Abstract
Purpose
We investigated the relationship between the tertiary Gleason component in radical prostatectomy specimens and biochemical recurrence in what is to our knowledge the largest single institution cohort to date.
Materials and Methods
We evaluated data on 3,230 men who underwent radical prostatectomy at our institution from 2000 to 2005. Tertiary Gleason component was defined as Gleason grade pattern 4 or greater for Gleason score 6 and Gleason grade pattern 5 for Gleason score 7 or 8.
Results
Biochemical recurrence curves for cancer with tertiary Gleason component were intermediate between those of cancer without a tertiary Gleason component in the same Gleason score category and cancer in the next higher Gleason score category. The only exception was that Gleason score 4 + 3 = 7 with a tertiary Gleason component behaved like Gleason score 8. The tertiary Gleason component independently predicted recurrence when factoring in radical prostatectomy Gleason score, radical prostatectomy stage and prostate specific antigen (HR 1.45, p = 0.029). Furthermore, the magnitude of the tertiary Gleason component effect on recurrence did not differ by Gleason score category (p = 0.593).
Conclusions
Although the tertiary Gleason component is frequently included in pathology reports, it is routinely omitted in other situations, such as predictive nomograms, research studies and patient counseling. The current study adds to a growing body of evidence highlighting the importance of the tertiary Gleason component in radical prostatectomy specimens. Accordingly consideration should be given to a modified radical prostatectomy Gleason scoring system that incorporates tertiary Gleason component in intuitive fashion, including Gleason score 6, 6.5 (Gleason score 6 with tertiary Gleason component), 7 (Gleason score 3 + 4 = 7), 7.25 (Gleason score 3 + 4 = 7 with tertiary Gleason component), 7.5 (Gleason score 4 + 3), 8 (Gleason score 4 + 3 with tertiary Gleason component or Gleason score 8), 8.5 (Gleason score 8 with tertiary Gleason component), 9 (Gleason score 4 + 5 or 5 + 4) and 10.
Keywords: prostate, prostatic neoplasms, prostatectomy, surgical pathology, prognosis
The Gleason grading system introduced more than 40 years ago describes the architectural patterns of tumor cells and categorizes the most prevalent pattern (primary grade) and the second most prevalent pattern (secondary grade), while adding the 2 grades gives the Gleason sum or GS.1,2 This system is well established as a prognostic tool for the pathological and clinical outcome after RP. However, since its introduction, many aspects of prostate cancer have changed, including the introduction of PSA testing, transrectal ultrasound guided prostate needle biopsy with greater sampling, immunohistochemistry for basal cells changing the classification of prostate cancer and the discovery of new prostate cancer variants, ie pseudohyper-plastic, foamy gland, mucinous and ductal. These dramatic changes in prostate cancer created the need to modify the Gleason grading system.
A consensus conference of international experts in urological pathology was recently convened to update the Gleason grading system.3 For needle biopsy specimens the panel agreed that tumors should not be graded by the primary and secondary pattern but by the primary and highest grade patterns, which in many cases represents TGC. For RP specimens the consensus was that a tertiary pattern should be commented on.3
To date there is relatively limited literature on the clinical significance of TGC in RP specimens and it is based on relatively small heterogeneous patient cohorts largely treated before 2000. However, existing studies are in agreement that TGC is significantly associated with other adverse pathological features and biochemical recurrence rates after RP.4–10
MATERIALS AND METHODS
We retrospectively reviewed the RP reports of clinically localized prostate cancer at our institution between 2000 and 2005 to identify TGC cases. All men underwent open radical prostatectomy and pelvic lymphadenectomy. RP specimens were processed at our institution. Prostates were inked to determine surgical margin status. The bladder neck margin was removed as a 1 mm thin shave margin and any tumor on the bladder neck margin slice was considered positive. The distal 5 to 8 mm of prostate were amputated and sectioned parallel to the urethra in 2 to 3 mm slices. Tumor at the inked perpendicular margins was considered a positive apical margin. After removing the apical and bladder neck margins the remaining prostate was sectioned at 2 to 3 mm intervals and entirely submitted for histological examination. The case GS was determined by the index tumor, which typically represented the largest and highest grade tumor nodule, as recommended by the Gleason consensus conference.3 Resection margins, extraprostatic extension extent and seminal vesicle invasion were defined according to our standard protocol.8
RP at our institution is diagnosed as having a tertiary component in 2 situations. The first situation is a third component of a Gleason pattern higher than the primary and secondary grades with the tertiary component visually estimated to be less than 5% of the whole tumor. When the third most common component is the highest grade and occupies greater than 5% of the tumor, it is recorded as the secondary pattern. The second situation is in cases of GS 3 + 3 = 6 and GS 4 + 4 = 8 when Gleason pattern 4/5 and Gleason pattern 5 is less than 5%, respectively. We restricted the study to the interval between 2000 and 2005 because TGC was not as consistently evaluated or recorded before 2000. This time frame also provided a contemporary cohort while still allowing sufficient followup for most recurrences to be identified. A total of 3,608 patients were identified, of whom 3,230 (90%) had complete data available on preoperative PSA, prostatectomy GS, pathological stage and followup for biochemical recurrence. They formed the patient sample for analysis.
Postoperative followup was obtained by routine serum PSA assay every 3 months for year 1, semiannually for year 2 and annually thereafter. Tumor progression was defined as a postoperative serum PSA increase of 0.2 ng/ml or greater and no included patients received adjuvant therapy before recurrence. Study data were obtained and analyzed according to an approved institutional review board protocol.
Univariate comparisons between participants with and without TGC were based on the t test for continuous variables and the chi-square test for categorical variables. The log rank test was used to compare Kaplan-Meier BRFS probabilities between groups with vs without TGC. Proportional hazards models were used to determine the HR for comparisons with vs without TGC. To determine whether the association of any given GS with BRFS was modified by TGC, we tested for interaction by adding cross-product terms between TGC and dummy variables for GS score categories and evaluating the change in the likelihood ratio. The overall accuracy of models was evaluated using the concordance index. All analyses were performed using SAS®, version 9.1 and Prism®, version 4.
RESULTS
Table 1 shows study cohort clinical and pathological characteristics. Overall 333 patients (10.3%) had TGC. The prevalence of nonorgan confined disease (extraprostatic extension, seminal vesicle involvement or lymph node metastasis) and positive surgical margins was twice as high in men with TGC (47% and 18%) as in men without TGC (23% and 8%, respectively). There were 166 recurrences (5.7%) in men without TGC and 57 (17.1%) in men with TGC.
Table 1.
Clinical and demographic characteristics in men who underwent radical prostatectomy from 2000 to 2005
| Characteristic | No TGC | TGC | p Value | ||
|---|---|---|---|---|---|
| No. pts | 2,897 | 333 | |||
| Mean ± SD age at surgery | 57.2 ± 6.62 | 57.9 ± 6.47 | 0.071 | ||
| No. race (%): | 0.044 | ||||
| White nonHispanic | 2,567 | (88.8) | 281 | (84.6) | |
| Black | 212 | (7.3) | 37 | (11.1) | |
| Other | 112 | (3.9) | 14 | (4.2) | |
| Mean ng/ml preop PSA (range) | 6.5 (0.1–62.2) | 7.9 (0.6–64.8) | < 0.0001 | ||
| No. postop GS (%):* | < 0.0001 | ||||
| 6 or Less | 2,005 | (69.2) | 178 | (53.5) | |
| 3 + 4 | 622 | (21.5) | 64 | (19.2) | |
| 4 + 3 | 166 | (5.7) | 60 | (18.0) | |
| 8 | 65 | (2.2) | 31 | (9.3) | |
| 9–10 | 39 | (1.4) | 0 | ||
| No. pathological stage (%): | < 0.0001 | ||||
| Organ confined | 2,225 | (76.8) | 176 | (52.9) | |
| Extraprostatic extension | 570 | (19.7) | 128 | (38.4) | |
| Seminal vesical involvement | 72 | (2.5) | 20 | (6.0) | |
| Lymph node metastasis | 30 | (1.0) | 9 | (2.7) | |
| No. surgical margin (%): | < 0.0001 | ||||
| Neg | 2,655 | (91.7) | 275 | (82.8) | |
| Pos | 239 | (8.3) | 57 | (17.2) | |
TGC definition restricted to GS 8 or less since GS 9 or 10 already includes pattern 5 component.
Median followup in censored study population cases was 2 years (range less than 1 to 7). Figures 1 to 4 each show 3 Kaplan-Meier BRFS curves for a specific GS without and with TGC, and the next highest GS without TGC. These figures show that the BRFS rate for cancer with TGC was intermediate between that of cancer without TGC in the same GS category and cancer in the next higher GS category. An exception was tumors with GS 4 + 3 = 7 with TGC 8, which showed a rate comparable to that of tumors with GS 8 without TGC (fig. 3). In a given GS category tumors with TGC had statistically significant lower BRFS than those without TGC (table 2). When comparing tumors with TGC to tumors of the next higher GS without TGC, there were no statistically significant differences in BRFS (table 2).
Figure 1.
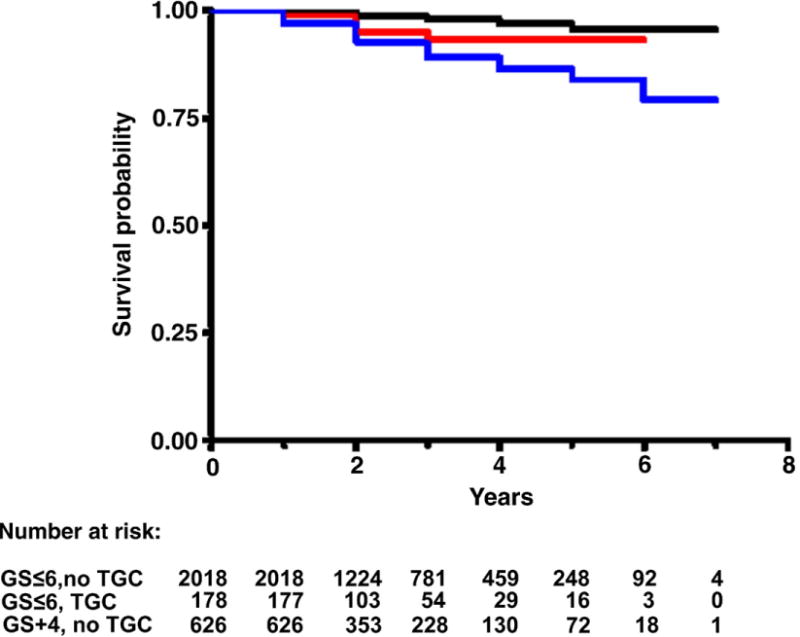
BRFS in men with GS 6 or less without TGC (black curve), 6 or less with TGC (red curve) and 3 + 4 without TGC (blue curve). Log rank chi-square (2 df) 68.7, p < 0.0001.
Figure 4.
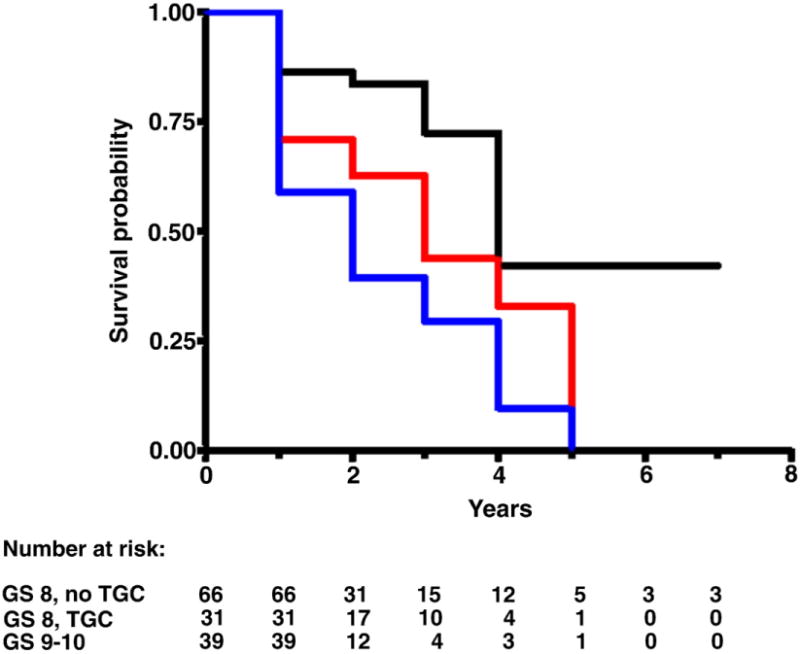
BRFS in men with GS 8 without TGC (black curve), 8 with TGC (red curve) and 9 to 10 without TGC (blue curve). Log rank chi-square (2 df) 16.6, p = 0.0003.
Figure 3.
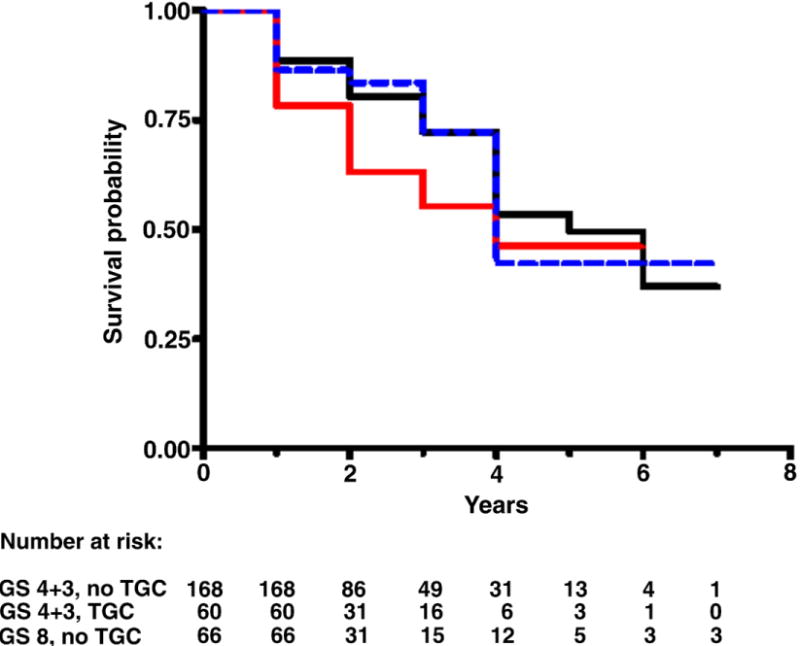
BRFS in men with GS 4 + 3 without TGC (black curve), 4 + 3 with TGC (red curve) and 8 without TGC (blue curve). Log rank chi-square (2 df) 1.8, p = 0.409.
Table 2.
BRFS probability in men without vs with TGC
| Log Rank p Value* (GS) |
No. at Risk† | BRFS (No. at risk)‡
|
Median BRFS (yrs) | |||
|---|---|---|---|---|---|---|
| 2 Yrs | 3 Yrs | |||||
| 0.015/0.094: | ||||||
| 6 Without TGC | 2,018 | 0.99 | (1,224) | 0.98 | (781) | Not attained |
| 6 With TGC | 178 | 0.95 | (103) | 0.93 | (54) | — |
| 3 + 4 Without TGC | 626 | 0.93 | (353) | 0.89 | (228) | — |
| 0.008/0.180: | ||||||
| 3 + 4 Without TGC | 626 | 0.93 | (353) | 0.89 | (228) | — |
| 3 + 4 With TGC | 64 | 0.89 | (42) | 0.85 | (23) | 5 |
| 4 + 3 Without TGC | 168 | 0.80 | (86) | 0.72 | (49) | 5 |
| 0.049/0.161: | ||||||
| 4 + 3 Without TGC | 168 | 0.80 | (86) | 0.72 | (49) | 5 |
| 4 + 3 With TGC | 60 | 0.63 | (31) | 0.55 | (16) | 4 |
| 8 Without TGC | 66 | 0.84 | (31) | 0.72 | (15) | 4 |
| 0.022/0.098: | ||||||
| 8 Without TGC | 66 | 0.84 | (31) | 0.72 | (15) | 4 |
| 8 With TGC | 31 | 0.63 | (17) | 0.44 | (10) | 3 |
| 9–10 Without TGC | 39 | 0.39 | (12) | 0.29 | (4) | 2 |
With vs without TGC in same GS/TGC vs next highest GS without TGC.
At beginning of followup
At beginning of period
Table 3 shows the results of a proportional hazards model of the impact of GS and TGC on BRFS, adjusting for overall GS, PSA, pathological stage and surgical margin status. Surgery year was tested in the models but was not statistically significant (data not shown). TGC was associated with a statistically significant 45% increase in the risk of biochemical recurrence independent of GS and other prognostic factors. Although TGC was a statistically significant independent prognostic factor in the model, it did not add to the overall accuracy of the multivariable model with a concordance index of 0.792 and 0.793 for models without and with TGC, respectively. The relative increase in the recurrence risk associated with TGC was comparable across GS categories (data not shown).
Table 3.
Multivariate proportional hazards model to predict biochemical recurrence in 3,226 men
| Variable | HR (95% CI) | p Value | |
|---|---|---|---|
| PSA (ng/ml): | |||
| 0–4.0 (referent) | 1.0 | ||
| 4.1–10.0 | 1.70 | (1.02, 2.83) | 0.0416 |
| 10.1–20.0 | 2.44 | (1.38, 4.32) | 0.0021 |
| Greater than 20.0 | 3.42 | (1.80, 6.52) | 0.0002 |
| Pathology stage: | |||
| Organ confined (referent) | 1.0 | ||
| Focal extraprostatic extension | 2.34 | (1.43, 3.85) | 0.0008 |
| Nonfocal extraprostatic extension | 3.57 | (2.42, 5.27) | < 0.0001 |
| Seminal vesicle involvement | 5.70 | (3.53, 9.20) | < 0.0001 |
| Lymph node metastasis | 5.82 | (3.24, 10.46) | < 0.0001 |
| Surgical margin status (pos vs neg) | 1.47 | (1.06, 2.04) | 0.0211 |
| Postop GS: | |||
| 6 or Less (referent) | 1.0 | ||
| 3 + 4 | 3.01 | (1.99, 4.56) | < 0.0001 |
| 4 + 3 | 6.82 | (4.38, 10.63) | < 0.0001 |
| 8 | 6.56 | (3.88, 11.07) | < 0.0001 |
| 9–10 | 18.45 | (10.38, 32.80) | < 0.0001 |
| TGC (yes vs no) | 1.45 | (1.04, 2.02) | 0.0285 |
DISCUSSION
The importance of commenting on TGC in RP specimens dates back to 2000 in a study from our institution of 114 RP cases with TGC from 1986 to 1995. This study suggested that TGC had an adverse impact on biological behavior, although multivariate analysis was not performed.3,7 Subsequent studies verified the prognostic importance of TGC in RP specimens. Rasiah et al noted that patients with Gleason 4 + 3 and tertiary Gleason 5 pattern had significantly worse biochemical progression-free survival than patients with primary Gleason 4 and no TGC.11 However, only 17 cases had a tertiary pattern. Similar results were reported by Hattab et al, who found that patients with Gleason 7 (4 + 3 and 3 + 4) had significantly higher biochemical recurrence when a tertiary Gleason 5 pattern was present.5 Similar to the study of Rashia et al, limitations included small sample size with a tertiary pattern, that is 37 and 13 patients with Gleason 4 + 3 + 5 and 3 + 4 + 5, respectively, and the lack of comparison to higher grade groups (Gleason 8 to 10).
van Oort et al investigated the impact of a tertiary Gleason pattern without thorough stratification for primary and secondary Gleason data, stating that a tertiary pattern was associated with worse biochemical outcome.6 More recently Sim et al reported 509 RPs with GS 7, including 66 with TGC.9 On multivariate analysis TGC was associated with biochemical recurrence. On subgroup analysis comparing patients with Gleason sum 3 + 4 + 5 and 4 + 3 + 5 to respective reference groups without TGC the TGC groups tended toward a higher progression rate. Finally, Whittemore et al analyzed 214 cases of GS 7, including 36 with TGC.10 Patients with GS 7 and TGC had significantly decreased BRFS than those with GS without TGC, although this was marginally nonsignificant on multivariate analysis (p = 0.053).10
We expanded on these findings in what is to our knowledge the largest, most contemporary series to date in 3,230 men, including 333 with TGC. TGC in the RP specimen was associated with an increased risk of biochemical recurrence regardless of GS and it typically increased the recurrence risk to a level intermediate between that of cancer without TGC in the same GS category and that of cancer in the next higher GS category. Recurrence was significantly higher for TGC in a GS category but there was no significant difference between lower GS with TGC compared to the next higher GS without TGC. This suggests that the behavior of tumors with TGC is more similar to that of tumors in the next highest GS than to that of tumors without TGC but with the same GS.
TGC was associated with a statistically significant doubling of the risk of positive margins and nonorgan confined disease (table 1). Similar findings were reported in prior studies.8–10 However, multivariate analysis revealed that the 45% increase in the recurrence risk associated with TGC was independent of stage, margin status and GS. Although TGC was a statistically significant independent prognostic factor, it did not add to overall model predictive accuracy, as indicated by the minimal change in the concordance index when TGC was added to other established prognostic factors. This reflects the fact that TGC correlates with stage and GS so that, when added to the model, it replaces some of the predictive ability already inherent in those factors. This does not negate the observation that in our cohort TGC was an indication of biologically more aggressive behavior that should be noted by the pathologist.
Although TGC has typically been added to pathology reports since the consensus conference, it is routinely omitted in practice since there is no simple way to incorporate it in predictive nomograms/tables, research studies and patient counseling. Thus, consideration should be given to incorporating TGC into a modified RP Gleason scoring system in intuitive fashion (see Appendix). An option would be to report GS with TGC as is currently performed along with the modified GS, ie GS 3 + 3 = 6 with tertiary pattern 4/5 (GS 6.5). Currently urologists at our institution typically counsel a patient with GS 6 and TGC 4 that GS is between 6 and 7. As such, formalizing this information with a modified GS 6.5 would not cause a dramatic change in practice. Although it may be argued that the modified GS system for RP is more complicated using various fractions of a grade, it is less complex and more intuitive than adding TGC to each GS.
A potential criticism of our study is that GS 3 + 3 = 6 with less than 5% pattern 4 should be diagnosed as GS 3 + 4 = 7 and not as GS 6 with TGC pattern 4. Similarly GS 4 + 4 = 8 with minor pattern 5 should be diagnosed as GS 4 + 5 = 9. Stamey et al were some of the first to note that an increasing percent of pattern 4/5 is associated with an adverse prognosis.12 As our data show, pattern 4 TGC worsens the prognosis of GS 6 but not to the level of GS 3 + 4 = 7, such that GS 6.5 is a more accurate grade. Similarly GS 8 with pattern 5 TGC does not behave as aggressively as GS 9. Our modified Gleason system also incorporates into the grade the well recognized prognostic difference at RP between GS 3 + 4 = 7 and GS 4 + 3 = 7, ie different proportions of greater than 5% Gleason pattern 4 by assigning a GS of 7 and 7.5, respectively.13
Further support for modifying the Gleason system as a result of TGC comes from a large study of the importance of TGC on needle biopsy. Patel et al noted that patients with GS 7 and tertiary grade 5 on prostate biopsy were at higher risk for an adverse outcome after RP and radiotherapy than patients with GS 7 and no tertiary pattern but at comparable risk for biochemical recurrence compared to patients with GS 8 to 10.14 They concluded that in patients with biopsy GS 7 and TGC 5 the GS should be calculated by adding the most prevalent Gleason grade pattern and the highest Gleason grade pattern, resulting in a GS of 8 or 9. Their data concur with the recently published consensus conference on grading prostate cancer.3,7 This change in the grading system in TGC cases on needle biopsy is a major departure from the original Gleason system, which was derived by summing the most prevalent and second most prevalent patterns. Our proposal incorporating fractions of a grade to account for TGC in RP specimens is not as fundamental a departure from the original Gleason system.
Our study has some limitations. 1) The median followup was only 2 years. Although this is relatively short, most biochemical recurrences develop within 3 years of surgery. Moreover, given the large sample size and number of recurrence events, we believe that these results will hold with longer followup. This potential limitation may be offset by our use of a contemporary cohort not confounded by era effects,11 in which a consistent approach was used for Gleason grade assessment and TGC evaluation. 2) Because of its retrospective nature, the study is susceptible to possible selection bias. Although we noted that TGC was an independent prognostic factor when known confounding factors were controlled for, this does not correct for unknown selection factors that could influence the composition of the analysis cohort. Thus, it is important that our findings be confirmed in prospective studies.
CONCLUSIONS
Recurrence-free survival curves for cancer with TGC are intermediate between those of cancer without TGC in the same GS category and cancer in the next higher GS category. To facilitate the use of TGC in clinical practice we recommend a modified Gleason system for RP specimens that incorporate TGC into GS.
Figure 2.
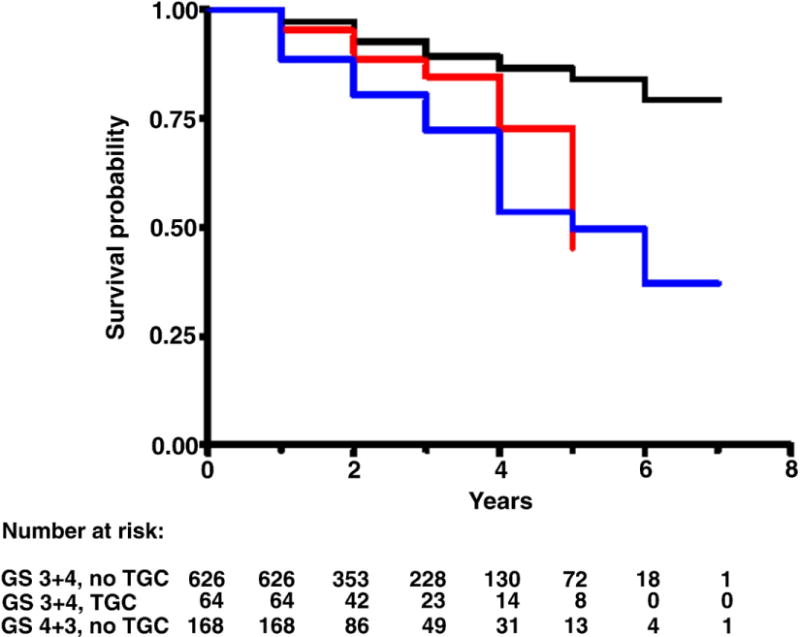
BRFS in men with GS 3 + 4 without TGC (black curve), 3 + 4 with TGC (red curve) and 4 + 3 without TGC (blue curve). Log rank chi-square (2 df) 52.5, p < 0.0001.
Abbreviations and Acronyms
- BRFS
biochemica recurrence-free surviva
- GS
Gleason score
- PSA
prostate specific antigen
- RP
radical prostatectomy
- TGC
tertiary Gleason component
APPENDIX
Proposed Modified RP GS System Incorporating TGC
| Current GS | Modified GS |
|---|---|
| GS 3 + 3 = 6 | GS 6 |
| GS 3 + 3 = 6 with TGC | GS 6.5 |
| GS 3 + 4 = 7 | GS 7 |
| GS 3 + 4 = 7 with TGC | GS 7.25 |
| GS 4 + 3 = 7 | GS 7.5 |
| GS 4 + 3 = 7 with TGC, GS 8 | GS 8 |
| GS 8 with TGC | GS 8.5 |
| GS 9 | GS 9 |
| GS 10 | GS 10 |
References
- 1.Mellinger GT, Gleason D, Bailar J., 3rd The histology and prognosis of prostatic cancer. J Urol. 1967;97:331. doi: 10.1016/S0022-5347(17)63039-8. [DOI] [PubMed] [Google Scholar]
- 2.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JI, Allsbrook WC, Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 4.Harnden P, Shelley MD, Coles B, et al. Should the Gleason grading system for prostate cancer be modified to account for high-grade tertiary components? A systematic review and meta-analysis. Lancet Oncol. 2007;8:411. doi: 10.1016/S1470-2045(07)70136-5. [DOI] [PubMed] [Google Scholar]
- 5.Hattab EM, Koch MO, Eble JN, et al. Tertiary Gleason pattern 5 is a powerful predictor of biochemical relapse in patients with Gleason score 7 prostatic adenocarcinoma. J Urol. 2006;175:1695. doi: 10.1016/S0022-5347(05)00998-5. [DOI] [PubMed] [Google Scholar]
- 6.van Oort IM, Schout BM, Kiemeney LA, et al. Does the tertiary Gleason pattern influence the PSA progression-free interval after retropubic radical prostatectomy for organ-confined prostate cancer? Eur Urol. 2005;48:572. doi: 10.1016/j.eururo.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Pan CC, Potter SR, Partin AW, et al. The prognostic significance of tertiary Gleason patterns of higher grade in radical prostatectomy specimens: a proposal to modify the Gleason grading system. Am J Surg Pathol. 2000;24:563. doi: 10.1097/00000478-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Mosse CA, Magi-Galluzzi C, Tsuzuki T, et al. The prognostic significance of tertiary Gleason pattern 5 in radical prostatectomy specimens. Am J Surg Pathol. 2004;28:394. doi: 10.1097/00000478-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Sim HG, Telesca D, Culp SH, et al. Tertiary Gleason pattern 5 in Gleason 7 prostate cancer predicts pathological stage and biochemical recurrence. J Urol. 2008;179:1775. doi: 10.1016/j.juro.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Whittemore DE, Hick EJ, Carter MR, et al. Significance of tertiary Gleason pattern 5 in Gleason score 7 radical prostatectomy specimens. J Urol. 2008;179:516. doi: 10.1016/j.juro.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 11.Rasiah KK, Stricker PD, Haynes AM, et al. Prognostic significance of Gleason pattern in patients with Gleason score 7 prostate carcinoma. Cancer. 2003;98:2560. doi: 10.1002/cncr.11850. [DOI] [PubMed] [Google Scholar]
- 12.Stamey TA, McNeal JE, Yemoto CM, et al. Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999;281:1395. doi: 10.1001/jama.281.15.1395. [DOI] [PubMed] [Google Scholar]
- 13.Chan TY, Partin AW, Walsh PC, et al. Prognostic significance of Gleason score 3 + 4 vs Gleason score 4 + 3 tumor at radical prostatectomy. Urology. 2000;56:823. doi: 10.1016/s0090-4295(00)00753-6. [DOI] [PubMed] [Google Scholar]
- 14.Patel AA, Chen MH, Renshaw AA, et al. PSA failure following definitive treatment of prostate cancer having biopsy Gleason score 7 with tertiary grade 5. JAMA. 2007;298:1533. doi: 10.1001/jama.298.13.1533. [DOI] [PubMed] [Google Scholar]


