Abstract
In a previous study tolerance to amphotericin B (AMB) was found among Candida parapsilosis and C. dubliniensis strains by seeding the whole volumes of wells used for MIC determinations, and minimum fungicidal concentrations (MFC) for non-C. albicans Candida strains were demonstrated to be above the levels safely achievable in serum. As an extension of that study, we performed time-kill assays with 26 blood culture isolates (6 C. albicans, 5 C. parapsilosis, 5 C. krusei, 4 C. glabrata, 3 C. lusitaniae, and 3 C. tropicalis isolates), 3 oropharyngeal C. dubliniensis isolates, 3 AMB-susceptible isolates (ATCC 90028, ATCC 22019, ATCC 6254), and 6 AMB-resistant isolates (ATCC 200955, ATCC 200956, ATCC 200950, ATCC 200951, ATCC 200952, ATCC 200953) using RPMI 1640 medium and 0.12 to 32 μg of AMB per ml and determined the numbers of CFU per milliliter at 0, 2, 4, 8, 12, 24, and 48 h. MFCs and time-kill patterns were species specific (MFCs, ≤1 μg/ml for all C. dubliniensis and C. albicans isolates except AMB-resistant strain ATCC 200955; MFCs, 2 to >16 μg/ml for the other isolates). The times required to reach the fungicidal endpoint (99.9% killing) at four times the MIC were 2 h for C. albicans and C. dubliniensis, 16 h for C. glabrata, 24 h for C. parapsilosis and C. lusitaniae, and ≥40 h for C. tropicalis and C. krusei. The killing rate increased as the AMB concentration was increased up to 2 μg/ml. The highest killing rates were achieved for C. albicans, C. dubliniensis, and C. lusitaniae, while viable C. tropicalis, C. krusei, and C. parapsilosis cells were present after 48 h (MICs, ≤2 μg/ml) when AMB was used at 2 μg/ml. Time-kill curves and MFCs can detect viable cells after 48 h when AMB is used at ≥2 μg/ml. The failure of AMB treatment could be due to its poor killing activity against some species at the concentrations reached in patients' serum.
Amphotericin B deoxycholate has been considered the “gold standard” for the treatment of invasive fungal infections since its introduction in the 1950s. Although the toxicity of this drug has somewhat limited its use, clinical experience has demonstrated that its less toxic lipid formulations can be used as suitable alternatives to the parental drug (18). Therefore, it is still important to obtain a clearer understanding of the killing patterns of amphotericin B. In general, the pharmacodynamic characteristics of amphotericin B have mainly been evaluated with Candida albicans, and scarce data are available for other Candida spp. In a prior study (4), by using an inoculum larger than that recommended in NCCLS document M27-A2 (16) and subculturing the total volume in the well used for MIC determination, we have shown that the difference between the fungistatic activities and the fungicidal activities could be species dependent among Candida spp. Tolerance to amphotericin B (by using the minimum fungicidal concentration [MFC] determination procedure described above) has been reported among C. parapsilosis and C. dubliniensis strains (4, 27), as have MFCs for non-C. albicans Candida isolates that are above the safe levels achievable in serum (4). Several investigators (17, 26, 32) have postulated that the MFCs and time-kill curve study results could be more clinically relevant than the MICs of amphotericin B. Because time-kill curves provide a quantitative assessment of fungicidal activity as well as the rapidity of killing over time, they provide more information than MIC or MFC endpoints.
Our previous evaluation of the MICs versus the MFCs of amphotericin B deoxycholate suggested that the same MIC may correspond to different killing activities, depending on the species or the strain tested. The purpose of the present study was (i) to examine the killing patterns (by species and strains) of amphotericin B deoxycholate for seven Candida spp., (ii) to develop a mathematical model for the identification of the time (in hours) required for its fungicidal activity, and (iii) to examine the value of the MFC endpoint as an alternative means of detecting the fungicidal activity of amphotericin B deoxycholate.
MATERIALS AND METHODS
Drugs.
Amphotericin B deoxycholate (Squibb Industria Farmacéutica, S.A. Grupo Bristol-Myers Squibb, Madrid, Spain) was dissolved in dimethyl sulfoxide to obtain a stock solution of 1,600 μg/ml. Further dilutions were made in standard RPMI 1640 medium (RPMI; Sigma Aldrich, Madrid, Spain), as recommended in NCCLS document M27-A2 (16).
Test isolates.
A total of 38 strains were selected for testing. These strains comprised 26 isolates recovered from blood cultures (6 C. albicans, 5 C. parapsilosis, 5 C. krusei, and 4 C. glabrata isolates and 3 strains each of C. lusitaniae and C. tropicalis) and 3 C. dubliniensis isolates recovered from patients with oropharyngeal infections. In addition, three amphotericin B-susceptible isolates (C. albicans ATCC 90028, C. parapsilosis ATCC 22019, and C. krusei ATCC 6254) and 6 amphotericin B-resistant isolates (C. albicans ATCC 200955; C. tropicalis ATCC 200956; and C. lusitaniae ATCC 200950, ATCC 200951, ATCC 200952, and ATCC 200953) were evaluated (25).
MIC and MFC determinations.
MICs and MFCs were determined as described previously (4). Briefly, MICs were evaluated by the NCCLS M27-A2 broth microdilution method by using two final inocula (2.5 × 103 and 2.5 × 104 CFU/ml). MFCs were determined by seeding the entire volumes from all clear MIC wells with the highest inoculum onto Sabouraud dextrose agar (SDA) plates. The MFC was the lowest drug concentration that killed 99.9% (with less than five colonies remaining) of the final inoculum.
Time-kill curve studies.
Before the time-kill curve studies were performed, the antifungal carryover effect was determined by the method described by Klepser et al. (12). Additionally, the aliquots were deposited as a spot onto the SDA plate and allowed to soak. After the plate had dried, streaking was performed as described by Moore et al. (15); no carryover antifungal effect was detected. Time-kill curve studies were performed in standard RPMI (16) by the method described by Klepser et al. (12, 13). Before the tests were performed, the isolates were subcultured at least twice and grown for 24 h at 35°C on SDA plates. The inoculum was adjusted spectrophotometrically to the density of a 0.5 McFarland turbidity standard at 530 nm. The adjusted inoculum suspension was diluted 1:20 in RPMI containing the appropriate concentrations of amphotericin B, and tubes with the test solution were incubated at 35°C without agitation; the final volume was 5 ml. This procedure yielded an initial inoculum ranging from 2 × 105 to 8 × 105 CFU/ml and amphotericin B concentrations of 0.12, 0.5, 2, 8, and 32 μg/ml. At predetermined time points (0, 2, 4, 6, 12, 24, and 48 h), a 0.1-ml aliquot was removed from both the control tube (drug free) and each tube with a test solution and serially diluted in sterile water. Volumes of 0.1 ml, 30 μl, or 10 μl (depending on the dilution and the concentration of the drug) were spread onto SDA plates and incubated at 35°C for 24 to 48 h to determine the numbers of CFU per milliliter. When the colony counts were expected to be less than 1,000 CFU/ml, samples of 30 or 10 μl were taken directly from the test solution and plated without dilution. The lower limit of accurate and reproducible detectable colony counts is 100 CFU/ml. All time-kill curve studies were conducted in duplicate and on two separate occasions.
Mathematical model for fungicidal activity.
The killing kinetics of the fungicidal activity were analyzed by fitting the mean data at each time point to an exponential equation: Nt = N0 × e−Kt, where Nt expresses the number of viable yeasts at time t, N0 is the number of viable yeasts at the beginning of the experiment, K is the killing (or lethality) rate, and t is the incubation time. The exponential equation was transformed into a line by applying natural logarithms (log Nt = log N0 + Kt). The goodness of fit of the data was determined from the correlation coefficient (r2; range, 0.8 to 0.98). The fungicidal activities were compared by use of the K values, positive values of which indicate growth and negative values of which indicate killing. Thus, the seven time points on each killing curve were reduced to one value, K. The following parameters were derived from the killing equation: the mean times to achieve reductions in the proportions of viable cells of 50% (t50 = 0.30103/K), 90% (t90 = 1/K), and 99% (t99 = 2/K) and the time to reach the fungicidal endpoint (t99.9 = 3/K) for each amphotericin B concentration and each strain (3).
RESULTS
Antifungal susceptibility results.
The MICs and MFCs of amphotericin B for the test isolates are shown in Table 1. Except for amphotericin B-resistant strain C. albicans ATCC 200955, MFCs were below 1 μg/ml for the C. albicans and C. dubliniensis isolates. For the strains belonging to other species, the MFCs were in the range of 2 to >16 μg/ml for all except two isolates: one C. parapsilosis isolate (MIC = 1 μg/ml) and C. krusei ATCC 6258 (MFC = 0.5 μg/ml). The MICs for the strains previously defined as resistant to amphotericin B were 1 to 8 μg/ml and the MFCs were 2 to >16 μg/ml, while for the other strains, the MICs were 0.06 to 2 μg/ml and the MFCs were 0.25 to >16 μg/ml.
TABLE 1.
MICs and MFCs of amphotericin B for test isolates
| Species (no. of isolates) | MIC range (μg/ml) | MFC range (μg/ml) |
|---|---|---|
| C. albicans (6) | 0.06-0.25 | 0.25 |
| C. parapsilosis (5) | 0.25-1 | 1-8 |
| C. lusitaniae (3) | 0.06-0.5 | 2 |
| C. tropicalis (3) | 1 | 2 |
| C. krusei (5) | 1-2 | 2->16 |
| C. dubliniensis (3) | 0.06-0.5 | 0.5 |
| C. glabrata (4) | 0.5 | 2 |
| C. albicans ATCC 90028 | 0.12 | 0.25 |
| C. albicans ATCC 200955a | 2 | 8 |
| C. parapsilosis ATCC 22019 | 0.12 | 2 |
| C. lusitaniae ATCC 200950a | 1 | 32 |
| C. lusitaniae ATCC 200951a | 1 | 8 |
| C. lusitaniae ATCC 200952a | 2 | 2 |
| C. lusitaniae ATCC 200953a | 1 | 2 |
| C. tropicalis ATCC 200956a | 8 | >32 |
| C. krusei ATCC 6258 | 0.5 | 0.5 |
Amphotericin B-resistant strains.
Killing curve studies.
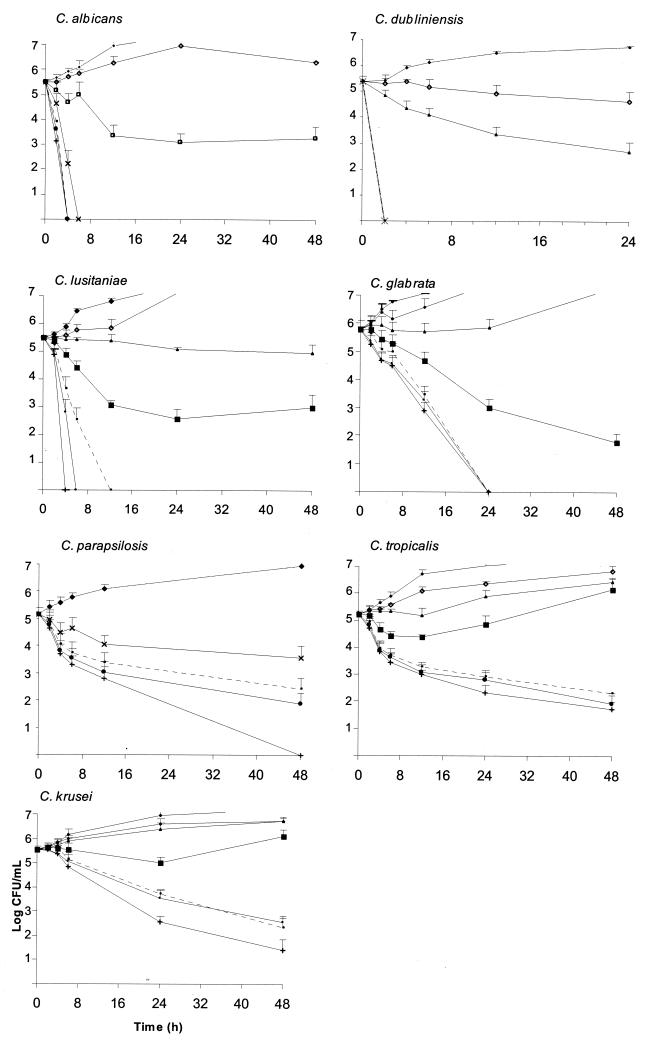
The killing activity of amphotericin B for each species (mean and standard deviation) is represented in Fig. 1. The fungicidal activity of amphotericin B was very fast (2 to 4 h at concentrations equal to two times the MIC) against C. albicans and C. dubliniensis; the reduction in the number of CFU per milliliter was greater than 3 log units (99.9%). On the contrary, for C. parapsilosis the fungicidal endpoint was reached after 48 h of incubation at four times the MIC, and for C. glabrata it was reached after either 16 to 32 h at the MIC or 24 h at four times the MIC; the effect was strain dependent for the latter species. The killing activity of amphotericin B against C. lusitaniae was also strain dependent (either very fast or slow); 4 or 12 h was required for amphotericin B at eight times the MIC to be fungicidal against the three clinical strains assayed. There was a rapid decrease in the numbers of CFU per milliliter in the first 12 h, with little killing after that point for C. tropicalis. The results were similar for C. krusei; 48 h was required to reach the fungicidal endpoint for both species.
FIG. 1.
Representative time-kill plots for Candida species following exposure to amphotericin B. Average datum points and standard deviations are provided for six C. albicans, three C. dubliniensis, five C. parapsilosis, five C. krusei, four C. glabrata, three C. tropicalis, and three C. lusitaniae strains at the following concentrations: no drug (control; ♦), 0.03 μg/ml (⋄), 0.12 μg/ml (▴), 0.25 μg/ml (□), 0.5 μg/ml (▪), 1 μg/ml (×), 2 μg/ml (dashed line), 8 μg/ml (•), and 32 μg/ml (+). As the curves for some concentrations overlap, see Fig. 2 for a better visualization of the effects of the different concentrations.
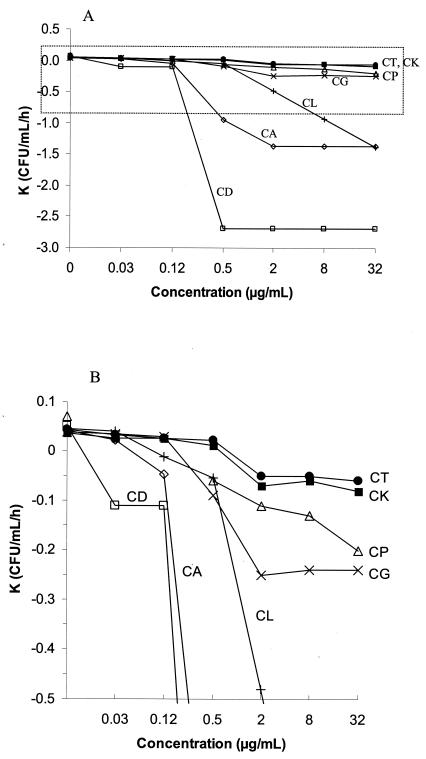
The relationship between amphotericin B concentrations and the lethality rate (K value) for the seven species of Candida is represented in Fig. 2. The highest K values were obtained for C. dubliniensis, C. albicans, and C. lusitaniae (−2.7, −1.4, and −1.4 CFU/ml/h, respectively, with amphotericin B concentrations of ≤0.5, 2, and 32 μg/ml, respectively). The lowest K value was obtained for C. tropicalis and C. krusei (−0.05 CFU/ml/h at an amphotericin B concentration of ≥2 μg/ml). With the exception of C. dubliniensis (K value, ≤0.5 μg/ml) and C. lusitaniae and C. parapsilosis (K values, 32 μg/ml), the K values increased as the amphotericin B concentration increased to 2 μg/ml.
FIG. 2.
(A) Relationship of amphotericin B concentrations and K values calculated from the regression line of survival times for C. dubliniensis (CD), C. albicans (CA), C. lusitaniae (CL), C. parapsilosis (CP), C. glabrata (CG), C. krusei (CK), and C. tropicalis (CT). (B) Amplification of the area marked with dotted lines in panel A.
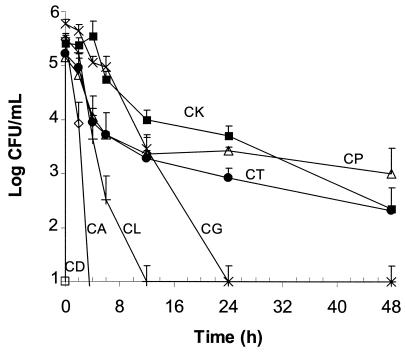
Figure 3 summarizes the killing patterns of amphotericin B at 2 μg/ml for each species. Although the maximum K values for most of the species were obtained at this concentration, viable C. parapsilosis, C. krusei, and C. tropicalis cells were observed after 48 h of incubation with amphotericin B at concentrations of >8 μg/ml (corresponding MICs, ≤2 μg/ml). Table 2 summarizes the times required to kill 50, 90, 99, and 99.9% of the initial inoculum for all strains tested (including American Type Culture Collection [ATCC] isolates) with 2 μg of the drug per ml. The mean times required to kill 50% of the cells ranged from 6 and 10 min for C. dubliniensis and C. albicans, respectively, to 5.8 h for C. tropicalis. However, the killing of 99.9% of C. parapsilosis, C. krusei, and C. tropicalis cells required longer incubation times (24 to >40 h) than those required for the other species (1 to 12 h).
FIG. 3.
Representative plots of mean ± standard deviation time-kill curves for several Candida species with 2 μg of amphotericin B per ml: C. dubliniensis (CD; □), C. albicans (CA; ⋄), C. lusitaniae (CL; +), C. parapsilosis (CP; ▵), C. glabrata (CG; ×), C. krusei (CK; ▪), and C. tropicalis (CT; •).
TABLE 2.
Times to achieve 50, 90, 99, and 99.9% reductions in growth from starting inoculum with 2 μg of amphotericin B per ml
| Isolate (no. of isolates) |
t50 (h)
|
t90 (h)
|
t99 (h)
|
t99.9 (h)
|
||||
|---|---|---|---|---|---|---|---|---|
| Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | |
| C. albicans (6) | 0.10-0.22 | 0.16 ± 0.06 | 0.36-0.74 | 0.55 ± 0.19 | 0.72-1.47 | 1.09 ± 0.39 | 1.08-2.20 | 1.64 ± 0.58 |
| C. dubliniensis (3) | 0.10-0.11 | 0.11 ± 0.005 | 0.36-0.39 | 0.122 ± 0.43 | 0.72-0.78 | 0.75 ± 0.03 | 1.08-1.17 | 1.13 ± 0.05 |
| C. glabrata (4) | 1.23-0.11 | 0.56 ± 0.47 | 0.36-4.10 | 1.85 ± 1.57 | 0.72-8.21 | 3.74 ± 3.12 | 1.07-12.32 | 5.61 ± 4.68 |
| C. krusei (5) | 2.55-5.47 | 4.27 ± 1.45 | 8.49-18.18 | 14.18 ± 4.84 | 16.99-36 | 28.37 ± 9.69 | 25.48-54.55 | 42.55 ± 14.53 |
| C. lusitaniae (3) | 0.21-0.61 | 0.35 ± 0.23 | 0.72-2.04 | 1.16 ± 0.76 | 1.44-4.08 | 2.32 ± 1.51 | 2.16-6.11 | 3.48 ± 2.27 |
| C. parapsilosis (5) | 0.81-4.83 | 2.71 ± 1.53 | 2.71-16.07 | 8.99 ± 5.09 | 5.43-32.13 | 17.99 ± 10.19 | 8.15-48.20 | 26.99 ± 15.29 |
| C. tropicalis (3) | 5.28-6.15 | 5.82 ± 0.47 | 17.85-20.46 | 19.33 ± 1.56 | 35.10-40.92 | 38.67 ± 3.12 | 52.65-61.38 | 58.01 ± 4.68 |
| C. lusitaniae ATCC 200950a | 5.04 | 16.7 | 33.5 | >48 | ||||
| C. lusitaniae ATCC 200951a | 4.42 | 14.7 | 29.4 | >48 | ||||
| C. lusitaniae ATCC 200952a | 3.78 | 12.38 | 24.4 | >48 | ||||
| C. lusitaniae ATCC 200953a | 0.33 | 1.10 | 22 | >48 | ||||
| C. albicans ATCC 200955a | 4.99 | 16.6 | 33.21 | >48 | ||||
| C. tropicalis ATCC 200956a | >48 | >48 | >48 | >48 | ||||
| C. albicans ATCC 90028 | 0.65 | 2.17 | 4.34 | 6.5 | ||||
| C. krusei ATCC 6254 | 1.3 | 4.34 | 8.69 | 13.1 | ||||
| C. parapsilosis ATCC 22019 | 0.7 | 1.36 | 4.72 | 7.08 | ||||
Amphotericin B-resistant strains.
The mean generation time determined from the growth control curves for each strain and species was 3.1 ± 0.5 h (range, 2.3 to 3.8 h), and the logarithmic phase lasted 12 h.
DISCUSSION
Plasma amphotericin B deoxycholate concentrations above 2 μg/ml have been associated with toxic effects and drug discontinuation, but such concentrations can be more safely achieved by using the less toxic lipid-associated formulations at increased dosages. The mean maximum concentrations of amphotericin B deoxycholate in serum (Cmaxs) reported after the administration of doses of 0.6 to 3 mg/kg of body weight/day are 1.1 to 3.6 μg/ml; for liposomal amphotericin B, however, Cmaxs are 17.2 to 83 μg/ml (after the administration of 3 to 5 mg/kg/day) and the Cmaxs of the lipid complex amphotericin B formulation are 22.9 μg/ml (after the administration of 2 mg/kg/day) (1, 7, 9, 28, 31). Furthermore, in the presence of serum, amphotericin B loses its killing activity (about 2 orders of magnitude); but the presence of serum does not affect its fungistatic activity (MIC for C. albicans), and it could have the same effect on other species (34). The method described in NCCLS guidelines is not reliable for the detection of resistance to amphotericin B in vitro, which has precluded the establishment of breakpoints. Although the Etest is used to determine the MICs of amphotericin B, the clinical value of Etest results needs to be determined.
Unlike earlier studies of fungicidal activity that only examined the 24-h killing effect of amphotericin B (8, 12, 13, 26), our investigation has identified the time required for the actual fungicidal activity (t50 to t99.9) for each species and/or strain (Table 2 and Fig. 1) and has included a larger range of amphotericin B concentrations (0.03 to 32 μg/ml) than those previously studied with 3 C. albicans isolates (0.5 to 16 μg/ml [12] and 0.03 to 4 μg/ml [2]) and 11 C. lusitaniae isolates (2 μg/ml [8]). Furthermore, we have included amphotericin B-resistant and -susceptible strains (ATCC strains) for which well-documented data from in vivo and in vitro studies are available (25).
Since in vitro testing with amphotericin B lipid-associated formulations can be influenced by the differences in drug release related to the lipid carrier, testing was performed with amphotericin B deoxycholate. The inclusion of a wide range of amphotericin B concentrations in the present study yielded a large number of curves, which precluded an easy comparison of the results. However, by mathematical calculation of the K value, the time-kill curve achieved with each concentration was reduced to one point, thus making it easier to visualize the killing pattern for each species (Fig. 2).
In our study, the killing pattern of amphotericin B was species and strain dependent; the fastest killing rate activity was achieved against C. dubliniensis and C. albicans, even in the presence of subinhibitory concentrations (one-half the MIC). Cells were killed by amphotericin B at concentrations below 1 μg/ml, as demonstrated by the absence of colonies and the absence of turbidity in the tube(s) used for the killing curve study. These results are in agreement with the results of 24-h killing curve studies reported previously (2, 12, 13).
Traditionally, C. lusitaniae has been considered intrinsically resistant to amphotericin B or prone to the development of resistance to this drug during the course of treatment (11, 14, 19, 21). Our data suggest that the rate of killing of C. lusitaniae by amphotericin B increases as the drug concentration increases (up to 32 μg/ml) and that the killing is strain dependent. Three of our recent clinical isolates were killed by 2 μg of amphotericin B per ml at 2 to 12 h, in agreement with the MFC (2 μg/ml). Other investigators have reported similar killing rates for strains of this species with 2 μg of amphotericin B per ml (8).
The frequency of C. glabrata as the cause of severe fungal infections has increased worldwide. C. glabrata is the Candida species that is the second most frequently associated with candidemia in the United States (5, 22). For this species, the fungicidal endpoint is reached after 16 h at four times the MIC. High Etest MICs (>1 μg/ml; 53% of strains) (22, 23) and MFCs >2 μg/ml (MFC for 90% of strains tested, 16 μg/ml) have been reported for this species (4).
C. parapsilosis is the Candida species that is the second most frequently recovered from children (5, 10, 20, 29) as well as a frequent cause of endocarditis among parenteral drug abusers (33). Although the mortality rate for infections caused by C. parapsilosis is lower than that caused by other non-C. albicans Candida species, the rate of mortality from C. parapsilosis endocarditis is comparable to that from invasive C. albicans infections (33). In the present study, the killing rate for C. parapsilosis was slower and increased slightly with the drug concentration. At least ≥2 μg of the drug per ml is required to reach the fungicidal endpoint within 24 h when viable cells are present, a phenomenon previously reported by Vazquez et al. (30).
Amphotericin B had the lowest level of fungicidal activity against C. krusei and C. tropicalis (endpoints at four times the MIC after 48 and 40 h, respectively). The maximum rate of killing of these species was reached with the drug at 2 μg/ml and hardly increased at higher concentrations. As reported by Vazquez et al. (30), viable cells are observed after 24 h of exposure to 2 μg of amphotericin B per ml; those investigators concluded that a fungicidal endpoint could perhaps have been achieved if the time of incubation had been extended beyond 24 h. However, in our study viable cells were still observed after an extended incubation time (48 h). The slow killing rate and the presence of viable cells after exposure to 2 μg of amphotericin B per ml could explain the lack of clinical response to amphotericin B treatment for certain infections caused by these two species (6, 24; J. Pemán, E. Cantón, A. Espinel-Ingroff, I. Jarque, M. Salvert, A. Querol, R. De Llanos, and M. Gobernado, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1025, 2003). High MICs by Etest (>2 μg/ml) have also been reported for C. krusei (93% of strains) and C. tropicalis (43%) of strains (23). Interestingly, the killing activity against the C. krusei reference strain (ATCC 6258) was faster (fungicidal endpoint after 13 h at four times the MIC) than that against other isolates of this species (48 h).
The killing activity of amphotericin B against the previously defined amphotericin B-resistant species varied by strain. Amphotericin B showed killing activity at 0.5 μg/ml (90% killing) only against C. lusitaniae ATCC 200953, and the maximum killing rate was reached with 2 μg/ml. For the other amphotericin B-resistant C. lusitaniae and C. albicans strains, the killing activity began at 2 μg/ml and increased with the concentration, with 22 to 33.5 h required to kill 99% of the initial inoculum at this concentration. Viable C. lusitaniae ATCC 200950 cells were still present after 48 h of incubation with 32 μg of amphotericin B per ml, in accordance with the MFCs. No killing activity against C. tropicalis ATCC 200956 was observed at any concentration assayed.
In summary, both amphotericin B time-kill curves and determination of the MFCs for Candida spp., as described in the present study, can detect viable cells after 48 h of incubation with ≥2 μg of amphotericin B per ml. This effect is strain dependent. The time required for the fungicidal activity (99.9% killing) of amphotericin B was also species and concentration dependent. The speed at which amphotericin B killed C. albicans (K values) was faster than that at which it killed the other species tested (3, 6, 13, 21, and 30 times more rapid killing compared with that for C. lusitaniae, C. glabrata, C. parapsilosis, C. krusei, and C. tropicalis, respectively). Nevertheless, the killing rate for C. dubliniensis was twice as fast as that for C. albicans. Therefore, since the performance of time-kill curve studies is cumbersome for routine use in the laboratory, the more practical determination of MFCs could become an alternative choice for the detection of the fungicidal activity of amphotericin B against those deep fungal infections for which determination of fungicidal activity could be clinically relevant. Clinical studies to determine the correlation between the in vivo response and the in vitro MFCs of amphotericin B are under way.
REFERENCES
- 1.Bekersky, I., R. M. Fielding, D. E. Dressler, J. W. Lee, D. N. Buell, and T. J. Walsh. 2002. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (Ambisome) and amphotericin B deoxycholate in humans. Antimicrob. Agents Chemother. 46:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burguess, S. D., R. W. Hasting, K. K. Summers, T. C. Harding, and M. G. Rinaldi. 2000. Pharmacodynamics of fluconazole, itraconazole, and amphotericin B against Candida albicans. Diagn. Microbiol. Infect. Dis. 36:13-18. [DOI] [PubMed] [Google Scholar]
- 3.Cantón, E., and J. Pemán. 1999. Curvas de letalidad en antifúngicos. Rev. Iberoam. Micol. 16:82-85. [PubMed] [Google Scholar]
- 4.Cantón, E., J. Pemán, A. Viudes, G. Quindós, M. Gobernado, and A. Espinel-Ingroff. 2003. Minimum fungicidal concentrations of amphotericin B for bloodstream Candida species. Diagn. Microbiol. Infect. Dis. 45:203-206. [DOI] [PubMed] [Google Scholar]
- 5.Diekeman, D. J., M. A. Pfaller, R. N. Jones, and SENTRY Participants Group. 2002. Age-related trends in pathogen frequency and antimicrobial susceptibility of bloodstream isolates in North America: SENTRY Antimicrobial Surveillance Program, 1997-2000. Int. J. Antimicrob. Agents 20:412-418. [DOI] [PubMed] [Google Scholar]
- 6.Druz, D. J., and R. I. Lebrer. 1978. Development of amphotericin B-resistant Candida tropicalis in a patient with defective leukocyte function. Am. J. Med. Sci. 276:77-92. [PubMed] [Google Scholar]
- 7.Dupont, B. 2002. Overview of the lipid formulations of amphotericin B. J. Antimicrob. Chemother. 49(Suppl. S1):31-36. [DOI] [PubMed] [Google Scholar]
- 8.Ernst, E. J., K. Yodoi, E. E. Roling, and M. E. Klepser. 2002. Rates and extents of antifungal activities of amphotericin B, flucytosine, fluconazole, and voriconazole against Candida lusitaniae determined by microdilution, Etest, and time-kill methods. Antimicrob. Agents Chemother. 46:578-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fields, B. T., J. H. Bates, and R. S. Abernathy. 1970. Amphotericin B serum concentrations during therapy. Appl. Microbiol. 19:955-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grillot, R., O. Faure, J. Fruit, B. Sendid, P. Rispail, A. Datry, et al. 2000. Working group: ECMM candidemia. ECMM prospective epidemiological survey of candidemia in Europe: report from France (682 cases). Rev. Iberoam. Micol. 17:S144. [Google Scholar]
- 11.Guinet, R., J. Chanas, A. Goullier, G. Bonnefoy, and P. Ambroise-Thomas. 1983. Fatal septicemia due to amphotericin B-resistant Candida lusitaniae. J. Clin. Microbiol. 18:443-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klepser, M.-E., E. J. Wolfe, R. N. Jones, C. H. Nightingale, and M. A. Pfaller. 1997. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob. Agents Chemother. 41:1392-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klepser, M.-E., E. J. Renst, R. E. Lewis, M. E. Ernst, and M. A. Pfaller. 1998. Influence of test conditions on antifungal time-kill curve results for standardized methods. Antimicrob. Agents Chemother. 42:1207-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merz, W. G. 1984. Candida lusitaniae: frequency of recovery, colonization, infection, and amphotericin B resistance. J. Clin. Microbiol. 20:1194-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore C. B., C. M. Walls, and D. W. Denning. 2001. In vitro activities of terbinafine against Aspergillus species in comparison with those of itraconazole and amphotericin B. Antimicrob. Agents Chemother. 45:1882-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 2nd ed. NCCLS document M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.Nguyen, M. H., C. J. Clancy, V. L. Yu, Y. C. Yu, A. J. Morris, D. R. Snydman, D. A. Sutton, and M. G. Rinaldi. 1998. Do the in vitro susceptibility data predict the microbiologic response to amphotericin B? Results of a prospective study of patients with Candida fungemia. J. Infect. Dis. 177:425-430. [DOI] [PubMed] [Google Scholar]
- 18.Ostrosky-Zeichner L., K. A. Marr, J. H. Rex, and S. H. Cohen. 2003. Amphotericin B: time for a new “gold standard.” Clin. Infect. Dis. 37:415-425. [DOI] [PubMed] [Google Scholar]
- 19.Pappagianis, D., M. S. Collins, R. Hector, and J. Remington. 1979. Development of resistance to amphotericin B in Candida lusitaniae infecting a human. Antimicrob. Agents Chemother. 16:123-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pemán, J., A. Cercós, M. C. Peñalver, A. Viudes, E. Cantón, A. Planes, E. Cañes, A. Del Palacio, J. Sevillano, J. Viñuelas, M. Elía, M. Jiménez, M. Pazo, J. J. García, J. J. Palomar, J. M. Hernández, E. Mínguez, J. A. Martínez, Y. Fernández, J. Martínez, C. Tapiol, M. S. Cuétara, and S. Giner. 2000. Epidemiological survey of candidemia in Spain. Rev. Iberoam. Micol. 17:S143. [Google Scholar]
- 21.Pfaller, M. A., M. A. Messer, and R. J. Hollis. 1994. Strain delineation and antifungal susceptibilities of epidemiologically related and unrelated isolates of Candida lusitaniae. Diagn. Microbiol. Infect. Dis. 32:223-227. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller, M. A., D. J. Diekema, R. N. Jones, S. A. Messer, R. J. Hollis, and the SENTRY Participants Group. 2002. Trends in antifungal susceptibility of Candida spp. isolated from pediatric and adult patients with bloodstream infections: SENTRY Antimicrobial Surveillance Program, 1997 to 2000. J. Clin. Microbiol. 40:852-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, R. J. Hollis, R. N. Jones, and the International Fungal Surveillance Participant Group. 2003. In vitro activities of voriconazole, posaconazole, and four licensed systemic antifungal agents against Candida species infrequently isolated from blood. J. Clin. Microbiol. 41:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powderly, W. G., G. S. Kobayashi, G. P. Herzig, and G. Medoff. 1988. Amphotericin B-resistant yeast infection in severely immunocompromised patients. Am. J. Med. 84:826-832. [DOI] [PubMed] [Google Scholar]
- 25.Rex, J. H., C. R. Cooper, Jr. W. G. Merz, J. N. Galgiani, and E. J. Anaissie. 1995. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob. Agents Chemother. 39:906-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodero, L., S. Córdoba, P. Cahn, M. Soria, M. Lucarini, G. Davel, S. Kaufman, C. Canteros, and L. Guelfand. 2000. Timed-kill curves for Cryptococcus neoformans isolated from patients with AIDS. Med. Mycol. 38:201-207. [DOI] [PubMed] [Google Scholar]
- 27.Seidenfeld, S. M., B. H. Cooper, J. W. Smith, J. P. Luby, and P. A. Mackowiak. 1983. Amphotericin B tolerance: a characteristic of Candida parapsilosis not shared by other Candida species. J. Infect. Dis. 147:116-119. [DOI] [PubMed] [Google Scholar]
- 28.Tollemar, J., L. Klingspor, and O. Ringden. 2001. Liposomal amphotericin B (Ambisome) for fungal infections in immunocompromised adults and children. Clin. Microbiol. Infect. 7(Suppl. 2):68-79. [DOI] [PubMed] [Google Scholar]
- 29.Tortorano, A. M., M. A. Viviani, A. L. Rigoni, E. Biraghi, M. Cogliati, et al. 2000. ECMM survey of candidemia in Europe. Report from Italy. Rev. Iberoam. Micol. 17:S143. [Google Scholar]
- 30.Vazquez, J. A., M. T. Arganoza, D. Boikov, S. Yoon, J. D. Sobel, and R. A. Akins. 1998. Stable phenotypic resistance of Candida species to amphotericin B conferred by preexposure to subinhibitory levels of azoles. J. Clin. Microbiol. 36:2690-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viscoli, C., and E. Castagnola. 1999. Emerging fungal pathogens, drug resistance, and the role of the lipid formulations of amphotericin B in the treatment of fungal infections in cancer patients: a review. Int. J. Infect. Dis. 3:109-118. [DOI] [PubMed] [Google Scholar]
- 32.Walsh, T. J., G. P. Melcher, M. G. Rinaldi, J. Lecciones, D. A. McGough, P. Kelly, J. Lee, D. Callender, M. Rubin, and P. A. Pizzo. 1990. Trichosporon beigelii, an emerging pathogen resistant to amphotericin B. J. Clin. Microbiol. 28:1616-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weems, J. J., Jr. 1992. Candida parapsilosis: epidemiology, pathogenicity, clinical manifestations, and antimicrobial susceptibility. Clin. Infect. Dis. 14:756-766. [DOI] [PubMed] [Google Scholar]
- 34.Zhanel, G. G., D. G. Saunders, D. J. Hoban, and J. A. Karlowsky. 2001. Influence of human serum on antifungal pharmacodynamics with Candida albicans. Antimicrob. Agents Chemother. 45:2018-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]