Abstract
The signals required to generate long-lived plasma cells remain unresolved. One widely cited model posits that long-lived plasma cells derive from germinal centers (GCs) in response to T-cell dependent (TD) antigens. Thus, T-cell independent (TI) antigens, which fail to sustain GCs, are considered ineffective at generating long-lived plasma cells. However, we show that long-lived hapten-specific plasma cells are readily induced without formation of GCs. Long-lived plasma cells developed in T-cell deficient mice after single immunization with haptenated LPS, a widely-used TI antigen. Long-lived plasma cells also formed in response to TD antigen when the GC response was experimentally prevented. These observations establish that long-lived plasma cells are induced in both TI and TD responses, and can arise independently of B-cell maturation in GCs.
Keywords: B cells, antibodies, cell differentiation, memory, plasma cells
Introduction
Antibodies play pivotal roles in host defense, and can cause disease when specific for self-antigens. Plasma cells, the main source of antibodies, are thought to consist of two pools: short-lived cells that secrete low affinity IgM antibodies and form extrafollicular foci in the spleen or lymph node; and long-lived cells that secrete high affinity, isotype switched antibodies found mainly in the bone marrow (BM)2 (1). Whereas short-lived plasma cells die within 3–5 days (2), long-lived plasma cells are thought to persist for months or years in mice and perhaps decades in people (3–6). Sustained serum antibody titers due to long-lived plasma cells are essential for protective immunity against many pathogens (3, 7–9). Therefore, defining the events underlying the generation of long-lived plasma cells is essential for understanding how antibody-mediated immunity is established and maintained.
Why some plasma cells achieve longevity whereas others are short-lived is not known. One current and widely cited model posits that long-lived plasma cells emanate chiefly from germinal centers (GCs) in response to T-cell dependent (TD) antigens (4, 10). GCs are microanatomical structures enriched with antigen-stimulated B cells undergoing class switch recombination (CSR), somatic hypermutation (SHM), and affinity based selection (11). Indeed, it was proposed recently that long-lived plasma cells are generated preferentially within the GC to favor the continuous secretion of high-affinity and therefore more protective antibodies (12). This viewpoint is supported by data showing that the bulk of BM plasma cells induced by TD antigen secrete high-affinity class-switched antibodies (13). However, the notion that long-lived plasma cells must derive from GCs appears incompatible with recent data showing that low affinity IgM antibodies can mediate long-term protection against certain microbial pathogens (14).
Classically, antigens that do not engage helper T cells are thought to generate transient IgM responses that reflect the activity of short-lived plasma cells (2, 15–17). These T-cell independent (TI) antigens may also generate short-lived GCs that fail to engender SHM and affinity maturation (18, 19). However, several recent studies challenge the long-standing paradigm that TI antibody responses are always short-lived. For instance, immunization of T cell deficient mice with the spirochete Borrelia hermsii induces long-term IgM-dependent protection (20, 21). IgM antibodies induced by the intracellular bacteria Franciscella tularensis and Ehrlichia muris also mediate long-term protection, however these responses may reflect the continuous generation of short-lived antibody-secreting cells (22, 23). More recently, E. muris and the encapsulated bacterium Streptococcus pneumoniae were shown to elicit a long-standing pool of antigen-specific BM plasma cells in T cell sufficient mice (24–26). While the latter work provides evidence that typical TD antigens are not unique in their ability to induce long-term antibody responses, these studies did not address whether long-lived plasma cells can be generated in the absence of T cells. Therefore, whether T cell-derived signals are strictly required to generate long-lived plasma cells remains unclear.
Our work addresses the capacity of TI and TD antigens to induce long-lived plasma cells during the earliest phases of plasma cell differentiation. We show that haptenated LPS, a classic type 1 TI antigen, readily induces a long-standing pool of BM plasma cells in mice that lack T cells. These antibody-secreting cells persist for more than 100 days after a single immunization, exhibit a half-life of 45–55 days, and arise despite an inability to detect antigen-induced GC B cells. These data challenge the long-standing notion that type 1 TI antigens fail to induce the formation of long-lived plasma cells, while also suggesting that long-lived plasma cells need not arise from GCs. Similarly, we show that long-lived plasma cells also form in response to a standard TD antigen without undergoing maturation and selection in GCs, as plasma cells secreting low affinity IgM antibodies persisted for at least 100 days in mice in which we prevented GC formation early in these responses. These findings indicate that maturation in GCs is not requisite for achieving longevity in the plasma cell lineage, while also suggesting that competence to enter long-lived plasma cell pools is achieved early in TI and TD antibody responses.
Materials and Methods
Mice
C57BL/6 (B6), B6.TcRβ−/−δ−/−, and MD4 Ig transgenic (anti-HEL) females (age 8–10 weeks) were obtained from Jackson Laboratories. AID−/− mice were provided by Dr. Nina Papavasiliou (Rockefeller University). All animal procedures were approved by the University of Pennsylvania Office of Regulatory Affairs.
Chimeras
Hosts were exposed to whole body radiation (900R) five days post immunization, and then given 1 × 106 BM cells intravenously (i.v.) from MD4 IgH+L transgenic mice in which all B cells are specific for Hen Egg Lysozyme (27). BM cells were depleted of CD3ε+ cells using a MACs LD column before i.v. transfer. All chimeras were maintained on antibiotic water for at least 6 weeks post radiation. Immunization of separate MD4-into-B6 chimeras with NP-CGG or NP-LPS failed to elicit detectable NP-specific plasma cells as determined by ELISPOT (not shown).
Immunizations
8–10 week old mice were immunized intraperitoneally (i.p.) with 50μg NP16-CGG in alum or 50μg NP0.6-LPS in PBS.
ELISPOT
Multiscreen HTS plates (Millipore) were coated with 10μg/well of either Goat anti-Mouse Ig(H+L) (Southern Biotech), or NP33-BSA, or NP4-BSA (BioSearch) in sodium bicarbonate buffer, and then blocked with 2% BSA/PBS. Cells were serially diluted across the plate, and then incubated for 4–6 hr at 37°C. Biotin-Goat anti-Igλ, Goat-anti-IgM, or Goat-anti-IgG1 (Southern Biotech) diluted in block buffer was added, followed by three washes with 0.1% Tween-20 detergent, and a secondary incubation with ExtrAvidin-alkaline phosphatase (Sigma). Spots were detected using BCIP/NBT (Sigma) and scanned and counted with an ImmunoSpot Analyzer (Cellular Technology Ltd.).
Flow cytometry and cell sorting
Spleen and BM cells were harvested and stained with optimal dilutions of the indicated antibodies as described (28). Unless noted otherwise all of the following reagents were purchased from eBiosciences: FITC-anti-Igλ1–3 (R26–46, Pharmingen), and PNA (Sigma); phycoerythrin (PE)-anti-CD138 (281–2, Pharmingen); PE-TexasRed-anti-B220 (RA3–6B2); PE-Cy7-anti-CD4 (RM4–5), anti-CD8α (53–6.7), anti-Gr-1 (RB6–8C5), anti-F4/80 (BM8), and anti-TER119; allophycocyanin (APC)-Cy5.5-anti-CD19 (1D3); Alexa405 anti-IgD (11–26), and Biotin-anti-CD138 (281–2, Pharmingen). Biotinylated antibodies were revealed with Streptavidin-APC-Cy7 (Pharmingen). NP-APC was conjugated in our laboratory using standard methods. Nonviable cells were eliminated from all analyses with the UV-excited DNA dye DAPI (Molecular Probes), and doublets were excluded from all analyses using the combined width parameter of the forward and side scatter parameters. Flow cytometry was performed on a BD LSRII and cell sorting was performed on a four laser Aria (Becton Dickinson). Analysis was done using FlowJo8.8 (Tree Star, Inc.).
BrdU labeling
B6 mice were immunized with NP-LPS and fed BrdU in the drinking water (0.5 mg/ml with 1% dextrose) for 3 days beginning immediately after immunization. Spleen and BM cells were stained with appropriate antibodies, fixed and permeabilized (Fix & Perm, Invitrogen), treated with 225 μg/ml DNase (DN25, Sigma), stained intracellularly with FITC-anti-BrdU antibodies and NP-APC, and washed twice before analysis on an LSR2 flow cytometer. Multiple files per sample were concatenated before data analysis using FlowJo8.8.
CD40-CD154 blockade
On days 5, 7, and 9 post-immunization NP-CGG/alum immunized mice were given i.v. inoculations (300μg/injection) of anti-CD154 (MR-1) or control Hamster IgG (both from BioXcell) as described by Takahashi et al. (29).
Statistical Analysis
Significances in differences in plasma cell frequencies between two experimental groups were evaluated with the unpaired two-tailed t-test using Excel software.
Results
Persistence of LPS-induced T-cell independent plasma cells
We immunized C57BL/6 (B6) or T cell deficient B6.TcRβ−/−δ−/− adults with the hapten (4-hydroxy-3-nitrophenyl)acetyl (NP) conjugated to LPS or chicken γ-globulin (CGG). We studied the antibody response to NP in B6 background mice for two reasons. First, the kinetics of both plasma cell differentiation and GC-dependent somatic hypermutation and selection in response to NP are well established (29, 30). Second, NP-responsive B-lineage cells are readily identified by flow cytometry with NP-conjugated fluorescent proteins, and such cells can be resolved in conventional inbred mice without using Ig transgenes to increase frequencies of antigen-responsive B cells (31).
We quantified NP-specific plasma cell frequencies in the spleen and BM at several time points after a single inoculation of B6 or B6.TcRβ−/−δ−/− mice with NP-LPS. Because the antibody response to NP-conjugates in B6-background mice is dominated by λ+ B cells (30, 32), we assayed frequencies of plasma cells secreting λ+ NP-binding antibodies. Although as expected splenic NP-specific plasma cell frequencies increased and decreased exponentially between days 1 and 5 (15), NP-specific plasma cells were readily observed in the spleen and BM for at least 200 days in B6 mice and at least 91 days in B6.TcRβ−/−δ−/− mice (Fig. 1A, 1B). Furthermore, although in the spleen background levels increased substantially for mice immunized with NP-LPS more than 300 days previously, obscuring hapten-specific splenic plasma cells, in the BM we easily detected λ+ NP-specific plasma cells well above background in mice that were immunized more than 400 days previously (Supplemental Fig. 1). Of note, as shown in Figure 1B, on day 91 post-immunization frequencies of NP-specific plasma cells appeared to be greater in B6.TcRβ−/−δ−/− mice compared to B6 mice, although whether this difference in meaningful is presently unclear. We conclude that a single immunization with NP-LPS is sufficient to induce the generation of hapten-specific BM plasma cells that are readily detected more than 400 days later.
Figure 1. Kinetics of the plasma cell response after NP-LPS immunization.

(A) NP-specific λ+ plasma cells (PCs) in the spleen and BM were quantified by ELISPOT after B6 adults were immunized once with NP-LPS in PBS. (B) B6 (circles, solid line) or B6.TcRβ−/−δ−/− (squares, dashed line) adults were immunized and evaluated as in (A). For (A) and (B) all symbols represent data from an individual mouse. Note: On day 91 BM PC frequencies were significantly different between B6 and B6.TcRβ−/−δ−/− mice as indicated by the * (p=0.002). At all other times points there were no significant differences in PC frequencies between these groups. (C) Representative ELISPOT wells from the BM (d0, d90) or spleen (d3) of B6.TcRβ−/−δ−/− mice.
Decay rate of radioresistant T-independent plasma cells
Because residual antigen may induce naïve B-cells to contribute to plasma cell pools after immunization, we sought to estimate the decay rate of hapten-specific BM plasma cells following radiation-induced ablation of the functional naïve B-cell pool. In this regard, though past experiments show that long-lived plasma cells in the BM are resistant to apoptosis induced by ionizing radiation (IR) (6), it was unclear whether IR resistance is also a property of early plasma cells in the spleen. Significantly, whereas naïve B-cells readily died upon direct exposure to 200R, the vast majority of very early splenic plasma cells were resistant to IR-induced apoptosis, regardless of whether they were induced by immunizing with NP-LPS or NP-CGG and exposed to doses up to 800R (Fig. 2). It should be noted, consistent with the past observation that cultured GC B cells die rapidly without stimulation of CD40 (33), in our hands GC B cells died immediately in culture, with or without IR (not shown). To assess the capacity of NP-LPS induced plasma cells to persist without input from naïve B-cells, we quantified NP-specific plasma cells out to 190 days after NP-LPS immunized B6 mice were exposed to 900R whole body IR. Notably, past experiments using this approach indicate that the t1/2 of BM plasma cells induced after acute infection with Lymphocytic choriomeningitis virus (LCMV), a complex TD viral antigen, is 94 days (range 85–105 days) (6). Figure 3 shows that NP-specific plasma cells in NP-LPS immunized and irradiated/reconstituted mice declined with an estimated t1/2 of 55 days (range 49–63 days). Therefore, by these measurements the half-life of NP-LPS induced plasma cells is only modestly less than that observed during acute LCMV infection.
Figure 2. Early NP-specific plasma cells are radioresistant.

(A) Flow cytometric approach for sorting NP-specific CD138+ plasma cells (PCs) from spleens of mice immunized 7 days previously with NP-CGG/alum. Cells from the CD138+ gate were sorted for subsequent irradiation experiments. In addition to NP-binding plasma cells, radio-sensitivity was also determined for naive IgD+ NP− B220+ CD19+ B cells sorted from alum and NP-CGG immunized mice (gate not shown). (B) Alum or NP-CGG/alum immunized mice were used as donors for the indicated cell populations including NP-binding CD138+ plasma cells. Sorted cells were either rested (top) or subjected to 200R (bottom) before resting. Nine hours later viability was determined by flow cytometry using DAPI. (C) Frequencies of cells secreting NP-specific antibodies were determined by ELISPOT using freshly sorted (open), sorted and cultured for 9 hours (black), or sorted, exposed to 200R IR, and then rested for 9 hours (gray) CD138+ NP-binding cells. (D) Separate experiment in which B6 adults immunized with NP-LPS in PBS or NP-CGG in alum were sacrificed on day 3 post-immunization. Single splenocyte suspensions were exposed to 0, 200, 600, or 800 rads, rested 9 hours, and then transferred to NP-coated ELISPOT plates to assay frequencies of NP-specific λ+ PCs. Non-irradiated samples are shown with closed symbols; irradiated samples with open symbols. Differences in PC frequencies in C and D were not statistically significant.
Figure 3. Decay rate for NP-LPS induced plasma cells.

Cohorts of NP-LPS immunized B6 adults were left untouched or irradiated/reconstituted as described in Materials and Methods. (A) Shown are ELISPOT results for NP-specific λ+ plasma cells (PCs) in the BM comparing non-irradiated controls (circles with solid line) versus irradiated (squares with dashed line) B6 mice. Lines are drawn through the mean for each group. On day 190 BM PC frequencies were significantly different between irradiated and control mice as indicated by the * (p=0.05). At all other times points there were no significant differences in PC frequencies between these groups. (B) The decay rate NP-LPS induced PCs was illustrated by dividing the number of NP-specific PCs in each irradiated mouse by the average number of NP-specific PCs in 3–5 controls for each indicated time point as previously described by Slifka et al (6). The half-life (t1/2) of BM plasma cells post-IR was calculated as follows: t1/2 = (T × log 2) / log (starting quantity / ending quantity) where T= elapsed time.
Given that whole body IR may change microenvironments in the BM and elsewhere, potentially altering plasma cell lifespan, we also adopted an in vivo BrdU pulse-chase protocol to assess the decay rate of BrdU+ NP-specific BM plasma cells without exposing mice to IR. This general approach was used previously to gauge the half-life of antigen-specific plasma cells induced by a TD antigen (5). We fed NP-LPS immunized mice BrdU for 3 days beginning at the time of immunization, and then assessed the fraction of BrdU+ NP-specific BM plasma cells at multiple time points later by flow cytometry. As shown in Figure 4, NP-specific BrdU+ plasma cells were readily apparent in the BM between 34 and 94 days of the chase period. Therefore, many plasma cells formed within the first three days of the response to NP-LPS were able to enter and persist in the BM for at least 94 days. However, consistent with the data in Figure 3, the fraction of NP-specific BM plasma cells that were BrdU+ declined progressively between days 34 and 94 of the chase period, resulting in an estimated t1/2 of 47 days (range 41–59 days) (Fig. 4B). Interestingly, although surface expression of B220 is often used to identify early plasma cells (34), many hapten-specific BrdU+ plasma cells continued to exhibit low but detectable surface levels of B220, even on day 94 of the chase period (Supplemental Fig. 2), suggesting that surface levels of B220 are not consistently down-regulated on all plasma cells during the earliest phases of plasma cell differentiation. Altogether these data, along with the analyses in Figure 3, indicate that NP-LPS induced plasma cells possess a half-life of approximately 50 days.
Figure 4. Pulse-chase kinetics for hapten-specific NP-LPS induced plasma cells.
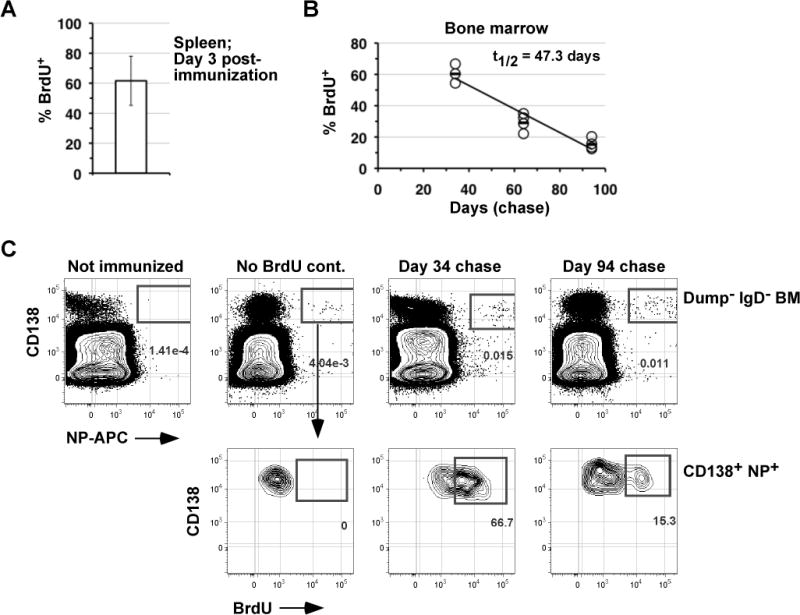
NP-LPS immunized B6 adults were fed BrdU in the drinking water for 3 days post-immunization. (A) The frequency of NP-binding splenic plasma cells that were BrdU+ on day 3 post-immunization is shown. Data are the mean of four mice. (B) Frequencies of NP-binding BM plasma cells that were BrdU+ on days 34, 64, and 94 days of the chase are shown. Each circle represents an individual mouse, and the mean for each time point is indicated by a solid line. The half-life (t1/2) of BM plasma cells post-IR was calculated as described for Figure 3. (C) Representative flow cytometric data illustrating the identification of NP-binding BrdU+/− plasma cells in the BM at the indicated time points of the chase period. Note that BrdU+ cells were identified by comparing immunized controls that were not exposed to BrdU. The “Dump” channel includes antibodies to CD4, CD8, F4/80, TER-119 and Gr-1. Each plots was derived from files containing 7–9 × 106 events.
Lack of evidence for induction of GCs in T-cell deficient mice
Past work with carbohydrate-enriched (type 2) TI antigens indicate that these antigens are sufficient to induce the transient development of GC-like structures. These B cell clusters, however, resolve quickly, fail to support SHM, and require high-dose antigen for their formation (18, 19). However, the capacity of LPS-based antigens to induce GC formation was less clear. To address whether TI GCs are induced by NP-LPS immunization, we quantified antigen-specific GCs in B6 or B6.TcRβ−/−δ−/− mice immunized in parallel with this type 1 TI antigen and compared these results with B6 mice immunized with NP-CGG. Hapten-specific GC B cells were identified as NP-binding CD19+ PNAhigh cells that lacked surface IgD expression (31, 35). Consistent with past data (36), NP-specific GC B cells were not detected in B6 mice immunized with NP-CGG until after day 5 post-immunization (Fig. 5B). By contrast, low frequencies of GC B cells were identified in B6 mice inoculated with NP-LPS three days post-immunization, but not at later time points. However, it should be noted that frequencies of NP-specific CD19+ PNAhigh B cells in B6 mice immunized with NP-LPS were highly variable, perhaps owing to the inefficiency of such responses in the absence of cognate T cell help. In contrast, we were unable to detect GC B cells in NP-LPS immunized B6.TcRβ−/−δ−/− mice at all time points examined (Fig. 5A, B). These data, together with the data in Figures 1, 3, and 4, establish that long-lived TI plasma cells can form independently of B cell maturation in GCs.
Figure 5. Failure to detect GCs in immunized T cell deficient mice.

B6 or B6.TcRβ−/−δ−/− adults were immunized with either NP-LPS/PBS or NP-CGG/alum, and frequencies of NP-binding CD19+ PNAhigh GC B cells in the spleen determined by flow cytometry. (A) Flow cytometric data for the indicated mice, representative of 3–6 mice per group. Left-most plots are pre-gated on viable (DAPI−) IgD− cells. 106 events were collected for each sample. Antibodies used for the “Dump” channel are as described for Figure 4. (B) Numbers of NP-specific GC B cells at 3, 5, or 7 days were calculated by multiplying the frequency of hapten-binding GC B cells using the gating strategy shown in (A) by the total number of splenocytes harvested in each mouse. Results are expressed as means +/− SEM of 3–6 mice per group performed over two separate experiments. Absolute numbers for days 3, 5, and 7 are graphed separately to better reveal the small number of PNA-binding B cells in NP-LPS immunized mice at early time points, which fell below detection levels in all mice after day 5.
Longevity of pre-GC plasma cells in T-cell dependent responses
Given that many NP-LPS induced plasma cells become long-lived without maturing in GCs, we also examined whether plasma cells induced with a TD antigen achieve longevity independently of the GC response. In this regard, several past studies suggest that B cell maturation in GCs is associated tightly with the formation of long-lived plasma cells in TD antibody responses. For instance, the BM is highly enriched for plasma cells synthesizing high-affinity isotype switched antibodies (13), and high-affinity GC B cells preferentially generate plasma cells (37). However these studies do not exclude the possibility that low affinity pre-GC plasma cells possess the potential to seed long-lived plasma cell pools in the BM. Furthermore, though B cells lacking the transcriptional repressor BCL6 fail to generate both GCs and long-lived BM plasma cells (38, 39), BCL6 mutation may lead to secondary effects that preempt the generation of long-lived plasma cells during the early phases of B cell differentiation before down-regulation of BCL6 expression during adoption of the plasma cell fate.
For these experiments we sought to prevent the GC response on day 5 post-immunization with NP-CGG, before the exponential increase observed in responding GC B cells (Fig. 5B), and three days before Ig somatic mutation events are detected in these cells (36). First, we abrogated the GC response via whole body IR. As observed for naïve B cells (Fig. 2), antigen-activated GC B cells in vivo were also sensitive to IR-induced cell death, as the NP-specific GC B cell pool was readily depleted upon exposure of NP-CGG immunized mice to 900R (Supplemental Figure 3). Figure 6A illustrates the frequency of NP-specific plasma cells in the BM of mice irradiated with 900R and reconstituted 5 days after immunization with NP-CGG. As shown, Igλ+ NP-specific plasma cells were readily detected in the BM for at least 105 days post-IR. Consistent with the absence of GC B-cell maturation and selection, NP-specific plasma cells in irradiated/reconstituted mice secreted low affinity IgM+ antibodies (Fig. 6B). Notably however, a readily detectable number of IgM+ plasma cells were observed in the BM of control (non-irradiated) mice. Second, we inhibited the generation of antigen-induced GCs by blocking B cell-T cell interactions with anti-CD154 blocking antibodies beginning on day 5 post-immunization. As shown, despite prevention of GC formation via this strategy (Supplemental Fig. 4A, 4B), NP-specific BM plasma cells were readily detected for at least 91 days post-immunization (Fig. 6C), and these cells did not secrete high affinity IgG1 antibodies (Fig. 6D). Significantly, we did not detect NP-specific GC B cells in any mouse given anti-CD154 antibodies throughout this study (Supplemental Fig. 4B). Of note, frequencies of BM λ+ NP-specific plasma cells were decreased significantly at the earliest time point (day 14) analyzed, perhaps suggesting suboptimal clonal expansion due to CD40 blockade. Regardless, these results suggest that maturation and selection of high affinity B cells in GCs is not requisite for propagating long-lived plasma cells in TD antibody responses. Further supporting this notion, we also readily detected hapten-specific plasma cells in the BM of NP-CGG immunized mice lacking activation-induced deaminase (AID), which is strictly required for the generation of high-affinity B cells by SHM (40) (Supplemental Fig. 4C). Collectively these data suggest that competence to enter the long-lived BM plasma cell pool occurs in early plasma cells and is not associated with maturation and affinity-based selection in the GC.
Figure 6. Long-lived T-cell dependent plasma cells without maturation in GCs.

(A) Frequencies of NP-specific λ+ plasma cells (PCs) post-IR in the BM of B6 adults immunized with NP-CGG/alum and then left untouched (closed) or irradiated and reconstituted (open) on day 5 post-immunization (see Methods). (B) BM cells from the mice in (A) on day 105 post-IR were assayed by ELISPOT using either NP33-BSA coated plates evaluated with anti-IgM specific antibodies (left) or NP4-BSA coated plated evaluated with anti-IgG1 specific antibodies. Differences in λ+ and IgM+ PC frequencies between irradiated and control groups were not significant. The p value for differences in frequencies of NP-specific IgG1+ PCs is shown on the graph. (C, D) NP-CGG/alum immunized B6 adults were given three injections of anti-CD154 (MR-1) antibodies (open) or hamster IgG (closed) beginning 5 days post-immunization (see Methods). (C) Shown are BM NP-specific λ+ PCs assayed with NP33-BSA. Differences between MR1 treated and control mice were significantly different on day 14 (p= 0.02) and day 190 (p=0.04). At all other times points there were no significant differences between these groups. (D) NP-specific IgM+ (left) PCs were assayed with NP33-BSA (left) or NP-specific IgG1+ PCs were evaluated with NP4-BSA (right). p values for statistically significant differences are shown on graphs. All results are expressed as means +/− SEM of 3–4 mice per group.
Discussion
T-independent antigens have long been deemed incapable of inducing the generation of long-lived plasma cells (1). However, our results show that many early plasma cells including those induced with an LPS-based conjugate persist for extended periods in the BM at frequencies that mirror TD responses, despite the significant contraction in antigen-specific plasma cell frequencies observed early in such responses (Fig. 1A) (15). Thus many early plasma cells achieve competence to enter long-lived BM plasma cell pools early in TI responses and in the absence of T cell derived signals associated with the generation of long-lived immunity. These findings contrast with the recently proposed “imprinting model” positing that TD antigens are unique in their capacity to induce B cells to yield long-lived plasma cells (12). Indeed, our findings suggest that TLR4 and T cell derived signals such as those provided by CD40 activation on B cells may provide comparable signals required for inducing plasma cell longevity.
We employed two different approaches to estimate the decay rate of hapten-specific NP-LPS induced plasma cells in the BM. We were surprised to learn that, although hapten-specific plasma cells were readily detected in the BM for more than 400 days following a single immunization with NP-LPS (Fig. 1), hapten-specific BM plasma cells exhibited a half-life of approximately 50 days (Figs. 3, 4). Whereas these calculations contrast substantially with the past notion that early plasma cells in TI responses fail to survive beyond 5 days of their generation (1, 2, 41), they also suggest that the long-term maintenance of serum antibodies specific for such antigens cannot be explained solely by the generation of exceptionally long-lived plasma cells generated early in such responses. Accordingly, we favor the notion that the BM plasma cell pool consists of both short- and long-lived cells. This viewpoint is supported by recent work from Racine et al., who characterized a surface IgM+ BM plasmablast population with a potential role in maintaining antibody titers to the intracellular bacterium E. muris (24). Further evidence for heterogeneity within the BM plasma cell pool stems from our analysis of BrdU-retaining hapten-specific BM plasma cells. Thus, though surface B220 expression is typically considered a trait restricted to recently formed plasma cells (34), we found that many long-lived BM plasma cells exhibited a bimodal pattern for surface B220 expression (Supplemental Figure 2). Together these findings underscore the need for increased understanding of the cellular dynamics of BM plasma cells and the potential influence of antigen on maintenance of the plasma cell pool.
Although TI pathogens were previously thought to be ineffectual inducers of long-term protective immunity, recent results including those shown here challenge this long-standing paradigm. TI antibody responses mediated by B1 B cells, for example, were sufficient to provide long-term IgM-dependent protection against B. hermsii (20, 21). However, whereas these investigators concluded that long-term protection to this spirochete was achieved through the continual induction of short-lived plasma cells, an alternative possibility supported by our data is that a significant fraction of plasma cells induced by B. hermsii infection are long-lived. This perspective may apply to other TI pathogens such as S. pneumoniae and E. muris. Indeed, recent work shows that plasma cells induced via immunization of conventional mice with S. pneumoniae or E. muris colonize and persist in the BM for extended periods (24–26). Whereas the cellular basis underlying long-term protection in many of these systems is not fully understood, our data reveal the possibility that long-lived plasma cells formed in the complete absence of T cells may play critical roles in responses to TI pathogens.
These recent studies, together with the conclusions drawn here, contrast substantially with the currently dominant model, which posits that T cell regulated GC maturation and selection events are associated with the propagation of long-lived plasma cells (4, 10, 12). Why then were TI antigens thought to induce only short-lived plasma cells? As reported by Fidler (15), following a robust clonal burst the number of hapten-specific NP-LPS induced plasma cells in the spleen declines by approximately 90% by day 5 in this response (see Fig. 1A), suggesting that the vast majority of early NP-LPS induced plasma cells are indeed short-lived. However, this observation does not exclude the possibility that a meaningful number of TI plasma cells enter the BM and persist for extended periods. Indeed, other past studies have documented small numbers of hapten-specific BM plasma cells 3 weeks after immunization with haptenated-LPS, but these workers did not address whether these plasma cells were maintained beyond 21 days, nor did they seek to measure the half-life of these cells (42). Furthermore, although the majority of early plasma cells induced by either TI or TD antigens may die before exiting the spleen (2, 41, 43, 44), only 9% of early splenic plasma cells in a TD response show clear signs of apoptosis (43). By quantifying frequencies of hapten-specific plasma cells in the BM following immunization with LPS or protein-based hapten conjugates, we have found that plasma cells persist in the BM for extended periods in both response to both TI and TD antigens and without undergoing GC maturation and selection.
Our data also suggest that autoantibodies to TI self-antigens, such as rheumatoid factor antibodies induced by DNA-chromatin complexes (45), might arise from long-lived plasma cells despite a fully tolerant T cell compartment. This notion emphasizes the importance of central and peripheral B cell tolerance mechanisms, especially in scenarios where such self-antigens are sufficient to induce somatic hypermutation without engaging helper T cells (46). The notion that most antigens are capable of inducing long-lived plasma cells may also apply to TI self-antigens underlying the formation of polyreactive natural antibodies, which have been shown to be protective against several pathogens (47).
We employed resistance to radiation-induced cell death as a tool to investigate plasma cell longevity in the context of TI and TD immune responses. In this context, we were surprised to find that radioresistance in plasma cells is established exceptionally early during these responses. It should be noted that at present the underlying mechanisms allowing plasma cells to avoid death despite widespread DNA damage are not understood. However, other studies have shown that anti-apoptotic DNA-repair pathways are induced by TLR signaling in memory CD4+ T cells (48). Hence TLR signaling may also play a similar role in activated B cells.
In sum, we propose that LPS-based antigens readily elicit the formation of a long-standing pool of plasma cells secreting low affinity but high avidity IgM antibodies that may play fundamental roles in combating certain pathogens. Accordingly, we suggest that the role of such plasma cells in host protection be re-evaluated. In addition, the control of gene expression patterns in short- and long-lived plasma cells, the signals required to induce the formation of long-lived cells, and conversely the mechanisms underlying the apparent loss of many plasma cells during early phases of antibody responses all remain important areas of investigation.
Supplementary Material
Acknowledgments
We thank Dr. Avinash Bhandoola for helpful discussions and critically reviewing this manuscript and Dr. Nina Papavasiliou for generously providing mice. We also gratefully acknowledge the expert technical support provided by Charles Pletcher, Drew Bantley, and other members of Abramson Cancer Center Flow Cytometry and Cell Sorting Shared Resource Laboratory.
Footnotes
Conflict of Interest: None
This work was supported by the NIH (T32 AI070099 to A.B., and R21 AI090700 to D.A.) and the DOD (W81WWXWH-10-1-0185 to M.P.C).
Abbreviations used in this paper: AID, Activation-induced deaminase; BCL6, B cell lymphoma gene-6; BM, bone marrow; BrdU, 5-bromo-2′-deoxyuridine; CGG, chicken γ-globulin; CSR, class switch recombination; GC, germinal center; IR, ionizing radiation; NP, 4-hydroxy-3-nitrophenyl)acetyl; SHM, somatic hypermutation; TD, T-cell dependent; TI, T-cell independent; TLR, toll-like receptor
References
- 1.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 2.Ho F, Lortan JE, MacLennan IC, Khan M. Distinct short-lived and long-lived antibody-producing cell populations. Eur J Immunol. 1986;16:1297–1301. doi: 10.1002/eji.1830161018. [DOI] [PubMed] [Google Scholar]
- 3.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 4.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11:681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- 5.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 6.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 7.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 8.Kjeldsen K, Simonsen O, Heron I. Immunity against diphtheria 25–30 years after primary vaccination in childhood. Lancet. 1985;1:900–902. doi: 10.1016/s0140-6736(85)91675-7. [DOI] [PubMed] [Google Scholar]
- 9.Simonsen O, Kjeldsen K, Heron I. Immunity against tetanus and effect of revaccination 25–30 years after primary vaccination. Lancet. 1984;2:1240–1242. doi: 10.1016/s0140-6736(84)92796-x. [DOI] [PubMed] [Google Scholar]
- 10.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarlinton DM. Evolution in miniature: selection, survival and distribution of antigen reactive cells in the germinal centre. Immunol Cell Biol. 2008;86:133–138. doi: 10.1038/sj.icb.7100148. [DOI] [PubMed] [Google Scholar]
- 12.Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236:125–138. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith KG, Light A, Nossal GJ, Tarlinton DM. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 1997;16:2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Racine R, Winslow GM. IgM in microbial infections: taken for granted? Immunol Lett. 2009;125:79–85. doi: 10.1016/j.imlet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidler JM. In vivo immune response to TNP hapten coupled to thymus-independent carrier lipopolysaccharide. Cell Immunol. 1975;16:223–236. doi: 10.1016/0008-8749(75)90114-8. [DOI] [PubMed] [Google Scholar]
- 16.Burlen O, Coutinho A, Freitas AA. Long-lasting thymus-independent immune responses to anti-idiotype lipopolysaccharide conjugates require continuous B cell renewal. Eur J Immunol. 1988;18:1433–1439. doi: 10.1002/eji.1830180920. [DOI] [PubMed] [Google Scholar]
- 17.Garcia de Vinuesa C, O’Leary P, Sze DM, Toellner KM, MacLennan IC. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur J Immunol. 1999;29:1314–1323. doi: 10.1002/(SICI)1521-4141(199904)29:04<1314::AID-IMMU1314>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Lentz VM, Manser T. Cutting edge: germinal centers can be induced in the absence of T cells. J Immunol. 2001;167:15–20. doi: 10.4049/jimmunol.167.1.15. [DOI] [PubMed] [Google Scholar]
- 19.de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M, Wabl M, Klaus GG, MacLennan IC. Germinal centers without T cells. J Exp Med. 2000;191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, Leong JM. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol. 2003;170:3819–3827. doi: 10.4049/jimmunol.170.7.3819. [DOI] [PubMed] [Google Scholar]
- 21.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Bitsaktsis C, Nandi B, Racine R, MacNamara KC, Winslow G. T-Cell-independent humoral immunity is sufficient for protection against fatal intracellular ehrlichia infection. Infect Immun. 2007;75:4933–4941. doi: 10.1128/IAI.00705-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole LE, Yang Y, Elkins KL, Fernandez ET, Qureshi N, Shlomchik MJ, Herzenberg LA, Vogel SN. Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. Proc Natl Acad Sci U S A. 2009;106:4343–4348. doi: 10.1073/pnas.0813411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Racine R, McLaughlin M, Jones DD, Wittmer ST, MacNamara KC, Woodland DL, Winslow GM. IgM production by bone marrow plasmablasts contributes to long-term protection against intracellular bacterial infection. J Immunol. 2011;186:1011–1021. doi: 10.4049/jimmunol.1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taillardet M, Haffar G, Mondiere P, Asensio MJ, Gheit H, Burdin N, Defrance T, Genestier L. The thymus-independent immunity conferred by a pneumococcal polysaccharide is mediated by long-lived plasma cells. Blood. 2009;114:4432–4440. doi: 10.1182/blood-2009-01-200014. [DOI] [PubMed] [Google Scholar]
- 26.Foote JB, Mahmoud TI, Vale AM, Kearney JF. Long-term maintenance of polysaccharide-specific antibodies by IgM-secreting cells. J Immunol. 2012;188:57–67. doi: 10.4049/jimmunol.1100783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self- reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 28.Lindsley RC, Thomas M, Srivastava B, Allman D. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109:2521–2528. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J Exp Med. 1998;187:885–895. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McHeyzer-Williams MG, McLean MJ, Lalor PA, Nossal GJ. Antigen-driven B cell differentiation in vivo. J Exp Med. 1993;178:295–307. doi: 10.1084/jem.178.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jack RS, Imanishi-Kari T, Rajewsky K. Idiotypic analysis of the response of C57BL/6 mice to the (4-hydroxy-3-nitrophenyl)acetyl group. Eur J Immunol. 1977;7:559–565. doi: 10.1002/eji.1830070813. [DOI] [PubMed] [Google Scholar]
- 33.Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan IC. Mechanism of antigen-driven selection in germinal centres. Nature. 1989;342:929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- 34.Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD, Nutt SL. Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J Exp Med. 2004;200:967–977. doi: 10.1084/jem.20040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McHeyzer-Williams LJ, Cool M, McHeyzer-Williams MG. Antigen-specific B cell memory: expression and replenishment of a novel b220(−) memory b cell compartment. J Exp Med. 2000;191:1149–1166. doi: 10.1084/jem.191.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacob J, Przylepa J, Miller C, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J Exp Med. 1993;178:1293–1307. doi: 10.1084/jem.178.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, Brink R. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med. 2006;203:2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997;276:589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 39.Toyama H, Okada S, Hatano M, Takahashi Y, Takeda N, Ichii H, Takemori T, Kuroda Y, Tokuhisa T. Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells. Immunity. 2002;17:329–339. doi: 10.1016/s1074-7613(02)00387-4. [DOI] [PubMed] [Google Scholar]
- 40.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 41.Sze DM, Toellner KM, Garcia de Vinuesa C, Taylor DR, MacLennan IC. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J Exp Med. 2000;192:813–821. doi: 10.1084/jem.192.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koch G, Lok BD, van Oudenaren A, Benner R. The capacity and mechanism of bone marrow antibody formation by thymus-independent antigens. J Immunol. 1982;128:1497–1501. [PubMed] [Google Scholar]
- 43.Smith KG, Hewitson TD, Nossal GJ, Tarlinton DM. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol. 1996;26:444–448. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- 44.Garcia De Vinuesa C, Gulbranson-Judge A, Khan M, O’Leary P, Cascalho M, Wabl M, Klaus GG, Owen MJ, MacLennan IC. Dendritic cells associated with plasmablast survival. Eur J Immunol. 1999;29:3712–3721. doi: 10.1002/(SICI)1521-4141(199911)29:11<3712::AID-IMMU3712>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 45.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 47.Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel RM. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 48.Zheng L, Asprodites N, Keene AH, Rodriguez P, Brown KD, Davila E. TLR9 engagement on CD4 T lymphocytes represses gamma-radiation-induced apoptosis through activation of checkpoint kinase response elements. Blood. 2008;111:2704–2713. doi: 10.1182/blood-2007-07-104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


