Abstract
Purpose
We report on the natural history and factors influencing the prognosis of a cohort of hormone naïve, prostate specific antigen era patients in whom metastatic prostate cancer developed after radical prostatectomy who were followed closely and treated with deferred androgen deprivation therapy at the time of metastasis.
Materials and Methods
A total of 3,096 men underwent radical prostatectomy performed by a single surgeon at Johns Hopkins Hospital between 1987 and 2005. Of these men 422 had prostate specific antigen failure. Distant metastasis developed in 123 patients, of whom 91 with complete data formed the study cohort initially treated during the prostate specific antigen era (1987 to 2005) and receiving androgen deprivation therapy after documented metastasis. A total of 41 men died of prostate cancer. Median survival times were estimated by Kaplan-Meier analysis. Prognostic impact was estimated as the hazard ratio derived from the Cox proportional hazards model.
Results
Median followup from radical prostatectomy was 120 months (range 24 to 216). Kaplan-Meier median (range) times to failure were 24 months (12 to 144) from radical prostatectomy to prostate specific antigen failure, 36 months (0 to 132) from prostate specific antigen failure to metastasis, 84 months (12 to 180) from metastasis to death and 168 months (24 to 216) from radical prostatectomy to death. Statistically significant univariate risk factors for prostate cancer specific mortality at the time of metastasis were pain at diagnosis of metastases (p = 0.002), time from radical prostatectomy to metastasis (p = 0.024) and prostate specific antigen doubling time less than 3 months during the 24 months before metastasis (p = 0.016). Multivariable analysis demonstrated independent predictors of prostate cancer specific mortality at the time of metastasis, namely pain (HR 3.5, p = 0.003) and prostate specific antigen doubling time less than 3 months (HR 3.4, p = 0.017).
Conclusions
Men treated with deferred androgen deprivation therapy for the development of metastasis after radical prostatectomy may have a long life span, 169 months after radical prostatectomy (range 24 to 216). The presence of pain and short prostate specific antigen doubling time predicted an unfavorable outcome.
Keywords: prostatic neoplasms, prostatectomy, neoplasm metastasis, androgens, pain
Radical prostatectomy has been demonstrated to be an effective treatment for prostate cancer, especially when the disease is organ confined.1–3 As our experience with treating men with RP has increased, we have also learned that many men who are not cured by surgery still may have good outcomes even when evidence of increasing PSA develops.4–6 Androgen signaling events have for many years been known to control CaP cell growth and differentiation, and androgen deprivation therapy has long been a mainstay for the treatment of advanced CaP.7,8
In the last several years the pattern of practice of ADT for CaP in most of the Western world has been characterized by implementation of treatment before evidence of metastasis. Practitioners frequently extrapolate the benefits of ADT determined in advanced disease9–11 and apply those same principles to patients with PSA recurrence after RP.12 However, the efficacy of immediate administration of ADT after PSA recurrence vs deferring ADT until evidence of metastatic disease has not been well established and is a subject of great controversy,13 while the deleterious effects of long-term ADT are well-known.14 We demonstrate CaP specific survival and analyze factors influencing it in a contemporary group of hormone naïve men with metastasis (stage D2) after RP, followed closely from the time of PSA increase.
MATERIALS AND METHODS
From January 1987 to July 2005 during a median followup of 120 months (range 24 to 216), 422 of 3,096 men (14%) who had undergone RP by a single surgeon (PCW) at Johns Hopkins Hospital experienced biochemical recurrence, defined as a single postoperative PSA of 0.2 ng/ml or higher. Of these men 123 (4%) had evidence of metastatic disease (visible on bone scan, computerized tomography or magnetic resonance imaging). A total of 32 patients were excluded from analysis for several reasons. There were 21 patients who received ADT before metastasis and 5 had missing data on ADT. Patients were censored at last followup, excluding 6 who were lost to followup after the diagnosis of metastasis. This resulted in a study population of 91 patients, all of whom first received ADT at the time of clinically evident distant metastasis. A total of 18 (20%) patients were given external beam radiation therapy after RP. There were 15 patients who received salvage XRT and 3 who received adjuvant XRT. A total of 41 men died of CaP and 5 died of other causes. Race was categorized using patient reported data and is reported for baseline demographic information purposes only. Because our study population was predominantly white, we did not include race in any statistical analyses (table 1). The institutional review board at Johns Hopkins approved this study and, when required, written informed consent was obtained from all study participants.
TABLE 1.
Clinical and pathological characteristics of men with metastasis after RP
| No. pts | 91 | ||
| Median pt age at surgery (range), (IQR) | 60 | (38–75), (57–64) | |
| No. white race (%) | 89 | (97.8) | |
| Median preop ng/ml PSA (range), (IQR) | 10.2 | (1.5–129),* (6.4–19) | |
| No. biopsy Gleason score (%): | |||
| 2–6 | 35 | (40) | |
| 7 (3 + 4) | 22 | (25) | |
| 4 + 3 or Greater | 30 | (34) | |
| No. clinical stage (%): | |||
| T1b | 2 | (2) | |
| T1c | 11 | (12) | |
| T2a | 29 | (32) | |
| T2b | 30 | (33) | |
| T2c | 8 | (9) | |
| T3a | 10 | (11) | |
| D0 | 1 | (1) | |
| No. pathological Gleason score (%): | |||
| 2–6 | 4 | (4) | |
| 7 (3 + 4) | 20 | (22) | |
| 4 + 3 or Greater | 67 | (73) | |
| No. pos margins (%) | 31 | (34) | |
| No. extraprostatic extension (%) | 82 | (90) | |
| No. seminal vesicle invasion (%) | 44 | (48) | |
| No. lymph node involvement (%) | 42 | (47) | |
| Median yrs followup after RP (range) | 10 | (2–18) | |
| Median time to failure (range): | |||
| Mos RP to PSA failure | 24 | (12–144) | |
| Mos PSA failure to metastasis | 32 | (2–129) | |
| Mos metastasis to death | 82 | (7–181) | |
| Median mos RP to death (range) | 168 | (24–216) | |
Includes data from 89 patients.
Followup
Patients were followed postoperatively with PSA determinations and rectal examinations every 3 months for the first year, semiannually for the second year and annually thereafter. After biochemical progression PSA was measured semiannually and bone scans were recommended to be performed yearly. At every visit a pain history was elicited and if present a bone scan was performed out of sequence. Pain was documented as present only if the examining physician noted pain in the record. Absence of pain was recorded if the physician noted no pain on examination or the patient was noted to be without complaints. Pain data were recorded as missing if there was no documentation of pain or patient well-being. Comorbidities (DM, CAD) at the diagnosis of metastasis were recorded, as well as hematocrit documented within 12 months of the diagnosis of metastasis. The location of metastasis, axial vs appendicular or visceral, was noted as well as Eastern Cooperative Oncology Group performance status (as recorded by the examining physician or calculated based on information from the physician’s note). If standard criteria used to determine ECOG performance status were missing or unclear, then performance status was treated as missing.
All 91 patients received ADT at the documentation of metastasis. Following progression after ADT, patients returned to the care of their primary oncologist for treatment and followup, and the manner and method by which ADT was administered were left to their discretion. PCSM was defined as death in any patient with metastasis showing any progression following ADT. Because of the limited number of nonCaP deaths (5) in this cohort, PCSM approached all-cause mortality, so data are only reported for PCSM. When the 5 men with nonCaP deaths were excluded vs censored at the time of death, the results of the multivariable analyses did not materially change. Therefore, these men were censored at the time of death and included in the analyses.
Determination of PSADT
Prostate specific antigen doubling time was calculated using the natural log of 2 (0.693) divided by the slope of the linear regression of logPSA vs time. We calculated PSADT using 2 different methods including all PSA values within 24 months after biochemical recurrence (greater than 0.2 ng/ml)5 and including all PSA values within 24 months before radiographic documentation of metastasis. Patients (9 for PSADT in the 24 months after biochemical recurrence, 1 for PSADT in the 24 months before metastasis) with a negative or undefined PSADT (no PSA increase) were assigned a value of 1,000 months for ease of calculations. All PSA values were taken before subsequent ADT, which might have affected PSADT calculation.
Statistical Methods
PSADTs were dichotomized as 3 or more months vs less than 3 months and time from surgery to metastasis was dichotomized as 5 or less years vs more than 5 years because a binary variable has easier clinical interpretation. These forms have also been prognostic in previous studies.5,6 Median failure times were estimated with the Kaplan-Meier method. Potential prognostic factors for time from radiographic documentation of metastasis to CaP specific mortality were examined using log rank tests and univariate proportional hazards regression. Variables exhibiting p ≤ 0.15 in univariate analyses were entered manually in Cox proportional hazards models in a forward stepwise fashion. Variable retention was based on the likelihood ratio test and change in estimated hazard ratios for variables already present. Final models were developed using multiple imputation and imputing missing values with a Markov Chain Monte Carlo (MCMC) method with 5 imputations, implemented in PROC MI and PROC MIANALYZE in SAS®. Imputation of missing values for the model variables is based on dependent variables in the model (survival time and death status), variables are correlated with model variables and variables correlated with the missingness of model variables. The proportional hazards assumption was verified for candidate prognostic factors by plotting Martingale residuals vs survival time and the Kolmogorov supremum test.15 Statistical analyses were performed using SAS® v9.1.
RESULTS
The clinical and pathological characteristics of the 91 patients analyzed in this study are shown in table 1. Most patients had clinically palpable, pathologically extraprostatic, high Gleason grade disease. Median preoperative PSA in these patients was 10.2 ng/ml (range 1.5 to 129, IQR 6.4 to 19). Median followup from RP was 120 months (range 24 to 216). Kaplan-Meier median failure times were 24 months (range 12 to 144) from RP to PSA failure, 32 months (range 2 to 129) from PSA failure to metastasis, 132 months (range 12 to 204) from PSA failure to death, 82 months (range 7 to 181) from metastasis to death and 168 months (range 24 to 216) from RP to death (fig. 1).
FIG. 1.

Median (range) failure times in hormone naïve men with metastasis after radical prostatectomy
Median PSA at the time of metastasis was 31.4 ng/ml (range 0 to 679, IQR 9.1 to 92.7, table 2). One patient with a PSA of 0 ng/ml had an isolated lung metastasis discovered during an evaluation for cough, verified by bronchoscopic biopsy. The median number of PSA values obtained from PSA failure to metastasis was 4 (range 0 to 12). Median PSADT calculated from the time of PSA recurrence was 5.8 vs 5.0 months during the 24 months before the documentation of metastasis. Most patients had few comorbidities in that 3% had DM, 18% had CAD and 93% exhibited a performance status of 0 to 1. Of the patients 67% presented with appendicular or soft tissue visceral metastasis and 34% presented with pain (table 2).
TABLE 2.
Clinical characteristics of men at diagnosis of metastasis after RP
| 1987–2005 | No. With Data | |||
|---|---|---|---|---|
| Median ng/ml PSA at metastasis (range), (IQR) | 31.4 | (0–679), (9.1–92.7) | 76 | |
| Median PSADT | 5.8 | 60 | ||
| Median PSADT at metastasis | 5.0 | 59 | ||
| Median hematocrit at metastasis (range), (IQR) | 41.8 | (27.2–47.1), (40–43.8) | 30 | |
| No. comorbid DM (%) | 2 | (3) | 80 | |
| No. comorbid CAD (%) | 14 | (18) | 80 | |
| No. presence of appendicular metastases (%) | 51 | (67) | 76 | |
| No. presence of pain (%) | 25 | (34) | 74 | |
| No. ECOG performance status (%): | 75 | |||
| 0 | 46 | (61) | ||
| 1 | 24 | (32) | ||
| 2 | 4 | (5) | ||
| 3 | 1 (3) | |||
| 4 | 0 | |||
Several variables were statistically significant prognostic factors for PCSM in univariate Cox proportional hazards analysis such as pain at diagnosis of metastases (HR 2.97, 95% CI 1.45–6.06, p = 0.002), time from RP to metastasis (continuous variable) (HR 0.99, 95% CI 0.98–0.998, p = 0.024), hematocrit at metastasis (HR 0.83, 95% CI 0.7–0.96, p = 0.007) and PSADT during the 24 months before metastasis (categorical variable less than 3 months) (HR 3.01, 95% CI 1.26–7.91, p = 0.016, table 3). Kaplan-Meier analysis revealed similar findings (fig. 2). Figure 2A, shows a log rank test revealing patients with no pain had a survival advantage compared to those with pain (p = 0.002). A similar survival advantage was seen in those with PSADTs of 3 or more months in figure 2B, (p = 0.009) and in those in whom metastasis took more than 5 years to develop (p = 0.041, fig. 2C).
TABLE 3.
Univariate Cox proportional hazards model predicting PCSM for men with metastasis after RP
| HR | 95% CI | p Value | No. With Data | |
|---|---|---|---|---|
| Time from RP to metastasis | 0.99 | 0.98–0.998 | 0.024 | 91 |
| Time to PSA failure | 0.99 | 0.97–1.00 | 0.100 | 91 |
| Log (preop PSA) | 1.07 | 0.75–1.53 | 0.704 | 89 |
| Age | 1.03 | 0.95–1.06 | 0.897 | 91 |
| Preop Gleason grade 4 + 3 or greater | 1.44 | 0.67–3.09 | 0.340 | 87 |
| Pathological Gleason grade 4 + 3 or greater | 1.92 | 0.92–3.99 | 0.083 | 91 |
| Organ confined disease | 0.73 | 0.22–2.36 | 0.594 | 91 |
| Pos surgical margins | 1.36 | 0.73–2.52 | 0.334 | 91 |
| PSADT at PSA failure less than 3 mos | 1.45 | 0.57–3.57 | 0.441 | 60 |
| PSADT before metastasis less than 3 mos | 3.01 | 1.26–7.19 | 0.016 | 59 |
| ECOG performance status greater than 1 | 1.36 | 0.41–4.50 | 0.621 | 75 |
| Presence of pain | 2.97 | 1.45–6.06 | 0.002 | 74 |
| Presence of appendicular metastases | 1.42 | 0.65–3.12 | 0.378 | 76 |
| Comorbid CAD | 1.21 | 0.42–3.50 | 0.716 | 80 |
| Absolute PSA at metastasis 100 ng/ml or greater | 1.54 | 0.67–3.48 | 0.294 | 76 |
FIG. 2.
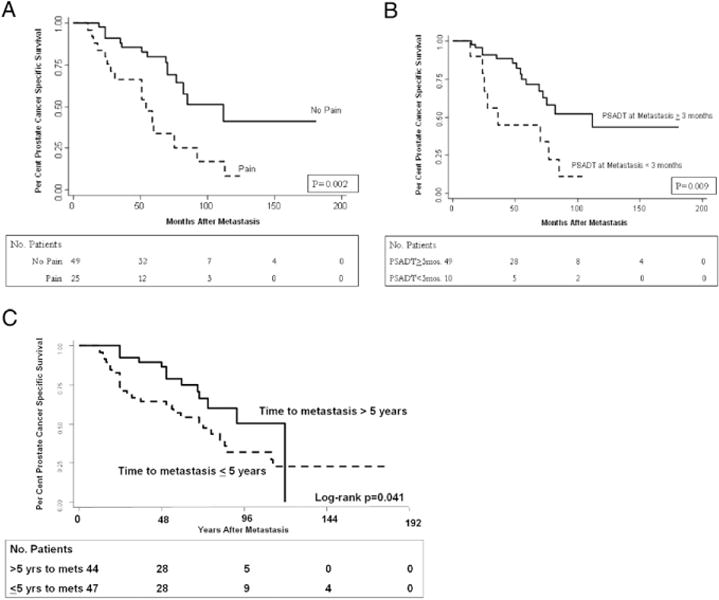
A, prostate cancer specific survival by pain at metastasis. B, prostate cancer specific survival by PSADT at metastasis. C, prostate cancer specific survival by time to metastasis.
A multivariable Cox proportional hazards model demonstrated that the independent predictors of PCSM are pain at diagnosis of metastasis (HR 7.9, 95% CI 2.5–24.7, p < 0.001) and PSADT before metastasis less than 3 months (HR 4.6, 95% CI 1.7–12.4, p = 0.003, table 4). Only 53 patients had data available for both covariates. We repeated the modeling process using multiple imputation to simulate missing data, resulting in an analysis representing all 91 patients. This analysis also resulted in a final model with pain at diagnosis of metastasis (HR 3.5, 95% CI 1.6–7.7, p = 0.003) and PSADT before metastasis of less than 3 months (HR 3.4, 95% CI 1.3–8.5, p = 0.017) (table 4). Testing Martingale residuals revealed no departure from the proportional hazards assumption (p = 0.379 and 0.548 for pain and PSADT before metastasis, respectively).15 PSADT determined in the 24 months immediately after PSA failure (HR 1.0, p = 0.371) and time from RP to metastasis (HR 1.0, p = 0.804) were not independent predictors of PCSM. Hematocrit obtained within 12 months before or after metastasis was not evaluated in a multivariable model because only 30 patients had data available. Kaplan-Meier analysis stratifying patients simultaneously by pain and short PSADT confirms the prominent role of pain as a predictor of PCSM (p < 0.001). The groups in order of longest to shortest survival are men without pain and with a long PSADT, then men without pain with a short PSADT, then men with pain and a long PSADT, and finally men with pain and a short PSADT (fig. 3).
TABLE 4.
Multivariate Cox proportional hazards models of risk factors for PCSM in men with metastasis after RP
| Prognostic Factor | HR (95% CI) | p Value |
|---|---|---|
| Model deleting subjects with missing data (53 subjects, 20 deaths): | ||
| Presence of pain at metastasis (yes vs no) | 7.9 (2.5–24.7) | < 0.001 |
| PSADT before metastasis (less than 3 vs 3 or more mos) | 4.6 (1.7–12.4) | 0.003 |
| Model with missing data simulated by multiple imputation (91 subjects, 41 deaths): | ||
| Presence of pain at metastasis (yes vs no) | 3.5 (1.6–7.7) | 0.003 |
| PSADT before metastasis (less than 3 vs 3 or more mos) | 3.4 (1.3–8.5) | 0.017 |
FIG. 3.

Prostate cancer specific survival by pain and PSADT at metastasis
DISCUSSION
The natural history of CaP following biochemical recurrence can be long,5,6 as can the natural history of CaP after the development of metastasis.5 While the timing of ADT is controversial, to our knowledge there are no studies describing the natural history of men treated with deferred ADT after the development of a PSA increase after RP. Nor are there studies evaluating the role of early vs deferred administration of ADT after the development of biochemical recurrence.13 Current practice patterns suggest that most patients in the United States receive ADT soon after PSA increase, before the development of metastatic disease. As a result the proportion of patients presenting with hormone naïve, metastatic CaP has decreased substantially in the last 2 decades.12,16
Here we describe a contemporary cohort of patients in whom metastatic CaP developed after RP, and who were treated with close observation and deferred ADT. We demonstrate that the survival of these hormone naïve patients can be long, and that the presence of pain and a short PSADT during the 24 months preceding metastasis are independent risk factors for PCSM in this cohort. We also found no independent association between absolute PSA at diagnosis of metastasis and PCSM. The wide range of PSA levels at metastasis demonstrates the difficulty in relying solely on this test as a predictor of metastasis and under-scores the need to perform bone scans routinely, even in men with low PSA.
Eisenberger et al examined prognostic factors in men presenting with stage D2 CaP enrolled in a National Cancer Institute Intergroup sponsored double-blind, placebo controlled, prospective randomized trial comparing treatment of hormone naïve patients with leuprolide alone vs leuprolide plus flutamide.17 Multivariable analysis demonstrated that the extent of bone involvement on bone scan, presence of pain, anorexia and DM were independent prognostic factors for PCSM. In that cohort the median time from diagnosis of unstaged prostate cancer to study entry was 6 weeks, implying these men already had metastatic CaP at presentation.17 The survival of contemporary patients with stage D2 CaP is markedly different from those of the prePSA and early PSA periods. This is due in large part to lead time bias from earlier detection of CaP.
The first outcomes data for men with metastasis after RP were published by Pound et al.5 In a subset of 103 men with metastasis after RP from 1982 to 1997 there were 44 men who died of CaP. Analysis revealed that time from surgery to the development of metastasis was the only significant independent risk factor for PCSM.
In our cohort of men in whom metastasis developed after RP, which incorporates many patients diagnosed after 1987 from the series evaluated by Pound et al,5 time to the development of metastasis is also significant in univariate analysis but is not independently prognostic in multivariable analyses including pain and PSADT 24 months before diagnosis of metastasis. Our cohort also has longer followup than the study by Pound et al and is more relevant to patients treated in the modern era, as PSA screening and followup were routinely available at Johns Hopkins Hospital by 1987. Our results differed from those observed in the trial by Eisenberger et al17 in that disease severity (determined by extent of bone scan involvement), anorexia at the time of diagnosis and performance status were not statistically significant risk factors for PCSM.16 Too few patients had data available on hematocrit or history of diabetes to evaluate their prognostic influence.
Our study has several limitations. It is a single center, retrospective analysis of a small number of patients. There were 21 patients who received ADT before the documentation of metastasis who were excluded from analysis. These patients may have received early ADT because they had more aggressive disease than those who received deferred ADT. In addition, this population of men who have undergone RP enjoys overall better health than patients from previous eras, such as those in the Intergroup trial who had stage D2 disease at initial diagnosis.16 This could be due to the selection bias for surgical candidates (only relatively healthy men can tolerate surgery), the schedule of frequent followup for surgical patients or a possible18 (although controversial19) protective effect of surgical control of the primary tumor on PCSM. Our study also evaluates patients who ultimately had metastasis after surgical treatment, who thus have highly aggressive disease. The prognostic factors that we identified are determined at the time of, not before, metastasis. Thus, our results may not be generalizable to evaluating risk for the average patient at the time of CaP diagnosis, after RP or at the time of biochemical failure. Finally, prognostic factor data were missing for a number of patients, requiring the use of multiple imputation for a more valid analysis. However, it is encouraging that the multivariate analyses identified the same 2 prognostic factors in analyses with and without multiple imputation, suggesting that these are not chance associations.
The data we present here do not specifically evaluate the difference between immediate and deferred ADT. However, they do demonstrate that hormone naïve patients with metastasis after RP, when closely followed in the interval after biochemical failure, may have an excellent response to deferred ADT and a long survival. When treating patients who may survive longer than a decade after surgery, the complications of ADT become significant.14,20 We have identified prognostic factors (presence of pain and short PSADT at the time of metastasis) which predict worse outcome. Patients with these negative prognostic indicators may benefit from therapies in addition to ADT at the time of metastasis.
CONCLUSIONS
Optimal timing for the initiation of ADT remains controversial. Our data in patients followed from the time of RP indicate that deferred ADT initiated at the radiographic documentation of metastasis may still be associated with long survival time. The outcome of stage D2 CaP in a contemporary series is different from the pre or early PSA era. PSADT immediately before the development of metastasis and the presence of pain are important predictors of PCSM.
Acknowledgments
Supported by the National Institutes of Health/National Cancer Institute SPORE Grant P50CA58236, The Prostate Cancer Foundation and Early Detection Research Network/National Cancer Institute/National Institutes of Health Grant U01-CA86323.
Abbreviations and Acronyms
- ADT
androgen deprivation therapy
- CAD
coronary artery disease
- CaP
prostate cancer
- DM
diabetes mellitus
- ECOG
Eastern Cooperative Oncology Group
- IQR
interquartile range
- PCSM
prostate cancer specific mortality
- PSA
prostate specific antigen
- PSADT
prostate specific antigen doubling time
- RP
radical prostatectomy
- XRT
external beam radiotherapy
Footnotes
Study received institutional review board approval.
References
- 1.Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 2.Blute ML, Nativ O, Zincke H, Farrow GM, Therneau T, Lieber MM. Pattern of failure after radical retropubic prostatectomy for clinically and pathologically localized adenocarcinoma of the prostate: influence of tumor deoxyribonucleic acid ploidy. J Urol. 1989;142:1262. doi: 10.1016/s0022-5347(17)39051-1. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JI, Pizov G, Walsh PC. Correlation of pathologic findings with progression after radical retropubic prostatectomy. Cancer. 1993;71:3582. doi: 10.1002/1097-0142(19930601)71:11<3582::aid-cncr2820711120>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Khan MA, Partin AW, Mangold LA, Epstein JI, Walsh PC. Probability of biochemical recurrence by analysis of pathologic stage, Gleason score, and margin status for localized prostate cancer. Urology. 2003;62:866. doi: 10.1016/s0090-4295(03)00674-5. [DOI] [PubMed] [Google Scholar]
- 5.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 6.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 7.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 8.Huggins C, Stevens RE, Hodges CV. Studies on prostatic cancer. II. The effect of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209. [Google Scholar]
- 9.Blackard CE, Byar DP, Jordan WP., Jr Orchiectomy for advanced prostatic carcinoma. A reevaluation. Urology. 1973;1:553. doi: 10.1016/0090-4295(73)90515-3. [DOI] [PubMed] [Google Scholar]
- 10.Byar DP, Corle DK. Hormone therapy for prostate cancer: results of the Veterans Administration Cooperative Urological Research Group studies. NCI Monogr. 1988;7:165. [PubMed] [Google Scholar]
- 11.Kirk D. Immediate vs deferred hormone treatment for prostate cancer: how safe is androgen deprivation? BJU Int. 2000;86:220. [Google Scholar]
- 12.Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22:2141. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh PC, DeWeese TL, Eisenberger MA. A structured debate: immediate versus deferred androgen suppression in prostate cancer-evidence for deferred treatment. J Urol. 2001;166:508. doi: 10.1016/s0022-5347(05)65972-1. [DOI] [PubMed] [Google Scholar]
- 14.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 15.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 199380:557. [Google Scholar]
- 16.Freedland SJ, Sutter ME, Naitoh J, Dorey F, Csathy GS, Aronson WJ. Clinical characteristics in black and white men with prostate cancer in an equal access medical center. Urology. 2000;55:387. doi: 10.1016/s0090-4295(99)00461-6. [DOI] [PubMed] [Google Scholar]
- 17.Eisenberger MA, Crawford ED, Wolf M, Blumenstein B, McLeod DG, Benson R, et al. Prognostic factors in stage D2 prostate cancer; important implications for future trials: results of a cooperative intergroup study (INT. 0036). The National Cancer Institute Intergroup Study #0036. Semin Oncol. 1994;21:613. [PubMed] [Google Scholar]
- 18.Thompson IM, Tangen C, Basler J, Crawford ED. Impact of previous local treatment for prostate cancer on subsequent metastatic disease. J Urol. 2002;168:1008. doi: 10.1016/S0022-5347(05)64562-4. [DOI] [PubMed] [Google Scholar]
- 19.Halabi S, Vogelzang NJ, Ou SS, Small EJ. The impact of prior radical prostatectomy in men with metastatic castration recurrent prostate cancer: a pooled analysis of 9 Cancer and Leukemia Group B Trials. J Urol. 2007;177:531. doi: 10.1016/j.juro.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 20.Fowler FJ, Jr, McNaughton Collins M, Walker Corkery E, Elliott DB, Barry MJ. The impact of androgen deprivation on quality of life after radical prostatectomy for prostate carcinoma. Cancer. 2002;95:287. doi: 10.1002/cncr.10656. [DOI] [PubMed] [Google Scholar]


