Abstract
Introduction
The aim of present study was to profile the glucose-dependent and glutamine- dependent metabolism in pancreatic cancer.
Methods
We performed Immunohistochemical staining of GLUT1, CAIX, BNIP3, p62, LC3, GLUD1, and GOT1. Based on the expression of metabolism-related proteins, the metabolic phenotypes of tumors were classified into two categories, including glucose- and glutamine-dependent metabolism. There were Warburg type, reverse Warburg type, mixed type, and null type in glucose-dependent metabolism, and canonical type, non-canonical type, mixed type, null type in glutamine-dependent metabolism.
Results
Longer overall survival was associated with high expression of BNIP3 in tumor (p = 0.010). Shorter overall survival was associated with high expression of GLUT1 in tumor (P = 0.002) and GOT1 in tumor (p = 0.030). Warburg type of glucose-dependent metabolism had a highest percentage of tumors with nerve infiltration (P = 0.0003), UICC stage (P = 0.0004), and activated autophagic status in tumor (P = 0.0167). Mixed type of glucose-dependent metabolism comprised the highest percentage of tumors with positive marginal status (P<0.0001), lymphatic invasion (P<0.0001), and activated autophagic status in stroma (P = 0.0002). Mixed type and Warburg type had a significant association with shorter overall survival (P = 0.018). Non-canonical type and mixed type of glutamine-dependent metabolism comprised the highest percentage of tumors with vascular invasion (p = 0.0073), highest percentage of activated autophagy in tumors (P = 0.0034). Moreover, these two types of glutamine-dependent metabolism were significantly associated with shorter overall survival (P<0.001). Further analysis suggested that most of tumors were dependent on both glucose- and glutamine-dependent metabolism. After dividing the tumors according to the number of metabolism, we found that the increasing numbers of metabolism subtypes inversely associated with survival outcome.
Conclusion
Warburg type, non-canonical type and mixed types of glucose- and glutamine-dependent metabolism comprised of more metabolically active, biologically aggressive and poor prognostic tumors. Moreover, the increasing subtypes and categories of the metabolism in each tumor significantly associated with poor prognosis.
Introduction
Aberrant metabolism is now considered as one of the hallmarks of cancer [1]. Notably, tumor cells exhibit high levels of glycolysis even under the adequate oxygen supply, a remarkable phenomenon termed Warburg effect. The pioneering work of Otto Warburg has been supported by reports in various tumor types, and is now exploited in the clinic for diagnostic purposes. However, this view is challenged by emerging evidences. In his theory, Warburg supposed that cancer cells tended to generate ATP through glycolysis instead of oxidative phorsphorylation following the mitochondrial dysfunction [2]. Nevertheless, mounting evidences indicated that primary defects in mitochondrial enzymes or complexes in oxidative phorsphorylation were not frequently observed in cancers [3]. The relatively low number of metabolic inhibitors were introduced in cancer treatment and only a few of them have entered clinical development [4], which partly reflected the heterogeneity and complexity in tumor metabolism. Moreover, study conducted by Zu and his colleague showed that the amount of ATP derived from glycolysis does not exceed 50–60% of total ATP production [5,6]. A new paradigm named “reverse Warburg effect” in which aerobic glycolysis took place in cancer-associated fibroblasts, rather than tumor cells, also emerged in breast cancer [7,8]. Recent data showed that pancreatic cancer cells utilize glutamine in an unusual way, which is dependent on glutamic-oxaloacetic transaminase rather than glutamate dehydrogenase [9]. These findings put a great emphasis on significant heterogeneity in cancer metabolism suggesting that each cancer has its own metabolic features, which needs to be taken into consideration before exploring new targets in cancer metabolism.
Pancreatic cancer is the fourth leading cause of cancer-related deaths in the United States and Europe. It is still one of the most intractable and fatal disease because of its late stage when diagnosed, invasive phenotype, resistant to current therapies [10,11]. Targeting cancer metabolism is a promising strategy in improving pancreatic cancer treatment. To pave the way for development of metabolism therapeutic treatments, metabolism phenotypes in pancreatic cancers are needed to be elucidated. To our best knowledge, there are no studies investigating the metabolic phenotypes in pancreatic cancer. Therefore, the aim of our present study was conducted to profile the glucose-dependent and glutamine-dependent metabolism, mitochondrial, and autophagic status in pancreatic cancer.
Materials and Methods
Patients’ selection
Samples of 106 pancreatic cancer tissues were derived from patients undergoing radical operations in a retrospective study cohort between Jan. 2000 and Dec. 2012 at Sun Yat-sen Memorial Hospital of Sun Yat-sen University and a prospective cohort from Jun.2012 to May 2013 (registration number: ChiCTR-TRC-12002548). This study complied with the Helsinki Declaration and was approved by the Institutional Ethics Committee of Sun Yat-sen Memorial Hospital. All patients signed consent forms indicating their willingness to participate and their understanding of the procedure and general aim of the study. All of the included pathological and histological confirmed patients with pancreatic cancer met the following criteria: no history of any other malignant tumor, without neoadjuvant therapy prior to the surgery. None of them died during the perioperative period because of post-operative complications. All patients consent was obtained prior to collection of specimens, and all study procedures were approved by the Ethic Committee Sun Yat-sen Memorial Hospital of Sun Yat-sen University. Data items were extracted through medical record system as following: age, gender, status of glucose metabolism, tumor size, tumor location, histological grade, marginal status, nerve infiltration, vascular invasion, UICC Stage (Tumor pathological stage was classified according to the Union for International Cancer Control (UICC)), and lymphatic invasion. Overall Survival (OS) was defined as the time interval from the date of operation to death or the last follow-up in May 2013.
Immunohistochemistry
Immunohistochemical analysis was carried out as following: Tissue samples were fixed in neutral-buffered formalin, and then embedded by paraffin. Endogenous peroxidase was quenched with 3% hydrogen peroxide for 30min at room temperature. Slides were then washed in PBS and incubated for 30min at 37°C with trypsin buffer. After adequate wash in PBS, antigen retrieval of 5-μm thick sections of 106 pancreatic cancer tissues was carried out in citrate buffer (PH6.0). When the slides were cooled down to room temperature, nonspecific protein binding were block for 60min by normal goat serum at room temperature. Subsequently, primary antibody (listed in Table 1 in S2 File) for immunohistochemistry was applied overnight at 4°C. Next, Elivision Super DAB kit was applied as manufactory instructions. The primary antibody incubation was omitted as negative controls. Positive controls were chosen as suggested in antibody instructions or previous data [12,13]: glucose transporter 1 (GLUT1): esophageal carcinoma; carbonic anhydrase IX (CAIX): renal carcinoma; BCL2/adenovirus E1B 19-kDa interacting protein 3 (BNIP3), kidney tissue; Beclin-1: breast tissue; microtubule-associated protein 1 light chain 3 (MAPLC3): nerve tissue in each specimens; p62: spleen tissue; glutamic-oxaloacetic transaminase (GOT1): kidney tissue; glutamate dehydrogenase 1 (GLUD1): heart tissue (Figure A in S1 File). Immunoreactivity-scoring (IRS) system was employed for semiquantitative analysis. In brief, the IRS score for each specimen was obtained by two independent pathologists and the mean value of the scores was the final score we used for further analysis. Any Inconsistencies between pathologists’ data were resolved by discussion with a third pathologist until consistency was reached. Scores for expression of metabolism-related protein were evaluated according to intensity and proportion. The intensity score was determined as 0 (no staining), 1 (weaker staining than positive control), 2(equal staining as positive control), and 3 (stronger staining than positive control). The proportion score was determined as 0(negative), 1 (<30% of tumor cells) and 2 (≧30% of tumor cells). The intensity score and proportion score were multiplied together for a total score. Total scores were categorized as follows: 0–1 (negative) and 2–6 (positive).
Classification of tumor metabolic phenotypes
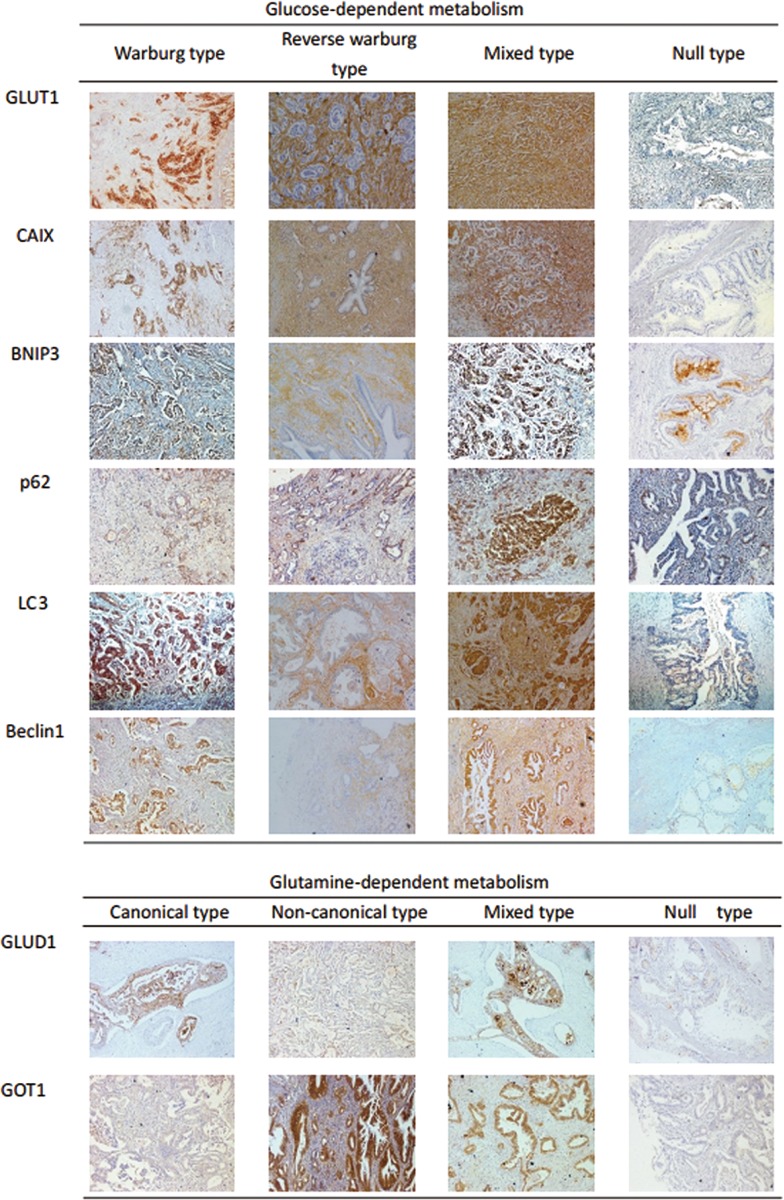
We classified the cases into phenotypes according to the immunohistochemical staining for these metabolism-related proteins as previous report [12,13]: glycosis types: GLUT1- and/or CAIX-positive; nonglycolysis type: GLUT1- and CAIX-negative; dysfunctional mitochondrial type: BNIP3-positive; functional mitochondrial type: BNIP3-negative; activated autophagy types: two or more autophagic markers from Beclin1, LC3, p62; inactivated autophagy type: positive for less than two autophagic markers from Beclin1, LC3, p62; canonical glutamine metabolism type: GLUD1-positive and GOT1 positive; non-canonical glutamine metabolism type: GLUD1-negative and GOT1 positive. Metabolism in pancreatic cancer was divided into two major categories: glucose-dependent metabolism and glutamine-dependent metabolism [9,14]. Glucose-dependent metabolism phenotype of individual pancreatic cancer specimen is classified as follow according to previous reports [12,13]: Warburg type: tumor (glycolysis type), stroma(non-glycolysis type, oxidative phosphorylation); reverse Warburg type: tumor(non-glycolysis type, oxidative phosphorylation), stroma (glycolysis type); mixed type: tumor (glycolysis type), stroma (glycolysis); and null type: tumor (non-glycolysis type, oxidative phosphorylation), stroma (non-glycolysis type, oxidative phosphorylation). Glutamine-dependent metabolism phenotype of individual pancreatic cancer specimen is classified as follow: canonical type (GLUD1-positive and GOT1-negative), non-canonical type (GOT1-positive and GLUD1-negative), mixed type (GLUD1-positive and GOT1-positive), null type (GLUD1-negative and GOT1-negative) of glutamine metabolism.
Quantitative Real-time Reverse Transcription-PCR Assay
We assessed the mRNA expression of the target genes with qRT-PCR. Total RNA was isolated from tissues and cell lines using Trizol reagent (Invitrogen), and the concentrations were measured with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). The first strand of the cDNA was synthesized with PrimeScript® 1st Strand cDNA Synthesis Kit (Takara). QRT-PCR was applied according to the manufacturer's instruction. QRT-PCR was then performed at 95°C for 10 minutes, at 95°C for 15 seconds, and at 60°C for 15 seconds, with a total of 40 cycles. Each reaction was performed in triplicate and all the primers used were listed in supplemental data. Primers were designed based on the sequence of each gene by using Premier 5.0. The mRNA levels were normalized to beta-actin mRNA according to the following formula: [2̂ − (CT Target − CT Actin)] × 100%, where CT is the threshold cycle.
Statistical analyses
Data was analyzed using SPSS for Windows version 16.0 software (SPSS Inc, Chicago, IL, USA). Categorical variables were compared using Fisher exact test. The median survival times were evaluated by Kaplan Meier survival curves, and the differences were further analyzed with the log-rank test. When P value was less than 0.05, significance was assumed unless marked. When P value was less than 0.1 in univariate analysis and these variables were further analyzed in multivariate analysis through cox proportional hazards models.
Results
Clinicopathological characteristics of included patients
The present cohort consisted of 106 patients including 57 men and 49 women. The mean age at operation was 60.42 years, with a median age of 61.00 years and a range from 31 years to 77 years. At the time of last clinical follow-up (May 2013), 29 (27.4%) patients were alive. The median overall survival of the 106 patients was 21.9 months, with 1-, 3-, and 5-year survival rates of 56.8%, 13.2%, and 6.2%, respectively. The majority of patients (80.2%) had tumors located at the head of pancreas, and sizes of most tumors (80.2%) were more than 20 mm in greatest diameter. Most tumors were moderately differentiated (37.7%), followed by well differentiated (35.9%), and poorly differentiated (26.4%). Twenty-three (21.7%) patients got a positive margin. Nerve infiltration was presented in 84 patients (79.2%), vascular invasion in 82 (77.4%) patients, and lymphatic invasion in 68 (64.2%) patients. Most patients (73.6%) presented with the Union for International Cancer Control (UICC) stage IIB disease, and the remaining (26.4%) patients presented with UICC stage IA, IB and IIA disease. No patients presented with UICC stage III and IV disease.
Correlation between the expression of metabolism-related proteins and clinicopathological parameters
The correlation between metabolism-related proteins and clinicopathological parameters were summarized in Table 1 to Table 4. We found that increased tumor expression of GLUT1 was associated with nerve infiltration (P = 0.0180), lymphatic invasion (P<0.0001), UICC stage (P = 0.0044), whereas stromal expression of GLUT1 was associated with tumor dedifferentiation (P = 0.0155), positive marginal status (P = 0.0063), nerve infiltration (P = 0.0005), lymphatic invasion (P = 0.0065) and UICC stage (P = 0.0010) (Table 1). Tumor expression of GLUD1 was associated with larger tumor size (P = 0.0086). Tumor expression of GOT1 was significantly linked with tumor differentiation (P = 0.0238), vascular invasion (P = 0.0384), lymphatic invasion (P = 0.0367). There was a significant association between stromal expression of GOT1 and nerve infiltration (P = 0.0087) (Table 2). Tumor expression of BNIP3 was associated with tumor dedifferentiation (P = 0.0155), whereas stromal expression of BNIP3 was associated with positive marginal status (P = 0.0010) (Table 3). Cytoplasmic p62 expression in tumor was associated with positive marginal status (P = 0.0132), while nuclear p62 expression in stroma was associated with larger tumor size (P = 0.0173) (Table 3). Tumor expression of LC3 was linked with impaired glucose metabolism (P = 0.0205), larger tumor size (P = 0.0453), tumor dedifferentiation (P = 0.0040), vascular invasion (P = 0.0044), lymphatic invasion (P = 0.0385), while stromal expression of LC3 associated with tumor dedifferentiation (P<0.0001), positive marginal status (P = 0.0010), negative vascular invasion (P = 0.0015) (Table 4). There was a significantly association between cytoplasmic expression of Beclin-1 in tumor and lymphatic invasion (P = 0.0175), whereas nuclear Beclin-1 expression in stroma was associated with positive marginal status (P = 0.0379) (Table 4).
Table 1. Associations of GLUT1, CAIX expression with clinicopathological parameters *.
| Parameters | No. of cases (%) | GLUT1 in tumor | GLUT1 in stroma | CAIX in tumor | CAIX in stroma | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative (n = 44) (%) | Positive (n = 62) (%) | P-value | Negative (n = 75) (%) | Positive (n = 31) (%) | P-value | Negative (n = 82) (%) | Positive (n = 24) (%) | P-value | Negative (n = 95) (%) | Positive (n = 11) (%) | P-value | ||
| Age | 1.0000 | 0.5272 | 0.4905 | 0.5342 | |||||||||
| <61 years | 50(47.2) | 21(47.7) | 29(46.8) | 37(49.3) | 13(41.9) | 37(45.1) | 13(54.2) | 46(48.4) | 4(36.4) | ||||
| ≥61 years | 56(52.8) | 23(52.3) | 33(53.2) | 38(50.7) | 18(58.1) | 45(54.9) | 11(45.8) | 49(51.6) | 7(63.6) | ||||
| Gender | 0.5568 | 0.5248 | 0.8165 | 1.0000 | |||||||||
| Male | 57(53.8) | 22(50.0) | 35(56.5) | 42(56.0) | 15(48.4) | 45(54.9) | 12(50.0) | 51(53.7) | 6(54.5) | ||||
| Female | 49(46.2) | 22(50.0) | 27(43.5) | 33(44.0) | 16(51.6) | 37(45.1) | 12(50.0) | 44(46.3) | 5(45.5) | ||||
| Diabetes mellitus or hyperglycemia | 0.3048 | 0.2578 | 0.6269 | 1.0000 | |||||||||
| No | 35(33.0) | 12(27.3) | 23(37.1) | 22(29.3) | 13(41.9) | 26(31.7) | 9(37.5) | 31(32.6) | 4(36.4) | ||||
| Yes | 71(67.0) | 32(72.7) | 39(62.9) | 53(70.7) | 18(58.1) | 56(68.3) | 15(62.5) | 64(67.4) | 7(63.6) | ||||
| Tumor Size | 0.8887 | 0.0590 | 0.2439 | 0.4526 | |||||||||
| ≤2.0cm | 21(19.8) | 9(20.5) | 12(19.4) | 11(14.7) | 10(32.3) | 14(17.1) | 7(29.2) | 18(18.9) | 3(27.3) | ||||
| >2.0cm | 85(80.2) | 35(79.5) | 50(80.6) | 64(85.3) | 21(67.7) | 68(82.9) | 17(70.8) | 77(81.1) | 8(72.7) | ||||
| Tumor Location | 0.6228 | 0.4217 | 0.0588 | 0.4526 | |||||||||
| Head | 85(80.2) | 34(77.3) | 51(82.3) | 62(82.7) | 23(74.2) | 13(15.9) | 8(33.3) | 77(81.1) | 8(72.7) | ||||
| Body/tail | 21(19.8) | 10(22.7) | 11(19.7) | 13(17.3) | 8(25.8) | 69(84.1) | 16(66.7) | 18(18.9) | 3(27.3) | ||||
| Differentiation | 0.8254 | 0.0155 | 0.9765 | 0.9948 | |||||||||
| Well | 38(35.9) | 15(34.1) | 23(37.1) | 31(41.3) | 7(22.6) | 29(35.4) | 9(37.5) | 34(35.8) | 4(36.4) | ||||
| Moderate | 40(37.7) | 16(36.4) | 24(38.7) | 30(40.0) | 10(32.2) | 31(37.8) | 9(37.5) | 36(37.9) | 4(36.4) | ||||
| Poor | 28(26.4) | 13(29.5) | 15(24.2) | 14(18.7) | 14(45.2) | 22(26.8) | 6(25.0) | 25(26.3) | 3(27.2) | ||||
| Marginal status | 0.0898 | 0.0063 | 0.1159 | 0.2483 | |||||||||
| R0 | 83(78.3) | 38(86.4) | 45(72.6) | 64(85.3) | 19(61.3) | 67(81.7) | 16(66.7) | 76(80.0) | 7(63.6) | ||||
| R1 | 23(21.7) | 6(13.6) | 17(27.4) | 11(14.7) | 12(38.7) | 15(18.3) | 8(33.3) | 19(20.0) | 4(36.4) | ||||
| Nerve Infiltration | 0.0180 | 0.0005 | 0.0941 | 1.0000 | |||||||||
| Negative | 22(20.8) | 14(31.8) | 8(12.9) | 9(12.0) | 13(41.9) | 14(17.1) | 8(33.3) | 20(21.1) | 2(18.2) | ||||
| Positive | 84(79.2) | 30(68.2) | 54(87.1) | 66(88.0) | 18(58.1) | 68(82.9) | 16(66.7) | 75(78.9) | 9(81.8) | ||||
| Vascular invasion | 0.8144 | 0.3121 | 0.0933 | 0.7091 | |||||||||
| No | 24(22.6) | 9(20.5) | 15(24.2) | 15(20.0) | 9(29.0) | 22(14.6) | 2 (50.0) | 21(22.1) | 3(27.3) | ||||
| Yes | 82(77.4) | 35(79.5) | 47(75.8) | 60(80.0) | 22(71.0) | 60(85.4) | 22(50.0) | 74(77.9) | 8(72.7) | ||||
| Lymphatic invasion | <0.0001 | 0.0065 | 0.8139 | 1.0000 | |||||||||
| Negative | 38(35.8) | 26(59.1) | 12(19.4) | 33(44.0) | 5(16.1) | 30(36.6) | 8(33.3) | 34(35.8) | 4(36.4) | ||||
| Positive | 68(64.2) | 18(40.1) | 50(80.6) | 42(56.0) | 26(83.9) | 52(63.4) | 16(66.7) | 61(64.2) | 7(63.6) | ||||
| UICC stage | 0.0044 | 0.0010 | 0.1912 | 0.4757 | |||||||||
| IA/IB/IIA | 28(26.4) | 18(40.9) | 10(16.1) | 13(17.3) | 15(48.4) | 19(23.2) | 9(37.5) | 24(25.3) | 4(36.4) | ||||
| IIB | 78(73.6) | 26(59.1) | 52(83.9) | 62(82.7) | 16(51.6) | 63(76.8) | 15(62.5) | 71(74.7) | 7(63.6) | ||||
* A P<0.05 was considered statistically significant.
Table 4. Associations of LC3, Beclin-1 expression with clinicopathological parameters.
| Parameters | No. of cases (%) | LC3 in tumor | LC3 in stroma | Cytoplasmic Beclin-1 in tumor | Nuclear Beclin-1 in tumor | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative (n = 43) (%) | Positive (n = 63) (%) | P-value | Negative (n = 87) (%) | Positive (n = 19) (%) | P-value | Negative (n = 80) (%) | Positive (n = 26) (%) | P-value | Negative (n = 75) (%) | Positive (n = 31) (%) | P-value | ||
| Age | 0.8440 | 0.8005 | 0.8226 | 1.0000 | |||||||||
| <61 years | 50(47.2) | 21(48.8) | 29(46.0) | 42(48.3) | 8(42.1) | 37(46.2) | 13(50.0) | 35(46.7) | 15(48.4) | ||||
| ≥61 years | 56(52.8) | 22(51.2) | 34(54.0) | 45(51.7) | 11(57.9) | 43(53.8) | 13(50.0) | 40(53.3) | 16(51.6) | ||||
| Gender | 0.1651 | 1.0000 | 0.4975 | 0.1370 | |||||||||
| Male | 57(53.8) | 27(62.8) | 30(47.6) | 47(54.0) | 10(52.6) | 45(56.2) | 12(46.2) | 44(58.7) | 13(41.9) | ||||
| Female | 49(46.2) | 16(37.2) | 33(52.4) | 40 (46.0) | 9(47.4) | 35(43.8) | 14(53.8) | 31(41.3) | 18(58.1) | ||||
| Diabetes mellitus or hyperglycemia | 0.0205 | 0.2869 | 0.4837 | 1.0000 | |||||||||
| No | 35(33.0) | 20(46.5) | 15(23.8) | 31(35.6) | 4(21.1) | 28(35.0) | 7(26.9) | 25(33.3) | 10(32.3) | ||||
| Yes | 71(67.0) | 23(53.5) | 48(76.2) | 56(64.4) | 15(78.9) | 52(65.0) | 19(73.1) | 50(66.7) | 21(67.7) | ||||
| Tumor Size | 0.0453 | 0.1125 | 1.0000 | 0.7892 | |||||||||
| ≤2.0cm | 21(19.8) | 13(30.2) | 8(12.7) | 20(23.0) | 1(5.3) | 16(20.0) | 5(19.2) | 14(18.7) | 7(22.6) | ||||
| >2.0cm | 85(80.2) | 30(69.8) | 55(87.3) | 67(77.0) | 18(94.7) | 64(80.0) | 21(80.8) | 61(81.3) | 24(77.4) | ||||
| Tumor Location | 0.3209 | 0.7592 | 1.0000 | 0.6041 | |||||||||
| Head | 85(80.2) | 37(86.0) | 48(76.2) | 69(85.1) | 16(57.9) | 64(80.0) | 21(80.8) | 59(78.7) | 26(83.9) | ||||
| Body/tail | 21(19.8) | 6(14.0) | 15(23.8) | 18(14.9) | 3(42.1) | 16(20.0) | 5(19.2) | 16(21.3) | 5(16.1) | ||||
| Differentiation | 0.0040 | < 0.0001 | 0.5450 | 0.3704 | |||||||||
| Well | 38(35.9) | 23(53.5) | 15(23.8) | 34(39.1) | 4(21.1) | 30(37.5) | 8(30.8) | 30(40.0) | 8(25.8) | ||||
| Moderate | 40(37.7) | 14(32.5) | 26(41.3) | 33(37.9) | 7(36.8) | 31(38.8) | 9(34.6) | 26(34.7) | 14(45.2) | ||||
| Poor | 28(26.4) | 6(14.0) | 22(34.9) | 20(23.0) | 8(42.1) | 19(23.7) | 9(34.6) | 19(25.3) | 9(29.0) | ||||
| Marginal status | 0.6338 | 0.0010 | 0.2722 | 0.0379 | |||||||||
| R0 | 83(78.3) | 35(81.4) | 48(76.2) | 74(85.1) | 9(47.4) | 65(81.3) | 18(69.2) | 63(84.0) | 20(64.5) | ||||
| R1 | 23(21.7) | 8(18.6) | 15(23.8) | 13(14.9) | 10(52.6) | 15(18.8) | 8(30.8) | 12(16.0) | 11(35.5) | ||||
| Nerve Infiltration | 0.1501 | 0.2187 | 0.5813 | 0.7954 | |||||||||
| Negative | 22(20.8) | 12(27.9) | 10(15.8) | 16(18.4) | 6(31.6) | 18(22.5) | 4(15.4) | 15(20.0) | 7(22.6) | ||||
| Positive | 84(79.2) | 31(72.1) | 53(84.1) | 71(81.6) | 13(68.4) | 62(77.5) | 22(84.6) | 60(80.0) | 24(77.4) | ||||
| Vascular invasion | 0.0044 | 0.0015 | 0.7896 | 0.4445 | |||||||||
| No | 24(22.6) | 16(37.2) | 8(12.7) | 14(16.1) | 10(52.6) | 19(23.8) | 5(19.2) | 19(25.3) | 5(16.1) | ||||
| Yes | 82(77.4) | 27(62.8) | 55(87.3) | 73(83.9) | 9(47.4) | 61(76.2) | 21(80.8) | 56(74.7) | 26(83.9) | ||||
| Lymphatic invasion | 0.0385 | 0.0633 | 0.0175 | 0.1883 | |||||||||
| Negative | 38(35.8) | 10(23.3) | 28(44.4) | 35(40.2) | 3(15.8) | 34(42.5) | 4(15.4) | 30(40.0) | 8(25.8) | ||||
| Positive | 68(64.2) | 33(76.7) | 35(55.6) | 52(59.8) | 16(84.2) | 46(57.5) | 22(84.6) | 45(60.0) | 23(74.2) | ||||
| UICC stage | 1.0000 | 1.0000 | 0.3103 | 0.4684 | |||||||||
| IA/IB/IIA | 28(26.4) | 11(25.6) | 17(27.0) | 23(26.4) | 5(26.3) | 19(23.8) | 9(34.6) | 18(24.0) | 10(32.3) | ||||
| IIB | 78(73.6) | 32(74.4) | 46(73.0) | 64(73.6) | 14(73.7) | 61(76.2) | 17(65.4) | 57(76.0) | 21(67.7) | ||||
Table 2. Associations of GLUD1, GOT1 expression with clinicopathological parameters.
| Parameters | No. of cases (%) | GLUD1 in tumor | GLUD1 in stroma | GOT1 in tumor | GOT1 in stroma | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative (n = 83) (%) | Positive (n = 23) (%) | P-value | Negative (n = 78) (%) | Positive (n = 28) (%) | P-value | Negative (n = 27) (%) | Positive (n = 79) (%) | P-value | Negative (n = 14) (%) | Positive (n = 92) (%) | P-value | ||
| Age | 0.9432 | 1.0000 | 0.3744 | 0.4024 | |||||||||
| <61 years | 50(47.2) | 39(47.0) | 11(47.8) | 37(47.4) | 13(46.4) | 15(55.56) | 35(44.3) | 5(35.7) | 45(48.9) | ||||
| ≥61 years | 56(52.8) | 44(53.0) | 12(52.2) | 41(52.6) | 15(53.6) | 12(44.44) | 44(55.7) | 9(64.3) | 47(51.1) | ||||
| Gender | 0.7651 | 0.8253 | 0.8270 | 0.7814 | |||||||||
| Male | 57(53.8) | 44(53.0) | 13(56.5) | 43(55.1) | 14(50.0) | 14(81.5) | 43(54.4) | 7(50.0) | 50(54.3) | ||||
| Female | 49(46.2) | 39(47.0) | 10(43.5) | 35(44.9) | 14(50.0) | 13(18.5) | 36(45.6) | 7(50.0) | 42(45.7) | ||||
| Diabetes mellitus or hyperglycemia | 0.8389 | 1.0000 | 0.4786 | 0.3792 | |||||||||
| No | 35(33.0) | 27(32.5) | 8(34.8) | 26(33.3) | 9(32.1) | 7(25.9) | 28(35.4) | 3(21.4) | 32(34.8) | ||||
| Yes | 71(67.0) | 56(67.5) | 15(65.2) | 52(66.7) | 19(67.9) | 20(74.1) | 51(64.6) | 11(78.6) | 60(65.2) | ||||
| Tumor Size | 0.0086 | 0.4199 | 0.7814 | 0.1463 | |||||||||
| ≤2.0cm | 21(19.8) | 12(14.5) | 9(39.1) | 14(17.9) | 7(25.0) | 6(22.22) | 15(19.0) | 5(35.7) | 16(17.4) | ||||
| >2.0cm | 85(80.2) | 71(85.5) | 14(60.9) | 64(82.1) | 21(75.0) | 21(77.78) | 64(81.0) | 9(64.3) | 76(82.6) | ||||
| Tumor Location | 0.1486 | 0.7878 | 0.4049 | 0.7316 | |||||||||
| Head | 85(80.2) | 69(83.1) | 16(69.6) | 63(80.7) | 22(78.6) | 20(74.1) | 65(82.3) | 12(85.7) | 73(79.3) | ||||
| Body/tail | 21(19.8) | 14(16.9) | 7(30.4) | 15(19.3) | 6(21.4) | 7(25.9) | 14(17.7) | 2(14.3) | 19(20.7) | ||||
| Differentiation | 0.8045 | 0.9473 | 0.0238 | 0.9773 | |||||||||
| Well | 38(35.9) | 31(37.3) | 7(30.4) | 28(35.9) | 10(35.7) | 7(25.9) | 31(39.2) | 5(35.7) | 33(35.9) | ||||
| Moderate | 40(37.7) | 31(37.3) | 9(39.1) | 30(38.5) | 10(35.7) | 9(33.3) | 31(39.2) | 5(35.7) | 35(38.0) | ||||
| Poor | 28(26.4) | 21(25.4) | 7(30.5) | 20(25.6) | 8(28.6) | 11(40.7) | 17(21.5) | 4(28.6) | 24(26.1) | ||||
| Marginal status | 0.5639 | 0.6038 | 0.1078 | 0.0743 | |||||||||
| R0 | 83(78.3) | 66(79.5) | 17(73.9) | 62(79.5) | 21(75.0) | 18(66.7) | 65(82.3) | 8(57.1) | 75(81.5) | ||||
| R1 | 23(21.7) | 17(20.5) | 6(26.1) | 16(20.5) | 7(25.0) | 9(33.3) | 14(17.7) | 6(42.9) | 17(18.5) | ||||
| Nerve Infiltration | 1.0000 | 0.2796 | 1.0000 | 0.0087 | |||||||||
| Negative | 22(20.8) | 17(20.5) | 5(21.7) | 14(17.9) | 8(28.6) | 5(18.5) | 17(21.5) | 7(50.0) | 15(16.3) | ||||
| Positive | 84(79.2) | 66(79.5) | 18(78.3) | 64(82.1) | 20(71.4) | 22(81.5) | 62(78.5) | 7(50.0) | 77(83.7) | ||||
| Vascular invasion | 0.5846 | 0.4331 | 0.0384 | 0.3005 | |||||||||
| No | 24(22.6) | 20(24.1) | 4(17.4) | 16(20.5) | 8(28.6) | 10(37.0) | 14(17.7) | 5(35.7) | 19(20.7) | ||||
| Yes | 82(77.4) | 63(75.9) | 19(82.6) | 62(79.5) | 20(71.4) | 17(63.0) | 65(82.3) | 9(64.3) | 73(79.3) | ||||
| Lymphatic invasion | 0.6282 | 0.1780 | 0.0367 | 0.0812 | |||||||||
| Negative | 38(35.8) | 31(37.3) | 7(30.4) | 31(39.7) | 7(25.0) | 5(18.5) | 33(41.8) | 2(14.3) | 36(39.1) | ||||
| Positive | 68(64.2) | 52(62.7) | 16(69.6) | 47(60.3) | 21(75.0) | 22(81.5) | 46(58.2) | 12(85.7) | 56(60.9) | ||||
| UICC stage | 0.6212 | 0.2312 | 0.3449 | 0.1342 | |||||||||
| IA/IB/IIA | 28(26.4) | 21(25.3) | 7(30.4) | 23(29.5) | 5(17.9) | 9(33.3) | 19(24.1) | 6(42.9) | 22(23.9) | ||||
| IIB | 78(73.6) | 62(74.7) | 16(69.6) | 55(70.5) | 23(82.1) | 18(66.7) | 60(75.9) | 8(57.1) | 70(76.1) | ||||
Table 3. Associations of BNIP3, p62 expression with clinicopathological parameters.
| Parameters | No. of cases (%) | BNIP3 in tumor | BNIP3 in stroma | Cytoplasmic p62 in tumor | Nuclear p62 in tumor | Nuclear p62 in stroma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative (n = 75) (%) | Positive (n = 31) (%) | P-value | Negative (n = 90) (%) | Positive (n = 16) (%) | P-value | Negative (n = 39) (%) | Positive (n = 67) (%) | P-value | Negative (n = 68) (%) | Positive (n = 38) (%) | P-value | Negative (n = 72) (%) | Positive (n = 34) (%) | P-value | ||
| Age | 0.6696 | 1.0000 | 0.3191 | 1.0000 | 0.6829 | |||||||||||
| <61 years | 50(47.2) | 34(45.3) | 16(51.6) | 42(46.7) | 8(50.0) | 21(53.8) | 29(43.3) | 32(47.1) | 18(47.4) | 35(48.6) | 15(44.1) | |||||
| ≥61 years | 56(52.8) | 41(54.7) | 15(48.4) | 48(53.3) | 8(50.0) | 18(46.2) | 38(56.7) | 36(52.9) | 20(52.6) | 37(51.4) | 19(55.9) | |||||
| Gender | 1.0000 | 0.5883 | 0.6920 | 0.2229 | 0.6779 | |||||||||||
| Male | 57(53.8) | 40(53.3) | 17(54.8) | 47(52.2) | 10(62.5) | 22(56.4) | 35(52.2) | 40(58.8) | 17(44.7) | 40(55.6) | 17(50.0) | |||||
| Female | 49(46.2) | 35(46.7) | 14(45.2) | 43(47.8) | 6(37.5) | 17(43.6) | 32(47.8) | 28(41.2) | 21(55.3) | 32(44.4) | 17(50.0) | |||||
| Diabetes mellitus or hyperglycemia | 0.4975 | 0.7746 | 0.3969 | 0.3892 | 0.3810 | |||||||||||
| No | 35(33.0) | 23(30.7) | 12(38.7) | 29(32.2) | 6(37.5) | 15(38.5) | 20(29.9) | 20(29.4) | 15(39.5) | 26(36.1) | 9(26.5) | |||||
| Yes | 71(67.0) | 52(69.3) | 19(61.3) | 61(67.8) | 10(62.5) | 24(61.5) | 47(70.1) | 48(70.6) | 23(60.5) | 46(63.9) | 25(73.5) | |||||
| Tumor Size | 0.7892 | 0.5172 | 0.6150 | 0.6115 | 0.0173 | |||||||||||
| ≤2.0cm | 21(19.8) | 14(18.7) | 7(22.6) | 17(18.9) | 4(25.0) | 9(23.1) | 12(17.9) | 13(19.1) | 8(21.1) | 19(13.9) | 2(32.4) | |||||
| >2.0cm | 85(80.2) | 61(81.3) | 24(77.4) | 73(81.1) | 12(75.0) | 30(76.9) | 55(82.1) | 55(80.9) | 30(78.9) | 53(86.1) | 32(67.6) | |||||
| Tumor Location | 0.1791 | 0.0835 | 1.0000 | 0.8047 | 1.0000 | |||||||||||
| Head | 85(80.2) | 63(84.0) | 22(71.0) | 75(83.3) | 10(62.5) | 31(79.5) | 54(80.6) | 55(80.9) | 30(78.9) | 58(80.6) | 27(79.4) | |||||
| Body/tail | 21(19.8) | 12(16.0) | 9(29.0) | 15(16.7) | 6(37.5) | 8(20.5) | 13(19.4) | 13(19.1) | 8(21.1) | 14(19.4) | 7(20.6) | |||||
| Differentiation | 0.0155 | 0.2015 | 0.9109 | 0.2332 | 0.2560 | |||||||||||
| Well | 38(35.9) | 31(41.3) | 7(22.6) | 33(36.7) | 5(31.2) | 15(38.5) | 23(34.3) | 28(41.2) | 10(26.3) | 29(40.3) | 9(26.5) | |||||
| Moderate | 40(37.7) | 30(40.0) | 10(32.2) | 31(34.4) | 9(56.3) | 14(35.9) | 26(38.8) | 25(36.8) | 15(39.5) | 27(37.5) | 13(38.2) | |||||
| Poor | 28(26.4) | 14(18.7) | 14(45.2) | 26(28.9) | 2(12.5) | 10(25.6) | 18(26.9) | 15(22.0) | 13(34.2) | 16(22.2) | 12(35.3) | |||||
| Marginal status | 0.6055 | 0.0010 | 0.0132 | 0.8069 | 1.0000 | |||||||||||
| R0 | 83(78.3) | 60(80.0) | 23(74.2) | 76(84.4) | 7(45.8) | 25(64.1) | 58(86.6) | 54(79.4) | 29(76.3) | 56(77.8) | 27(79.4) | |||||
| R1 | 23(21.7) | 15(20.0) | 8(25.8) | 14(15.6) | 9(56.3) | 14(35.9) | 9(13.4) | 14(20.6) | 9(23.7) | 16(22.2) | 7(20.6) | |||||
| Nerve Infiltration | 0.7954 | 0.1830 | 0.2137 | 0.1390 | 0.4418 | |||||||||||
| Negative | 22(20.8) | 15(20.0) | 7(22.6) | 21(23.3) | 1(6.3) | 11(28.2) | 11(16.4) | 11(20.6) | 11(28.9) | 17(23.6) | 5(14.7) | |||||
| Positive | 84(79.2) | 60(80.0) | 24(77.4) | 69(76.7) | 15(93.7) | 28(71.8) | 56(83.6) | 57(79.4) | 27(71.1) | 55(76.4) | 29(85.3) | |||||
| Vascular invasion | 0.1358 | 0.3518 | 0.6336 | 0.3331 | 0.3207 | |||||||||||
| No | 24(22.6) | 14(18.7) | 10(32.3) | 19(21.1) | 5(31.3) | 10(25.6) | 14(20.9) | 13(19.1) | 11(28.9) | 14(22.2) | 10(29.4) | |||||
| Yes | 82(77.4) | 61(81.3) | 21(67.7) | 71(78.9) | 11(68.7) | 29(74.4) | 53(79.1) | 55(80.9) | 27(71.1) | 58(77.8) | 24(70.6) | |||||
| Lymphatic invasion | 0.8241 | 0.7823 | 1.0000 | 1.0000 | 0.6685 | |||||||||||
| Negative | 38(35.8) | 26(34.7) | 12(38.7) | 33(36.7) | 5(31.3) | 14(35.9) | 24(35.8) | 24(35.3) | 14(36.8) | 27(37.5) | 11(32.3) | |||||
| Positive | 68(64.2) | 49(65.3) | 19(61.3) | 57(63.3) | 11(68.7) | 25(64.1) | 43(64.2) | 44(64.7) | 24(63.2) | 45(62.5) | 23(67.6) | |||||
| UICC stage | 0.0649 | 0.2751 | 0.1719 | 0.8186 | 0.4797 | |||||||||||
| IA/IB/IIA | 28(26.4) | 16(21.3) | 12(38.7) | 22(24.4) | 6(37.5) | 7(17.9) | 21(31.3) | 19(27.9) | 9(23.7) | 21(29.2) | 7(20.6) | |||||
| IIB | 78(73.6) | 59(78.7) | 19(61.3) | 68(75.6) | 10(62.5) | 32(82.1) | 46(68.7) | 49(72.1) | 29(76.3) | 51(70.8) | 27(79.4) | |||||
Correlation between tumor metabolic phenotypes and clinicopathological parameters, mitochondrial function and autophagic status
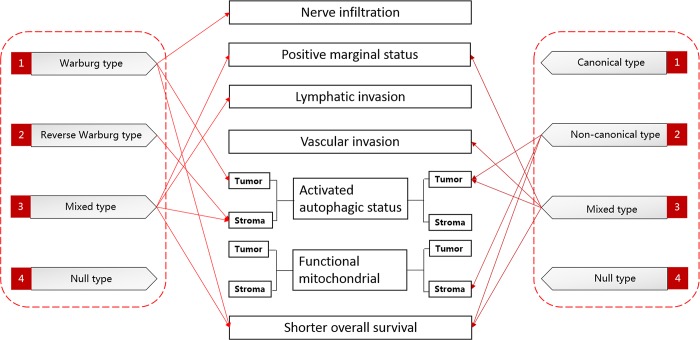
Classifications of tumor metabolic phenotypes were summarized according to previous reports (Table 2 in S2 File) and representative immunohistochemical labeling profiles were shown in Fig. 1 [13,14]. Warburg type refers to a phenotype in which only tumor cells show increased glycolysis and glycolysis takes place in stroma not tumor in reverse Warburg type. Both tumor cells and stromal cells are glycolytic in mixed type and nonglycolytic in null type. The correlations between the metabolic phenotypes of pancreatic cancer and clinicopathological parameters were summarized in Table 5. The total 106 tumor samples were divided into following four types according to their glycolysis-related proteins expression: Warburg type (n = 46, 43.4%), reverse Warburg type (n = 15, 14.2%), mixed type (n = 16, 15.1%), null type (n = 29, 27.3%). Warburg type of glucose-dependent metabolism had a highest percentage of tumors with nerve infiltration (P = 0.0003), UICC stage (P = 0.0004). Mixed type of glucose-dependent metabolism comprised the highest percentage of tumors located in the body/tail (P = 0.0041), with positive marginal status (P<0.0001), and with lymphatic invasion (P<0.0001). Reverse Warburg type had a highest percentage of tumors located in head (P = 0.0041), with absence of nerve infiltration (P = 0.0003). Null type of glucose-dependent metabolism comprised highest percentage of tumors with negative marginal status (P<0.0001) and absence of lymphatic invasion (P<0.0001).
Fig 1. Typical immunohistochemical labeling of each metabolism-related proteins according to metabolic phenotypes in pancreatic cancer.
Table 5. Clinicopathological characteristics of patients according to metabolic phenotype.
| Parameters | No. of cases (%) | Glucose-dependent metabolism | Glutamine-dependent metabolism | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Warburg type (n = 46) (%) | Reverse Warburgtype (n = 15 (%) | Mixed type (n = 16) (%) | Null type (n = 29) (%) | P value | Canonical type (n = 18) (%) | Non-canonical type (n = 74) (%) | Mixed type(n = 5) (%) | Null type (n = 9) (%) | P-value | |||
| Age | 0.8886 | 0.2230 | ||||||||||
| <61 years | 50(47.2) | 22(47.8) | 6(40.0) | 7(43.8) | 15(51.7) | 8(44.4) | 32(43.2) | 3(60.0) | 7(77.8) | |||
| ≥61 years | 56(52.8) | 24(52.2) | 9(60.0) | 9(56.2) | 14(48.3) | 10(55.6) | 42(56.8) | 2(40.0) | 2(22.2) | |||
| Gender | 0.3488 | 0.0544 | ||||||||||
| Male | 57(53.8) | 25(54.3) | 5(33.3) | 10(62.5) | 17(58.6) | 8(44.4) | 38(51.3) | 5(100.0) | 6(66.7) | |||
| Female | 49(46.2) | 21(45.7) | 10(66.7) | 6(37.5) | 12(41.4) | 10(55.6) | 36(48.7) | 0(0) | 3(33.3) | |||
| Diabetes mellitus or hyperglycemia | 0.1948 | 0.5164 | ||||||||||
| No | 35(33.0) | 14(30.4) | 4(26.7) | 9(56.3) | 8(27.6) | 5(27.8) | 25(33.8) | 3(60.0) | 2(22.2) | |||
| Yes | 71(67.0) | 32(69.6) | 11(73.3) | 7(43.7) | 21(72.4) | 13(72.2) | 49(66.2) | 2(40.0) | 7(77.8) | |||
| Tumor Size | 0.1153 | 0.0163 | ||||||||||
| ≤2.0cm | 21(19.8) | 8(17.4) | 6(40.0) | 4(25.0) | 3(10.3) | 6(33.3) | 12(16.2) | 3(60.0) | 0(0) | |||
| >2.0cm | 85(80.2) | 38(82.6) | 9(60.0) | 12(75.0) | 26(89.7) | 12(66.7) | 62(83.8) | 2(40.0) | 9(100.0) | |||
| Tumor Location | 0.0041 | 0.4739 | ||||||||||
| Head | 85(80.2) | 42(91.3) | 14(93.3) | 9(56.3) | 20(69.0) | 12(66.7) | 61(82.4) | 4(80.0) | 8(88.9) | |||
| Body/tail | 21(19.8) | 4(8.7) | 1(6.7) | 7(43.7) | 9(31.0) | 6(33.3) | 13(17.6) | 1(20.0) | 1(11.1) | |||
| Differentiation | 0.2009 | 0.4849 | ||||||||||
| Well | 38(35.9) | 19(41.3) | 3(20.0) | 4(25.5) | 12(41.4) | 5(27.8) | 29(39.2) | 2(40.0) | 2(22.2) | |||
| Moderate | 40(37.7) | 19(41.3) | 5(33.3) | 5(31.3) | 11(37.9) | 7(38.9) | 29(39.2) | 2(40.0) | 2(22.2) | |||
| Poor | 28(26.4) | 8(17.4) | 7(46.7) | 7(43.2) | 6(20.7) | 6(33.3) | 16(21.6) | 1(20.0) | 5(55.6) | |||
| Marginal status | <0.0001 | <0.0001 | ||||||||||
| R0 | 83(78.3) | 36(78.3) | 13(86.7) | 6(37.5) | 28(96.6) | 17(94.4) | 65(87.8) | 0(0) | 1(11.1) | |||
| R1 | 23(21.7) | 10(21.7) | 2(13.3) | 10(62.5) | 1(3.4) | 1(5.6) | 9(12.2) | 5(100.0) | 8(88.9) | |||
| Nerve Infiltration | 0.0003 | 0.7601 | ||||||||||
| Negative | 22(20.8) | 4(8.7) | 9(60.0) | 4(25.0) | 5(17.2) | 3(16.7) | 15(20.3) | 2(40.0) | 2(22.2) | |||
| Positive | 84(79.2) | 42(91.3) | 6(40.0) | 12(75.0) | 24(82.8) | 15(83.3) | 59(79.7) | 3(60.0) | 7(77.8) | |||
| Vascular invasion | 0.4958 | 0.0073 | ||||||||||
| Negative | 24(22.6) | 9(19.6) | 3(20.0) | 6(37.5) | 6(20.7) | 4(22.2) | 14(18.9) | 0(0) | 6(66.7) | |||
| Positive | 82(77.4) | 37(80.4) | 12(80.0) | 10(62.5) | 23(79.3) | 14(77.8) | 60(81.1) | 5(100.0) | 3(33.3) | |||
| Lymphatic invasion | <0.0001 | 0.0802 | ||||||||||
| Negative | 38(35.8) | 10(21.7) | 3(20.0) | 2(12.5) | 23(79.3) | 5(27.8) | 31(41.9) | 2(40.0) | 0(0) | |||
| Positive | 68(64.2) | 36(78.3) | 12(80.0) | 14(87.5) | 6(20.7) | 13(72.2) | 43(58.1) | 3(60.0) | 9(100.0) | |||
| UICC stage | 0.0004 | 0.4847 | ||||||||||
| IA/IB/IIA | 28(26.4) | 3(6.5) | 8(53.3) | 7(43.8) | 10(34.5) | 5(27.8) | 17(23.0) | 2(40.0) | 4(44.4) | |||
| IIB | 78(73.6) | 43(93.5) | 7(46.7) | 9(56.2) | 19(65.5) | 13(72.2) | 57(77.0) | 3(60.0) | 5(55.6) | |||
Mitochondrial function and autophagic status were further assessed according to subtype of glucose-dependent metabolism. We found that Warburg type comprised a highest percentage of tumors with activated autophagy (P = 0.0167), mixed type and reverse Warburg type with activated autophagic status in stroma (P = 0.0002), reverse Warburg type with nonacitivated autophagic status in tumor (P = 0.0167), and null type with nonactivated autophagic status in stroma (P = 0.0002) (Table 6).
The total 106 tumor samples were also divided into four subtypes of glutamine-dependent metabolism, including canonical type (n = 18, 17.0%), non-canonical type (n = 74, 69.8%), mixed type (n = 5, 4.7%) and null type (n = 9, 8.5%) (Table 5). The canonical type refers to a phenotype in which tumors rely on glutamate dehydrogenase (GLUD1) for glutamine utilization; whereas non-canonical type refers to a phenotype in which tumor rely on aspartate transaminase (GOT1) for glutamine utilization [9,15]. The mixed type of glutamine-dependent metabolism refers to a phenotype in which tumors metabolize glutamine through GLUD1 and GOT1, and null type refers to a phenotype in which tumors metabolize glutamine by neither GLUD1 nor GOT1. Canonical type had a highest percentage of tumors with negative marginal status (P<0.0001), whereas mixed type of glutamine-dependent metabolism comprised the highest percentage of tumors with positive marginal status (P<0.0001), vascular invasion (p = 0.0073). A significant association was found between the null type of glutamine-dependent metabolism and tumors with larger size (>2.0cm) (p = 0.0163). Moreover, tumors of non-canonical type and mixed type had a significantly activated autophagic status in tumor (P = 0.0034), and non-canonical type comprised the highest percentage of stroma with functional mitochondrial status (P = 0.0046) (Table 6).
Table 6. Mitochondrial function and autophagic status according to metabolic phenotype.
| Parameters | No. of cases (%) | Glucose-dependent metabolism | Glutamine-dependent metabolism | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Warburg type (n = 46) (%) | Reverse Warburg type (n = 15 (%) | Mixed type (n = 16) (%) | Null type (n = 29) (%) | P value | Canonical type (n = 18) (%) | Non-canonical type (n = 74) (%) | Mixed type(n = 5) (%) | Null type (n = 9) (%) | P-value | |||
| Tumor mitochondrial status | 0.2642 | 0.0999 | ||||||||||
| Dysfunctional | 31(29.2) | 12(26.1) | 7(46.7) | 6(37.5) | 6(20.7) | 8(44.4) | 17(23.0) | 1(20.0) | 5(55.6) | |||
| Functional | 75(70.8) | 34(73.9) | 8(53.3) | 10(62.5) | 23(79.3) | 10(55.6) | 57(77.0) | 4(80.0) | 4(44.4) | |||
| Stroma mitochondrial status | 0.1356 | 0.0046 | ||||||||||
| Dysfunctional | 16(15.1) | 4(8.7) | 5(33.3) | 2(12.5) | 5(17.2) | 3(16.7) | 6(8.1) | 3(60.0) | 4(44.4) | |||
| Functional | 90(84.9) | 42(91.3) | 10(66.7) | 14(87.5) | 24(82.8) | 15(83.3) | 68(91.9) | 2(40.0) | 5(55.6) | |||
| Tumor autophagic status | 0.0167 | 0.0034 | ||||||||||
| Activated | 57(53.8) | 30(65.2) | 3(20.0) | 7(43.7) | 17(58.6) | 5(27.8) | 44(59.5) | 5(100.0) | 3(33.3) | |||
| Nonactivated | 49(46.2) | 16(34.8) | 12(80.0) | 9(56.3) | 12(41.4) | 13(72.2) | 30(40.0) | 0(0) | 6(66.7) | |||
| Stroma autophagic status | 0.0002 | 0.1629 | ||||||||||
| Activated | 18(17.0) | 5(10.9) | 6(37.5) | 7(43.7) | 0(0) | 4(11.1) | 13(17.6) | 1(20.0) | 0(0) | |||
| Nonactivated | 88(83.0) | 41(89.1) | 10(62.5) | 9(56.3) | 29(100.0) | 14(88.9) | 61(82.4) | 4(80.0) | 9(100.0) | |||
Impact of clinicopathological parameters and metabolism-related proteins on patient prognosis
Univariate analysis was performed to explore the relation of the clinicopathological parameters/metabolism-related proteins and overall survivals (Table 7 and Figure B in S1 File). Higher histological grade (P = 0.041) was significantly associated with shorter overall survival. Longer overall survival was associated with high expression of BNIP3 in tumor (p = 0.010). Shorter overall survival was associated with high expression of GLUT1 in tumor (P = 0.002), GOT1 in tumor (p = 0.030), and LC3 in tumor (p = 0.009). There were borderline significant differences in nerve infiltration with regard to overall survival (P = 0.075).
Table 7. Clinicopathological Parameters/the expression of metabolism-related proteins and overall survival by log-rank test.
| Variables | Number of patients/death | Overall survival | Variables | Number of patients/death | Overall survival | ||
|---|---|---|---|---|---|---|---|
| Mean survival(95% CI), months | P-value | Mean survival(95% CI), months | P-value | ||||
| Age | 0.272 | Marginal status | 0.314 | ||||
| <61 years | 50/35 | 17(13 to 21) | R0 | 83/63 | 20(16 to 25) | ||
| ≥61 years | 56/44 | 22(17 to 28) | R1 | 23/16 | 17(10 to 24) | ||
| Gender | 0.268 | Nerve Infiltration | 0.075 | ||||
| Male | 57/45 | 18(13 to 22) | Negative | 22/18 | 14(10 to 17) | ||
| Female | 49/34 | 23(17 to 29) | Positive | 84/61 | 22(17 to 26) | ||
| Diabetes mellitus or hyperglycemia | 0.840 | Vascular invasion | 0.523 | ||||
| No | 35/25 | 20(13 to 27) | No | 24/19 | 18(16 to 27) | ||
| Yes | 71/54 | 20(16 to 25) | Yes | 82/60 | 24(17 to 29) | ||
| Tumor Size | 0.562 | Lymphatic invasion | 0.373 | ||||
| ≤2.0cm | 21/17 | 23(14 to 31) | Negative | 38/30 | 18(12 to 24) | ||
| >2.0cm | 85/62 | 19(15 to 23) | Positive | 68/49 | 21(16 to 25) | ||
| Tumor Location | 0.243 | UICC Stage | 0.012 | ||||
| Head | 85(80.2) | 21(15 to 28) | IA/IB/IIA | 28/17 | 20(15 to 27) | ||
| Body/tail | 21(19.8) | 25(19 to 33) | IIB | 78/62 | 13(8 to 15) | ||
| Differentiation | 0.041 | GLUT1 in tumor | 0.002 | ||||
| Well | 38/28 | 21(18 to27) | Negative | 44/29 | 28(22 to 34) | ||
| Moderate | 40/27 | 16(10 to 22) | Positive | 62/50 | 17(13 to 21) | ||
| Poor | 28/24 | 14(9 to 19) | |||||
| GLUT1 in stroma | 0.520 | Cytoplasmic p62 in tumor | 0.310 | ||||
| Negative | 75/56 | 23(18 to 28) | Negative | 39/29 | 23(17 to 30) | ||
| Positive | 31/23 | 19(14 to 24) | Positive | 67/50 | 21(17 to 26) | ||
| CAIX in tumor | 0.807 | Cytoplasmic p62 in tumor | 0.698 | ||||
| Negative | 82/61 | 22(15 to 30) | Negative | 39/29 | 23(17 to 30) | ||
| Positive | 24/18 | 23(15 to 30) | Positive | 67/50 | 21(17 to 26) | ||
| CAIX in stroma | 0.234 | Nuclear p62 in stroma | 0.983 | ||||
| Negative | 95/69 | 22(18 to 26) | Negative | 72/53 | 21(16 to 28) | ||
| Positive | 11/10 | 17(8 to 26) | Positive | 34/26 | 22(17 to 27) | ||
| GLUD1 in tumor | 0.783 | LC3 in tumor | 0.009 | ||||
| Negative | 83/60 | 25(18 to 33) | Negative | 43/28 | 28(17 to 35) | ||
| Positive | 23/19 | 21(17 to 26) | Positive | 63/51 | 17(13 to 20) | ||
| GLUD1 in stroma | 0.990 | LC3 in stroma | 0.691 | ||||
| Negative | 78/57 | 22(18 to 27) | Negative | 87/66 | 21(17 to 25) | ||
| Positive | 28/22 | 21(14 to 28) | Positive | 19/13 | 25(14 to 36) | ||
| GOT1 in tumor | 0.030 | BNIP3 in tumor | 0.010 | ||||
| Negative | 79/60 | 33(25 to 40) | Negative | 75/58 | 21(16 to 25) | ||
| Positive | 27/19 | 18(14 to 22) | Positive | 31/21 | 24(17 to 32) | ||
| GOT1 in stroma | 0.449 | BNIP3 in stroma | 0.991 | ||||
| Negative | 14/8 | 29(15 to 42) | Negative | 75/58 | 22(18 to 26) | ||
| Positive | 92/71 | 21(17 to 25) | Positive | 31/21 | 23(15 to 31) | ||
Impact of metabolism phenotypes on patient prognosis
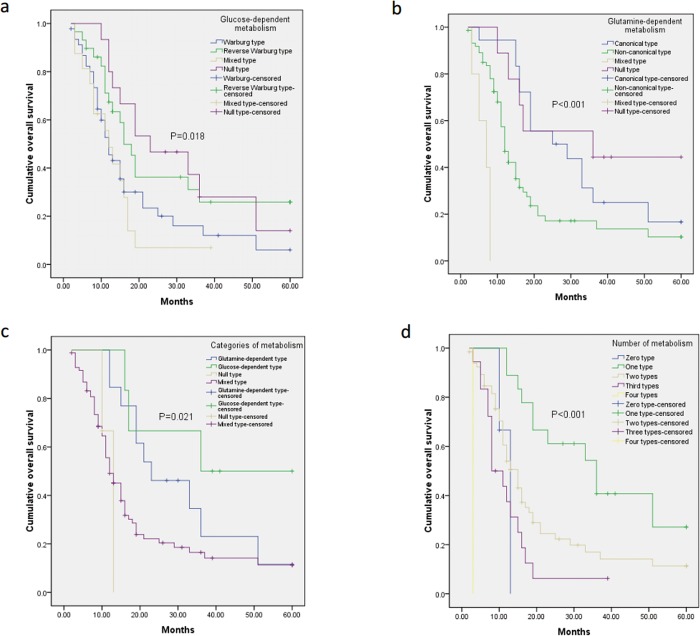
The results of univariate analysis using log-rank tests of the metabolism phenotypes in relation to overall survival are presented in Table 8. Glucose-dependent metabolism status was significantly associated with overall survival (P = 0.018) (Fig. 2A). Then we performed pairwise comparison among the glucose-dependent metabolism subtypes, and found that Warburg type and mixed type of glucose-dependent metabolism were significantly associated with shorter overall survival (Warburg type vs. reverse Warburg type, P = 0.0340; Warburg type vs. Null type, P = 0.0480; Mixed type vs. reverse Warburg type, P = 0.0060; Mixed type vs. Null type, P = 0.0350). There was no difference between other pairs (Warburg type vs. Mixed type, P = 0.4880; Reverse Warburg type vs. null type, P = 0.648) (Table 3 in S2 File). We also found that glutamine-dependent metabolism status was significantly associated with overall survival (P<0.001) (Fig. 2B and Table 8). After pairwise test was performed, we found that canonical type was significantly associated with longer overall survival (Canonical type vs. Non-canonical type, p = 0.009; Canonical type vs. Mixed type, P<0.001). Non-canonical type was significantly associated with longer overall survival than mixed type (P<0.001), but shorter than null type (P = 0.028) and the canonical type (P = 0.0009). The mixed type of glutamine-dependent metabolism was significantly associated with shorter overall survival than null type (P<0.001). There was no difference between canonical type and null type (P = 0.453) (Table 4 in S2 File) (Fig. 3).
Table 8. Univariate analysis of metabolism phenotypes in pancreatic cancers and overall survival by log-rank test.
| Metabolism phenotypes | Number of patients/death | Overall survival | |
|---|---|---|---|
| Mean survival(95% CI), months | P-value | ||
| Glucose-dependent metabolic status | 0.018 | ||
| Warburg type | 46/36 | 18(13 to 23) | |
| Reverse Warburg type | 15/11 | 30(21 to 40) | |
| Mixed type | 16/14 | 13(9 to 18) | |
| Null type | 29/18 | 27(19 to 36) | |
| Glutamine-dependent metabolic status | <0.001 | ||
| Canonical type | 18/14 | 31(23 to 39) | |
| Non-canonical type | 74/55 | 19(14 to 23) | |
| Mixed type | 5/5 | 6(4 to 8) | |
| Null type | 9/5 | 37(23 to 51) | |
| Categories of metabolism* | 0.021 | ||
| Glutamine-dependent metabolism | 26/17 | 29(21 to 41) | |
| Glucose-dependent metabolism | 6/4 | 37(22 to 45) | |
| Mixed type | 71/57 | 19(15 to 23) | |
| Null type | 3/1 | 51(39 to 65) | |
| No. of metabolism types # | <0.001 | ||
| Zero type | 3/1/ | 51(39 to 65) | |
| One type | 31/20 | 29(17 to 35) | |
| Two types | 53/41 | 21(16 to 26) | |
| Three types | 18/16 | 12(8 to 16) | |
| Four types | 1/1 | 3(3 to 3) | |
* Glutamine-dependent type (tumors only rely on glutamine-dependent metabolism), glucose-dependent type (tumors only rely on glucose-dependent metabolism), mixed type (tumors rely on both glucose- and glutamine-dependent metabolism), and null type (tumors show no sign of glucose- or glutamine-dependent metabolism)
#Metabolism types refer to cross combination among glucose- and glutamine-dependent metabolisms. Mixed types were composed of two main types of metabolism (i.e. Warburg effect and Reverse Warburg effect, Canonical type and Non-canonical type), and the null types were not composed of zero type in glucose- and glutamine-dependent metabolism (i.e. neither Warburg effect nor Rev erse Warburg effect, neither Canonical type nor Non-canonical type).
Fig 2. Overall survival curves according to the metabolic phenotypes of pancreatic cancer, including glucose-dependent metabolism (a), glutamine-dependent metabolism (b), combination of the two metabolism categories including glucose- and glutamine-dependent metabolism (c), and numbers of metabolism subtypes (d).
We assumed that mixed types composed of two main types within glucose- and glutamine-dependent metabolism (i.e. Warburg effect and Reverse Warburg effect, Canonical type and Non-canonical type), and the null types were not composed of any of the two main types in glucose- and glutamine-dependent metabolism (i.e. neither Warburg effect nor Reverse Warburg effect, neither Canonical type nor Non-canonical type.
Fig 3. Schematic representation summing up the associations of various metabolic categories and subtypes with the specific associated parameters.
A solid line with arrow means metabolic categories or subtypes have the highest percentage of the specific associated parameters.
In order to compare the two categories of metabolism in parallel, we classified the tumors into following groups according to the two categories of metabolism: glucose-dependent type (tumors only rely on glucose-dependent metabolism and have no sign of glutamine-dependent metabolism), glutamine-dependent type (tumors only rely on glutamine-dependent metabolism and show no sign of glucose-dependent metabolism), mixed type (tumors rely on both glucose- and glutamine-dependent metabolism), and null type (tumors show no sign of glucose- or glutamine-dependent metabolism) (Table 8). Most (n = 71, 67.0%) of the included tumors in present study were mixed type, followed by glutamine-dependent metabolism (n = 26, 24.5%), glucose-dependent metabolism (n = 6, 5.7%), and null type (n = 3, 2.8%). It suggested that most tumors utilized both glucose- and glutamine-dependent metabolism to survive under shortage of nutrients and oxygen. Then we conducted Kaplan-Meier survival analysis and found that categories of metabolism were significantly associated with overall survival of patients (P = 0.021) (Fig. 2C). However, after pairwise test was done, only the null type significantly linked with longer overall survival than glutamine- and glucose-dependent metabolism. Notably, there was no significant difference in overall survival between glucose-dependent metabolism and glutamine-dependent metabolism (P = 0.279). However, careful consideration should be given to these results due to lack of data in null type and glucose-dependent type (Table 5 in S2 File). We hypothesized that the tumor with mixed type or Warburg type of glucose-dependent metabolism are highly glycolytic, and tumors with non-canonical type and mixed type of glutamine-dependent metabolism are highly glutaminolytic. We found that highly glycolytic tumors were also more dependent on glutamine-dependent metabolism and vice versa (P<0.0001).
Next, we hypothesized that mixed types were composed of two main subtypes in glucose- and glutamine-dependent metabolism (i.e. Warburg type and Reverse Warburg type, Canonical type and Non-canonical type), and the null types were not composed of any of the two main types in glucose- and glutamine-dependent metabolism (i.e. neither Warburg type nor Reverse Warburg type, neither Canonical type nor Non-canonical type). Therefore, based on the types of glucose-dependent and glutamine-dependent metabolism, we classified the patients into 4 groups: zero type (n = 3, 2.8%), one type (n = 31, 29.2%), two types (n = 53, 50.0%), three types (n = 18, 17.0%), four types (n = 1, 0.9%) (Table 8). As indicated in Kaplan-Meier survival analysis, the overall survival of one-type group was significantly longer than two-type group (P = 0.039) and three-type group (P = 0.005) (Fig. 2D). Moreover, two-type group show a relatively longer overall survival than three-type group (P = 0.024). Statistical analysis comparing zero type or four type and other types was also performed, but the results needed to be taken careful consideration due to small number of patients. To sum up, the increasing numbers of the metabolism types robustly reflected major differences in survival outcome (Table 8 and Table 6 in S2 File).
Multivariate analysis of relation between prognostic factors and patient survival
Finally, multivariate analysis was also performed when P value in univariate analysis was less than 0.1. Prognostic factors including tumor differentiation, nerve infiltration, expression of GLUT1 in tumor, GOT1 in tumor, expression of LC3 in tumor, expression of BNIP3 in tumor, glucose-dependent metabolism, glutamine-dependent metabolism, categories of metabolism and numbers of metabolism types in tumor were analyzed in Multivariate models using Cox proportional hazards analysis. The results showed that independent factors for overall survival were higher histological grade (hazard ration 1.734, P = 0.015), GLUT1 in tumor (hazard ration 1.781, P = 0.003), GOT1 in tumor (hazard ration 1.982, P = 0.001), LC3 in tumor (hazard ration 3.321, P = 0.001), glutamine-dependent metabolism (hazard ration 1.471, P = 0.041), glucose-dependent metabolism (hazard ration 1.689, P = 0.022), numbers of metabolism type (hazard ratio 1.806, P- = 0.031). However, expression of BNIP3 in tumor was significantly associated with better prognosis (hazard ration 0.049, P = 0.008) (Table 9).
Table 9. Multivariate Analysis of Prognostic Factors for Overall Survival.
| Variable | Hazard Ratio | 95% Confidence Limit | P-value | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Tumor differentiation | 1.734 | 1.231 | 2.563 | 0.015 |
| Nerve infiltration | 0.823 | 0.554 | 1.678 | 0.062 |
| GLUT1 in tumor | 1.781 | 1.024 | 2.843 | 0.003 |
| GOT1 in tumor | 1.982 | 1.237 | 2.561 | 0.001 |
| LC3 in tumor | 3.321 | 1.660 | 6.642 | 0.001 |
| BNIP3 in tumor | 0.409 | 0.233 | 0.718 | 0.008 |
| Glutamine-dependent metabolism | 1.417 | 1.014 | 1.979 | 0.041 |
| Glucose-dependent metabolism | 1.689 | 1.005 | 2.641 | 0.022 |
| Categories of metabolism | 1.245 | 0.746 | 1.892 | 0.384 |
| No. of metabolism types | 1.806 | 1.055 | 3.090 | 0.031 |
Transcript level of metabolism-related genes in tumor according to tumor phenotype
In order to further confirm results from IHC above, QRT-PCR was performed to investigate expression of metabolism-related genes (Table 7 in S2 File) in tumor according to the tumor phenotype. The expression of SLC2A1(gene of GLUT1), BNIP3, BECN1(gene of Beclin1) and MAP1LC3A(gene of LC3) was higher in Warburg types, whereas the expression of GLUT1, CAIX, BNIP3, BECN1, MAP1LC3A and SQSTM1(gene of p62) were higher in mixed type of glucose-dependent metabolism than in controls. The expression of GLUD1 was higher in canonical type than controls, and expression of GOT1 was higher in non-canonical type and mixed type (Figure C in S1 File).
Discussion
The prognosis of pancreatic cancer patients is still dismal, and the disease is obviously resistant to current therapy[16]. Therefore, there is a growing need for new strategies to enhance the antitumor effect of gemcitabine in pancreatic cancer, which is the standard chemotherapeutic agent in pancreatic cancer. Recently, there has been growing interest in understanding cancer altered metabolism and targeting cancer metabolism holds a promising strategy for cancer treatment[4,9,17–19]. Before such effective metabolic drugs come into use, metabolism phenotypes in individual patient with pancreatic cancer need to be clearly identified due to obvious heterogeneity in pancreatic cancer [20,21]. However, little is known about metabolic features in pancreatic cancer.
Therefore, the present study was conducted to profile the glucose-dependent and glutamine-dependent metabolism in patients with pancreatic cancer. To our best knowledge, our study was the first study that focused on profiling metabolism phenotypes in pancreatic cancer. In order to identify the metabolism phenotype of individual tumor specimen, we firstly investigated the differential expression of metabolism-related protein markers not only in tumor cells but also in stromal cells. Besides, we also investigate the autophagic status through p62, LC3, Beclin1 expression and mitochondrial function by BNIP3 expression. It was reported that p62 is shuttled between the nucleus and the cytosol continuously. p62 is required to recruit the large phosphoinositide-binding protein ALFY (autophagy-linked FYVE protein, also known as WDFY3),which is predominantly located at nuclear, to cytoplasmic p62 bodies. ALFY, as well as p62, is required for formation and autophagic degradation of cytoplasmic ubiquitin-positive inclusions [22,23]. BNIP3 is a pro-apoptotic member of the Bcl-2 family induced under hypoxia and was employed to assess the mitochondrial function. It was recently shown that BNIP3 mediates mitochondrial dysfunction through activation of Bax or Bak, and induced mitochondrial permeability transition via its carboxy terminal tail. Therefore, overexpression of BNIP3 indicates the dysfunctional mitochondria [24,25]. Of note, stromal cells metabolize through glycolysis due to dysfunctional mitochondria and enhanced autophagy, while tumor cells metabolize through oxidative phosphorylation according to the theory of reverse Warburg effect [7,26,27]. As there is no data reporting the reverse Warburg effect in pancreatic cancer, the present study was carried out to verify the existence of reverse Warburg effect in pancreatic cancer in the meantime. We found that increased tumor expression of GLUT1 was associated with nerve infiltration, lymphatic invasion, UICC stage, whereas stoma expression of GLUT1 was associated with tumor dedifferentiation, positive marginal status, nerve infiltration, lymphatic invasion and UICC stage. Tumor expression of GLUD1 was associated with larger tumor size, while tumor expression of GOT1 was significantly linked with tumor dedifferentiation, vascular invasion, and lymphatic invasion. The results above proved the existence of reverse Warburg type in pancreatic cancer (defined when tumor is oxidative phosphorylation type and stroma is glycolytic with enhanced autophagy and dysfunctional mitochondrial status). According to the definition of Warburg and other types of glucose-dependent metabolism in breast cancer elsewhere [13,14], glucose-dependent metabolism phenotypes in pancreatic cancer were Warburg type>null type> mixed type > reverse Warburg type in order of frequency. Further, we found that each glucose-dependent metabolism subtypes has its own characteristics. Warburg type of glucose-dependent metabolism had a highest percentage of tumors with nerve infiltration, UICC stage, and activated autophagic status in tumor. Mixed type of glucose-dependent metabolism comprised the highest percentage of tumors located in the body/tail, with positive marginal status, with lymphatic invasion, with activated autophagic status in stroma. Reverse Warburg type had a highest percentage of tumors located in head, with absence of nerve infiltration, and with nonacitivated autophagic status in tumor. Null type of glucose-dependent metabolism comprised highest percentage of tumors with negative marginal status, absence of lymphatic invasion, nonactivated autophagic status in stroma. Moreover, Warburg type and mixed type had a significantly poorer prognosis than reverse Warburg and null type. In summary, the Warburg type and mixed type comprised of more metabolically active, biologically aggressive and poorer prognostic tumors, whereas the reverse Warburg type and null type consisted of metabolically inactive, biologically nonaggressive and better prognostic tumors.
Besides glucose-dependent metabolism, we also assessed the glutamine-dependent metabolism, which has been proved to be critical for pancreatic cancer cell growth. As the most abundant amino acid in cytoplasm, glutamine plays an important part in marcromolecular synthesis by fueling TCA cycle and ATP production, nitrogen for nucleotide, nonessential amino acid, and hexosamine biosynthesis [18,28]. Recent evidence showed that pancreatic cancer cells mostly utilizes glutamic-oxaloacetic transaminase (including GOT1) (Non-canonical type) other than glutamate dehydrogenase (GLUD1) (Canonical type) for converting into alpha-ketoglutarate to fuel TCA cycle, which is obviously different from other kinds of tumors and normal cells. Son and his colleagues had proved that GOT1 mediates the utilization of glutamine in pancreatic cancer; oncogenic KRAS coordinates the shift from canonical type to non-canonical type of glutamine-dependent metabolism by increasing expression of GOT1 and decreasing expression of GLUD1 [9,15,29,30]. To our best knowledge, the profiling of glutamine-dependent metabolism still lacked in pancreatic cancer. Therefore, we evaluated this kind of metabolism in human samples. The present study consisted of highest percentage of non-canonical type, followed by canonical type, mixed type and null type. Mixed type of glutamine-dependent metabolism comprised the highest percentage of tumors with positive marginal status, vascular invasion. Tumors of non-canonical type and mixed type had a significantly activated autophagic status in tumor, while non-canonical type comprised the highest percentage of stroma with functional mitochondrial status. The mixed type and non-canonical type of glutamine-dependent metabolism were significantly associated with shorter overall survival than the other two types (P<0.001). To sum up, the mixed type and non-canonical type of glutamine-dependent metabolism in pancreatic cancer were more metabolic active, biologically aggressive, and had a relatively poorer prognosis than the other two types.
Next, we classified the tumors into following types according to the two categories of metabolism: glucose-dependent type, glutamine-dependent type, mixed type, and null type. Results above suggested that most tumors utilized both glucose- and glutamine-dependent metabolism to survive under shortage of nutrients and oxygen. We also found that highly glycolytic tumors were more dependent on glutamine-dependent metabolism and vice versa, which may be helpful for tumor growth and survival. Further, we divided the patients into groups according to the numbers of metabolic types and found that the overall survival was significantly longer in one-type group than two-type or three-type group. Moreover, two-type group show a relatively better prognosis than three-type group. Results above suggested that the increasing numbers of metabolism types inversely associated with survival outcome. The more subtypes and categories of metabolism pancreatic cancer utilizes, the worse outcome it is. It is reasonable that pancreatic cancers can reprogram metabolism and rely on two or more metabolism types and categories to adapt themselves to environmental pressures and support their rapid growth.
Indeed, various clinicopathological parameters, including tumor size, tumor location, tumor histological grade, lymphatic/vascular invasion, and resection margin status, have an impact on prognosis of patients with pancreatic cancer after tumor resection [31,32]. However, the relation between clinicopathological parameters and prognosis are obscure and hard to grasp. Therefore, some more adequate preoperative identification methods of patients with poor prognosis are desirable to aid appropriate clinical decision making. We demonstrated here that expression of metabolism-related proteins, status of glutamine-dependent metabolism, subtypes and categories of metabolism which pancreatic cancer utilizes may be good prognostic factors. By assessing the status of glucose-dependent and glutamine-dependent metabolism through endoscopic ultrasound-fine needle aspiration in a prospective manner, classification of pancreatic cancer patients may be achievable and more adequate clinical decisions will be made.
We acknowledge that our present study has following limitations. First, the study design is partly retrospective and the number of patients is not large enough. Second, though all of the patients receive the same surgery (pancreaticoduodenectomy) and received gemcitabine chemotherapy after surgery, the other postoperative treatment of these patients varied. But results above appeared to be not affected by those different postoperative treatments. Some results (i.e. the relation of lymphatic invasion and overall survival) in this study were inconsistent with previous data, which may be attributed to possible differences in ethnic incidence.
Conclusion
Pancreatic cancer is heterogeneous in its metabolic phenotypes, and classification into various metabolic phenotypes may be helpful for more adequate clinical decision of therapy targeting metabolism and improving prognosis. To sum up, Warburg type, non-canonical type and mixed types of these two metabolisms consisted of metabolically active, biologically aggressive and poor prognosis tumors. Further, we identified for the first time that increasing numbers of the metabolism subtypes and categories robustly reflected major differences in survival outcome. The more subtypes and categories of metabolism pancreatic cancers employ, the worse outcome it is.
Supporting Information
Figure A in S1 File. Typical immunohistochemical labeling of positive control of various metabolism-related proteins. Figure B in S1 File. Kaplan-Meier survival curves for patients after surgery for pancreatic ductal adenocarcinoma demonstrating relationships of metabolism-related proteins with postoperative overall survival. Figure C in S1 File. Transcript levels of various genes including SLC2A1(solute carrier family 2 (facilitated glucose transporter), member 1), CAIX (carbonic anhydrase IX), BNIP3 (BCL2/adenovirus E1B 19kDa interacting protein 3), BECN1 (beclin 1, autophagy related), MAP1LC3A (microtubule-associated protein 1 light chain 3 alpha), SQSTM1 (sequestosome 1), GLUD1 (glutamate dehydrogenase 1), GOT1 (glutamic-oxaloacetic transaminase 1) were determined by quantitative qRT-PCR, results are means ± SD of 2 independent experiments done in triplicate. Three normal pancreas tissues were chosen as negative controls. *P<0.05, **P<0.01, ***P<0.005 versus controls.
(PDF)
(PDF)
Funding Statement
This work was supported by National Natural Scientific Foundation of China (no. 81000917 and no. 81370059). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 2. Warburg O (1956) On the origin of cancer cells. Science 123: 309–314. [DOI] [PubMed] [Google Scholar]
- 3. Frezza C, Gottlieb E (2009) Mitochondria in cancer: not just innocent bystanders. Semin Cancer Biol 19: 4–11. 10.1016/j.semcancer.2008.11.008 [DOI] [PubMed] [Google Scholar]
- 4. Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G (2013) Metabolic targets for cancer therapy. Nat Rev Drug Discov 12: 829–846. 10.1038/nrd4145 [DOI] [PubMed] [Google Scholar]
- 5. Zu XL, Guppy M (2004) Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun 313: 459–465. [DOI] [PubMed] [Google Scholar]
- 6. Zheng J (2012) Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review). Oncol Lett 4: 1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, et al. (2009) The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8: 3984–4001. [DOI] [PubMed] [Google Scholar]
- 8. Martinez-Outschoorn UE, Lin Z, Trimmer C, Flomenberg N, Wang C, et al. (2011) Cancer cells metabolically "fertilize" the tumor microenvironment with hydrogen peroxide, driving the Warburg effect: implications for PET imaging of human tumors. Cell Cycle 10: 2504–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Son J, Lyssiotis CA, Ying H, Wang X, Hua S, et al. (2013) Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496: 101–105. 10.1038/nature12040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pannala R, Basu A, Petersen GM, Chari ST (2009) New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol 10: 88–95. 10.1016/S1470-2045(08)70337-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghaneh P, Costello E, Neoptolemos JP (2007) Biology and management of pancreatic cancer. Gut 56: 1134–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim S, Kim do H, Jung WH, Koo JS (2013) Metabolic phenotypes in triple-negative breast cancer. Tumour Biol 34: 1699–1712. 10.1007/s13277-013-0707-1 [DOI] [PubMed] [Google Scholar]
- 13. Choi J, Kim DH, Jung WH, Koo JS (2013) Metabolic interaction between cancer cells and stromal cells according to breast cancer molecular subtype. Breast Cancer Res 15: R78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guillaumond F, Leca J, Olivares O, Lavaut MN, Vidal N, et al. (2013) Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc Natl Acad Sci U S A 110: 3919–3924. 10.1073/pnas.1219555110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lyssiotis CA, Son J, Cantley LC, Kimmelman AC (2013) Pancreatic cancers rely on a novel glutamine metabolism pathway to maintain redox balance. Cell Cycle 12: 1987–1988. 10.4161/cc.25307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamada S, Masamune A, Shimosegawa T (2013) Novel therapeutic strategies targeting tumor-stromal interactions in pancreatic cancer. Front Physiol 4: 331 10.3389/fphys.2013.00331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blum R, Kloog Y (2014) Metabolism addiction in pancreatic cancer. Cell Death Dis 5: e1065 10.1038/cddis.2014.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hensley CT, Wasti AT, DeBerardinis RJ (2013) Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest 123: 3678–3684. 10.1172/JCI69600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamanaka RB, Chandel NS (2012) Targeting glucose metabolism for cancer therapy. J Exp Med 209: 211–215. 10.1084/jem.20120162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vander Heiden MG (2013) Exploiting tumor metabolism: challenges for clinical translation. J Clin Invest 123:3648–51. 10.1172/JCI72391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hidalgo M (2012) New insights into pancreatic cancer biology. Ann Oncol 23:x135–8. [DOI] [PubMed] [Google Scholar]
- 22. Knaevelsrud H, Simonsen A (2010) Fighting disease by selective autophagy of aggregate-prone proteins. FEBS Lett 584:2635–45. 10.1016/j.febslet.2010.04.041 [DOI] [PubMed] [Google Scholar]
- 23. Clausen TH, Lamark T, Isakson P, Finley K, Larsen KB, et al. (2010) p62/SQSTM1 and ALFY interact to facilitate the formation of p62 bodies/ALIS and their degradation by autophagy. Autophagy 6:330–44. [DOI] [PubMed] [Google Scholar]
- 24. Nicolle B, Teralee RB, Elizabeth SH, Coleen OJ, Michelle B, et al. (2011) Truncated forms of BNIP3 act as dominant negatives inhibiting hypoxia-induced cell death. Biochim Biophys Acta 1812: 302–311. 10.1016/j.bbadis.2010.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim JY, Cho JJ, Ha J, Park JH (2002) The carboxy terminal C-tail of BNip3 is crucial in induction of mitochondrial permeability transition in isolated mitochondria. Arch Biochem Biophys 398:147–52. [DOI] [PubMed] [Google Scholar]
- 26. Hart I (2011) Eating for two: how stromal fibroblasts might nurture adjacent carcinoma cells. Cell Cycle 10: 2829–2830. [PubMed] [Google Scholar]
- 27. Salem AF, Whitaker-Menezes D, Lin Z, Martinez-Outschoorn UE, Tanowitz HB, et al. (2012) Two-compartment tumor metabolism: autophagy in the tumor microenvironment and oxidative mitochondrial metabolism (OXPHOS) in cancer cells. Cell Cycle 11: 2545–2556. 10.4161/cc.20920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dang CV (2012) Links between metabolism and cancer. Genes Dev 26: 877–890. 10.1101/gad.189365.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou W, Capello M, Fredolini C, Racanicchi L, Piemonti L, et al. (2012) Proteomic analysis reveals Warburg effect and anomalous metabolism of glutamine in pancreatic cancer cells. J Proteome Res 11:554–63. 10.1021/pr2009274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ward PS, Thompson CB (2012) Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21:297–308. 10.1016/j.ccr.2012.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cai SW, Yang SZ, Gao J, Pan K, Chedn JY, et al. (2011) Prognostic significance of mast cell count following curative resection for pancreatic ductal adenocarcinoma. Surgery 149:576–84. 10.1016/j.surg.2010.10.009 [DOI] [PubMed] [Google Scholar]
- 32. Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, et al. (2006) 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 10:1199–1210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A in S1 File. Typical immunohistochemical labeling of positive control of various metabolism-related proteins. Figure B in S1 File. Kaplan-Meier survival curves for patients after surgery for pancreatic ductal adenocarcinoma demonstrating relationships of metabolism-related proteins with postoperative overall survival. Figure C in S1 File. Transcript levels of various genes including SLC2A1(solute carrier family 2 (facilitated glucose transporter), member 1), CAIX (carbonic anhydrase IX), BNIP3 (BCL2/adenovirus E1B 19kDa interacting protein 3), BECN1 (beclin 1, autophagy related), MAP1LC3A (microtubule-associated protein 1 light chain 3 alpha), SQSTM1 (sequestosome 1), GLUD1 (glutamate dehydrogenase 1), GOT1 (glutamic-oxaloacetic transaminase 1) were determined by quantitative qRT-PCR, results are means ± SD of 2 independent experiments done in triplicate. Three normal pancreas tissues were chosen as negative controls. *P<0.05, **P<0.01, ***P<0.005 versus controls.
(PDF)
(PDF)