Abstract
Objective
The etiology of major depressive disorder (MDD) is likely to be heterogeneous, but postpartum depression (PPD) is hypothesized to represent a more homogenous subset of MDD. We use genome-wide SNP data to explore this hypothesis.
Method
We assembled a total cohort of 1,420 self-report cases of PPD and 9,473 controls with genome-wide genotypes from Australia, the Netherlands, Sweden and the United Kingdom. We estimated the total variance attributable to genotyped variants. We used association results from the Psychiatric Genomics Consortia (PGC) of Bipolar Disorder (BPD) and MDD to create polygenic scores in PPD and related MDD data sets to estimate the genetic overlap between the disorders.
Results
We estimated that the percentage of variance on the liability scale explained by common genetic variants to be 0.22 with a standard error of 0.12, p = 0.02. The R2 from a logistic regression of PPD case-control status in all four cohorts on a SNP profile score weighted by PGC-BPD association results was small (0.1%) but significant (p= 0.004) indicating a genetic overlap between BPD and PPD. The results were highly significant in the Australian and Dutch cohorts (R2 > 1.1%, p < 0.008), where the majority of cases met criteria for MDD. The genetic overlap between BPD and MDD was not significant in larger Australian and Dutch MDD case-control cohorts after excluding PPD cases (R2 =0.06%, p= 0.08), despite the larger MDD group affording more power.
Conclusions
Our results suggest empirical genetic evidence for a more important shared genetic etiology between BPD and PPD, than between BPD and MDD.
Keywords: depression, postpartum, bipolar
Introduction
Postpartum depression (PPD) is defined as a sub-type of major depression occurring within the first 3 months postpartum and it can have far-reaching consequences for the woman, her children and family (Gaynes et al., 2005, Marmorstein et al., 2004, Flynn et al., 2004). PPD is associated with poorer maternal-infant attachment (Stein et al., 1991) and parenting behavior (Gavin et al., 2005, Britton, 2007). Treatment options include antidepressants and cognitive behavioural therapy, and many women experience improvement in symptoms (Miller, 2002).
Differences in assessment criteria and the length of time that subjects have been followed have given rise to some inconsistency regarding the prevalence of PPD, with estimates ranging from 10–20% (O’Hara and Swain, 1996). PPD has partial genetic etiology with heritability of postpartum depressive symptoms estimated to be 0.38 (Treloar et al., 1999) (estimated in a sample that partly overlaps with one used in the present study), implying both genetic and environmental risk factors but with evidence of a genetic component partially distinct from MDD. A number of studies have demonstrated that women with a prior history of bipolar disorder (BPD) or unipolar depression are at elevated risk of postpartum mood episodes. Similarly, women with siblings with BPD or MDD are also at increased risk of postpartum episodes, indicating that there are likely shared genetic risk factors between mood disorders and postpartum depression.
The advent of reasonably cheap genotyping chips that can survey a large proportion of the common genetic variants in the genome has led to a number of new insights into the genetic underpinnings of psychiatric disorders. Genome-wide association studies (GWAS) that test each genetic variant for association with the disorder of interest have been successful in identifying individual variants associated with psychiatric disorders (Sullivan et al., 2012), most notably for schizophrenia (SCZ) (Ripke et al., 2013) and BPD (Sklar et al., 2011). Specifically for BPD, 3 distinct regions have been reliably identified as associated with the disorder, implicating the ANK3, CACNA1C and ODZ4 genes However, each identified common variant has had an OR of 1.2 or less, and thus very large samples are required to detect them. Studies that have examined many SNPs in aggregate rather than just one at a time have shown that many common (Minor Allele Frequency (MAF) > 0.01) SNPs of small effect account for a large proportion of the overall heritability of psychiatric disorders.(Lee et al., 2012b, Sullivan et al., 2012). Moreover, when analysing large numbers of SNPs, it has been shown that many common variants that increase risk to disease are shared between disorders (Purcell et al., 2009). Genetic risk profiles constructed using results from schizophrenia GWAS studies were shown to be significantly associated with case/control status in an independent bipolar dataset, indicating that they share common genetic risk alleles.
Genome-wide association studies of MDD have not proven to be as successful in identifying genetic risk variants (Sullivan et al., 2009, Shi et al., 2011, Muglia et al., 2010, Shyn et al., 2011, Lewis et al., 2010, Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium, 2012). This may reflect, in part, the phenotypic, genetic and environmental heterogeneity that characterizes this disorder. PPD may represent a more homogenous subset of MDD that is more amenable to genetic analysis (females only, age-banded, all exposed to the same bio-psychosocial event). The existing GWAS studies of MDD have included PPD cases among the MDD cases, aiming to identify SNPs associated with the broad diagnostic class of MDD. Yet evidence from a twin study suggests that a proportion of the genetic risk to PPD is distinct from MDD (Treloar et al., 1999). Furthermore, there is an increased rate of conversion to bipolar disorder in PPD cases relative to women with MDD with onset that was not after childbirth, implying that there are clinical differences between the two disorders.
To investigate the genetic architecture of PPD, we assembled a total cohort of 1,420 self-report cases of PPD and 9,473 controls with genome-wide genotypes from Australia, the Netherlands, Sweden, and the United Kingdom. We sought to investigate whether there is evidence for differences in genetic risk factors between PPD and MDD without postpartum onset, and specifically if there is evidence that BPD shares more genetic risk factors with PPD than with MDD. We used SNP association results from the Psychiatric Genomics Consortia (PGC) of BPD and MDD to create polygenic scores in PPD and related MDD data sets and estimate the proportion of variance (R2) explained in case-control status by the scores. We also conducted a GWAS but the study was underpowered to detect common risk variants with effect sizes typical of those found for other psychiatric disorders. Our study was adequately powered for performing multi-SNP profile scoring analyses.
Methods
The Australian QIMR Sample
Phenotypic information was obtained from seven studies undertaken at the Queensland Institute of Medical Research (QIMR). Participants were drawn from the Australian Twin Registry and also included relatives of the twin pairs. Studies were carried out between 1980 and 2001 and consisted of mailed health questionnaires and follow-up telephone interviews. In some studies participants were asked ‘Have you ever had a period of at least two weeks when you were feeling depressed or down most of the day nearly every day?’ (‘Yes/No’) and if ‘Yes’, female participants were asked ‘Did this depression occur around the time of childbirth?’ Later studies included a comprehensive psychiatric interview designed to assess MDD and other psychiatric disorders according to DSM-IIIR and DSM-IV criteria. In other studies participants were asked ‘Did you feel depressed after the birth of any of your children?’ (‘Yes/No’), and if ‘Yes’ ‘How many weeks did this go on for?’ PPD cases were defined as those endorsing depressed feelings around the time of childbirth for a period of two or more weeks and who had at least one child (n= 1,856). Participants with no recorded history of MDD, who had at least one child, did not qualify for diagnoses of PPD, and did not have a sister who met the PPD criteria were selected as controls (n= 2,621). A summary of cases drawn from each study is shown in Supplementary Table 1. A more detailed description of the samples and data collection is given in Treloar et al (Treloar et al., 1999). Across a 10-year period, the test-retest reliability of reporting depressive symptoms after live birth was high (r = 0.75 S.E. = 0.06)
After removing those who had not been genotyped, a total of 564 cases and 1,571 controls remained of which 486 cases and 1,056 controls were unrelated and used in genetic analyses (Supplementary Table 1). Depending upon the genotyping study in which they were included, participants were genotyped either on the Illumina 317K, 370K, or 610K platform (Supplementary Methods).
The QIMR PDD case-control sample partly overlaps with the QIMR samples included in the PGC-MDD study(Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium, 2012) (Table 1). The PGC-MDD-QIMR cases and controls had all completed full diagnostic psychiatric interviews. Some PPD cases not in the PGC-MDD-QIMR sample had a relative in the PGC-MDD sample (either case or control). The PPD controls are a partially overlapping subset of the PGC-MDD-QIMR controls. The PGC-MDD-QIMR cases that did not report PPD were used in additional profile scoring analyses to compare how well the PGC association results can predict MDD without PPD versus MDD with PPD.
Table 1.
Profile scoring target sets from the QIMR and GAIN samples and the number of cases and controls in each set.
| Analysis Group | Analysis Name | Description | Sex | QIMR | NESDA/NTR | STR | ALSPAC |
|---|---|---|---|---|---|---|---|
| Group 1 | PPD | PPD cases | F | 484 | 208 | 104 | 616 |
| PPD screened controls | F | 1024 | 761 | 1,351 | 6311 | ||
| Group 2 | MDD | MDD cases | M+F | 1450 | 1699 | ||
| MDD screened controls | M+F | 1703 | 1765 | ||||
| Group 3 | MDD ex PPD | MDD cases ex. PPD | M+F | 1103 | 1491 | ||
| MDD screened controls | M+F | 1703 | 1765 | ||||
| Group 4 | PPD_Allcontrols | PPD cases | F | 484 | 208 | ||
| MDD screened controls | M+F | 1703 | 1765 | ||||
| Group 5 | MDD_Female | MDD female cases | F | 932 | 1180 | ||
| MDD screened female controls | F | 988 | 1095 |
The Dutch NESDA/NTR Sample
The NESDA/NTR sample is a subset of the GAIN MDD cohort (Boomsma et al., 2008, Sullivan et al., 2009, Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium, 2012), which draws participants from the Netherlands Twin Registry (NTR)(Boomsma et al., 2006) and the Netherlands Study of Depression (NESDA)(Penninx et al., 2008). For this analysis, PPD cases came from the NESDA study, controls came from both NESDA and NTR studies. Details of genotyping and quality control procedures in the sample have been described in detail elsewhere (Boomsma et al., 2008, Sullivan et al., 2009), and the imputation procedure is described in the PGC-MDD study(Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium, 2012). Lifetime MDD was assessed using the Composite International Diagnostic Interview (CIDI, version 2.1)(Wittchen et al., 1991). Cases of PPD were selected using a modified retrospective version of the Edinburgh Postnatal Depression Scale (EPDS)(Cox et al., 1987) that was only administered to the NESDA cohort. The EPDS is commonly used to assess current symptoms of depression and anxiety in the post-partum period. A score of >11 is considered as a cut-off for identifying those most likely to meet the criteria for a depression diagnosis (Cox et al., 1987, Wisner et al., 2002). The EPDS was expanded to include two initial screening questions: 1) At any point in your childbearing, did you experience symptoms of depression or anxiety that began during pregnancy or postpartum? 2) Were you ever diagnosed or treated for PND? Those women who answered yes to either question were asked to complete the EPDS based on the symptoms they experienced in the worst episode. Women scoring more than 11 were considered to be cases. Women from NTR with no history of mood disorders, a low factor score based on indices of depression, neuroticism and anxiety (Boomsma et al., 2008) and who reported having at least one child were selected as controls. A total of 214 cases and 755 controls were included in the analysis. All of these participants were included in the PGC-MDD GWAS analysis. NESDA/NTR MDD cases from the PGC-MDD study that did not have PPD were also included in separate profile scoring analyses.
The Swedish STR Sample
The Swedish sample consisted of participants drawn from the Swedish Twin Registry (STR) (Magnusson et al., 2013). The phenotypic information was obtained from paper-questionnaire self reports from the SALTY study initiated in 2007. Target population was twins born in Sweden 1943–1958. The first requests for participation in the SALTY study were sent out in early 2009 and the data collection was completed in the summer of 2010 when a total of 24,916 twins had been contacted. The survey was answered by 11,372 respondents that gave informed consent (46%) and 54.3% were female (Magnusson et al., 2013). PPD cases were those scoring > 11 on the retrospective EPDS. A total of 104 PPD cases and 1,351 controls had available genome wide genotype data (Illumina OmniExpress platform) of which 100 cases and 1,209 controls were unrelated. Controls were not screened for psychiatric disorders such as MDD, but did not report depression after childbirth.
The ALSPAC Sample
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a prospective birth cohort in which all pregnant women in the former county of Avon, in the South West of England, with an expected date of delivery between 1 April 1991 and 31 December 1992 were eligible to take part. The cohort has been described in detail elsewhere (Fraser et al., 2013). Phenotype information was obtained from the EPDS, which was administered to the mothers at several time points during pregnancy and post-partum to monitor depression. Scores recorded at 8 weeks post-partum were used to establish presence of PPD, with women scoring >12 classified as cases. The data reported in this paper include 6,927 women (616 cases and 6,311 controls) with both genome-wide SNP data and EPDS scores at 8 weeks post-partum. Genotyping was performed on the Illumina Human660W-quad array and imputation to HapMap 2 was performed.
Further information on genotyping and QC in each cohort is provided in the Supplementary Methods.
The study was approved by the ethics board of each of the participating institutions.
Association Analyses
Genome-wide association analyses were conducted in each cohort, and a fixed-effect meta-analysis was performed in PLINK (Purcell et al., 2007). Our study was under-powered and no genome-wide significant associations were found. Details are presented in the supplementary material (Supplementary Methods, Supplementary Tables 1–5, Supplementary Figures 1–3) and full results are available from the authors to allow future meta-analyses.
GREML analysis
We estimated the proportion of variance explained by the common SNPs together in the Australian, Dutch and Swedish samples using the GREML (genomic relationship matric restricted maximum liklehood method) implemented in GCTA (Yang et al., 2011). The combined GREML analysis requires access to the raw genotypes of each cohort, and this access was unavailable for the ALSPAC sample. ALSPAC was therefore not included in the GREML analysis. We removed at random one of any pair of individuals with genetic relatedness >0.025 (n = 312). A total of 739 cases and 2,739 controls were included in the analysis. Study cohort and ancestry principal components were included as covariates.
Polygenic Profile Score Analyses
Despite being underpowered for association analysis, our sample is well-powered as a target sample in a polygenic profile score analysis, in which the sample size of the discovery sample (in which associated SNPs are identified and their effect sizes estimated) is more critical. Assuming a significance threshold of 0.05, we have 100% power to detect whether the polygenic scores explain 0.1% or more of the variance in PPD case/control status in our sample (Dudbridge, 2013)
Analysis groups for polygenic profile scoring
The aim of the profile scoring was to test for overlap in common genetic risk factors between BPD and PPD and MDD and PPD. We then compared the overlap in genetic risk factors for BPD and PPD, and for BPD and MDD respectively to test if BPD shares more genetic risk with PPD than with MDD. We therefore conducted 5 different profile scoring analyses to compare the genetic overlap of BPD and PPD and BPD and MDD without PPD. The groups for profile scoring analyses are as follows (Table 1):
Group 1 (PPD)
This group includes all PPD cases and controls. Information on PPD case/control status was available in all of the cohorts. Profile scoring was conducted in each of the cohorts separately and the results were combined to give an estimate of how well the BPD genetic risk score can predict PPD case/control status.
The remaining groups included only QIMR and NTR/NESDA samples as they provided information on MDD case/control status. No diagnostic information on MDD was available in the STR and ALSPAC studies.
Group 2 (MDD)
All MDD cases and controls from the QIMR and NESDA/NTR study that were included in the PGC-MDD study. This group allowed for estimation of the genetic overlap between BPD and MDD in these two cohorts regardless of whether an episode of MDD occurred postpartum or not. The NESDA/NTR PPD cases and controls are a direct subset of the NESDA/NTR cohort included in the PGC-MDD study and so they are included in this analysis, along with MDD cases without postpartum onset. The QIMR PPD case/control set is not strictly a subset of the QIMR cohort in the PGC-MDD cohort, although there is a partial overlap between the two datasets (Table 1).
Group 3 (MDD ex PPD)
PGC-MDD cohorts from QIMR and NESDA/NTR with the PPD cases removed. In the QIMR cohort, cases in PGC-MDD with a relative included as a PPD case in the present study were removed. This analysis allowed for testing of how well the bipolar polygene score can predict MDD case/control status in those who did not experience MDD postpartum (both males and females).
Group 4 (PPD_Allcontrols)
PPD cases compared to the controls from the PGC-MDD study. This set allows for comparing the predictive power of the bipolar polygene score in a group of PPD cases and a larger control group that has not been screened for a postpartum MDD episode. Not all of the controls are female and have not been screened for having children. This particular analysis was included because we wanted to compare to the results of using PPD cases and only women who have had children as controls. Naively, including more controls should improve the power and therefore increase the accuracy of the profile scores.
Group 5 (MDD Female)
Female cases and controls from the QIMR and NESDA/NTR PGC-MDD study. This analysis allowed for testing of whether results from predicting PPD case/control status using bipolar polygene scores are due to a sex-specific effect in females that is not attributable to PPD.
We used the PGC- BPD (Sklar et al., 2011) (downloaded from http://www.broadinstitute.org/mpg/ricopili/) and PGC- MDD (Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium, 2012) samples as the discovery cohorts, clumped based on LD with r2 <= 0.25. As both the QIMR and GAIN samples were part of the PGC MDD study, we re-analysed the PGC-MDD data with the QIMR samples and NESDA/NTR samples removed to obtain the MDD polygenic profile score. A total of 6,324 cases and 6,678 controls remained.
We used the profile score method (Purcell et al., 2009), constructing a score for each case and control in the target samples as the sum of the log odds ratios of the risk alleles weighted by the number of risk alleles. We used different MDD and PPD cohorts as target samples (Table 1). The MDD cases and controls from the Australian and Dutch samples that were used as target samples, were all included in the PGC-MDD study. Information on MDD case status was unavailable in the STR and ALSPAC samples, so comparison between MDD and PPD cases was not possible in those samples. Different SNP sets were used in the predictor based on the association p-values in the discovery sample. We report the Nagelkerke’s R2 attributable to the polygenic score after fitting covariates.
To combine results across cohorts, each individual’s profile score was transformed into a z-score within each cohort. The cohorts were then combined together, and a logistic regression of case/control status on the profile z-scores was performed.
Results
GREML analyses
We estimated that the percentage of variance on the liability scale explained by SNPs in the combined QIMR, GAIN and Swedish PPD case-control samples, assuming a prevalence of 0.13, was 0.22 with a standard error of 0.12, p = 0.02 (null hypothesis: percentage of variance on the liability scale explained by SNPs = 0).
Polygenic Profile Scoring Analyses
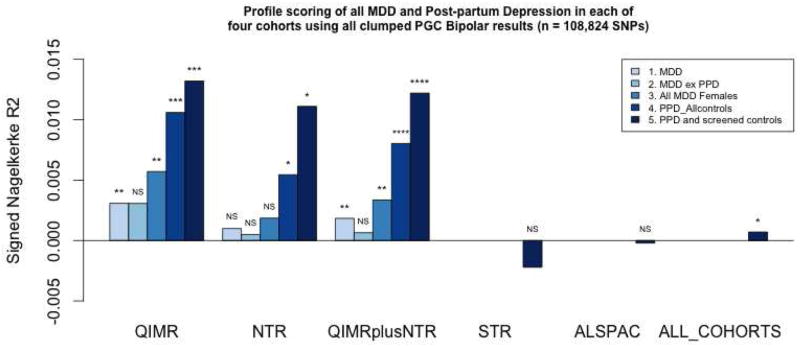
Profile scores based on the PGC-MDD (excluding QIMR and NESDA/NTR samples) association results were not significant in any of the four cohorts (see Supplementary Table 6). When all four samples were analysed together, profile scores based on PGC-BPD GWAS results explained a small but significant proportion of the variance across all samples. The most significant prediction of PPD case/control status came when using PGC-BPD SNPs with p < 0.1. The variance explained was 0.1% (p = 0.04, Supplementary Table 7). From analyzing the results by cohort, it is clear that the predictive signal of PPD case/control status is driven primarily by the QIMR and NESDA/NTR cohorts (Figure 1). The PGC-BPD profile scores significantly predicted PPD case/control status in both QIMR and NESDA/NTR and the direction of effect was such that carrying increasing numbers of BPD risk alleles, increased the chance of being a PPD case. The PGC-BPD profile scores were not significantly associated with PPD case-control status in the Swedish or UK samples, and in both cohorts the direction of the estimate of effect was in the opposite direction to that in QIMR and NESDA/NTR.
Figure 1.
Profile scoring results with polygenic scores constructed from PGC-BPD association results
**** = p < 0.00005, *** = p < 0.0005, ** = p < 0.005, * = p < 0.05, NS = p > 0.05
Note, negative R2 values represent negative effect sizes i.e. that higher profile scores increased likelihood of being a control rather than a case
Results for all SNP sets are provided in the Supplementary Table 7, and results based on all SNPs (n = 108,824 SNPs) are shown in Figure 1. The diagnostic interviews conducted in the QIMR and NESDA/NTR samples allowed for diagnosis of MDD, and hence comparison of profile scoring results when applied to MDD cases and controls and PPD cases and controls. BPD profile scores were applied to different QIMR and NESDA/NTR MDD or PPD cohorts (Table 1, Figure 1). The PGC-BPD profile score significantly predicts MDD, but explains more variance and is more significant when applied to the PPD cases and PPD controls (i.e. women who have had at least one child). The pattern of results was consistent in both cohorts.
We confirmed the significance levels via permutation analysis in the NESDA/NTR sample (Supplementary Material). The results indicated that the increased prediction of case/control status of PPD cases and controls when compared to MDD cases was unlikely to have occurred by chance (p = 0.02).
When analyzing the MDD datasets with the PPD cases removed, there was no significant prediction using PGC-BPD profile scores in the NESDA/NTR sample, implying that the prediction is mostly coming from the PPD cases. In the QIMR sample, removing the PPD cases from the overall MDD sample and trying to predict using PGC-BPD only gives significant results when using SNPs with p < 0.1 (R2= 0.4%, p = 0.02). By contrast, the R2 was 1.64%, p= 3.04 × 10−5 for the QIMR PPD case control sample for the same SNP set, despite a reduced sample size (Table 1).
Discussion
Despite the adverse impact of PPD on women and their newborns, little is understood about the genetic and environmental components affecting this disease (Mahon et al., 2009). Our estimate of variance explained by all SNPs (0.22 with a standard error of 0.12) provides direct evidence for a polygenic architecture for PPD although large sample sizes are needed to increase the accuracy of this estimate and to identify individual associated loci. This estimate is approximately half of the total heritability of PPD estimated in a twin study. The method used in this paper is not dependent upon the same assumptions as twin studies, and therefore supports the results from the twin analysis showing that PPD is heritable. The observation that approximately half of the heritability of liability to PPD is tagged by common variants is in line with the results from the same analyses of schizophrenia (Lee et al., 2012a) and BPD (Lee et al, 2013) (approximately one third of the heritability explained). Two previous studies have estimated the SNP-heritability of MDD, with estimates of 21% (Cross-Disorder Group of the Psychiatric Genomics et al., 2013) and estimated as 30% (Lubke et al., 2012) for the GAIN sample. The estimated SNP-heritability of PPD lies between those estimates, although it should be noted that some of the samples included in the analysis here were also included in those analyses. In terms of the contribution of common SNPs, PPD demonstrates similar genetic architecture to other psychiatric disorders. This implies that vastly increased sample sizes for GWAS will identify common variants that increase PPD risk in the population.
The profile scoring results show evidence that the genetic risk factors for PPD overlap with those for BPD and suggest a stronger genetic relationship (at least of common variants) between BPD and PPD than between BPD and MDD. These results replicate in the QIMR and NESDA/NTR cohorts. In both cohorts, the predictive power of the PGC-BPD profile scores is reduced when non-PPD MDD cases are included in the target sample. Predicting non-PPD MDD case status is also reduced when compared to predicting PPD case status, in spite of the larger sample size in the non-PPD MDD target sample. A permutation analysis where the same number of cases and controls from the NESDA/NTR set as were in the PPD analysis were randomly selected 1,000 times, but allowing cases to have either PPD or MDD, demonstrated that the stronger prediction when analyzing PPD cases and controls was unlikely to have occurred by chance.
While the overall amount of variance in PPD risk explained by the BPD scores across all cohorts is low (0.1%), this does not imply that the actual amount of genetic overlap between the disorders is small. Similarly, the negative results when using MDD profile scores from the PGC to predict PPD case/control status do not imply that there is no genetic correlation between them. The results of the profile scoring analysis depend on several factors, the primary one being that the estimates of the SNP effects in the discovery sample should be as accurate as possible. This accuracy depends upon the power. The power of the PGC-BPD study is likely greater than that of the PGC-MDD study. Since MDD is a more prevalent disorder, sample sizes 3–5 fold greater are needed for MDD compared to BPD to afford the same power (Wray et al., 2012). Greater heterogeneity in MDD may also contribute to lower accuracy of the polygenic predictor. The sample size in the target sample also affects the power. In general, the method of estimating the SNP effects one at a time, then summing their effects to generate a predictor, is not optimal owing to the errors in the estimation of the SNP effects (for a review of profile scoring see (Wray et al., 2013)). As an example of this, profile scoring was first used to demonstrate that a predictor based on a schizophrenia GWAS in a discovery sample could explain 3% of the variance in schizophrenia case/control status in an independent dataset (Purcell et al., 2009). The true genetic correlation between schizophrenia cases in one sample and another is of course much greater than this. In the same study, the schizophrenia profile score could explain approximately 1% of the variance in an independent BPD dataset. The genetic correlation between SCZ and BPD estimated from population data is 0.6 (Lichtenstein et al., 2009), while the estimate of genetic correlation based on common SNPs is 0.64 (Cross-Disorder Group of the Psychiatric Genomics et al., 2013). So while the estimates of variance explained using profile scoring are often small except in the case where very large sample sizes are used, they reflect genetic overlap that is far more substantial. An overall estimate of 0.1% variance explained by PGC-BPD scores in the independent PPD samples is therefore not trivial. The estimates of the variance explained in the QIMR and NESDA/NTR PPD case/control groups are greater than 1%, similar to what was found when SCZ profile scores were used to predict BPD.
In both QIMR and NESDA/NTR, comparing PPD cases to all controls (both PPD and MDD controls) reduces the predictive power of the bipolar polygenic score, despite the fact that, naively, the larger numbers of controls should afford more power. One potential explanation for this is that the additional controls include men and women who carry BPD risk alleles, but having not experienced the environmental trigger of being pregnant or giving birth, they are at reduced risk of developing a mood disorder. The observation that the predictive ability of the polygenic score is reduced when using all females compared to the PPD only sample (i.e., female controls with children) indicates that the PPD results cannot be explained as a sex-specific effect.
The results in the QIMR and NESDA/NTR cohorts support trends from previous epidemiological studies of postpartum mood disorders. A recent study using Danish population registries that followed up women who presented to a psychiatrist for the first time and who were not given a BPD diagnosis, found that almost 14% of women who presented shortly after childbirth went on to be given a BPD diagnosis in the next 15 years. This was a 3-fold increase over women who first presented to a psychiatrist and were not given a BPD diagnosis at a time other than after childbirth. The risk decreased with increasing number of days postpartum the patient presented (Munk-Olsen et al., 2012). Specifically, among those women given a diagnosis of unipolar depression upon first presentation, women who presented in the postpartum period had a relative risk of 2.88 (95% CI 1.51–5.92) of a subsequent BPD diagnosis, compared to women presenting at any other time. Another study that compared depressive symptoms in BPD patients to unipolar patients found a greatly increased reporting of a postpartum episode in BPD patients compared to unipolar (OR = 7.9 95% CI 0.8 – 378.1)(Ghaemi et al., 2004). However, the small sample size of the study meant that the null hypothesis of equality of postpartum episodes could not be rejected.
A limitation of our study was that PPD was defined by the use of self-reported data obtained from questionnaires (retrospective in 3 of the studies), which may be less homogenous and not representative of a clinically derived sample. However heritability for post-natal depressive symptoms screened in the QIMR sample has previously been reported (12), and the measures showed good test-retest reliability. The modified EPDS shows strong internal consistency (Cronbach’s alpha = 0.82 (Meltzer-Brody et al., 2013)) and may be used in the future as a screen for lifetime postpartum depression in a clinical setting. Our study did not assess postnatal mania, which is a distinct diagnosis from postnatal depression, but is much less common with a rate of approximately 1 in 1,000 births (Andrews-Fike, 1999, Kendell et al., 1987). Questionnaires administered to PPD cases in subsequent studies also did not assess criteria for BPD, so it was not possible to estimate how many women went on to a subsequent diagnosis of BPD. Future studies should systematically assess postpartum mania and should include followup of symptoms in the months and years after the postpartum mood episode, to get a clearer picture of the diagnosis.
Another source of heterogeneity in our study is the definition of cases being different in the Australian sample when compared to the Dutch, Swedish and UK samples. Out of the 486 cases in the Australian discovery sample, 354 had been qualified as MDD by DSM-IV lifetime criteria. Of the remaining 132 Australian PPD cases, only 53 had completed a diagnostic interview that allowed DSM classification. All of the cases in the NESDA/NTR sample met DSM-IV criteria for MDD as well as having an EPDS score above 11, and controls were screened for psychiatric disorders. The STR and ALSPAC samples used the EPDS to ascertain cases and controls, but no further information on psychiatric disorders such as MDD was available, and hence the controls were not screened. This heterogeneity in ascertainment may explain some of the differences in the profile scoring results seen across cohorts.
Further studies of the genetic relationship between BPD and PPD are warranted. Specifically, such studies should include cohorts where the controls have been fully screened for MDD and other psychiatric disorders. Studies investigating the prevalence of postpartum mood episodes in women with BPD showed that 67% experienced an episode within 1 month of delivery, and these were almost exclusively depressive episodes with no psychotic features (Freeman et al., 2002). Allied to this, another study showed that >50% of women given a diagnosis of PPD were misdiagnosed and were subsequently given a lifetime diagnosis of BPD (Sharma et al., 2008). Our results support the hypothesis that postpartum depression is more closely related to BPD, and highlight the need for proper screening for BPD in patients presenting with PPD. Increasing the accuracy of diagnosis could aid in selecting the best treatment and help to reduce the risk of adverse events for mother and child.
Supplementary Material
Acknowledgments
Australia: This research was diectly supported by the Australian Research Council (FT0991360) and the Australian National Health and Medical Research Council (613608). We acknowledge Professor Matthew Brown for genotyping undertaken at the University of Queensland Centre for Clinical Genomics, housed at UQ’s Diamantina Institute. A portion of the statistical analyses were carried out on the QIMR GenEpi Cluster, which is financially supported by contributions from grants from the Australian National Health and Medical Research Council (389892; 496682; 496688; 496739; 613672). We thank the ATR twins for their continued participation. We also thank Dixie Statham, Bronwyn Morris and Megan Ferguson for coordinating the data collection for the twins; David Smyth, Olivia Zheng and Harry Beeby for data management; Lisa Bowdler, Steven Crooks (DNA processing); Sarah Medland, Dale Nyholt and Scott Gordon (imputation and genotyping QC).
NTR/NESDA: The Netherlands Study of Depression and Anxiety (NESDA) and the Netherlands Twin Register (NTR) were funded by the Netherlands Organization for Scientific Research (MagW/ZonMW grants 904-61-090, 985-10-002,904-61-193,480-04-004, 400-05-717, 912-100-20; Spinozapremie 56-464-14192; Geestkracht program grant 10-000-1002); the Center for Medical Systems Biology (CMSB2; NWO Genomics), Biobanking and Biomolecular Resources Research Infrastructure (BBMRI-NL), VU University EMGO+ Institute for Health and Care Research and the Neuroscience Campus Amsterdam, NBIC/BioAssist/RK (2008.024); the European Science Foundation (EU/QLRT-2001-01254); the European Community’s Seventh Framework Program (FP7/2007-2013); ENGAGE (HEALTH-F4-2007-201413); and the European Science Council (ERC, 230374).). Part of the genotyping and analyses were funded by the Genetic Association Information Network (GAIN) of the Foundation for the National Institutes of Health, by the US National Institute of Mental Health (RC2 MH089951, PI Sullivan and 1RC2 MH089995 PI Hudziak) and as part of the American Recovery and Reinvestment Act of 2009. We acknowledge Rutgers University Cell and DNA Repository (NIMH U24 MH068457-06) and the Avera Institute, Sioux Falls, South Dakota (USA).
ALSPAC: A grant from the Wellcome Trust (WT088806) provided funds for the ALSPAC genome-wide data used in this study and for G.M’s salary at the time analyses with these data were undertaken. The UK Medical Research Council and the Wellcome Trust (Grant ref: 092731) and the University of Bristol provide core support for ALSPAC. H.M.S. was funded by a Wellcome Trust 4-year PhD studentship in molecular, genetic and life course epidemiology (WT099871MA). H.M.S, D.A.L, and D.M.E work in a Unit that receives research funds from the MRC. The authors are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses
STR: The Swedish Twin Registry is supported by the Swedish Department of Higher Education, the European Commission European Network for Genetic and Genomic Epidemiology [ENGAGE: 7th Framework Program (FP7/2007-2013)/Grant agreement HEALTH-F4-2007-201413; and GenomEUtwin: 5th Framework program “Quality of Life and Management of the Living Resources” Grant QLG2-CT-2002-01254]; the NIH (DK U01-066134); the Swedish Research Council (M-2005-1112 and 2009-2298); the Swedish Foundation for Strategic Research (ICA08-0047); the Jan Wallander and Tom Hedelius Foundation; and the Swedish Council for Working Life and Social Research.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- ANDREWS-FIKE C. A Review of Postpartum Depression. Prim Care Companion J Clin Psychiatry. 1999;1:9–14. doi: 10.4088/pcc.v01n0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOMSMA DI, DE GEUS EJ, VINK JM, STUBBE JH, DISTEL MA, HOTTENGA JJ, POSTHUMA D, VAN BEIJSTERVELDT TC, HUDZIAK JJ, BARTELS M, WILLEMSEN G. Netherlands Twin Register: from twins to twin families. Twin Res Hum Genet. 2006;9:849–57. doi: 10.1375/183242706779462426. [DOI] [PubMed] [Google Scholar]
- BOOMSMA DI, WILLEMSEN G, SULLIVAN PF, HEUTINK P, MEIJER P, SONDERVAN D, KLUFT C, SMIT G, NOLEN WA, ZITMAN FG, SMIT JH, HOOGENDIJK WJ, VAN DYCK R, DE GEUS EJ, PENNINX BW. Genome-wide association of major depression: description of samples for the GAIN Major Depressive Disorder Study: NTR and NESDA biobank projects. Eur J Hum Genet. 2008;16:335–42. doi: 10.1038/sj.ejhg.5201979. [DOI] [PubMed] [Google Scholar]
- BRITTON JR. Postpartum anxiety and breast feeding. J Reprod Med. 2007;52:689–95. [PubMed] [Google Scholar]
- COX JL, HOLDEN JM, SAGOVSKY R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- CROSS-DISORDER GROUP OF THE PSYCHIATRIC GENOMICS C. LEE SH, RIPKE S, NEALE BM, FARAONE SV, PURCELL SM, PERLIS RH, MOWRY BJ, THAPAR A, GODDARD ME, WITTE JS, ABSHER D, AGARTZ I, AKIL H, AMIN F, ANDREASSEN OA, ANJORIN A, ANNEY R, ANTTILA V, ARKING DE, ASHERSON P, AZEVEDO MH, BACKLUND L, BADNER JA, BAILEY AJ, BANASCHEWSKI T, BARCHAS JD, BARNES MR, BARRETT TB, BASS N, BATTAGLIA A, BAUER M, BAYES M, BELLIVIER F, BERGEN SE, BERRETTINI W, BETANCUR C, BETTECKEN T, BIEDERMAN J, BINDER EB, BLACK DW, BLACKWOOD DH, BLOSS CS, BOEHNKE M, BOOMSMA DI, BREEN G, BREUER R, BRUGGEMAN R, CORMICAN P, BUCCOLA NG, BUITELAAR JK, BUNNEY WE, BUXBAUM JD, BYERLEY WF, BYRNE EM, CAESAR S, CAHN W, CANTOR RM, CASAS M, CHAKRAVARTI A, CHAMBERT K, CHOUDHURY K, CICHON S, CLONINGER CR, COLLIER DA, COOK EH, COON H, CORMAND B, CORVIN A, CORYELL WH, CRAIG DW, CRAIG IW, CROSBIE J, CUCCARO ML, CURTIS D, CZAMARA D, DATTA S, DAWSON G, DAY R, DE GEUS EJ, DEGENHARDT F, DJUROVIC S, DONOHOE GJ, DOYLE AE, DUAN J, DUDBRIDGE F, DUKETIS E, EBSTEIN RP, EDENBERG HJ, ELIA J, ENNIS S, ETAIN B, FANOUS A, FARMER AE, FERRIER IN, FLICKINGER M, FOMBONNE E, FOROUD T, FRANK J, FRANKE B, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDBRIDGE F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9:e1003348. doi: 10.1371/journal.pgen.1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLYNN HA, DAVIS M, MARCUS SM, CUNNINGHAM R, BLOW FC. Rates of maternal depression in pediatric emergency department and relationship to child service utilization. General Hospital Psychiatry. 2004;26:316–322. doi: 10.1016/j.genhosppsych.2004.03.009. [DOI] [PubMed] [Google Scholar]
- FRASER A, MACDONALD-WALLIS C, TILLING K, BOYD A, GOLDING J, DAVEY SMITH G, HENDERSON J, MACLEOD J, MOLLOY L, NESS A, RING S, NELSON SM, LAWLOR DA. Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN MP, SMITH KW, FREEMAN SA, MCELROY SL, KMETZ GE, WRIGHT R, KECK PE., JR The impact of reproductive events on the course of bipolar disorder in women. J Clin Psychiatry. 2002;63:284–7. doi: 10.4088/jcp.v63n0403. [DOI] [PubMed] [Google Scholar]
- GAVIN NI, GAYNES BN, LOHR KN, MELTZER-BRODY S, GARTLEHNER G, SWINSON T. Perinatal depression - A systematic review of prevalence and incidence. Obstetrics and Gynecology. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- GAYNES BN, GAVIN N, MELTZER-BRODY S, LOHR KN, SWINSON T, GARTLEHNER G, BRODY S, MILLER WC. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ) 2005:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHAEMI SN, HSU DJ, KO JY, BALDASSANO CF, KONTOS NJ, GOODWIN FK. Bipolar spectrum disorder: a pilot study. Psychopathology. 2004;37:222–6. doi: 10.1159/000080717. [DOI] [PubMed] [Google Scholar]
- KENDELL RE, CHALMERS JC, PLATZ C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150:662–73. doi: 10.1192/bjp.150.5.662. [DOI] [PubMed] [Google Scholar]
- LEE SH, DECANDIA TR, RIPKE S, YANG J, SULLIVAN PF, GODDARD ME, KELLER MC, VISSCHER PM, WRAY NR SCHIZOPHRENIA PSYCHIATRIC GENOME-WIDE ASSOCIATION STUDY C, INTERNATIONAL SCHIZOPHRENIA C, MOLECULAR GENETICS OF SCHIZOPHRENIA C. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat Genet. 2012a;44:247–50. doi: 10.1038/ng.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE SH, DECANDIA TR, RIPKE S, YANG J, SULLIVAN PF, GODDARD ME, KELLER MC, VISSCHER PM, WRAY NR. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat Genet. 2012b;44:247–50. doi: 10.1038/ng.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS CM, NG MY, BUTLER AW, COHEN-WOODS S, UHER R, PIRLO K, WEALE ME, SCHOSSER A, PAREDES UM, RIVERA M, CRADDOCK N, OWEN MJ, JONES L, JONES I, KORSZUN A, AITCHISON KJ, SHI J, QUINN JP, MACKENZIE A, VOLLENWEIDER P, WAEBER G, HEATH S, LATHROP M, MUGLIA P, BARNES MR, WHITTAKER JC, TOZZI F, HOLSBOER F, PREISIG M, FARMER AE, BREEN G, CRAIG IW, MCGUFFIN P. Genome-wide association study of major recurrent depression in the U.K. population. Am J Psychiatry. 2010;167:949–57. doi: 10.1176/appi.ajp.2010.09091380. [DOI] [PubMed] [Google Scholar]
- LICHTENSTEIN P, YIP BH, BJORK C, PAWITAN Y, CANNON TD, SULLIVAN PF, HULTMAN CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–9. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUBKE GH, HOTTENGA JJ, WALTERS R, LAURIN C, DE GEUS EJ, WILLEMSEN G, SMIT JH, MIDDELDORP CM, PENNINX BW, VINK JM, BOOMSMA DI. Estimating the genetic variance of major depressive disorder due to all single nucleotide polymorphisms. Biol Psychiatry. 2012;72:707–9. doi: 10.1016/j.biopsych.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGNUSSON PK, ALMQVIST C, RAHMAN I, GANNA A, VIKTORIN A, WALUM H, HALLDNER L, LUNDSTROM S, ULLEN F, LANGSTROM N, LARSSON H, NYMAN A, GUMPERT CH, RASTAM M, ANCKARSATER H, CNATTINGIUS S, JOHANNESSON M, INGELSSON E, KLARESKOG L, DE FAIRE U, PEDERSEN NL, LICHTENSTEIN P. The Swedish twin registry: establishment of a biobank and other recent developments. Twin Res Hum Genet. 2013;16:317–29. doi: 10.1017/thg.2012.104. [DOI] [PubMed] [Google Scholar]
- MAHON PB, PAYNE JL, MACKINNON DF, MONDIMORE FM, GOES FS, SCHWEIZER B, JANCIC D, CORYELL WH, HOLMANS PA, SHI JX, KNOWLES JA, SCHEFTNER WA, WEISSMAN MM, LEVINSON DF, DEPAULO JR, ZANDI PP, POTASH JBD, NGIB CONSORTIUM B. Genome-Wide Linkage and Follow-Up Association Study of Postpartum Mood Symptoms. American Journal of Psychiatry. 2009;166:1229–1237. doi: 10.1176/appi.ajp.2009.09030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJOR DEPRESSIVE DISORDER WORKING GROUP OF THE PSYCHIATRIC GWAS CONSORTIUM. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMORSTEIN NR, MALONE SM, IACONO WG. Psychiatric disorders among offspring of depressed mothers: Associations with paternal psychopathology. American Journal of Psychiatry. 2004;161:1588–1594. doi: 10.1176/appi.ajp.161.9.1588. [DOI] [PubMed] [Google Scholar]
- MELTZER-BRODY S, BOSCHLOO L, JONES I, SULLIVAN PF, PENNINX BW. The EPDS-Lifetime: assessment of lifetime prevalence and risk factors for perinatal depression in a large cohort of depressed women. Arch Womens Ment Health. 2013 doi: 10.1007/s00737-013-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER LJ. Postpartum depression. JAMA. 2002;287:762–5. doi: 10.1001/jama.287.6.762. [DOI] [PubMed] [Google Scholar]
- MUGLIA P, TOZZI F, GALWEY NW, FRANCKS C, UPMANYU R, KONG XQ, ANTONIADES A, DOMENICI E, PERRY J, ROTHEN S, VANDELEUR CL, MOOSER V, WAEBER G, VOLLENWEIDER P, PREISIG M, LUCAE S, MULLER-MYHSOK B, HOLSBOER F, MIDDLETON LT, ROSES AD. Genome-wide association study of recurrent major depressive disorder in two European case-control cohorts. Mol Psychiatry. 2010;15:589–601. doi: 10.1038/mp.2008.131. [DOI] [PubMed] [Google Scholar]
- MUNK-OLSEN T, LAURSEN TM, MELTZER-BRODY S, MORTENSEN PB, JONES I. Psychiatric disorders with postpartum onset: possible early manifestations of bipolar affective disorders. Arch Gen Psychiatry. 2012;69:428–34. doi: 10.1001/archgenpsychiatry.2011.157. [DOI] [PubMed] [Google Scholar]
- O’HARA MW, SWAIN AM. Rates and risk of postpartum depression—a meta-analysis. International Review of Psychiatry. 1996;8:37–54. [Google Scholar]
- PENNINX BW, BEEKMAN AT, SMIT JH, ZITMAN FG, NOLEN WA, SPINHOVEN P, CUIJPERS P, DE JONG PJ, VAN MARWIJK HW, ASSENDELFT WJ, VAN DER MEER K, VERHAAK P, WENSING M, DE GRAAF R, HOOGENDIJK WJ, ORMEL J, VAN DYCK R. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int J Methods Psychiatr Res. 2008;17:121–40. doi: 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PURCELL S, NEALE B, TODD-BROWN K, THOMAS L, FERREIRA MA, BENDER D, MALLER J, SKLAR P, DE BAKKER PI, DALY MJ, SHAM PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PURCELL SM, WRAY NR, STONE JL, VISSCHER PM, O’DONOVAN MC, SULLIVAN PF, SKLAR P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIPKE S, O’DUSHLAINE C, CHAMBERT K, MORAN JL, KAHLER AK, AKTERIN S, BERGEN SE, COLLINS AL, CROWLEY JJ, FROMER M, KIM Y, LEE SH, MAGNUSSON PK, SANCHEZ N, STAHL EA, WILLIAMS S, WRAY NR, XIA K, BETTELLA F, BORGLUM AD, BULIK-SULLIVAN BK, CORMICAN P, CRADDOCK N, DE LEEUW C, DURMISHI N, GILL M, GOLIMBET V, HAMSHERE ML, HOLMANS P, HOUGAARD DM, KENDLER KS, LIN K, MORRIS DW, MORS O, MORTENSEN PB, NEALE BM, O’NEILL FA, OWEN MJ, MILOVANCEVIC MP, POSTHUMA D, POWELL J, RICHARDS AL, RILEY BP, RUDERFER D, RUJESCU D, SIGURDSSON E, SILAGADZE T, SMIT AB, STEFANSSON H, STEINBERG S, SUVISAARI J, TOSATO S, VERHAGE M, WALTERS JT, LEVINSON DF, GEJMAN PV, KENDLER KS, LAURENT C, MOWRY BJ, O’DONOVAN MC, OWEN MJ, PULVER AE, RILEY BP, SCHWAB SG, WILDENAUER DB, DUDBRIDGE F, HOLMANS P, SHI J, ALBUS M, ALEXANDER M, CAMPION D, COHEN D, DIKEOS D, DUAN J, EICHHAMMER P, GODARD S, HANSEN M, LERER FB, LIANG KY, MAIER W, MALLET J, NERTNEY DA, NESTADT G, NORTON N, O’NEILL FA, PAPADIMITRIOU GN, RIBBLE R, SANDERS AR, SILVERMAN JM, WALSH D, WILLIAMS NM, WORMLEY B, ARRANZ MJ, BAKKER S, BENDER S, BRAMON E, COLLIER D, CRESPO-FACORRO B, et al. MULTICENTER GENETIC STUDIES OF SCHIZOPHRENIA C, PSYCHOSIS ENDOPHENOTYPES INTERNATIONAL C. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–9. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARMA V, KHAN M, CORPSE C, SHARMA P. Missed bipolarity and psychiatric comorbidity in women with postpartum depression. Bipolar Disord. 2008;10:742–7. doi: 10.1111/j.1399-5618.2008.00606.x. [DOI] [PubMed] [Google Scholar]
- SHI J, POTASH JB, KNOWLES JA, WEISSMAN MM, CORYELL W, SCHEFTNER WA, LAWSON WB, DEPAULO JR, GEJMAN PV, SANDERS AR, JOHNSON JK, ADAMS P, CHAUDHURY S, JANCIC D, EVGRAFOV O, ZVINYATSKOVSKIY A, ERTMAN N, GLADIS M, NEIMANAS K, GOODELL M, HALE N, NEY N, VERMA R, MIREL D, HOLMANS P, LEVINSON DF. Genome-wide association study of recurrent early-onset major depressive disorder. Mol Psychiatry. 2011;16:193–201. doi: 10.1038/mp.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHYN SI, SHI J, KRAFT JB, POTASH JB, KNOWLES JA, WEISSMAN MM, GARRIOCK HA, YOKOYAMA JS, MCGRATH PJ, PETERS EJ, SCHEFTNER WA, CORYELL W, LAWSON WB, JANCIC D, GEJMAN PV, SANDERS AR, HOLMANS P, SLAGER SL, LEVINSON DF, HAMILTON SP. Novel loci for major depression identified by genome-wide association study of Sequenced Treatment Alternatives to Relieve Depression and meta-analysis of three studies. Mol Psychiatry. 2011;16:202–15. doi: 10.1038/mp.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKLAR P, RIPKE S, SCOTT LJ, ANDREASSEN OA, CICHON S, CRADDOCK N, EDENBERG HJ, NURNBERGER JI, JR, RIETSCHEL M, BLACKWOOD D, CORVIN A, FLICKINGER M, GUAN W, MATTINGSDAL M, MCQUILLIN A, KWAN P, WIENKER TF, DALY M, DUDBRIDGE F, HOLMANS PA, LIN D, BURMEISTER M, GREENWOOD TA, HAMSHERE ML, MUGLIA P, SMITH EN, ZANDI PP, NIEVERGELT CM, MCKINNEY R, SHILLING PD, SCHORK NJ, BLOSS CS, FOROUD T, KOLLER DL, GERSHON ES, LIU C, BADNER JA, SCHEFTNER WA, LAWSON WB, NWULIA EA, HIPOLITO M, CORYELL W, RICE J, BYERLEY W, MCMAHON FJ, SCHULZE TG, BERRETTINI W, LOHOFF FW, POTASH JB, MAHON PB, MCINNIS MG, ZOLLNER S, ZHANG P, CRAIG DW, SZELINGER S, BARRETT TB, BREUER R, MEIER S, STROHMAIER J, WITT SH, TOZZI F, FARMER A, MCGUFFIN P, STRAUSS J, XU W, KENNEDY JL, VINCENT JB, MATTHEWS K, DAY R, FERREIRA MA, O’DUSHLAINE C, PERLIS R, RAYCHAUDHURI S, RUDERFER D, HYOUN PL, SMOLLER JW, LI J, ABSHER D, THOMPSON RC, MENG FG, SCHATZBERG AF, BUNNEY WE, BARCHAS JD, JONES EG, WATSON SJ, MYERS RM, AKIL H, BOEHNKE M, CHAMBERT K, MORAN J, SCOLNICK E, DJUROVIC S, MELLE I, MORKEN G, GILL M, MORRIS D, QUINN E, MUHLEISEN TW, DEGENHARDT FA, MATTHEISEN M, et al. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43:977–83. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN A, GATH DH, BUCHER J, BOND A, DAY A, COOPER PJ. The relationship between postnatal depression and mother-child interaction. British Journal of Psychiatry. 1991;158:46–52. doi: 10.1192/bjp.158.1.46. [DOI] [PubMed] [Google Scholar]
- SULLIVAN PF, DALY MJ, O’DONOVAN M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13:537–51. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULLIVAN PF, DE GEUS EJ, WILLEMSEN G, JAMES MR, SMIT JH, ZANDBELT T, AROLT V, BAUNE BT, BLACKWOOD D, CICHON S, COVENTRY WL, DOMSCHKE K, FARMER A, FAVA M, GORDON SD, HE Q, HEATH AC, HEUTINK P, HOLSBOER F, HOOGENDIJK WJ, HOTTENGA JJ, HU Y, KOHLI M, LIN D, LUCAE S, MACINTYRE DJ, MAIER W, MCGHEE KA, MCGUFFIN P, MONTGOMERY GW, MUIR WJ, NOLEN WA, NOTHEN MM, PERLIS RH, PIRLO K, POSTHUMA D, RIETSCHEL M, RIZZU P, SCHOSSER A, SMIT AB, SMOLLER JW, TZENG JY, VAN DYCK R, VERHAGE M, ZITMAN FG, MARTIN NG, WRAY NR, BOOMSMA DI, PENNINX BW. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry. 2009;14:359–75. doi: 10.1038/mp.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRELOAR SA, MARTIN NG, BUCHOLZ KK, MADDEN PA, HEATH AC. Genetic influences on post-natal depressive symptoms: findings from an Australian twin sample. Psychol Med. 1999;29:645–54. doi: 10.1017/s0033291799008387. [DOI] [PubMed] [Google Scholar]
- WISNER KL, PARRY BL, PIONTEK CM. Clinical practice. Postpartum depression. N Engl J Med. 2002;347:194–9. doi: 10.1056/NEJMcp011542. [DOI] [PubMed] [Google Scholar]
- WITTCHEN HU, ROBINS LN, COTTLER LB, SARTORIUS N, BURKE JD, REGIER D. Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The Multicentre WHO/ADAMHA Field Trials. Br J Psychiatry. 1991;159:645–53. 658. doi: 10.1192/bjp.159.5.645. [DOI] [PubMed] [Google Scholar]
- WRAY NR, PERGADIA ML, BLACKWOOD DH, PENNINX BW, GORDON SD, NYHOLT DR, RIPKE S, MACINTYRE DJ, MCGHEE KA, MACLEAN AW, SMIT JH, HOTTENGA JJ, WILLEMSEN G, MIDDELDORP CM, DE GEUS EJ, LEWIS CM, MCGUFFIN P, HICKIE IB, VAN DEN OORD EJ, LIU JZ, MACGREGOR S, MCEVOY BP, BYRNE EM, MEDLAND SE, STATHAM DJ, HENDERS AK, HEATH AC, MONTGOMERY GW, MARTIN NG, BOOMSMA DI, MADDEN PA, SULLIVAN PF. Genome-wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Mol Psychiatry. 2012;17:36–48. doi: 10.1038/mp.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRAY NR, YANG J, HAYES BJ, PRICE AL, GODDARD ME, VISSCHER PM. Pitfalls of predicting complex traits from SNPs. Nat Rev Genet. 2013;14:507–15. doi: 10.1038/nrg3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG J, LEE SH, GODDARD ME, VISSCHER PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.