Summary
The signaling specificity conveyed by distinct combination of Notch receptors/ligands has remained elusive. In this issue of Cancer Discovery, through the development of ligand-specific Notch inhibitors, Kangsamaksin et al. uncovered unique signaling outcomes downstream of DLL- and JAG- receptor activation and demonstrated their effects in the suppression of tumor angiogenesis.
The vasculature of tumors arises from two processes: (1) co-option of existent vessels present within the tissue that harbors the tumor and (2) growth of new vessels, also referred to as angiogenesis. As an important hallmark of cancer, angiogenesis, has been the successful target of therapeutic approaches aiming at depriving tumor cells from nutrients and oxygen. During angiogenesis, vascular sprouts are initiated by the departure of endothelial cells (tip cells) from the vessel core (stalk cells). Tip endothelial cells are morphologically, molecularly and functionally distinct from stalk cells and their emergence is essential to the formation of a new capillary sprout. Tip cells are specified by an intricate crosstalk between the VEGF and the Notch signaling pathways, both targets of past and current anti-tumor therapies. In this issue of Cancer Discovery, Kangsamaksin and colleagues propose a novel therapeutic approach inhibiting Notch signaling through the use of ligand-selective decoy peptides (1).
The Notch signaling pathway is highly conserved across evolution and contributes to the development of multiple tissues and organs, including the vasculature. Mammals express four NOTCH receptors (NOTCH1 to 4) and five ligands (Delta-like: DLL1, DLL2, DLL3 and JAGGED: JAG1, JAG2). Canonical activation of the pathway requires binding of a transmembrane ligand from the signal-sending cell to a Notch receptor expressed on an adjacent signal-receiving cell. Formation of the ligand-receptor complex enables a series of three sequential proteolytic cleavages that ultimately generate the functionally active form of Notch, also known as Notch Intracellular Domain (NICD). NICD is subsequently translocated to the nucleus where it interacts with a complex composed of RBP-Jk/CSL and Mastermind-like proteins (MAML) to induce the transcription of target genes.
Activation of the Notch pathway has been shown to regulate multiple genes in a cell and context-dependent manner (2). Its critical role in development and homeostasis is highlighted by the broad number of anomalies and disorders that arise once the pathway goes awry. These disorders include vascular anomalies (CADASIL), cardiac malformations (Alagille Syndrome), and liver dysfunctions (Alagille Syndrome). In addition, deregulation of Notch signaling has been described in a variety of cancers, portraying functions as both oncogene or tumor suppressor (3). Thus, it is not surprising that Notch frequently emerges as a potential target for cancer therapy, as indicated by several past and currently ongoing clinical trials (4).
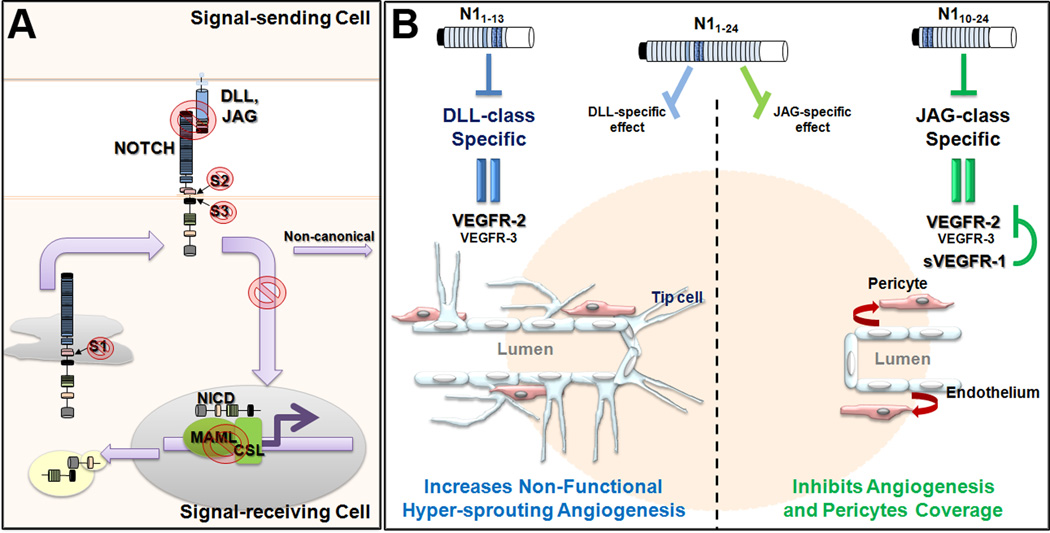
Our current understanding of the molecular events in canonical Notch signaling offers multiple avenues for inhibition of the pathway (Figure 1A). A frequent approach targets the γ-secretase complex, via the use of Gamma-Secretase Inhibitors (GSI). Blockade of γ-secretase prevents the release of Notch from the plasma membrane and subsequent generation of NICD, thus inhibiting Notch signaling. While GSI have shown great promise in some pathologies, their chronic use is often associated with a number of severe side effects including gastrointestinal toxicity. Nevertheless, GSI continue to be explored in clinical trials. The current focus is to optimize regimen doses, and reduce OFF-target effects though distinct formulations. Alternative therapies have explored the use of antibodies to block Notch ligand/receptor interactions. As observed with GSI, concurrent administration of antibodies directed against several Notch receptors has also led to gastrointestinal toxicity. This effect was reduced by targeting only one receptor at a time. However, a frequent problem associated with the use of antibodies against specific Notch receptors relates to the fact that it is not always clear what receptor is dominant. Consequently the use of ligand-directed antibodies has emerged as an attractive choice, as they could potentially block multiple receptors. In this regard, antibodies directed against DLL4 have been the most commonly used in clinical trials to disrupt tumor angiogenesis. However long term blockade of DLL4 in animal models has shown to promote pathological activation of endothelial cells with subsequent vascular neoplasm formation (5). Thus, given its impact in the vasculature, it is paramount to fully explore potential side-effects of Notch therapy.
Figure 1.
A- The knowledge acquired on canonical Notch signaling offers several targets to modulate the pathway. The generation of Notch Intracellular Domain (NICD) could be restricted by targeting the processing of Notch after cleavage by Furin (S1), metalloproteases (S2) and/or γ-secretase complex (S3). Modulation of Notch signaling can also be achieved by affecting the interaction of with the transcriptional complex (MAML/CSL). These approaches have caveats and OFF-targets effects. Disruption of Notch signaling can be accomplished by perturbation of Ligand/Receptor interactions. B- Blockade of Notch signaling by ligand-specific Notch decoy peptides. N1–13 specifically inhibits DLL binding to Notch leading to hyper-sprouting angiogenesis secondary to VEGFR-2 overexpression. In contrast, N10–24 binds and blocks JAG-mediated Notch signaling resulting in overexpression of VEGFR-2 together with soluble VEGFR-1 (sVEGFR1) that competes with VEGFR-2. As a result, endothelial proliferation and angiogenesis are blocked. In addition, specific blockade of JAG-mediated Notch signaling affects pericyte coverage and subsequent vascular integrity. Finally, N1–24 a pan-Notch inhibitor, have dominant ligand-effect depending on context. The use of either of the decoy Notch peptides blocks functional angiogenesis and decreases tumor growth.
Additional challenges of targeting the Notch pathway relate to the potential specific roles of multiple receptors and ligands. This highlights an unexplored level of complexity in signaling outcomes that might underline particular ligand-receptor pairs. While it might be intuitive that alternative signaling or transcriptional regulation would occur when different ligands-receptors are involved, a complete picture has been more difficult to attain. In relation to the vasculature, recent studies of global or conditional knock-out for Notch receptors or ligands revealed non redundant phenotypes. For example, while deletion of Dll4 leads to angiogenic hypersprouting in the retina, endothelial specific loss of Jag1 impairs retinal angiogenesis (6). This body of knowledge has been expanded in this issue of Cancer Discovery. Here, Kangsamaksin and colleagues elegantly showed that the Notch ligands DLL-type and JAG-type regulate physiological and tumor angiogenesis through distinct mechanisms (1). By developing ligand-specific inhibitors, the authors uncovered that blocking distinct ligand subtypes reduces tumor angiogenesis through alternative modes of vascular regulation. Their approach took advantage of information from ligand-receptor binding to generate ligand-specific decoy peptides, the outcome proved extremely fruitful with strong potential for translation.
The human NOTCH1 extracellular domain is composed of 36 EGF-like repeats that bind to the DSL domain of Notch ligands. EGF-like 11–12 are required for the interaction of Notch with all ligands (7). Yamamoto and colleagues have shown the specificity of Notch2 EGF repeat 8 for JAG1 but not DLL1 (8). In addition, the Notch1 EGF repeats 6 to 15 were shown to have more affinity for the DLL-type ligands (9). In the present study the authors developed soluble peptides composed of different Notch1 EGF-like repeats fused to IgG γ heavy chain (1). They uncovered unique domains of Notch1 that preferentially and specifically bind to and functionally block either DLL or JAG-type ligands (Figure 1B).
Decoy N11–13 preferentially blocked DLL-type ligands peptides whereas N110–24 was specific to JAG-type. In addition, a decoy N11–24 was able to inhibit both. As expected from previous studies, the use of DLL-type inhibitor N11–13 induced angiogenic hypersprouting in vitro, in the retina and in tumor models; the resulting non-functional vasculature being associated with a decrease in tumor growth. In contrast, when blocking JAG-induced Notch signaling via the N110–24 decoy, the authors were able to suppress vascular sprouting in vitro and in vivo. In tumor models, N110–24 also reduced vessels perfusion, pericyte coverage and tumor growth. N11–24 administration resulted in differential effects depending of the experimental model. Specifically, N11–24 exhibited a DLL-type dominant effect in retinal vascular sprouting; while this decoy presented a JAG-type dominant effect in vitro and in tumor xenografts.
Curiously, while blocking DLL- or JAG- ligands led to the same outcome, i.e. decreased vascular perfusion and suppression of tumor growth the mechanisms of action of each decoy appear to be distinct. As mentioned, Notch/Dll4 functions in angiogenesis to regulate tip cell emergence and to promote the formation of a vascular sprout. Tip cells exhibit high levels of Dll4 that activate Notch in adjacent cells, rendering them as stalk cells. Notch/Dll4 also represses VEGFR2, while inducing the competitive inhibitor VEGFR1. This effect enhances the stalk cell phenotype stabilizing the core emerging vessel (stalk) and still enabling a tip cell to expand the vascular tree. In accordance with these studies, the DLL-specific decoy promoted an increase in the expression of VEGFR2 and repressed the release of sVEGFR1/sFlt-1 facilitating endothelial cell proliferation. In contrast, the JAG-type decoy yield increase of sVEGFR1/sFlt-1 suppressing VEGF-mediated endothelial proliferation. Furthermore, they also observed defects in pericyte recruitment, an effect that was exclusive to the JAG-type decoy. Thus, through evaluation of these decoy receptors, Kangsamaksin and colleagues clarified that Jag/Notch promotes angiogenesis through the inhibition of sVEGFR1/Flt-1 and enhancement of mural/endothelial cells interaction.
Importantly, the authors also showed that Notch decoys had only moderate gastrointestinal toxicity when compared to pan-Notch inhibitors, such as GSI. Also, N11–13 induced minor sinusoidal dilation, significantly less severe than what was previously observed with chronic inhibition of DLL4. Thus the JAG-decoys display a better tolerance with less side-effects, while being as effective in the suppression of tumor angiogenesis.
While the work presented clearly demonstrates the promising effects of targeting JAG1 within the context of tumor angiogenesis, the approach might hold a broader anti-tumoral effect. Indeed JAG1 has been found to be over-expressed in multiple cancer types and its main role in tumor growth was attributed to its direct effect on tumor cells themselves. Jag1 has also been reported to promote “stemness” in cancer cells, protect against apoptosis and increase epithelial to mesenchymal transition through activation of Notch receptor presented at the surface of the tumor cells (10). Thus targeting Notch signaling using a Jag-specific approach might offer double benefits affecting both the vascular microenvironment and the tumor cell.
Current information in the literature indicates a low rate of mutations in Notch ligands in cancer, indicating that targeting the ligands might be a promising approach with low development of tumor-resistance. However, such treatment would not be effective against hyperactivation of Notch receptors that bypass ligand/receptor interactions, as observed in T-ALL. Nonetheless, given the variety of diseases in which Notch deregulation emerges as a relevant player, the development of alternative and specific approaches to modulate the pathway are of extreme value.
Acknowledgments
Grant Support: The work is supported by a grant by the National Institutes of Health (R01 HL114086) to MLIA.
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Kangsamaksin T, Murtomaki A, Kofler NM, Cuervo H, Chaudhri RA, Tattersall IW, et al. Notch decoys that selectively block dll/notch or jagged/notch disrupt angiogenesis by unique mechanisms to inhibit tumor growth. Cancer discovery. 2014 doi: 10.1158/2159-8290.CD-14-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson ER, Sandberg R, Lendahl U. Notch signaling: Simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 3.Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: A little bit of everything but not all the time. Nature reviews. Cancer. 2011;11:338–351. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- 4.Andersson ER, Lendahl U. Therapeutic modulation of notch signalling--are we there yet? Nature reviews. Drug discovery. 2014;13:357–378. doi: 10.1038/nrd4252. [DOI] [PubMed] [Google Scholar]
- 5.Yan M, Callahan CA, Beyer JC, Allamneni KP, Zhang G, Ridgway JB, Niessen K, Plowman GD. Chronic dll4 blockade induces vascular neoplasms. Nature. 2010;463:E6–E7. doi: 10.1038/nature08751. [DOI] [PubMed] [Google Scholar]
- 6.Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, et al. The notch ligands dll4 and jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Rebay I, Fleming RJ, Fehon RG, Cherbas L, Cherbas P, Artavanis-Tsakonas S. Specific egf repeats of notch mediate interactions with delta and serrate: Implications for notch as a multifunctional receptor. Cell. 1991;67:687–699. doi: 10.1016/0092-8674(91)90064-6. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto S, Charng WL, Rana NA, Kakuda S, Jaiswal M, Bayat V, et al. A mutation in egf repeat-8 of notch discriminates between serrate/jagged and delta family ligands. Science. 2012;338:1229–1232. doi: 10.1126/science.1228745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrawes MB, Xu X, Liu H, Ficarro SB, Marto JA, Aster JC, et al. Intrinsic selectivity of notch 1 for delta-like 4 over delta-like 1. The Journal of biological chemistry. 2013;288:25477–25489. doi: 10.1074/jbc.M113.454850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li D, Masiero M, Banham AH, Harris AL. The notch ligand jagged1 as a target for anti-tumor therapy. Frontiers in oncology. 2014;4:254. doi: 10.3389/fonc.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]