% B over a period of 25 min was run; 100% B was then pumped through for additional 5 min. The flow rate was 1.5 ml/min. Standards of dolichol 11 and dodecaprenol and a mixture of dolichols 18 and 19 were coinjected (4).
Hexane extracts of each parasite stage were analyzed for isoprenic side chains attached to benzoquinone rings. Whole hexane extracts from each stage were dried under a nitrogen stream, resuspended in methanol and coinjected with a mixture of known amounts of authentic Q7-10 standards. Hexane-methanol (75:25, vol/vol) was used as a solvent system at a flow of 1 ml/min. Standards were detected at 275 nm (7). Each 30-s fraction was collected, and aliquots were monitored for radioactivity. In order to compare the effect of terpenes and FTS on the biosynthesis of dolichol and derivates or on the isoprenic chains side of ubiquinones, the same quantities of treated or nontreated parasites were injected. Percentage inhibition was determined as follows: (cpm for control samples − cpm for treated samples)/cpm for control samples × 100.
A comparative statistical analysis of peak areas of HPLC chromatographs of samples treated with terpenes or FTS versus untreated samples were performed for both dolichol and ubiquinones. In order to determine the possible inhibitory effect of these compounds during treatment, an estimate of the degree of inhibition for each phase of the process was made. After evaluating the normality of the population, Student's t test was applied to the data, taking as null hypothesis (H0) the equality of the means between control and treated populations (95% confidence limits). The average inhibition was then estimated with a significance of 95% for each phase of the experiment.
RESULTS
Inhibition of P. falciparum development after treatment with terpenes and FTS.
To test the inhibitory effect of terpenes and FTS on P. falciparum growth, defined numbers of parasites were cultured in the absence or presence of increasing concentrations of each drug. Three independent determinations of the IC50 for terpenes and FTS demonstrated the precision of the assay. The results of parasitemia and the uptake of [G-3H]hypoxanthine by parasitized erythrocytes on the microtiter plates were similar. IC50 and IC90 determinations and confidence intervals are summarized in Table 1. The respective IC50s and IC90s were 64 μM (95% confidence interval of 47.755 to 65.262 μM) and 108 μM (95% confidence interval of 78.19 to 116.31 μM) for farnesol, 760 nM (95% confidence interval of 618.96 to 802.95 nM) and 2,189 nM (95% confidence interval of 1,759 to 2,307 nM) for nerolidol, 1.22 mM (95% confidence interval of 1.07 to 1.42 mM) and 2.27 mM (95% confidence interval of 1.99 to 2.70 mM) for limonene, 0.28 mM (95% confidence interval of 0.196 to 0.324 mM) and 0.64 mM (95% confidence interval of 0.633 to 1.152 mM) for linalool, and 14 μM (95% confidence interval of 13.61 to 19.18 μM) and 26 μM (95% confidence interval of 23.44 to 50.7 μM) for FTS. At the IC90, the parasites died after 3 h of treatment with each terpene or FTS.
TABLE 1.
In vitro IC50s and IC90s of terpenes and FTS for P. falciparuma
| Terpene | Mean IC50 ± SE (CI) | Mean IC90 ± SE (CI) |
|---|---|---|
| Farnesol | 64 ± 5.5 μM (47.755-65.262 μM) | 108 ± 6.5 μM (78.19-116.31 μM) |
| Nerolidol | 760 ± 23 nM (618.96-802.95 nM) | 2,189 ± 74 nM (1,759-2,307 nM) |
| Limonene | 1.22 ± 0.13 mM (1.07-1.42 mM) | 2.27 ± 0.15 mM (1.99-2.70 mM) |
| Linalool | 0.28 ± 0.03 mM (0.196-0.324 mM) | 0.64 ± 0.01 mM (0.633-1.152 mM) |
| FTS | 14 ± 1.3 μM (13.61-19.18 μM) | 26 ± 3 μM (23.44-50.7 μM) |
The IC50s and IC90s of terpenes and FTS were calculated by probit analysis as described in Material and Methods, item Inhibition tests. Student's t test was applied to the results. The results are significantly different at P < 0.05. Data are based on at least three independent experiments, which were performed in triplicate. CI, confidence interval.
The drug concentrations chosen to determine the effect of terpenes and FTS on the incorporation of isoprenyl precursors into dolichol, ubiquinones, or proteins were 25 μM farnesol, 500 nM nerolidol, 0.5 mM limonene, 0.1 mM linalool, and 5.5 μM FTS, because under these conditions only 10% of parasites died after 48 h of treatment and overall protein synthesis was not affected. No differences in incorporation of l-[35S]methionine into the trichloroacetic acid pellets were observed between parasites monitored for 48 h after treatment with terpenes and FTS at the concentrations mentioned above and untreated controls cultured for the same period of time (data not shown). Electrophoretic profiles of l-[35S]methionine-labeled proteins from terpenes and FTS (48-h)-treated or untreated parasites did not differ significantly in the three parasite stages (data not shown). The development of the intraerythrocytic stages of P. falciparum was analyzed over a period of 96 h (two cycles) in the presence of the above-listed concentrations of terpenes and FTS. Limonene and linalool arrested the development of schizont stages at 72 h of treatment. At this same time point farnesol and FTS treatment reduced the number of ring stages. Nerolidol caused reduction of ring stages at 55 h of treatment. This fact led us to search for specific factors involved in this process.
Inhibition of dolichol and ubiquinone biosynthesis in P. falciparum induced by treatment with terpenes and FTS.
The percent inhibition of dolichol and isoprenic chain side of ubiquinone biosynthesis, shown in Table 2, was determined by comparing average peak areas of HPLC chromatographs and a statistical analysis applied as described in Materials and Methods [see “Reverse-phase high-performance liquid chromatography (HPLC)”]. All the terpenes inhibited dolichol biosynthesis in the trophozoite and schizont stages when [3H]FPP was used as precursor. Linalool and nerolidol showed a similar effect on ring stages. Farnesol inhibited biosynthesis of dolichol in the trophozoite and schizont stages when [3H]GGPP was used as a precursor; this effect was also detected in schizont stages upon treatment with nerolidol. FTS showed a low inhibitory effect on dolichol biosynthesis in the schizont stages (Table 2). The inhibitory effects of terpenes on dolichol biosynthesis were similar when dolichyl-P and dolichyl-PP were analyzed (data not show).
TABLE 2.
Percent inhibitionb of the biosynthesis of dolichol and isoprenic chains of ubiquinone in treated P. falciparum
| Stage | Precursor | % Inhibition of P. falciparum treated witha:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Farnesol
|
Nerolidol
|
Limonene
|
Linalool
|
FTS
|
|||||||
| D | Q | D | Q | D | Q | D | Q | D | Q | ||
| Ring | GGPP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FPP | 0 | 41 | 58 | 0 | 0 | 34 | 54 | 0 | 0 | 0 | |
| Trophozoite | GGPP | 56 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FPP | 68 | 78 | 69 | 0 | 66 | 51 | 55 | 0 | 0 | 0 | |
| Schizont | GGPP | 30 | 62 | 42 | 88 | 0 | 0 | 0 | 22 | 0 | 0 |
| FPP | 66 | 84 | 62 | 87 | 43 | 40 | 91 | 87 | 23 | 21 | |
Abbreviations: FPP: [1(n)-3H]farnesyl triammonium pyrophosphate GGPP: [1(n)-3H]geranylgeranyl triammonium pyrophosphate D, dolichol of 55 and 60 carbons; Q, ubiquinone of eight isoprenic and nine isoprenic units.
Percent inhibition was determined as described in Materials and Methods [see “Reverse- phase high-performance liquid chromatography (HPLC)]. Student's t test was applied to the results. The results are significantly different at P < 0.05.
Concerning the biosynthesis of the isoprenic chain attached to the benzoquinone ring of ubiquinones, farnesol, nerolidol, and linalool showed a stronger inhibitory activity on the schizont stage. Farnesol was a more potent inhibitor than limonene in the ring and trophozoite stages when [3H]FPP was used as precursor. FTS caused low inhibition (21%) of the isoprenic chain side of ubiquinone biosynthesis in schizont stages (Table 2).
To determine whether the inhibitory effect of terpenes and FTS on dolichol and on the isoprenic side chain of ubiquinone biosynthesis could be taking place upstream of the metabolic pathway of isoprenoids, the incorporation of [14C]IPP was studied in untreated and treated schizont stages.
Figure 1 shows the effect of terpenes and FTS on P. falciparum isoprenoid biosynthesis. When we compared [14C]IPP incorporation between untreated and farnesol- or nerolidol-treated schizont stages, we observed no differences in two bands with Rf values equivalent to geraniol and farnesol; however, other bands corresponding to dolichol and ubiquinones showed lower intensity in treated parasites. On the other hand, when parasites were treated with limonene or linalool, the intensity of the bands with Rf values equivalent to those of geraniol, farnesol, dolichol, or ubiquinones was reduced. No differences were detected between parasites treated with FTS and untreated parasites. These results agree with the HPLC analysis, which showed that FTS is a poor inhibitor of ubiquinone and dolichol biosynthesis. There was no incorporation of [3H]FPP, [3H]GGPP, or [14C]IPP into uninfected erythrocytes submitted to identical experimental conditions (4).
FIG. 1.
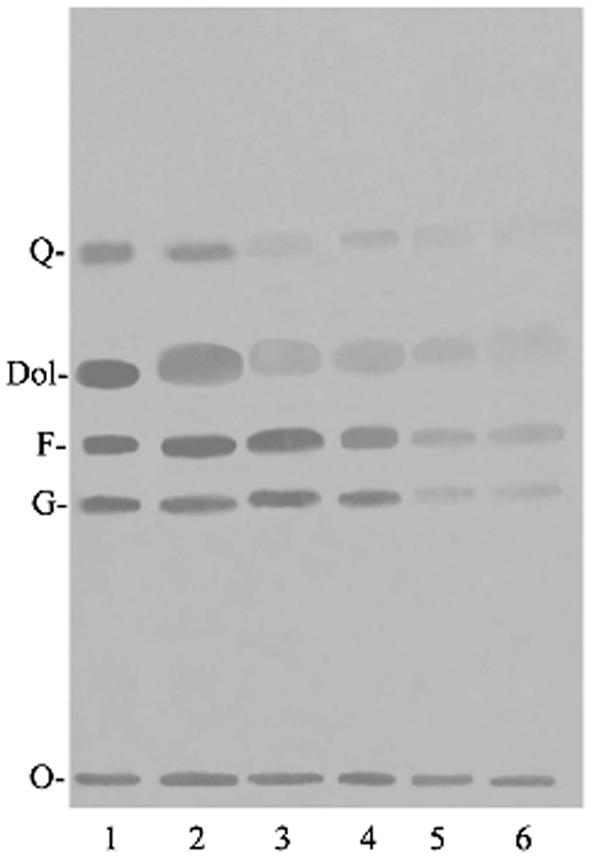
HPTLC analysis of hexane extracts of schizont stages treated for 48 h with terpenes and FTS and then metabolically labeled for 18 h with [14C]IPP at 3.125 μCi/ml. Solvent system: hexane-diethylether-acetic acid (80:20:1, vol/vol/vol). Lane 1, untreated parasites; lane 2, 5.5 μM FTS; lane 3, 25 μM farnesol; lane 4, 0.5 mM nerolidol; lane 5, 0.5 mM limonene; lane 6, 0.1 mM linalool. Abbreviations: O, origin; G, geraniol; F, farnesol; Dol, dolichol of 11 isoprenic units; Q, mixture of standards coenzyme Q7-10.
Treatment of parasites with terpenes and FTS decreases the incorporation of isoprenyl precursors in proteins.
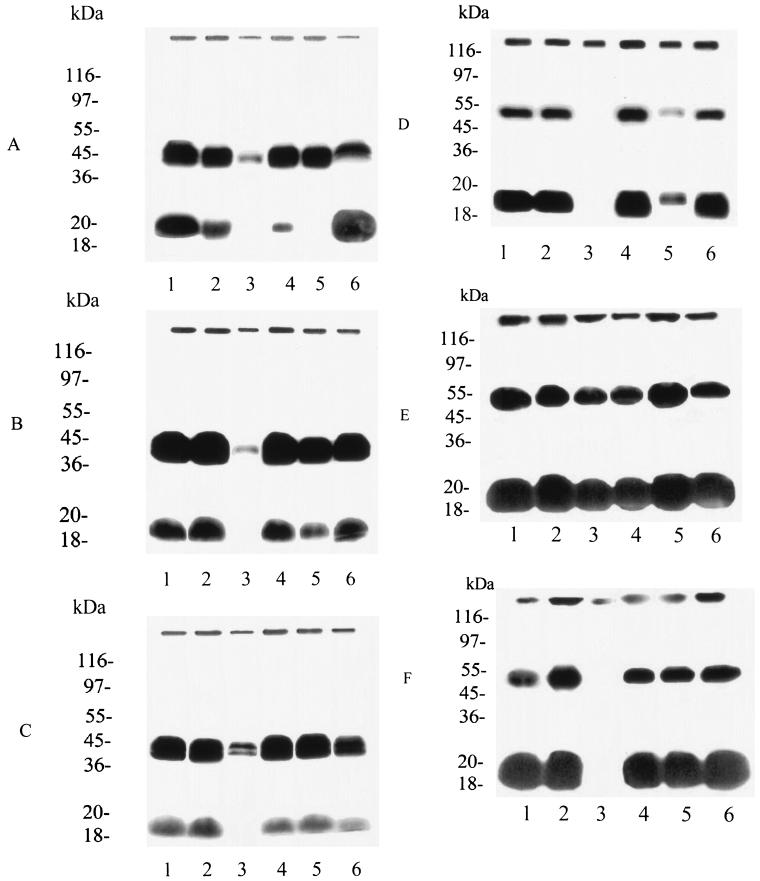
Treatment of parasites with terpenes and FTS during [3H]GGPP labeling showed difference in the incorporation of this precursor into the proteins, in the ring stages. The effect was most visible for the ∼20 kDa band in parasites treated with farnesol and limonene (Fig. 2A, lanes 3 and 5). The intensity of the ∼40-kDa band decreased only when parasites were treated with farnesol or linalool (Fig. 2A, lanes 3 and 6).
FIG. 2.
In vitro treatment of P. falciparum cultures with terpenes or FTS and metabolic labeling with [3H]GGPP and [3H]FPP. Parasites were untreated or treated for 48 h with terpenes and FTS and labeled for 18 h with a 3.125-μCi/ml concentration of [3H]GGPP (A to C) or [3H]FPP (D to F) in the presence of the drugs. The parasites were harvested and purified by Percoll gradient, lysed, and analyzed by SDS-PAGE and fluorography. (A and D) Ring forms; (B and E) trophozoite forms; (C and F) schizont forms. Lane 1, untreated parasites; lane 2, 5.5 μM FTS; lane 3, 25 μM farnesol; lane 4, 500 nM nerolidol; lane 5, 0.5 mM limonene; lane 6, 0.1 mM linalool. Molecular size standards are indicated on the left.
In the trophozoite and schizont stages treated with farnesol, a marked decrease in [3H]GGPP incorporation into proteins (∼40 and ∼20 kDa) was observed (Fig. 2B and C, lane 3). Limonene reduced labeling to some extent (∼40 and ∼20 kDa) in the trophozoite stages (Fig. 2B, lane 5). Linalool had a less pronounced effect (∼40 kDa) than farnesol did in the schizont stages (Fig. 2C, lane 6).
Of all the terpenes tested, farnesol was more effective at reducing [3H]FPP labeling. In the ring stage, farnesol actually inhibited [3H]FPP incorporation into ∼50- and ∼20-kDa proteins, while limonene had a partial effect (Fig. 2D, lanes 3 and 5). In the trophozoite stage, farnesol, nerolidol, and linalool had a low inhibitory effect on [3H]FPP incorporation into the ∼50-kDa protein (Fig. 2E, lanes 3, 4, and 6). In the schizont stage, only farnesol was effective, inhibiting the incorporation of the precursor into both the ∼50- and the ∼20-kDa proteins (Fig. 2F, lane 3).
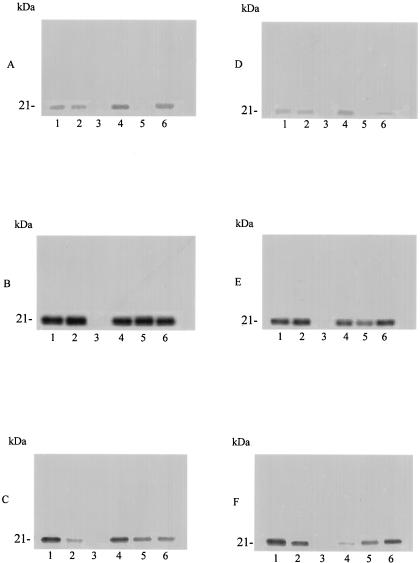
In order to characterize whether these terpenes and FTS specifically inhibit p21ras and p21rap of P. falciparum, we immunoprecipitated lysates of untreated or treated ring, trophozoite, and schizont forms previously labeled with [3H]FPP or [3H]GGPP with anti-p21ras or anti-p21rap antibodies, respectively.
Ras-like protein levels in the ring stages were inhibited by treatment with farnesol and limonene (Fig. 3A, lane 3 and lane 5). The band corresponding to Ras-like protein disappeared after farnesol treatment in the trophozoite and schizont stages (Fig. 3B and C, lane 3), whereas FTS, limonene, and linalool decreased the incorporation of the precursor in the schizont stages (Fig. 3C, lanes 2, 5, and 6).
FIG. 3.
Immunoprecipitation of p21ras and p21rap from P. falciparum parasites treated with terpenes or FTS and labeled with [3H]FPP or [3H]GGPP. Parasites were treated for 48 h in the absence or presence of terpenes or FTS and metabolically labeled with [3H]FPP (A to C) or [3H]GGPP (D to F) for 18 h in the presence of the drugs; harvested; purified on a Percoll gradient; lysed and immunoprecipitated with anti-p21ras or anti-p21rap antibody, respectively; and analyzed by SDS-PAGE and fluorography. (A and D) Ring stage; (B and E) trophozoite stage; (C and F) schizont stage. Lane 1, untreated parasites; lane 2, 5.5 μM FTS; lane 3, 25 μM farnesol lane 4, 500 nM nerolidol; lane 5, 0.5 mM limonene; lane 6, 0.1 mM linalool.
Rap-like protein levels in farnesol- and limonene-treated ring stages of the parasite were inhibited, while linalool treatment significantly reduced the intensity of the 21-kDa band compared with untreated samples (Fig. 3D, compare lanes 3, 5, and 6 and lane 1). In the trophozoite stage, incorporation of labeled precursor was inhibited by farnesol and reduced by limonene and nerolidol (Fig. 3E, lanes 3 to 5).
In the schizont stage, all the terpenes tested and FTS led to a decrease in intensity of the band corresponding to a p21rap protein and farnesol actually induced total inhibition (Fig. 3F).
DISCUSSION
In this report we demonstrate that farnesol, nerolidol, limonene, linalool, and FTS have antimalarial activities on intraerythrocytic stages of P. falciparum in vitro. In recent reports, we demonstrated that nerolidol inhibits the biosynthesis of the isoprenic chain attached to the benzoquinone ring in the intraerythrocytic stages of P. falciparum (7) and that limonene inhibits protein isoprenylation in the same stages of the parasite (29). In the present study we demonstrate that terpenes such as farnesol and linalool inhibit the biosynthesis of several intermediates and end products of the isoprenoids pathway.
The terpenes tested here may be acting through inhibition of the isoprenyl diphosphate synthases. This family of enzymes catalyzes consecutive 1′-4 condensations of isopentenyl-PP with the allylic substrate to form the linear backbone for all isoprenoid compounds, including prenylated proteins, prenylated quinones, and dolichol (41). In plants the isoprenoid pathway biosynthesizes only terpenes, while in P. falciparum this pathway generates other types of isoprenoids, e.g., dolichol, isoprenic side chains of ubiquinones, and isoprenic chain attached to proteins (4, 7, 9). Due to their structural similarity, terpenes might interfere with the parasite's biosynthesis of polyisoprenoids by competing with natural substrates in enzyme-substrate reactions or by interfering with the mechanisms of elongation of isoprenic chains.
It is known that farnesyl-PP and geranylgeranyl-PP are substrates of the enzymes prenyltransferases involved in the biosynthesis of dolichol, the isoprenic side chain of ubiquinones and isoprenic chains attached to proteins (41). In this report we used [3H]FPP or [3H]GGPP to determine if the terpenes assayed had specificity for one of these precursor. According to our results, these terpenes appear to have a widespread inhibitory action.
Farnesol had a strong inhibitory effect on the biosynthesis of dolichol and the isoprenic side chain of benzoquinone rings as well as on protein isoprenylation when [3H]FPP was used as precursor. Farnesol has a molecular structure similar to that of the precursors used for labeling these polyisoprenoids and could be interfering with chain elongation. When schizont stages treated with farnesol were labeled with [14C]IPP in the presence of this terpene, only the spots with Rf coincident with dolichol and ubiquinones were diminished (Fig. 1, lane 3). This fact would suggest that, since farnesyl-PP an intermediate product of the biosynthetic pathway of isoprenoids, the addition of external farnesol might interfere with the elongation of the isoprenic chains that contain more than 10 carbons in their molecule. Similar results were obtained in nerolidol-treated parasites (Fig. 1, lane 4). On the other hand, limonene and linalool might interfere in the synthesis of the first precursor, since all the spots corresponding to geraniol, farnesol, dolichol, and ubiquinones were reduced when parasites were treated with these terpenes (Fig. 1, lanes 5 and 6). Antimalarial activity by essential oils that contain nerolidol has been described previously by us and others (7, 12). The IC50 of nerolidol for isolate NF54, clone 3D7, of P. falciparum was quite different from that described by us for isolate S20 (7); however, both isolates presented identical inhibition of dolichol and ubiquinone biosynthesis without inhibiting of farnesol and geraniol. In other cell systems, farnesol has been found to inhibit the biosynthesis of dolichol and ubiquinones by degradation of 3-hydroxy-3-methylglutaryl-Co A reductase (3). However, this enzyme or the gene sequences related to 3-hydroxy-3-methylglutaryl-Co A reductase are not present in P. falciparum, where isoprenoids are biosynthesized via the DOXP/MEP pathway (17, 21). We thus suggest that farnesol and nerolidol act in P. falciparum by inhibiting the elongation of isoprenic chains.
The antimalarial activity of limonene through inhibition of protein isoprenylation previously described by us was confirmed here (29). This antimalarial effect could be more extensive. Monoterpenes like limonene or linalool probably inhibit the biosynthesis of dolichol and ubiquinones by interfering with the condensation between isopentenyl-PP and dimethylallyl-PP. This hypothesis is suggested by the results of HPTLC, when parasite labeling with [14C]IPP in the presence of limonene or linalool resulted in reduction of spots coinciding with geraniol, farnesol, dolichol, and ubiquinones. These inhibitory effects of limonene on the biosynthesis of dolichol and the isoprenic side chains of benzoquinone rings were first described in hepatoma and Neuro2A cells, respectively (22, 36). It has been proposed that linalool inhibits the biosynthesis of dolichol and ubiquinones. Although this effect has not been described to date, antileishmanial activity via increased nitric oxide production was recently reported in linalool-treated macrophages (11). Antifungal and antibacterial effects of linalool have also been reported but their mechanisms of action are not still known (32).
We believe that the inhibitory effects of nerolidol, farnesol, limonene, and linalool on isoprenoid biosynthesis demonstrated herein are specific because the concentrations of terpenes used did not affect overall protein biosynthesis.
FTS had a low inhibitory effect on the biosynthesis of dolichol and ubiquinones (23 and 21%, respectively). These results agree with data published for other cell systems (15). We used FTS as a control of a modified terpene, and the levels of inhibition of dolichol and ubiquinones biosynthesis obtained were very low. These results might be interpreted as an indication that the inhibitory effect of farnesol and the other terpenes (nerolidol, limonene, and linalool) tested in the present study is specific for dolichol and side chain of ubiquinones biosynthesis.
On the other hand, when parasites were treated with FTS, a reduced signal was detected in anti-Ras immunoprecipitates of the schizont stages, and parasite development was arrested at this stage. FTS inhibits the development of the human pancreatic tumor by dislodging Ras from its membrane-anchoring domains and accelerating its degradation (14, 16, 19).
Farnesol generated the widest range of inhibition of isoprenylation of Ras- and Rap-like proteins in the three stages of P. falciparum. The effect produced by farnesol has not been described in eukaryotic cells to date. We suggest that farnesol interferes with the linkage between the protein and a farnesyl-PP group or a geranylgeranyl-PP group. Linalool induced some inhibition of Ras-like protein isoprenylation in ring and schizont stages. A similar effect was described in the human erythroleukemia cell line K562, and the authors suggested that alcohols with an open chain are inactive or have low inhibitory activity on isoprenylation (20).
Farnesol was found to be the strongest inhibitor of isoprenoid biosynthesis at different points of the isoprenoid pathway and inhibited the development of the intraerythrocytic stages of P. falciparum at one of the lowest IC50s. The other terpenes assessed here also showed antimalarial activity in vitro and inhibited some steps of the isoprenoid pathway. This widespread antimalarial activity was more evident in the schizont stages, probably because during the differentiation from trophozoites to schizonts the transport of several molecules is facilitated (40).
The present report is the first demonstration that these terpenes have an inhibitory action on isoprenoid biosynthesis at different points of the metabolic pathway. Such wide range of action might override the appearance of resistance mechanisms in P. falciparum. FTS was the only compound to inhibit only one point target of the isoprenoid pathway; however, Ras-protein expression is essential for cell development. This effect could be seen in vitro because FTS arrests parasite development in the schizont stage.
Combinations of some terpenes or FTS tested in the present study with other antimalarial drugs that inhibit the enzyme DOXP reductoisomerase, like fosmidomycin, may become a new strategy for treatment of malaria. Synergist studies with these terpenes, FTS, and fosmidomycin are currently under development in our laboratory.
Acknowledgments
This work was supported by grants from FAPESP, CNPq, PRONEX, UNDP/World Bank/WHO (TDR), and Agencia Nacional de Promocion Científica y Tecnologica-PICT 0606545, Argentina. H.R.G. is supported by a doctoral fellowship from FAPESP.
A.S.C. is a member of the research council of Argentina (CONICET).
We thank Yoel Kloog, Department of Neurobiochemistry, The George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel-Aviv, Israel, for providing FTS.
REFERENCES
- 1.Braun-Breton, C., M. Jendoubi, E. Brunet, L. Perrin, J. Scaife, and L. Pereira da Silva. 1986. In vivo time course of synthesis and processing of major schizont membrane polypeptides in Plasmodium falciparum. Mol. Biochem. Parasitol. 20:33-43. [DOI] [PubMed] [Google Scholar]
- 2.Chojnacki, T., and G. Dallner. 1988. The biological role of dolichol. Biochem. J. 251:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correll, C. C., L. Ng, and P. A. Edwards. 1994. Identification of farnesol as the non-sterol derivative of mevalonic acid required for the accelerated degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. 269:17390-17393. [PubMed] [Google Scholar]
- 4.Couto, A. S., E. A. Kimura, V. J. Peres, M. L. Uhrig, and A. M. Katzin. 1999. Active isoprenoid pathway in the intra-erythrocytic stages of Plasmodium falciparum: presence of dolichols of 11 and 12 isoprene units. Biochem. J. 341:629-637. [PMC free article] [PubMed] [Google Scholar]
- 5.Cowan, M. M. 1999. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12:564-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowell, P. L. 1999. Prevention and therapy of cancer by dietary monoterpenes. J. Nutr. 129:775S-778S. [DOI] [PubMed] [Google Scholar]
- 7.de Macedo, C. S., M. L. Uhrig, E. A. Kimura, and A. M. Katzin. 2002. Characterization of the isoprenoid chain of coenzyme Q in Plasmodium falciparum. FEMS Microbiol. Lett. 207:13-20. [DOI] [PubMed] [Google Scholar]
- 8.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewick, P. M. 1999. The biosynthesis of C5-C25 terpenoid compounds. Nat. Prod. Rep. 16:97-130. [DOI] [PubMed] [Google Scholar]
- 10.Dewick, P. M. 2002. The biosynthesis of C5-C25 terpenoid compounds. Nat. Prod. Rep. 19:181-222. [DOI] [PubMed] [Google Scholar]
- 11.do Socorro, S. R. M. S., R. R. Mendonca-Filho, H. R. Bizzo, I. de Almeida Rodrigues, R. M. Soares, T. Souto-Padron, C. S. Alviano, and A. H. Lopes. 2003. Antileishmanial activity of a linalool-rich essential oil from Croton cajucara. Antimicrob. Agents Chemother. 47:1895-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duker-Eshun, G., J. W. Jaroszewski, W. A. Asomaning, F. Oppong-Boachie, C. E. Olsen, and S. B. Christensen. 2002. Antiplasmodial activity of labdanes from Aframomum latifolium and Aframomum sceptrum. Planta Med. 68:642-644. [DOI] [PubMed] [Google Scholar]
- 13.Eckstein-Ludwig, U., R. J. Webb, I. D. Van Goethem, J. M. East, A. G. Lee, M. Kimura, P. M. O'Neill, P. G. Bray, S. A. Ward, and S. Krishna. 2003. Artemisinins target the SERCA of Plasmodium falciparum. Nature 424:957-961. [DOI] [PubMed] [Google Scholar]
- 14.Egozi, Y., B. Weisz, M. Gana-Weisz, G. Ben-Baruch, and Y. Kloog. 1999. Growth inhibition of ras-dependent tumors in nude mice by a potent ras-dislodging antagonist. Int J. Cancer. 80:911-918. [DOI] [PubMed] [Google Scholar]
- 15.Elad, G., A. Paz, R. Haklai, D. Marciano, A. Cox, and Y. Kloog. 1999. Targeting of K-Ras 4B by S-trans,trans-farnesyl thiosalicylic acid. Biochim. Biophys. Acta 1452:228-242. [DOI] [PubMed] [Google Scholar]
- 16.Gana-Weisz, M., J. Halaschek-Wiener, B. Jansen, G. Elad, R. Haklai, and Y. Kloog. 2002. The Ras inhibitor S-trans,trans-farnesylthiosalicylic acid chemosensitizes human tumor cells without causing resistance. Clin. Cancer Res. 8:555-565. [PubMed] [Google Scholar]
- 17.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein, J. L., and M. S. Brown. 1990. Regulation of the mevalonate pathway. Nature 343:425-430. [DOI] [PubMed] [Google Scholar]
- 19.Haklai, R., M. G. Weisz, G. Elad, A. Paz, D. Marciano, Y. Egozi, G. Ben-Baruch, and Y. Kloog. 1998. Dislodgment and accelerated degradation of Ras. Biochemistry 37:1306-1314. [DOI] [PubMed] [Google Scholar]
- 20.Holstein, S. A., and R. J. Hohl. 2003. Monoterpene regulation of Ras and Ras-related protein expression. J. Lipid Res. 44:1209-1215. [DOI] [PubMed] [Google Scholar]
- 21.Jomaa, H., J. Wiesner, S. Sanderbrand, B. Altincicek, C. Weidemeyer, M. Hintz, I. Turbachova, M. Eberl, J. Zeidler, H. K. Lichtenthaler, D. Soldati, and E. Beck. 1999. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 285:1573-1576. [DOI] [PubMed] [Google Scholar]
- 22.Kawata, S., T. Nagase, E. Yamasaki, H. Ishiguro, and Y. Matsuzawa. 1994. Modulation of the mevalonate pathway and cell growth by pravastatin and d-limonene in a human hepatoma cell line (Hep G2). Br. J. Cancer. 69:1015-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellogg, B. A., and C. D. Poulter. 1997. Chain elongation in the isoprenoid biosynthetic pathway. Curr. Opin. Chem. Biol. 1:570-578. [DOI] [PubMed] [Google Scholar]
- 24.Kessler, S. W. 1975. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J. Immunol. 115:1617-1624. [PubMed] [Google Scholar]
- 25.Kimura, E. A., A. S. Couto, V. J. Peres, O. L. Casal, and A. M. Katzin. 1996. N-linked glycoproteins are related to schizogony of the intraerythrocytic stage in Plasmodium falciparum. J. Biol. Chem. 271:14452-14461. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 27.Lopes, N. P., M. J. Kato, E. H. Andrade, J. G. Maia, M. Yoshida, A. R. Planchart, and A. M. Katzin. 1999. Antimalarial use of volatile oil from leaves of Virola surinamensis (Rol.) Warb. by Waiapi Amazon Indians. J. Ethnopharmacol. 67:313-319. [DOI] [PubMed] [Google Scholar]
- 28.Marciano, D., G. Ben-Baruch, M. Marom, Y. Egozi, R. Haklai, and Y. Kloog. 1995. Farnesyl derivatives of rigid carboxylic acids-inhibitors of ras-dependent cell growth. J. Med. Chem. 38:1267-1272. [DOI] [PubMed] [Google Scholar]
- 29.Moura, I. C., G. Wunderlich, M. L. Uhrig, A. S. Couto, V. J. Peres, A. M. Katzin, and E. A. Kimura. 2001. Limonene arrests parasite development and inhibits isoprenylation of proteins in Plasmodium falciparum. Antimicrob. Agents Chemother. 45:2553-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer, D. N., D. R. Husbands, P. J. Winter, J. W. Blunt, and R. D. Jolly. 1986. Ceroid lipofuscinosis in sheep. I. Bis(monoacylglycerol)phosphate, dolichol, ubiquinone, phospholipids, fatty acids, and fluorescence in liver lipopigment lipids. J. Biol. Chem. 261:1766-1772. [PubMed] [Google Scholar]
- 31.Pasvol, G., R. J. Wilson, M. E. Smalley, and J. Brown. 1978. Separation of viable schizont-infected red cells of Plasmodium falciparum from human blood. Ann. Trop. Med. Parasitol. 72:87-88. [DOI] [PubMed] [Google Scholar]
- 32.Pattnaik, S., V. R. Subramanyam, M. Bapaji, and C. R. Kole. 1997. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios 89:39-46. [PubMed] [Google Scholar]
- 33.Pouter, C. D., P. L. Wiggins, and T. L. Plummer. 1981. Isolation and assay of dolichol and dolichyl phosphate. J. Org. Chem. 46:1532-1533. [Google Scholar]
- 34.Rohmer, M. 1999. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 16:565-574. [DOI] [PubMed] [Google Scholar]
- 35.Sacchettini, J. C., and C. D. Poulter. 1997. Creating isoprenoid diversity. Science 277:1788-1789. [DOI] [PubMed] [Google Scholar]
- 36.Shi, W., and M. N. Gould. 1995. Induction of differentiation in neuro-2A cells by the monoterpene perillyl alcohol. Cancer Lett. 95:1-6. [DOI] [PubMed] [Google Scholar]
- 37.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 38.Utzinger, J., M. Tanner, and B. H. Singer. 2001. The Internet: a valuable tool for Roll Back Malaria. Trends Parasitol. 17:159-161. [DOI] [PubMed] [Google Scholar]
- 39.Vial, H. J. 2000. Isoprenoid biosynthesis and drug targeting in the Apicomplexa. Parasitol. Today 16:140-141. [DOI] [PubMed] [Google Scholar]
- 40.Vial, H. J., M. L. Ancelin, J. R. Philippot, and M. J. Thuet. 1990. Biosynthesis and dynamics of lipids in Plasmodium-infected mature mammalian erythrocytes. Blood Cells 16:531-561. [PubMed] [Google Scholar]
- 41.Wang, K. C., and S. Ohnuma. 2000. Isoprenyl diphosphate synthases. Biochim. Biophys. Acta 1529:33-48. [DOI] [PubMed] [Google Scholar]
- 42.Wiesner, J., D. Henschker, D. B. Hutchinson, E. Beck, and H. Jomaa. 2002. In vitro and in vivo synergy of fosmidomycin, a novel antimalarial drug, with clindamycin. Antimicrob. Agents Chemother. 46:2889-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]