Deregulation of Axl in esophageal squamous cell carcinoma (OSCC) with potential therapeutic implications is described for the first time. This paper also sheds light on the understanding of how Axl regulates OSCC development in vitro and in vivo. Axl expression leads to an Akt-dependent regulation of glycogen synthase kinase 3β activity and the nucluear factor kappaB (NF-κB) pathway, affecting the epithelial–mesenchymal transition.
Abstract
The receptor tyrosine kinase Axl has been described as an oncogene, and its deregulation has been implicated in the progression of several human cancers. While the role of Axl in esophageal adenocarcinoma has been addressed, there is no information about its role in esophageal squamous cell carcinoma (OSCC). In the current report, we identified, for the first time, deregulation of Axl expression in OSCC. Axl is consistently overexpressed in OSCC cell lines and human tumor samples, mainly in advanced stages of the disease. Blockage of Axl gene expression by small interfering RNA inhibits cell survival, proliferation, migration, and invasion in vitro and esophageal tumor growth in vivo. Additionally, repression of Axl expression results in Akt-dependent inhibition of pivotal genes involved in the nuclear factor-kappaB (NF-κB) pathway and in the induction of glycogen synthase kinase 3β (GSK3β) activity, resulting in loss of mesenchymal markers and induction of epithelial markers. Furthermore, treatment of esophageal cancer cells with the Akt inhibitor wortmannin inhibits NF-κB signaling, induces GSK3β activity, and blocks OSCC cell proliferation in an Axl-dependent manner. Taken together, our results establish a clear role for Axl in OSCC tumorigenesis with potential therapeutic implications.
INTRODUCTION
Esophageal cancer is the eighth most common and the sixth leading cause of cancer-related deaths worldwide, with the majority of these deaths (86%) occurring in developing countries (Ferlay et al., 2010). The two most common types of esophageal cancer are esophageal squamous cell carcinoma (OSCC) and adenocarcinoma, and variability in the incidence of these cancer types is observed according to the geographic region and ethnic background. Adenocarcinoma is most prevalent in white populations, while OSCC is the most common esophageal cancer type occurring in nonwhite populations (Cooper et al., 2009; Ferlay et al., 2010; Somdyala et al., 2010). OSCC is mainly associated with environmental factors such as smoking and alcohol consumption, while adenocarcinoma is primarily associated with gastric reflux and Barret's esophagus (Gamliel, 2000). Despite the increase in incidence of adenocarcinoma over the last few decades, squamous cell carcinoma still represents a major problem in developing countries such as South Africa (Ferlay et al., 2010). In fact, esophageal cancer is the third most common cancer among South African men, who are twice as likely to get this cancer than women (Somdyala et al., 2010).
The receptor tyrosine kinase Axl belongs to the TAM (Tyro-3, Axl, and Mer) family. Members of this family are distinguished from one another by a conserved sequence within the kinase domain and adhesion molecule–like domains in the extracellular region (Janssen et al., 1991; O'Bryan et al., 1991). Activation of Axl occurs after the binding to the growth arrest–specific gene 6 (Gas6; Mark et al., 1996; McCloskey et al., 1997). Gas6 contains an N-terminal γ-carboxy-glutamic acid domain, whose carboxylation is dependent on vitamin K and is essential for its activity and receptor binding (Manfioletti et al., 1993; Hafizi and Dahlbäck, 2006a). In addition to Axl, Gas6 binds to other receptors of the TAM family and acts as a growth factor for many nontransformed cell types and Axl-transfected tumor cell lines (McCloskey et al., 1994, 1997; Mark et al., 1996). Rather than conferring a mitogenic signal, Axl has been implicated in cell survival, cellular adhesion, and chemotaxis and in vascular remodeling (Neubauer et al., 1994, 1997).
Axl phosphorylation and consequent activation have been linked to signaling pathways such as the phosphatidylinositol 3-OH kinase (PI3K) pathway, including its downstream targets S6K and Akt; the mitogen-activated protein kinases (MAPK) pathway; and the Jak/Stat and NF-κB signal transduction pathway cascades, which are closely related to progression and development of tumors and inhibition of apoptosis (Lee et al., 2002; Ruan and Kazlauskas, 2012; Paccez et al., 2013).
Previous reports have demonstrated the oncogenic activity of Axl (Janssen et al., 1991; McCloskey et al., 1994). This receptor tyrosine kinase is deregulated in several types of cancer, such as prostate (Shiozawa et al., 2010; Paccez et al., 2013), breast (Zhang et al., 2008), lung (Wimmel et al., 2001; Shieh et al., 2005; Linger et al., 2012), leukemia (Neubauer et al., 1993; Dirks et al., 1999), and esophageal adenocarcinomas (Hector et al., 2010), and it has been linked to a more aggressive phenotype, suggesting that Axl may be a relevant therapeutic target for cancer (Zhang et al., 2008; Hector et al., 2010; Rankin et al., 2010; Paccez et al., 2014). Additionally, Axl has been shown to play a role in the epithelial–mesenchymal transition (EMT), which is an important feature for the initiation of metastasis (Gjerdrum et al., 2010; Vuoriluoto et al., 2011; Byers et al., 2013; Asiedu et al., 2014).
Furthermore, Axl's expression predicts poor overall survival in breast and pancreatic cancer patients (Koorstra et al., 2009; Holland et al., 2010), and its overexpression is linked to an increase in resistance to various drug treatments (Liu et al., 2009; Zhang et al., 2012; Hong and Belkhiri, 2013; Hong et al., 2013). Increased Axl levels have been linked to imatinib-resistant gastrointestinal stromal tumors, nilotinib-resistant chronic myeloid leukemia cells, BMS-754087–resistant rhabdomyosarcoma, and lapatinib-resistant HER-2–positive breast tumor cells (Liu et al., 2009; Huang et al., 2010; Dufies et al., 2011; Gioia et al., 2011). In addition, previous reports demonstrated increased activation of Axl and evidence for EMT in epidermal growth factor receptor–mutant lung cancer models with acquired resistance to erlotinib (a tyrosine kinase inhibitor used for treatment of lung cancer) in vitro and in vivo (Zhang et al., 2012). Moreover, inhibition of Axl leads to restoration of sensitivity to erlotinib, indicating that Axl may represent a promising therapeutic target whose inhibition could prevent or overcome acquired resistance to certain drugs (Zhang et al., 2012).
While the role of Axl in esophageal adenocarcinoma progression and in resistance to therapy has already been described (Hector et al., 2010; Hong and Belkhiri, 2013; Hong et al., 2013), there is a complete lack of information about its role in OSCC. In this report, we demonstrate for the first time the importance of Axl in OSCC. We demonstrate that Axl is up-regulated in OSCC tumor samples from patients and OSCC cell lines. Furthermore, we reveal that blockage of Axl protein expression inhibits proliferation, invasion, and migration of OSCC cells and tumor formation in vivo. Interestingly, inhibition of Axl expression strikingly leads to reduction of Akt activation, leading to inhibition of IκBα phosphorylation and consequent blockage of NF-κB transcriptional activity. Concurrently, decreased Akt activity leads to activation of glycogen synthase kinase 3β (GSK3β) and results in loss of mesenchymal and induction of epithelial markers. Taken together, our data establish the importance of Axl in the development of OSCC and highlight this receptor tyrosine kinase as a potential therapeutic target.
RESULTS
Differential expression of Axl in OSCC cell lines and human cancer tissue
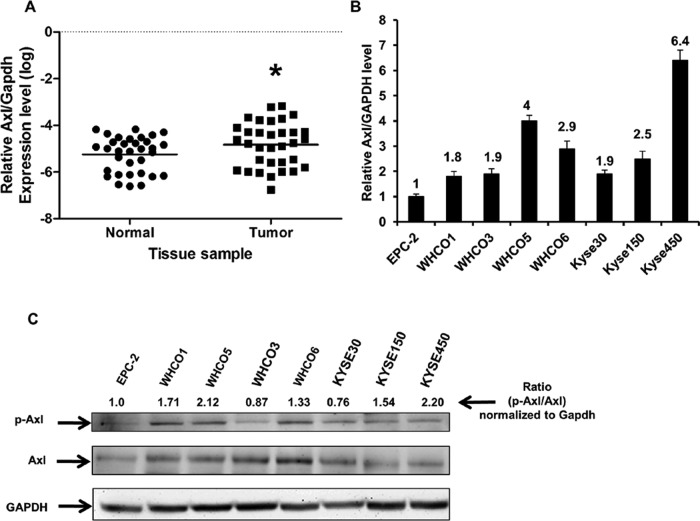
The receptor tyrosine kinase Axl is deregulated in several cancer types (Neubauer et al., 1993; Dirks et al., 1999; Wimmel et al., 2001; Shieh et al., 2005; Zhang et al., 2008; Hector et al., 2010; Shiozawa et al., 2010; Linger et al., 2012; Paccez et al., 2013). Axl expression and activity correlates with poor prognosis and therapy resistance in different cancer types, such as breast and lung cancer (Shieh et al., 2005; Gjerdrum et al., 2010). To determine the relevance of Axl expression in OSCC, we first evaluated mRNA expression levels in primary OSCC tumor tissue compared with normal tissue. We analyzed 66 patient samples consisting of matched pairs of normal and OSCC tumor tissue samples of 33 patients. As observed in Figure 1A, Axl expression levels in OSCC tissue are statistically significantly higher than in the matching normal esophageal tissue. Additionally, we analyzed the matched expression levels of Axl in each individual patient and observed that Axl is up-regulated in tumor as compared with matched normal tissue in 60% of patients, with fold induction varying from 1.7- to 56-fold. These findings clearly indicate that Axl is largely overexpressed during OSSC tumorigenesis. Analysis of tumor status indicates that, among our cohort, four tumor samples were well differentiated, 21 tumors were moderately differentiated, two were poorly differentiated, and six were not differentiated. As shown in Table 1, our analysis indicates that Axl expression is up-regulated in 52% of tumors in initial stages (well to moderately differentiated) and in 87.5% of tumors in more advanced stages (poorly to undifferentiated). These data clearly point toward a pivotal role of Axl in OSCC development and progression.
FIGURE 1:
Axl is up-regulated in OSCC human clinical samples and cell lines. (A) RT-PCR analysis of Axl in human samples. Total RNA was collected from human tissue consisting of paired normal and tumor samples and was analyzed for Axl expression levels. GAPDH was used as an internal control and for normalization of data. Statistics were performed to compare the significance of Axl expression in tumor vs. normal tissue, using the two-tailed, nonparametric Mann-Whitney test. Data are shown as the median values to account for outliers; *, p < 0.05. (B) RT-PCR analysis of Axl in OSCC cell lines. Total RNA was collected from WHCO1, WHCO3, WHCO5, WHCO6, Kyse30, Kyse150, and Kyse450. The normal esophageal EPC-2 cell line was used as a control. Normalization of each sample was carried out by measuring the amount of GAPDH cDNA. (C) Phosphorylation status of OSCC cell lines. Western blot analysis of Axl and phosphorylated Axl expression in OSCC lines was performed using specific antibodies, and GAPDH was used as the loading control. Ratios between p-Axl and Axl were calculated using the VisionWorks LS Image Acquisition and Analysis Software. The total amount of Axl protein present was normalized to the amount of GAPDH. Following this, the amount of p-Axl was normalized to this Axl/GAPDH ratio. The values represent the amount of phosphorylated Axl levels in different cell lines.
TABLE 1:
RT-PCR analysis of Axl gene expression in human OSCC tissue.
| Tumor status | Number of up-regulated samples/number of total samples (%) | Fold induction |
|---|---|---|
| Well to moderately differentiated | 13/25 (52) | 1.7–56 |
| Poor to undifferentiated | 7/8 (87.5) | 2–27 |
We also determined the expression levels of Axl in a panel of OSCC cell lines. Using real-time PCR (RT-PCR), we observed that Axl is up-regulated in OSCC cells when compared with normal esophageal epithelial cells (Figure 1B). As mRNA levels do not necessarily correspond with protein levels, we analyzed Axl protein levels in whole-cell extracts by Western blotting. As demonstrated in Figure 1C, most OSCC cell lines express higher levels of Axl protein than the normal EPC-2 cells. Additionally, to determine the activation status of Axl, we evaluated the levels of phosphorylated Axl in these cell lines. As observed in Figure 1C, the levels of phosphorylated Axl are consistently higher for most of the OSCC cell lines when compared with the noncancer cells, EPC-2. These findings strongly support our hypothesis that the Axl signaling pathway may play a critical role in OSCC.
Inhibition of Axl reduces migration, invasion, and proliferation in OSCC cells
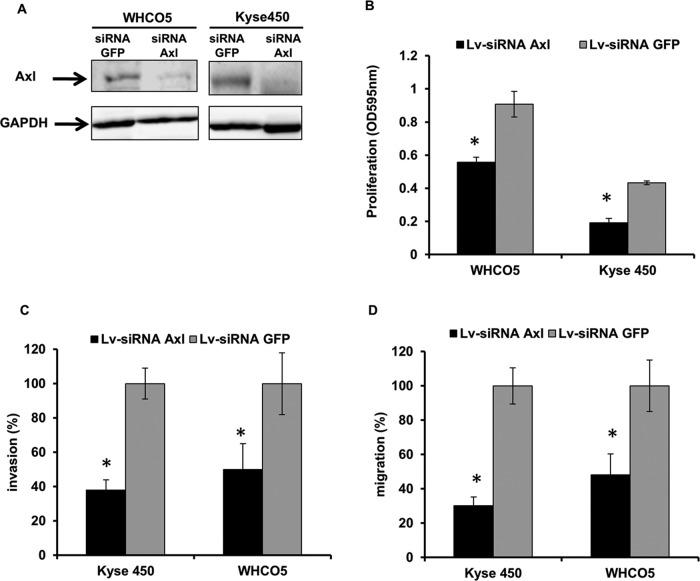
We established a model to further study the biological role of Axl in OSCC. Stable Axl knockdown cell lines were generated after infection of WHCO5 and Kyse450 cell lines with lentivirus expressing small interfering RNA against Axl (LV-siRNA Axl) or siRNA against green fluorescent protein (LV-siRNA GFP; control). Infection with LV-siRNA Axl led to significant repression of Axl expression in WHCO5 (reduction of 85%) and Kyse450 (reduction of 80%) when compared with the LV-siRNA GFP control (Figure 2A).
FIGURE 2:
Knockdown of Axl inhibits proliferation, invasion, and migration in OSCC cell lines. WHCO5 and Kyse450 cells were infected with LV-siRNA Axl and LV-siRNA GFP as control. (A) Western blot analysis of Axl expression in WHCO5 and Kyse450 after infection with lentiviruses. (B) Proliferation assays were performed 48-h postinfection in Kyse450 and WHCO5. Data shown are mean ± SD of triplicate independent experiments. (C) Invasion assays were measured 48-h postinfection. Invading cells were fixed and stained, and three to five random microscopic fields were counted. Values shown are mean ± SD from a representative experiment. (D) Migration assays were measured 48-h postinfection. Migrating cells were fixed and stained, and three to five random microscopic fields were counted. All values shown are mean ± SD from a representative experiment; *, p < 0.05.
The Axl signaling pathway is also related to the induction of malignant properties of cancer cells such as proliferation, migration, and invasion (Gjerdrum et al., 2010; Rankin et al., 2010; Paccez et al., 2013, 2014). To evaluate the role of Axl in cancer proliferation, we tested Kyse450 and WHCO5 cells infected with LV-siRNA Axl and LV-siRNA GFP by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Axl knockdown reduces cell proliferation by 55% in Kyse450 cells and by 43.3% in WHCO5 cells (Figure 2B). One of the main characteristics of metastatic cells is the ability to migrate and invade distant organs. To test the role of Axl in migration and invasion, we performed Transwell and wound-healing assays. As shown in Figure 2C, blockage of Axl reduces invasion of Kyse450 and WHCO5 cells by 62 and 50%, respectively. Additionally, reduction of migration by 70 and 52% is observed in Kyse450 cells and WHCO5 cells (Figure 2D), respectively. Together, these data point to Axl as an essential mediator of metastasis in OSCC.
Axl reduces OSCC tumor growth in vivo
For determining the effect of Axl blockage on tumor formation in vivo, Kyse450 cells infected either with LV-siRNA GFP (control) or LV-siRNA Axl were subcutaneously injected into MF-1 nude mice. Mice were observed on a daily basis for tumor development and, after 65 d, tumor weight and volume were determined. Mice implanted with Kyse450 siSRNA Axl cells developed tumors 60% lower in mass when compared with mice implanted with Kyse450 siRNA GFP control cells (Figure 3). Additionally, we observed lymph node metastasis only in mice implanted with Kyse450 siRNA GFP cells, while no metastases were observed in mice implanted with Kyse450 siRNA Axl (unpublished data), indicating that Axl expression plays an important role for tumor growth in vivo.
FIGURE 3:
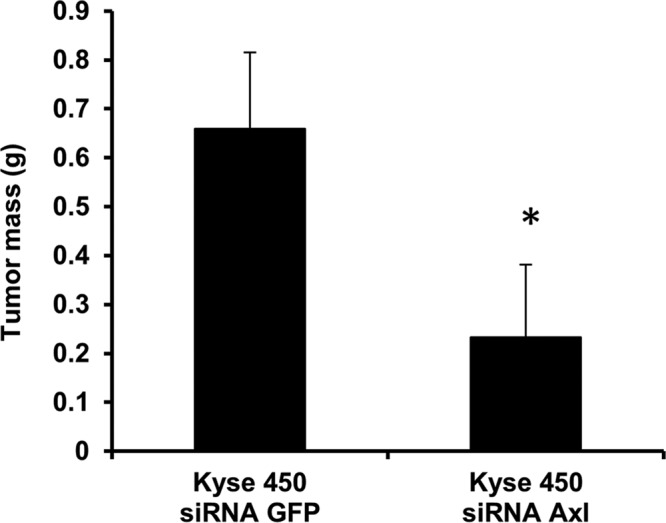
Blockage of Axl inhibits tumor formation in MF-1 nude mice. Kyse450 cells (5 × 106) infected with LV-siRNA Axl or LV-siRNA GFP were implanted subcutaneously into the right flank of MF-1 mice. Tumor weight was measured 45 d after implantation. Values are represented as mean ± SD of five individuals; *, p < 0.0001.
Blockage of Axl expression and activity reduces cell survival pathways
The PI3K signaling pathway has been shown to be a downstream effector of Axl in cancer cells, and its activation was described as correlating with higher levels of Axl expression (Lee et al., 2002; Ruan and Kazlauskas, 2012; Paccez et al., 2013). Activation of Akt by Axl leads to phosphorylation of IKKα and consequent phosphorylation, ubiquitination, and degradation of IκBα, triggering the activation of NF-κB (Gjerdrum et al., 2010; Rankin et al., 2010; Paccez et al., 2013). In fact, PI3K/Akt has been shown to stimulate the NF-κB pathway, enhancing tumor cell invasion in breast and ovarian cancers (Lee et al., 2002; Ruan and Kazlauskas, 2012; Paccez et al., 2013, 2014). The NF-κB pathway has been directly implicated in cancer cell survival and metastasis (Lee et al., 2002; Ruan and Kazlauskas, 2012; Paccez et al., 2013). Constitutive activation of NF-κB is frequently observed in various cancer types and is a critical step in the escape of cancer cells from apoptosis (Karin et al., 2002; Li and Verma, 2002; Zerbini et al., 2003). To evaluate the effects of Axl regulation on NF-κB in OSCC, we analyzed the expression and activation status based on phosphorylation of key proteins involved in the NF-κB pathway.
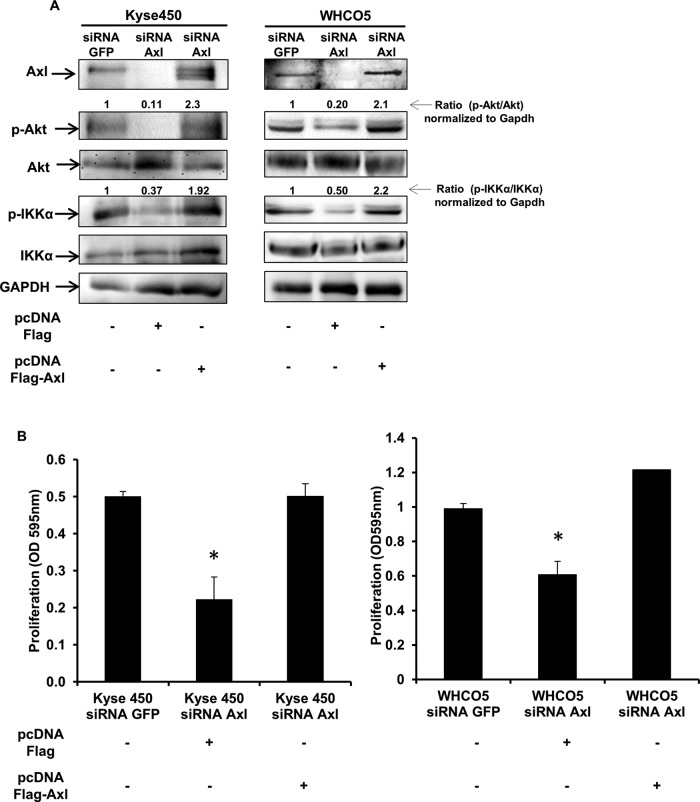
Western blot analysis of whole-cell extracts comparing Kyse450 and WHCO5 cells with Axl knockdown to the GFP knockdown cells revealed that inhibition of Axl leads to reduction of both Akt and IKKα phosphorylation and activity, which should result in inhibition of NF-κB (Figure 4A). To corroborate our findings and further evaluate the functional consequences of Axl regulation, we assessed the effect of Axl overexpression on IKKα and Akt activity and on cell proliferation in Axl knockdown cells. Control cells (Kyse450 siRNA GFP and WHCO5 siRNA GFP) and Axl knockdown cells (Kyse 450 siRNA Axl and WHCO5 siRNA Axl) were transfected with pcDNA Flag control or pcDNA Flag-Axl plasmids, and proliferation and IKKα and Akt activities were evaluated 48-h posttransfection. Our results clearly demonstrate that Axl overexpression in knockdown cells restores the activation of IKKα and Akt signaling pathways (Figure 4A) and proliferation (Figure 4B) back to the level of the control cells (siRNA GFP expressing the vector alone). These data indicate that Axl's critical role in the metastatic potential of OSSC cells may be mediated through the AKT/NF-κB pathway.
FIGURE 4:
Axl-mediated Akt activation is essential for proliferation of OSCC cells. (A) Kyse450 siRNA GFP and WHCO5 siRNA GFP and Axl knockdown cells Kyse 450 siRNA Axl and WHCO5 siRNA Axl were transfected with pcDNA Flag control or pcDNA Flag-Axl plasmids. Total-cell lysates were collected at 48 h posttransfection. The extracts were then analyzed by Western blotting using anti-Axl, anti–phospho-Akt, anti-Akt, anti–phospho-IKKα, anti-IKKα, and anti-GAPDH antibodies after restoration of Axl expression. (B) Reexpression of Axl protein restores proliferation in Kyse450 siRNA Axl and WHCO5 siRNA Axl. Control cells (Kyse450 siRNA GFP and WHCO5 siRNA GFP) and Axl knockdown cells (Kyse 450 siRNA Axl and WHCO5 siRNA Axl) were transfected with pcDNA Flag control or pcDNA Flag-Axl plasmids, and proliferation was evaluated 48-h posttransfection. Data shown are mean ± SD of triplicate independent experiments for each condition; *, p < 0.05.
Axl promotes esophageal cancer progression by repressing GSK3β activity through Akt activation
GSK3β is a ubiquitously expressed serine/threonine kinase that is involved in multiple processes, including cell fate determination and oncogenesis (Kim et al., 2007). GSK3β activity is regulated by site-specific phosphorylation of the Ser-9 residue, and deregulated phosphorylation has been linked to pathological conditions such as cancer (Doble and Woodgett, 2003). GSK3β is involved in growth factor–mediated signaling through protein kinase A (PKA) and Akt/PKB (Saito et al., 1994; Cross et al., 1995; Fang et al., 2000). Indeed, Akt phosphorylates the Ser-9 residue in GSK3β, leading to its inactivation (Cross et al., 1995; Fang et al., 2000; Doble and Woodgett, 2003; Iamaroon and Krisanaprakornkit, 2009; Wu et al., 2009).
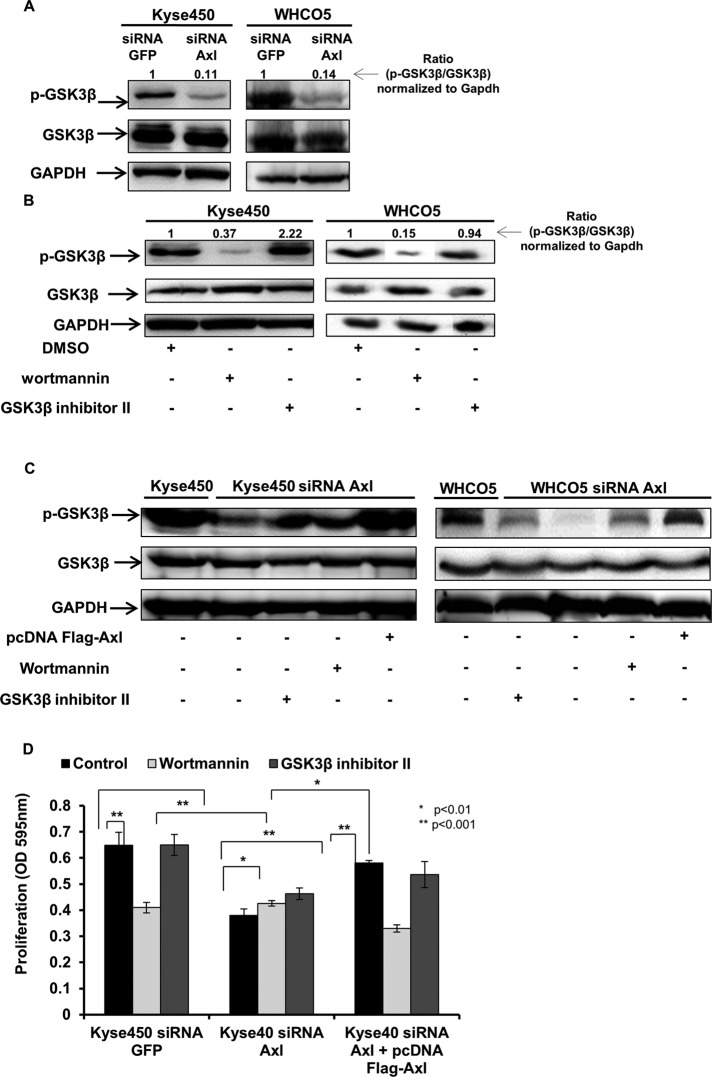
Because the PI3K/Akt pathway has been described to be deregulated in OSCC (Cross et al., 1995; Fang et al., 2000) and, based on our observations, Axl activates the Akt pathway, we investigated the association between Axl activation and GSK3β. First, we measured the expression levels of GSK3β and GSK3β phosphorylation in parental Kyse450 and WHCO5 cells and Kyse450 and WHCO5 Axl knockdown cells. As observed in Figure 5A, knockdown of Axl in both Kyse450 and WHCO5 cells inhibits the GSK3β Ser-9 phosphorylation, indicating that Axl leads to inactivation of GSK3β in OSSC. To gain more insights into the regulation of GSK3β by Axl, we utilized pharmacological inhibitors of Akt and GSK3β. We tested the effect of wortmannin and GSK3β inhibitor II in Kyse450 and WHCO5 cells. Wortmannin is a well-known Akt inhibitor, and the GSK3β inhibitor II is a 2-thio-[1,3,4]-oxadiazole-pyridyl derivative that acts as a potent inhibitor of glycogen synthase kinase-3β. Figure 5B demonstrates that GSK3β is activated after treatment of esophageal cancer cells with wortmannin, because wortmannin reduces its phosphorylation. No significant increase in the phosphorylation of GSK3β was observed in cells treated with GSK3β inhibitor II (Figure 5B). Furthermore, we evaluated the effect of GSK3β inhibitor II and wortmannin in Axl knockdown cells. A slight increase in the phosphorylation levels of GSK3β was seen in both Kyse450 siRNA Axl and WHCO5 siRNA Axl treated with GSK3β inhibitor II when compared with untreated cells, indicating its inactivation (Figure 5C). To establish the role of Axl in GSK3β regulation, we analyzed the activation status of GSK3β in Kyse450 siRNA Axl and WHCO5 siRNA Axl reconstituted with ectopic Axl expression. Restoration of Axl levels in Kyse450 siRNA Axl cells increases the phosphorylation of GSK3β (Figure 5C). Moreover, we analyzed the effect of wortmannin and GSK3β inhibitor II in Kyse450 siRNA Axl transfected with pcDNA Flag-Axl. As shown in Supplemental Figure 1, inactivation of GSK3β is more pronounced in Kyse450 siRNA Axl transfected with pcDNA Flag-Axl vector and treated with GSK3β inhibitor II. Interestingly, we observed reduction of phosphorylation of GSK3β in Kyse450 siRNA Axl transfected with pcDNA Flag-Axl vector after treatment with wortmannin, indicating that Axl expression indeed leads to activation of Akt and, consequently, inactivation of GSK3 β.
FIGURE 5:
Axl expression inhibits activation of GSK3β in OSCC cells. Western blot analysis of the phosphorylated form of GSK3β and total protein expression of GSK3 and GAPDH. (A) Western blot analysis of protein extracts obtained from Kyse450 and Kyse450 siRNA Axl cells (left) and WHCO5 and WHCO5 siRNA Axl (right). (B) Western blot analysis of protein extracts obtained from cells treated with GSK3β inhibitor II (200 nM) for 2 h or with wortmannin (1 mM) for 30 min. Left, a representative experiment performed with Kyse450 cells; right, a representative experiment performed with the WHCO5 cell line. (C) Western blot analysis of protein extracts from Axl knockdown cells treated with GSK3β inhibitor II (200 nM) for 2 h or with wortmannin (1 mM) for 30 min. Left, a representative experiment performed with Kyse450 siRNA Axl cells; right, a representative experiment performed with WHCO5 siRNA Axl cells. (D) Proliferation assay. Kyse450, Kyse450 siRNA Axl, and Kyse450 siRNA Axl cells were transfected with pCDNA Flag-Axl or parental control plasmid and treated with GSK3β inhibitor II (200 nM) for 2 h or with wortmannin (1 mM) for 30 min. Data shown are mean ± SD of triplicate independent experiments for each condition; *, p < 0.01; **, p < 0.001.
Next we evaluated the biological effects of GSK3β inhibition by Axl on proliferation of OSCC cells. Proliferation was measured in Axl knockdown cells (Kyse450 siRNA Axl and WHCO5 siRNA Axl), Kyse450 siRNA GFP, WHCO5 siRNA GFP, and Axl knockdown cells transfected with pcDNA Flag-Axl. Treatment of cells with wortmannin or GSK3β inhibitor II reveals that wortmannin reduces proliferation of OSCC cells and knockdown cells transfected with pcDNA Flag-Axl vector, but it does not significantly affect proliferation of cells lacking Axl (Figure 5D). On the other hand, the GSK3β inhibitor II induces proliferation in Axl knockdown cells but not in parental cells (Kyse450) or Axl knockdown cells transfected with pcDNA Flag-Axl. These data clearly indicate that Axl inactivates GSK3β via Akt activation, leading to OSCC cell proliferation.
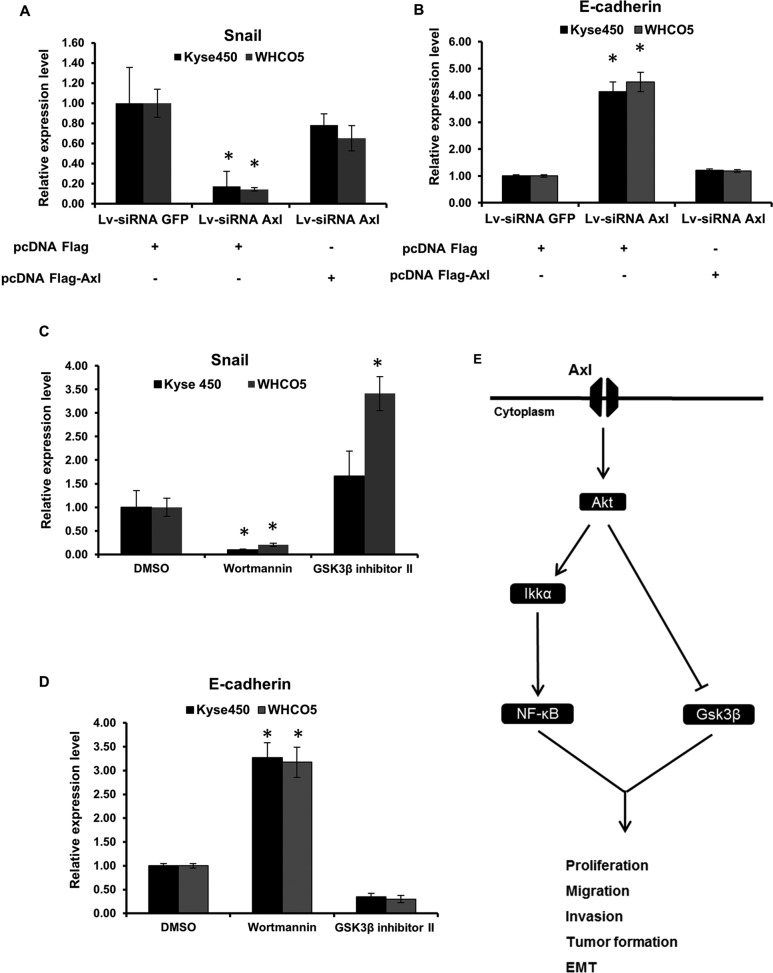
Blockage of Axl expression results in loss of the mesenchymal marker Snail and induction of the epithelial marker E-cadherin
GSK3β is known to repress the transcription of Snail, a repressor of E-cadherin and an inducer of EMT (Bachelder et al., 2005). EMT is a critical event in the progression toward cancer metastasis. Axl has been implicated in EMT in cancers such as breast and lung (Gjerdrum et al., 2010; Vuoriluoto et al., 2011; Byers et al., 2013; Vazquez-Martin et al., 2013; Asiedu et al., 2014). To test whether Axl is linked to EMT in OSCC, we first analyzed the expression of Snail in Kyse450, Kyse 450 siRNA Axl, WHCO5, and WHCO5 siRNA Axl cells. RT-PCR reveals that levels of Snail mRNA are drastically reduced in siRNA Axl when compared with control cells (Figure 6A). Levels of Snail mRNA were at least partially restored when Axl knockdown cells were transfected with pcDNA Flag-Axl (Figure 6A), indicating that Axl expression is important for Snail transcription. In addition, analysis of E-cadherin mRNA levels, an epithelial marker, demonstrates that cells lacking Axl and, consequently, expressing lower levels of Snail, have higher levels of E-cadherin mRNA when compared with control, and transfection of Axl knockdown cells with pcDNA Flag-Axl reduces E-cadherin expression (Figure 6B).
FIGURE 6:
Analysis of EMT markers regulated by Axl in OSCC. RT-PCR analysis of Snail (A) and E-cadherin (B) in OSCC. Analysis was performed in Kyse450 and WHCO5 cells and their knockdown pairs (Kyse450 siRNA Axl and WHCO5 siRNA Axl) transfected with pcDNA Flag-Axl or pcDNA plasmids as control. (C and D) Analysis of EMT markers regulated by Axl in OSCC treated with wortmannin and GSK3β inhibitor II. Kyse450 and WHCO5 cells were treated with GSK3β inhibitor II (200 nM) for 2 h or with wortmannin (1 mM) for 30 min. RT-PCR analysis of E-cadherin (C) and Snail (D) in Kyse450, Kyse450 siRNA Axl, and Kyse450 siRNA Axl transfected with pcDNA Flag-Axl. Total RNA was collected from cells, and normalization of each sample was carried out by measuring the amount of human GAPDH cDNA. Data shown are mean ± SD of triplicate independent experiments for each condition; *, p < 0.01. (E) Schematic representation of Axl pathway in OSCC.
We also evaluated the expression of mRNA levels of EMT markers in OSCC cells treated with wortmannin and GSK3β inhibitor II. Kyse450 and WHCO5 cells treated with wortmannin express lower levels of Snail mRNA (Figure 6C) and higher levels of E-cadherin mRNA (Figure 6D) when compared with control. In contrast, treatment with GSK3β inhibitor II increases Snail mRNA (Figure 6C) and reduces E-cadherin mRNA levels, respectively (Figure 6D). Our data clearly indicate GSK3β may play an important role during EMT and is a molecular target of the Axl pathway in OSCC via Akt activation.
DISCUSSION
Esophageal cancer is one of the 10 most common and fatal solid tumors diagnosed worldwide (Ferlay et al., 2010). The two types of esophageal cancer, OSCC and adenocarcinoma, vary in incidence in both geographic region and ethnic background. Owing to the relevance of squamous cell carcinoma in the developing world, an effort has been made to reveal its biology in order to understand how this type of cancer develops and also to identify new therapeutic targets. In this context, we demonstrate for the first time the role of the receptor tyrosine kinase Axl in OSCC. Our data indicate that Axl expression is up-regulated in OSCC tumors when compared with normal tissue. In addition, our findings point out that Axl expression is higher in advanced stages of the disease. In fact, Axl has been linked to poor prognosis and resistance to cancer therapy in several tumor types (Zhang et al., 2012). Interestingly, Axl has been shown to play an important role for adenocarcinomatous esophageal cancer (Hector et al., 2010; Hong et al., 2013); however, its role in the malignancy of OSCC has remained unknown up to now. In this report, we provide a detailed analysis of the role of Axl in OSCC. We demonstrate that Axl is up-regulated in both OSCC human cell lines and patient tissue samples.
Metastatic cancer cells are categorized by gain of motility, which allows attachment and growth at distant organs. Axl is associated with cancer migration and invasion, and Axl has been reported as an essential regulator for EMT in breast and prostate cancer metastasis (Gjerdrum et al., 2010; Asiedu et al., 2014). Our findings demonstrate that blockage of Axl expression significantly reduces migration, invasion, and proliferation of OSCC cells and inhibits tumor growth. Our data clearly indicate Axl is a crucial regulator of OSCC progression and metastasis and is a potential therapeutic target, since its inhibition leads to the reduction of metastatic properties such as proliferation, migration, and invasion.
The dissection of the Axl biological pathway in OSCC may provide more information about the mechanism involved in the metastatic potential of Axl. In this context, several reports have linked Axl to the PI3K/Akt and NF-κB pathways (Lee et al., 2002; Ruan and Kazlauskas, 2012; Paccez et al., 2013). The NF-κB pathway has been directly implicated in cancer cell survival and metastasis (Zerbini et al., 2003, 2004) and is known to be related to proliferation of cancer cells and to survival and resistance to therapy. In fibroblasts and endothelial cells, Axl phosphorylation activates the NF-κB transcription factor (Goruppi et al., 1997; Hafizi and Dahlbäck, 2006b; Ruan and Kazlauskas, 2012; Ammoun et al., 2013), and constitutive activation of NF-κB is observed in various cancer types, including OSCC (Li et al., 2009). Furthermore, inhibition of NF-κB induces apoptosis and blocks tumor growth (Zerbini et al., 2004).
Here we demonstrate that knockdown of Axl expression leads to the inactivation of NF-κB by inhibition of Akt and IKKα activity. IKKα is responsible for the abrogation of IκBα phosphorylation leading to IκBα escaping the ubiquitination process and, subsequently, protein degradation, impeding NF-κB translocation into the nucleus. In fact, the PI3K/Akt/NF-κB pathway is extremely important for OC progression, especially in OSCC (Li et al., 2007, 2009). Activation of Axl has been associated with poor prognosis in cancer patients (Gustafsson et al., 2009; Gjerdrum et al., 2010; Hector et al., 2010; Holland et al., 2010). In addition, the PI3K/AKT pathway plays an important role in promoting OSCC survival and proliferation (Li et al., 2007). NF-κB is activated in both primary tumor samples and OSCC lines, and its pharmacological inhibition leads to decreases in migration, invasion, and tumor formation (Li et al., 2007). In this context, we provide strong evidence of Axl regulation of the PI3K/Akt/NF-κB pathway in OSCC with impact on proliferation, migration, and invasion of OSCC cells. In addition, Axl has been linked to induction of EMT (Gjerdrum et al., 2010; Vuoriluoto et al., 2011; Byers et al., 2013; Asiedu et al., 2014). The EMT is a complex molecular program in which nonmotile epithelial cells convert into migratory mesenchymal cells with enhanced cell survival attributes (Thiery, 2002). Therefore our data provide further evidence that Axl regulates EMT factors via inhibition of the GSK3β pathway.
GSK3β is involved in signaling by different growth factors (Saito et al., 1994; Cross et al., 1995). GSK3β-mediated signaling in OSCC likely involves the activation of upstream pathways such as the PKA and Akt/PKB pathways (Cross et al., 1995; Fang et al., 2000). These kinases induce rapid inactivation of GSK3β activity by phosphorylation of Ser-9 (Doble and Woodgett, 2003). In fact, deregulation of these pathways has been reported in OSCC (Iamaroon and Krisanaprakornkit, 2009; Wu et al., 2009). We demonstrate now that inhibition of Axl expression has a pivotal effect on GSK3β activation. OSCC cells expressing Axl present enhanced Akt activation that leads to inactivation of GSK3β activity. In this regard, our observation that wortmannin leads to inhibition of proliferation in Kyse450 and WHCO5 cells but not in Kyse450 siRNA Axl or WHCO5 siRNA Axl is in line with the literature that describes Axl as an activator of the Akt pathway. Our findings were corroborated in Axl knockdown cells transfected with pcDNA Flag-Axl. Our analysis of proliferation using the GSK3β inhibitor II also helped to decipher the mechanistic interactions between the Axl and GSK3 pathways. In OSCC cells, expression of Axl activates Akt and leads to GSK3β inactivation via phosphorylation of its Ser-9. In this scenario, the use of GSK3β inhibitor II does not affect OSCC cell proliferation. However, in Axl knockdown cells, Akt activity is inhibited, leading to GSK3β activation and significant reduction of OSCC cell proliferation. Accordingly, the treatment of Axl knockdown cells with an inhibitor of GSK3β leads to increased cell proliferation.
We also demonstrate that Axl inhibition leads to induction of GSK3β and reduction of the expression of Snail, an important marker for EMT in cancer. Our observations are in line with previous reports describing that inhibition of GSK3β results in the up-regulation of Snail and down-regulation of E-cadherin (Bachelder et al., 2005). These observations strongly support the notion that Axl may represent an underexplored therapeutic target for OSCC and may induce EMT in OSCC via GSK3β inactivation. In Figure 6E, we summarize our findings in a schematic representation of Axl regulation of the Akt/NF-κB and Akt/GSK3β pathways in OSCC.
Inhibition of Axl has been explored as a new therapeutic modality. The development of specific inhibitors, as well as monoclonal antibodies, demonstrated effectiveness in the treatment of cancers such as breast and pancreatic cancer (Gjerdrum et al., 2010; Song et al., 2011). In this regard, our results corroborate these observations, as we validate Axl as an essential regulator of OSSC malignancy.
Our results establish Axl as a critical mediator of OSCC tumorigenesis via activation of the Akt/NF-κB and Akt/GSK3β signaling pathways. Moreover, we propose Axl as a new therapeutic entry point for this disease.
MATERIALS AND METHODS
Tissue samples
Histopathologically confirmed OSCC biopsies, together with corresponding adjacent normal tissue samples, were collected at the Groote Schuur Hospital, Cape Town, South Africa, between 2008 and 2011. Informed consent was obtained from all participants, and ethical approval for this study was obtained from the Ethics Committee of the University of Cape Town. Samples were stored in RNAlater solution (Qiagen, Valencia, CA ) at −80°C until nucleic acid extraction was performed.
Cell lines and culture
Six OSCC cell lines, WHCO1, WHCO3, WHCO5 (Veale and Thornley, 1984), Kyse30, Kyse150, and Kyse450 (obtained commercially from Deutsche Sammlung von Mikroorganismen und Zellkulturen), originally established from surgical specimens of primary OSCCs, were grown in DMEM (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 50 U of penicillin/ml, and 50 mg streptomycin/ml (Life Technologies, Carlsbad, CA). Primary normal esophageal epithelial cells (EPC2) established from normal human esophagus (Andl et al., 2003) were grown in keratinocyte-SFM medium (Life Technologies, Rockville, MD) containing bovine pituitary extract (40 mg/ml) (Life Technologies, Rockville, MD). All cells were maintained in a 5% CO2 humidified incubator at 37ºC. Cell lines were routinely frozen within two passages after receipt from the cell bank and used within less than 6 mo after resuscitation.
RT-PCR
Total RNA was harvested using QIAshredder (Qiagen) and the RNeasy mini-kit (Qiagen) from tissue samples or cells as recommended by the manufacturer. Primers used for real-time PCR, and conditions for RT-PCR were performed as previously described (Gu et al., 2007; Paccez et al., 2013).
siRNA lentiviral vectors
siRNA against Axl was obtained from Mission short hairpin RNA Lentiviral Transduction Particles from Sigma-Aldrich (St. Louis, MO). A control lentivirus encoding siRNA against GFP was cloned using the Advantage 2 PCR kit (Clontech, Mountain View, CA) as previously described (Zerbini et al., 2004; Paccez et al., 2013).
Plasmids and transfection assays
Transfection was performed by Lipofectamine plus reagent (Invitrogen) using pcDNA Flag and pCDNA Flag-Axl plasmids as previously described (Zerbini et al., 2004, 2006; Paccez et al., 2013).
Western blot analysis
Western blot analyses were performed as previously described (Zerbini et al., 2004, 2006; Paccez et al., 2013). The following primary antibodies were used: Axl, p-Akt, Akt, IKKα, p-IKKα, GSK3, p-GSK3, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Cell Signaling Technology, Beverly, MA). Antibodies against p-Axl were obtained from R&D Systems (Minneapolis, MN).
Quantification of protein
Ratios of protein in different cells, calculated using the VisionWorks LS Image Acquisition and Analysis Software (UVP), were the intensity of the band correlates to the amount of protein. The total amount of protein present in each sample was normalized to the amount of GAPDH. As a matter of comparison, the amount observed in control was set up as 100%.
Reagents
GSK3β inhibitor II (2-thio(3-iodobenzyl)-5-(1-pyridyl)-[1,3,4]-oxadiazole) was obtained from Santa Cruz, and wortmannin was obtained from Calbiochem (San Diego, CA). Final concentrations used were 200 nM (GSK3β inhibitor II), 1 mM (wortmannin), and 0.1% dimethyl sulfoxide as control.
Proliferation assay
Proliferation assay was performed using the Cell Proliferation Kit I (MTT; Roche, Basel, Switzerland) according to the manufacturer's protocol.
Invasion and migration assays
Cell migration and invasion assays were performed as previously described (Zerbini et al., 2003) using a modified Transwell chamber migration assay and invasion assay Matrigel-coated membrane (BD Biosciences, Bedford, MA).
Animal experiments
Eight-week-old male MF-1 nude mice, obtained from the University of Cape Town, were bred at the university's animal facility and housed in a pathogen-free environment. The animals were randomly divided into two groups (n = 6 per group).
Kyse450 cells (5 × 106) infected with lentivirus encoding siRNA against GFP (LV-siRNA GFP) and lentivirus encoding siRNA against Axl (LV-siRNA Axl) were used for subcutaneous implantation. Immediately before implantation, Kyse450 siRNA GFP and Kyse450 siRNA Axl cells were trypsinized and resuspended in DMEM with 10% FBS. Cell viability was determined by trypan blue exclusion, and a single cell suspension with 90% viability was used for implantation. Tumor size (volume) was measured on a daily basis after implantation. The experiment was concluded when tumor size in the control animals reached 200 mm. At the end of the experiment (65 d), the animals were killed, tumors were carefully dissected and weighed, and lymph nodes were analyzed to determine metastases. All procedures with animals were reviewed and approved by the Animal Research Ethics Committee of the University of Cape Town.
Statistical analysis
Statistical analysis was performed using Student's t test and the Mann-Whitney test as appropriate.
Supplementary Material
Acknowledgments
This work was supported by the International Centre for Genetic Engineering and Biotechnology (ICGEB). J.D.P. was the recipient of an ICGEB postdoctoral fellowship.
Abbreviations used:
- EMT
epithelial–mesenchymal transition
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GFP
green fluorescent protein
- GSK3β
glycogen synthase kinase 3β
- LV
lentivirus
- NF-κB
nuclear factor-kappaB
- OSCC
esophageal squamous cell carcinoma
- PI3K
phosphatidylinositol 3-OH kinase
- PKA/PKB
protein kinase A/B
- RT-PCR
real-time PCR
- siRNA
small interfering RNA
- TAM
Tyro-3, Axl, and Mer.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-04-0868) on January 7, 2015.
The authors declare no conflict of interest.
REFERENCES
- Ammoun S, Provenzano L, Zhou L, Barczyk M, Evans K, Hilton Da, Hafizi S, Hanemann CO. Axl/Gas6/NFκB signalling in schwannoma pathological proliferation, adhesion and survival. Oncogene. 2013;33:336–346. doi: 10.1038/onc.2012.587. [DOI] [PubMed] [Google Scholar]
- Andl CD, Mizushima T, Nakagawa H, Oyama K, Harada H, Chruma K, Herlyn M, Rustgi AK. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J Biol Chem. 2003;278:1824–1830. doi: 10.1074/jbc.M209148200. [DOI] [PubMed] [Google Scholar]
- Asiedu MK, Beauchamp-Perez FD, Ingle JN, Behrens MD, Radisky DC, Knutson KL. AXL induces epithelial-to-mesenchymal transition and regulates the function of breast cancer stem cells. Oncogene. 2014;33:1316–1324. doi: 10.1038/onc.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelder RE, Yoon S-O, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J Cell Biol. 2005;168:29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, Shen L, Fan Y, Giri U, Tumula PK, et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res. 2013;19:279–290. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SC, Day R, Brooks C, Livings C, Thomson CS, Trudgill NJ. The influence of deprivation and ethnicity on the incidence of esophageal cancer in England. Cancer Causes Control. 2009;20:1459–1467. doi: 10.1007/s10552-009-9372-5. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Dirks W, Rome D, Ringel F, Jäger K, MacLeod Ra, Drexler HG. Expression of the growth arrest-specific gene 6 (GAS6) in leukemia and lymphoma cell lines. Leuk Res. 1999;23:643–651. doi: 10.1016/s0145-2126(99)00075-2. [DOI] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufies M, Jacquel A, Belhacene N, Robert G, Cluzeau T, Luciano F, Cassuto JP, Raynaud S, Auberger P. Mechanisms of AXL overexpression and function in imatinib-resistant chronic myeloid leukemia cells. Oncotarget. 2011;2:874–885. doi: 10.18632/oncotarget.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Yu SX, Lu Y, Bast RC, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci USA. 2000;97:11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Gamliel Z. Incidence, epidemiology, and etiology of esophageal cancer. Chest Surg Clin N Am. 2000;10:441–450. [PubMed] [Google Scholar]
- Gioia R, Leroy C, Drullion C, Lagarde V, Etienne G, Dulucq S, Lippert E, Roche S, Mahon F-X, Pasquet J-M. Quantitative phosphoproteomics revealed interplay between Syk and Lyn in the resistance to nilotinib in chronic myeloid leukemia cells. Blood. 2011;118:2211–2221. doi: 10.1182/blood-2010-10-313692. [DOI] [PubMed] [Google Scholar]
- Gjerdrum C, Tiron C, Høiby T, Stefansson I, Haugen H, Sandal T, Collett K, Li S, McCormack E, Gjertsen BT, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci USA. 2010;107:1124–1129. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goruppi S, Ruaro E, Varnum B, Schneider C. Requirement of phosphatidylinositol 3-kinase-dependent pathway and Src for Gas6-Axl mitogenic and survival activities in NIH 3T3 fibroblasts. Mol Cell Biol. 1997;17:4442–4453. doi: 10.1128/mcb.17.8.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Zerbini LF, Otu HH, Bhasin M, Yang Q, Joseph MG, Grall F, Onatunde T, Correa RG, Libermann TA. Reduced PDEF expression increases invasion and expression of mesenchymal genes in prostate cancer cells. Cancer Res. 2007;67:4219–4226. doi: 10.1158/0008-5472.CAN-06-3689. [DOI] [PubMed] [Google Scholar]
- Gustafsson A, Martuszewska D, Johansson M, Ekman C, Hafizi S, Ljungberg B, Dahlbäck B. Differential expression of Axl and Gas6 in renal cell carcinoma reflecting tumor advancement and survival. Clin Cancer Res. 2009;15:4742–4749. doi: 10.1158/1078-0432.CCR-08-2514. [DOI] [PubMed] [Google Scholar]
- Hafizi S, Dahlbäck B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006a;273:5231–5244. doi: 10.1111/j.1742-4658.2006.05529.x. [DOI] [PubMed] [Google Scholar]
- Hafizi S, Dahlbäck B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006b;17:295–304. doi: 10.1016/j.cytogfr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Hector A, Montgomery EA, Karikari C, Canto M, Dunbar KB, Wang JS, Feldmann G, Hong S, Haffner MC, Meeker AK, et al. The Axl receptor tyrosine kinase is an adverse prognostic factor and a therapeutic target in esophageal adenocarcinoma. Cancer Biol Ther. 2010;10:1009–1018. doi: 10.4161/cbt.10.10.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W, Duan M, Torneros A, Yu J, Heckrodt TJ, et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010;70:1544–1554. doi: 10.1158/0008-5472.CAN-09-2997. [DOI] [PubMed] [Google Scholar]
- Hong J, Belkhiri A. AXL mediates TRAIL resistance in esophageal adenocarcinoma. Neoplasia. 2013;15:296–304. doi: 10.1593/neo.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Peng D, Chen Z, Sehdev V, Belkhiri A. ABL regulation by AXL promotes cisplatin resistance in esophageal cancer. Cancer Res. 2013;73:331–340. doi: 10.1158/0008-5472.CAN-12-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Hurlburt W, Greer A, Reeves Ka, Hillerman S, Chang H, Fargnoli J, Graf Finckenstein F, Gottardis MM, Carboni JM. Differential mechanisms of acquired resistance to insulin-like growth factor-i receptor antibody therapy or to a small-molecule inhibitor, BMS-754807, in a human rhabdomyosarcoma model. Cancer Res. 2010;70:7221–7231. doi: 10.1158/0008-5472.CAN-10-0391. [DOI] [PubMed] [Google Scholar]
- Iamaroon A, Krisanaprakornkit S. Overexpression and activation of Akt2 protein in oral squamous cell carcinoma. Oral Oncol. 2009;45:e175–e179. doi: 10.1016/j.oraloncology.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Janssen JW, Schulz AS, Steenvoorden AC, Schmidberger M, Strehl S, Ambros PF, Bartram CR. A novel putative tyrosine kinase receptor with oncogenic potential. Oncogene. 1991;6:2113–2120. [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li Z-W. NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Kim M, Datta A, Brakeman P, Yu W, Mostov KE. Polarity proteins PAR6 and aPKC regulate cell death through GSK-3β in 3D epithelial morphogenesis. J Cell Sci. 2007;120:2309–2317. doi: 10.1242/jcs.007443. [DOI] [PubMed] [Google Scholar]
- Koorstra JB, Karikari CA, Feldmann G, Bisht S, Rojas PL, Offerhaus GJ, Alvarez H, Maitra A. The Axl receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target. Cancer Biol Ther. 2009;8:618–626. doi: 10.4161/cbt.8.7.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WP, Wen Y, Varnum B, Hung M-C. Akt is required for Axl-Gas6 signaling to protect cells from E1A-mediated apoptosis. Oncogene. 2002;21:329–336. doi: 10.1038/sj.onc.1205066. [DOI] [PubMed] [Google Scholar]
- Li B, Cheung PY, Wang X, Tsao SW, Ling MT, Wong YC, Cheung ALM. Id-1 activation of PI3K/Akt/NFκB signaling pathway and its significance in promoting survival of esophageal cancer cells. Carcinogenesis. 2007;28:2313–2320. doi: 10.1093/carcin/bgm152. [DOI] [PubMed] [Google Scholar]
- Li B, Li YY, Tsao SW, Cheung ALM. Targeting NF-κB signaling pathway suppresses tumor growth, angiogenesis, and metastasis of human esophageal cancer. Mol Cancer Ther. 2009;8:2635–2644. doi: 10.1158/1535-7163.MCT-09-0162. [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Linger RMA, Cohen RA, Cummings CT, Sather S, Migdall-Wilson J, Middleton DHG, Lu X, Barón AE, Franklin WA, Merrick DT, et al. Mer or Axl receptor tyrosine kinase inhibition promotes apoptosis, blocks growth and enhances chemosensitivity of human non-small cell lung cancer. Oncogene. 2012:1–12. doi: 10.1038/onc.2012.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R, Halsey W, Sathe GM, Martin A-M, Gilmer TM. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res. 2009;69:6871–6878. doi: 10.1158/0008-5472.CAN-08-4490. [DOI] [PubMed] [Google Scholar]
- Manfioletti G, Brancolini C, Avanzi G, Schneider C, Cascade C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol Cell Biol. 1993;13:4976–4985. doi: 10.1128/mcb.13.8.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark MR, Chen J, Hammonds RG, Sadick M, Godowsk PJ. Characterization of Gas6, a member of the superfamily of G domain-containing proteins, as a ligand for Rse and Axl. J Biol Chem. 1996;271:9785–9789. doi: 10.1074/jbc.271.16.9785. [DOI] [PubMed] [Google Scholar]
- McCloskey P, Fridell YW, Attar E, Villa J, Jin Y, Varnum B, Liu ET. GAS6 mediates adhesion of cells expressing the receptor tyrosine kinase Axl. J Biol Chem. 1997;272:23285–23291. doi: 10.1074/jbc.272.37.23285. [DOI] [PubMed] [Google Scholar]
- McCloskey P, Pierce J, Koski RA, Varnum B, Liu ET. Activation of the Axl receptor tyrosine kinase induces mitogenesis and transformation in 32D cells. Cell Growth Differ. 1994;5:1105–1117. [PubMed] [Google Scholar]
- Neubauer A, Burchert A, Maiwald C, Gruss HJ, Serke S, Huhn D, Wittig B, Liu E. Recent progress on the role of Axl, a receptor tyrosine kinase, in malignant transformation of myeloid leukemias. Leuk Lymphoma. 1997;25:91–96. doi: 10.3109/10428199709042499. [DOI] [PubMed] [Google Scholar]
- Neubauer A, Fiebeler A, Graham DK, O'Bryan JP, Schmidt CA, Barckow P, Serke S, Siegert W, Snodgrass HR, Huhn D. Expression of axl, a transforming receptor tyrosine kinase, in normal and malignant hematopoiesis. Blood. 1994;84:1931–1941. [PubMed] [Google Scholar]
- Neubauer A, O'Bryan JP, Fiebeler A, Schmidt C, Huhn D, Liu ET. Axl, a novel receptor tyrosine kinase isolated from chronic myelogenous leukemia. Semin Hematol. 1993;30:34. [PubMed] [Google Scholar]
- O'Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R, Le Beau MM, Earp HS, Liu ET. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paccez JD, Vasques GJ, Correa RG, Vasconcellos JF, Duncan K, Gu X, Bhasin M, Libermann Ta, Zerbini LF. The receptor tyrosine kinase Axl is an essential regulator of prostate cancer proliferation and tumor growth and represents a new therapeutic target. Oncogene. 2013;32:689–698. doi: 10.1038/onc.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paccez JD, Vogelsang M, Parker MI, Zerbini LF. The receptor tyrosine kinase Axl in cancer: biological functions and therapeutic implications. Int J Cancer. 2014;134:1024–1033. doi: 10.1002/ijc.28246. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Fuh KC, Taylor TE, Krieg AJ, Musser M, Yuan J, Wei K, Kuo CJ, Longacre TA, Giaccia AJ. AXL is an essential factor and therapeutic target for metastatic ovarian cancer. Cancer Res. 2010;70:7570–7579. doi: 10.1158/0008-5472.CAN-10-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan G-X, Kazlauskas A. Axl is essential for VEGF-A-dependent activation of PI3K/Akt. EMBO J. 2012;31:1692–1703. doi: 10.1038/emboj.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Vandenheede JR, Cohen P. The mechanism by which epidermal growth factor inhibits glycogen synthase kinase 3 in A431 cells. Biochem J. 1994;303:27–31. doi: 10.1042/bj3030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh Y-S, Lai C-Y, Kao Y-R, Shiah S-G, Chu Y-W, Lee H-S, Wu C-W. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia. 2005;7:1058–1064. doi: 10.1593/neo.05640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y, Wang J, Zalucha S, Loberg RD, Pienta K, et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12:116–127. doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somdyala NI, Bradshaw D, Gelderblom WC, Parkin DM. Cancer incidence in a rural population of South Africa, 1998–2002. Int J Cancer. 2010;127:2420–2429. doi: 10.1002/ijc.25246. [DOI] [PubMed] [Google Scholar]
- Song X, Wang H, Logsdon CD, Rashid A, Fleming JB, Abbruzzese JL, Gomez HF, Evans DB. Overexpression of receptor tyrosine kinase Axl promotes tumor cell invasion and survival in pancreatic ductal adenocarcinoma. Cancer. 2011;117:734–743. doi: 10.1002/cncr.25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin AI, Cufí S, Oliveras-Ferraros C, Torres-Garcia VZ, Corominas-Faja B, Cuyàs El, Bonavia R, Visa J, Martin-Castillo B, Barrajón-Catalán E, et al. IGF-1R/epithelial-to-mesenchymal transition (EMT) crosstalk suppresses the erlotinib-sensitizing effect of EGFR exon 19 deletion mutations. Sci Rep. 2013;3:2560. doi: 10.1038/srep02560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veale RB, Thornley AL. Atypical cytokeratins synthesized by human oesophageal carcinoma cells in culture. S Afr J Sci. 1984;80:260–267. [Google Scholar]
- Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi J-P, Nevo J, Gjerdrum C, Tiron C, Lorens JB, Ivaska J. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2011;30:1436–1448. doi: 10.1038/onc.2010.509. [DOI] [PubMed] [Google Scholar]
- Wimmel A, Glitz D, Kraus A, Roeder J, Schuermann M. Axl receptor tyrosine kinase expression in human lung cancer cell lines correlates with cellular adhesion. Eur J Cancer. 2001;37:2264–2274. doi: 10.1016/s0959-8049(01)00271-4. [DOI] [PubMed] [Google Scholar]
- Wu H-T, Ko S-Y, Fong JH-J, Chang K-W, Liu T-Y, Kao S-Y. Expression of phosphorylated Akt in oral carcinogenesis and its induction by nicotine and alkaline stimulation. J Oral Pathol Med. 2009;38:206–213. doi: 10.1111/j.1600-0714.2008.00659.x. [DOI] [PubMed] [Google Scholar]
- Zerbini LF, Wang Y, Czibere A, Correa RG, Cho J, Ijiri K, Wei W, Joseph M, Gu X, Grall F, et al. NF-κB-mediated repression of growth arrest- and DNA-damage-inducible proteins 45α and γ is essential for cancer cell survival. Proc Natl Acad Sci USA. 2004;101:13618–13623. doi: 10.1073/pnas.0402069101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbini LF, Czibere A, Wang Y, Correa RG, Otu H, Joseph M, Takayasu Y, Silver M, Gu X, Ruchusatsawat K, et al. A novel pathway involving melanoma differentiation associated gene-7/interleukin-24 mediates nonsteroidal anti-inflammatory drug-induced apoptosis and growth arrest of cancer cells. Cancer Res. 2006;66:11922–11931. doi: 10.1158/0008-5472.CAN-06-2068. [DOI] [PubMed] [Google Scholar]
- Zerbini LF, Wang Y, Cho J-YY, Libermann TA. Constitutive activation of nuclear factor κB p50/p65 and Fra-1 and JunD is essential for deregulated interleukin 6 expression in prostate cancer. Cancer Res. 2003;63:2206–2215. [PubMed] [Google Scholar]
- Zhang Y-X, Knyazev PG, Cheburkin YV, Sharma K, Knyazev YP, Orfi L, Szabadkai I, Daub H, Kéri G, Ullrich A. AXL is a potential target for therapeutic intervention in breast cancer progression. Cancer Res. 2008;68:1905–1915. doi: 10.1158/0008-5472.CAN-07-2661. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, Abdel-Rahman M, Wang X, Levine AD, Rho JK, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44:852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.