Abstract
Endocytosis is a fundamental process of eukaryotic cells and fulfills numerous functions, most notably, that of macromolecular nutrient uptake. Malaria parasites invade red blood cells and during their intracellular development endocytose large amounts of host cytoplasm for digestion in a specialized lysosomal compartment, the food vacuole. In the present study we have examined the effects of artemisinin and the quinoline drugs chloroquine and mefloquine on endocytosis in Plasmodium falciparum. By using novel assays we found that mefloquine and artemisinin inhibit endocytosis of macromolecular tracers by up to 85%, while the latter drug also leads to an accumulation of undigested hemoglobin in the parasite. During 5-h incubations, chloroquine inhibited hemoglobin digestion but had no other significant effect on the endocytic pathway of the parasite, as assessed by electron microscopy, the immunofluorescence localization of hemoglobin, and the distribution of fluorescent and biotinylated dextran tracers. By contrast, when chloroquine was added to late ring stage parasites, followed by a 12-h incubation, macromolecule endocytosis was inhibited by more than 40%. Moreover, there is an accumulation of transport vesicles in the parasite cytosol, possibly due to a disruption in vacuole-vesicle fusion. This fusion block is not observed with mefloquine, artemisinin, quinine, or primaquine but is mimicked by the vacuole alkalinizing agents ammonium chloride and monensin. These results are discussed in the light of present theories regarding the mechanisms of action of the antimalarials and highlight the potential use of drugs in manipulating and studying the endocytic pathway of malaria parasites.
During their residence inside host erythrocytes (RBCs), malaria parasites are known to endocytose large amounts of the surrounding RBC cytoplasm through an invagination of the parasite plasma membrane known as the cytostome (40). The ingested cytoplasm is initially contained in typical double-membrane transport vesicles, where some proteolytic digestion of the contents may already have occurred (18). The outer membrane of the transport vesicle is presumably derived from the invaginated parasite plasma membrane and the inner membrane from the parasitophorous vacuole membrane that tightly envelops the parasite and shields it from the RBC cytoplasm. The endocytosed host cytoplasm is finally delivered to the food vacuole, a specialized parasite version of a mammalian lysosome, where it is digested at a pH of between 5.0 and 5.5 (16). Since the endocytosed RBC cytoplasm consists almost entirely of hemoglobin, proteolytic digestion in the food vacuole results in the release of large and potentially toxic amounts of the ferriprotoporphyrin IX prosthetic group of the protein (heme). The parasite solves this hazardous waste problem by crystallizing the heme monomers into an insoluble substance known as hemozoin (39). The food vacuole proteases responsible for digestion of endocytosed hemoglobin have been well characterized (3); however, little else is known regarding the organization or molecular machinery of the endocytic pathway in malaria parasites, apart from the broad morphological description provided above.
Despite their waning clinical usefulness due to toxicity concerns and growing parasite resistance, the quinoline antimalarials chloroquine and mefloquine are being intensely studied to determine their intracellular targets, modes of action, structure-function relationships, and resistance mechanisms (for a review, see reference 11). This information could be invaluable in producing novel, more efficacious derivatives and preempting or overcoming resistance. By contrast, the artemisinins are a very potent new class of endoperoxide antimalarials, derived from a traditional Chinese herbal remedy, that promise to overcome the resistance problems experienced with the quinoline drugs (22).
There is often a lack of general consensus regarding the precise mechanisms and intracellular effects of these three important antimalarials; however, it is clear that their modes of action in each case rely critically on the digestion of endocytosed hemoglobin in the parasite food vacuole (33). Chloroquine interferes with the crystallization of the free heme monomers released during hemoglobin proteolysis by binding to them, causing a buildup of toxic heme and chloroquine-heme complexes (5, 7, 23) that can potentially inhibit key enzymes as well as bind to the membrane and compromise membrane integrity (14, 36). Less certainty exists about the action of mefloquine, but studies suggest that it also has the ability to interfere with hemozoin formation, resulting in free heme accumulation (44). The endoperoxide bridge of artemisinin may be cleaved in a heme Fe2+-catalyzed reaction to yield a reactive radical species that can covalently modify various parasite proteins (31). In addition, artemisinin may also bind to the heme transiently available in the vacuole during hemoglobin digestion to form adducts that could inhibit the proteolytic enzymes in the vacuole (34). The latter findings are disputed, however, by a recent study which shows that artemesinin is activated by nonheme Fe2+ in the parasite cytoplasm and inhibits the parasite sarcoplasmic and endoplasmic reticulum Ca2+ ATPase (SERCA) by a mechanism independent of hemoglobin digestion (6).
Interestingly, antimalarials have been shown to have various effects on endocytosis and the endocytic pathway of mammalian cells. Both artemisinin and mefloquine inhibit phagocytosis in immune cells (28, 49), the 8-aminoquinoline primaquine perturbs endosome behavior in Hep-G2 cells (48), while chloroquine is widely used as a lysosomotropic agent to disrupt lysosome function and trafficking in the endocytic and secretory pathways (17, 32, 37, 41, 45). Although these effects are not usually achieved with concentrations in the parasiticidal ranges of these drugs, the extensive conservation of the basic cell biological machinery and processes in eukaryotic cells presents at least the formal possibility that the drugs could have similar effects in the parasite as well.
The actions of antimalarials on the endocytic pathway and compartments of malaria parasites have not been extensively investigated beyond the visualization of some superficial alterations in morphology by electron microscopy (19-21, 51). By sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) analysis it has been found that artemisinin leads to hemoglobin accumulation in the murine malaria species Plasmodium yoelii, possibly due to inhibition of vacuolar proteases by artemisinin-heme complexes (34). Similarly, chloroquine also leads to hemoglobin buildup in the murine species Plasmodium berghei and the FCR3 strain of Plasmodium falciparum (8, 9, 52). This effect has been ascribed to an inhibition of vacuolar hydrolases responsible for removing the inner membrane of endocytic vesicles following their fusion with the vacuole: there appears to be a buildup of hemoglobin-filled vesicles surrounded by a single membrane inside the vacuole of treated parasites when viewed by electron microscopy (51). In addition, a chloroquine-induced swelling of the vacuole has been reported (20), an effect that is also found in mammalian cells. Finally, a reduction in the hemoglobin content in parasites treated with mefloquine compared with that in controls and the fact that this drug antagonizes chloroquine toxicity in the FCR3 strain of P. falciparum have led to the proposal that mefloquine inhibits the actual endocytosis of hemoglobin by the parasite (8).
In this study, we found by using novel endocytosis assays that mefloquine strongly inhibits endocytosis in the chloroquine-sensitive D10 strain of P. falciparum. Artemisinin has a similar effect on endocytosis, in addition to its inhibition of hemoglobin digestion. Chloroquine produces a more moderate inhibition of endocytosis when it is added to the late ring stage, but it causes an apparent block in transport vesicle-vacuole fusion under these conditions, as assessed by electron and fluorescence microscopy, an effect that appears to be mimicked by ammonium chloride and monensin. Over shorter incubation periods, however, chloroquine has no visible effect on endocytosis or vacuole behavior, apart from inhibition of hemoglobin digestion.
MATERIALS AND METHODS
Materials.
RPMI 1640 powdered culture medium (with glutamine, without bicarbonate) was purchased from Highveld Biologicals (Johannesburg, South Africa). Albumax II was obtained from GIBCO BRL; type O-positive RBCs were obtained from the Western Province Blood Transfusion Service (Cape Town, South Africa); biotin-dextran (Mr, 70,000) and polyvinyl alcohol antifading mounting medium were obtained from Fluka (Sigma-Aldrich GmbH, Steinheim, Germany); Percoll, fluorescein isothiocyanate (FITC)-dextran (Mr, 70,000), and rabbit antihemoglobin antiserum were obtained from Sigma-Aldrich (St. Louis, Mo.); rhodamine goat anti-rabbit immunoglobulin G (IgG) was obtained from Calbiochem-Novabiochem (La Jolla, Calif.); peroxidase-conjugated goat anti-rabbit IgG was obtained from Kirkegaard & Perry Laboratories, Inc. (Gaithersburg, Md.); and streptavidin-peroxidase conjugate and recombinant streptavidin were obtained from Roche Diagnostics GmbH (Mannheim, Germany). Artemisinin, chloroquine, and monensin were supplied by Sigma-Aldrich.
Parasite culture and enrichment.
The D10 strain of P. falciparum was cultured in RPMI 1640 medium supplemented with 50 mM glucose, 0.65 mM hypoxanthine, 25 mM HEPES, 0.2% (wt/vol) NaHCO3, 0.048 mg of gentamicin per ml, 0.5% (wt/vol) Albumax II, and 2 to 4% (vol/vol) type O-positive RBCs under an atmosphere of 3% CO2-4% O2-93% N2. Parasites were synchronized by the sorbitol method (29). Late trophozoite- or schizont-infected RBCs were enriched by sorbitol-Percoll density centrifugation: 600 μl of a solution of 80% (vol/vol) Percoll in RPMI 1640 medium containing 5% (wt/vol) sorbitol was added to a microcentrifuge tube, and an equal volume of 60% Percoll was layered on top. Packed RBCs from a culture with 10 to 30% parasitemia (200 to 300 μl) was carefully pipetted onto the Percoll step gradient and centrifuged at 10,000 × g for 20 min in a microcentrifuge. Following centrifugation, RBCs containing mature stage parasites were recovered from the 80% Percoll-60% Percoll interface and washed in parasite culture medium.
Drug treatments.
Unless stated otherwise, drugs were added to parasite cultures at the following concentrations: chloroquine, 120 nM; mefloquine, 60 nM; artemisinin, 110 nM; primaquine, 15 μM; quinine, 1 μM; ammonium chloride, 20 mM; monensin, 30 nM. The 50% inhibitory concentrations of the various drugs for the D10 strain of P. falciparum were determined by a parasite lactate dehydrogenase assay (10) and were found to be as follows: chloroquine, 33 nM; mefloquine, 8 nM; artemisinin, 22 nM; primaquine, 1.5 μM; quinine, 210 nM; ammonium chloride, 3.4 mM; monensin, 1.8 nM. Drugs were added to parasite cultures for 12 to 14 h (i.e., they were added to parasites in the late ring stage) or 5 to 6 h (i.e., they were added in the early to parasites in the middle trophozoite stage), and the parasites were assayed in the middle to late trophozoite stage (i.e., before nuclear division takes place, as assessed by Giemsa staining of thin blood smears or fluorescence microscopy of 4′,6′-diamidino-2-phenylindole-stained parasites).
Electron microscopy.
Packed erythrocytes from a P. falciparum culture were washed in phosphate-buffered saline (PBS) and fixed in PBS containing 2.5% (wt/vol) glutaraldehyde for 4 h. Fixed cells were subsequently pelleted, immobilized in 1.5% low-melting-point agarose, and postfixed in 2% (wt/vol) OsO4 for 1 h. Agarose plugs containing fixed infected RBCs were dehydrated in a series of ascending ethanol concentrations and embedded in Spurr resin. Ultrathin sections were prepared with an ultramicrotome, contrasted with uranyl acetate and lead citrate, and viewed with a JEOL 100S transmission electron microscope.
FITC-dextran endocytosis assay.
Fresh RBCs (300 μl of packed cells) were preloaded with FITC-dextran as described previously (25). The cells were washed in parasite culture medium and added to 20 to 30 μl of enriched trophozoite- or schizont-infected RBCs in 15 ml of culture medium. After 40 h in culture (in the middle to late trophozoite stage), the cells were pelleted and resuspended in 0.25% (wt/vol) saponin in PBS to lyse the RBC membranes, release the intact parasites, and remove the excess hemoglobin. Under these conditions, more than 80% of the parasites are completely removed from their host RBCs and are free of surrounding RBC ghost membranes, as assessed by phase-contrast light microscopy and transmission electron microscopy. Parasites were washed in PBS, fixed in PBS containing 3% (wt/vol) paraformaldehyde and 0.25% (wt/vol) glutaraldehyde, mounted on a microscope slide in polyvinyl alcohol mounting medium containing DABCO (1,4-diazabicyclo[2.2.2]octane) antifading agent, and examined by fluorescence microscopy.
Immunofluorescence assay.
Glass coverslips were coated in polylysine and distributed into a 24-well plate containing PBS. Twenty microliters of infected RBCs was resuspended in 200 μl of PBS, and 40 μl of the suspension was pipetted into each plate well. The cells were pelleted onto the polylysine coverslips by centrifugation at 100 × g for 2 min. Excess, unbound RBCs were flushed from the coverslips by pipetting. RBC membranes were lysed and excess hemoglobin was removed by briefly rinsing the coverslips in PBS containing 0.04% (wt/vol) saponin and additional washing in PBS. The coverslips were fixed in methanol and incubated for 30 min in blocking solution (PBS containing 1 mM CaCl2, 1 mM MgCl2, 2% [wt/vol] bovine serum albumin [BSA], and 10% [vol/vol] fetal calf serum), followed by incubation for 1 h in blocking solution containing rabbit antihemoglobin antiserum (dilution, 1:500). After several washes in PBS, the coverslips were incubated in blocking solution containing rhodamine-conjugated goat anti-rabbit Ig secondary antibody (1:250), washed in PBS, rinsed in water, mounted in mounting medium, and examined by fluorescence microscopy.
Biotin-dextran endocytosis assay.
Fresh RBCs were preloaded with biotin-dextran as described above for the FITC-dextran endocytosis assay, except that the FITC-dextran in the hypotonic solution was replaced with 0.45 mg of biotin-dextran per ml. As described above, enriched trophozoite-infected parasitized RBCs (pRBCs) were added to the preloaded RBCs and placed into culture for 40 h. The amount of biotin-dextran associated with the parasites at the end of the assay was quantitated by a modified sandwich enzyme-linked immunosorbent assay approach. Parasites were released from the infected RBCs by suspending the latter in PBS containing 0.25% (wt/vol) saponin, pelleted at 1,500 × g for 3 min, and washed six times in PBS. The parasite pellet was lysed by adding 50 μl of TBS (20 mM Tris, 150 mM NaCl [pH 7.4]) containing 0.5% (vol/vol) Triton X-100 and vortexing, followed by the addition of an additional 350 μl of TBS containing 0.1% (vol/vol) Tween 20 and 1% (wt/vol) BSA (TBST-BSA). Four serial dilutions of this parasite lysate were performed by removing a 200-μl aliquot and adding an additional 200 μl of TBST-BSA. Each dilution of the parasite lysate was subsequently added in quadruplicate to a microtiter plate in which each well had been precoated for 30 min with 0.2 μg of streptavidin in 0.1 M NaHCO3 (pH 9.5) and blocked for 1 h in TBST-BSA. After an overnight incubation at 4°C, the parasite lysates were removed, and the plate wells were washed three times in TBST and incubated for 1.5 h in TBST-BSA containing peroxidase-conjugated streptavidin (1:1,000). Following four washes, the wells were developed in peroxidase substrate solution (0.1 M phosphate-citrate buffer [pH 4.8] containing 1 mg of o-phenylenediamine per ml and 0.015% H2O2). Color development was stopped by adding 50 μl of 2.5 N sulfuric acid to each well and was quantitated spectrophotometrically at 490 nm with an enzyme-linked immunosorbent assay plate reader. In addition to the parasite lysates, the microtiter plate was also incubated with serial dilutions of a biotin-dextran standard solution (0 to 37.5 μg/ml) in order to draw up a standard curve of the number of micrograms of biotin-dextran versus the A490. Parasite lysate dilutions which gave values that fell in the linear range of the standard curve were used to calculate the amount of biotin-dextran endocytosed by the original parasites by using GraphPad Prism (version 3) software.
Western blotting.
RBCs from a 10 ml-culture were suspended in 1 ml of 0.25% (wt/vol) saponin in PBS to lyse the RBC membranes. The released parasites were pelleted at 1,500 × g for 3 min and washed five times in cold PBS to remove excess hemoglobin. The parasite pellet was solubilized in 150 μl of reducing SDS-PAGE sample buffer, 10-μl were aliquots run on an SDS-11% polyacrylamide gel, and the resolved proteins transblotted onto Hybond ECL nitrocellulose membranes (Amersham Pharmacia Biotech). The membranes were incubated in blocking buffer (20 mM Tris and 0.15 M NaCl [pH 7.4] containing 0.1% [vol/vol] Tween 20, 1% [wt/vol] BSA, and 2% [wt/vol] fat-free milk powder), followed by incubation in blocking buffer containing rabbit antihemoglobin antiserum (1:5,000) and blocking buffer containing peroxidase-conjugated goat anti-rabbit IgG (1:5,000). The membranes were washed, soaked with an enhanced chemiluminescence Western blotting detection reagent (Amersham Pharmacia Biotech), and exposed to Kodak BioMax Light autoradiography film to detect bound secondary antibodies. Images of the developed autoradiographs were captured with a Kodak EDAS 290 gel documentation system that incorporates a Kodak DC290 digital camera. The images were analyzed, and the net intensities of individual bands were determined with Kodak 1D image analysis software (version 3.5).
RBC-free endocytosis assay.
RBC lysate was obtained by vortexing 4 ml of packed fresh RBCs in 4 ml of water. To prepare the endocytosis mixture, the lysate was added to 4.8 ml of 2× RPMI 1640 medium (supplemented with 100 mM glucose, 50 mM HEPES, 1.3 mM hypoxanthine, 0.1 mg of gentamicin per ml, and 0.2% [wt/vol] Albumax II), 224 μl of 10% (wt/vol) NaHCO3, and 800 μl of a stock FITC-dextran solution (10 mg/ml in water). RBC ghost membranes were removed by centrifugation at 1,000 × g for 5 min. Free trophozoite stage parasites were obtained by resuspending the RBCs from a 15-ml culture in 1 ml of RPMI 1640 wash medium (culture medium without Albumax II) containing 0.25% (wt/vol) saponin, pelleting the parasites at 1,500 × g for 3 min in a microcentrifuge, and washing once in wash medium. The parasites were resuspended in 8 ml of the endocytosis mixture. Aliquots of 1 ml were pipetted into culture flasks, gassed with a 3% CO2-4% O2-93% N2 atmosphere, and incubated in a shaking incubator at 37°C for 5 h. Following incubation, the parasites were pelleted at 1,500 × g for 3 min, washed twice in ice-cold wash medium, fixed in PBS containing 3% (wt/vol) paraformaldehyde and 0.25% (wt/vol) glutaraldehyde, washed in PBS, and mounted under a coverslip in antifading polyvinyl alcohol mounting medium for examination by fluorescence microscopy.
Fluorescence microscopy.
The microscope slides were examined with a Nikon Eclipse E600 fluorescence microscope fitted with a ×100 Apochromat objective. The images were captured with a Media Cybernetics CoolSNAP-Pro monochrome cooled charge-couple device camera. The mean intensity of the fluorescence in the parasite vacuoles was determined by using the histogram function of Adobe Photoshop (version 7.0) software.
RESULTS
Effect of drug treatment on hemoglobin levels in parasites.
Previous reports have suggested that chloroquine blocks hemoglobin degradation and should therefore produce an accumulation of undigested hemoglobin in treated parasites and, moreover, that quinoline antimalarials may differ in this regard (8, 52). To determine the effects of antimalarial drugs on the hemoglobin levels in the D10 strain of P. falciparum, parasites were incubated with chloroquine, mefloquine, and artemisinin for 12 h; released from the RBCs by saponin treatment, and washed extensively to remove extraneous hemoglobin. Parasite pellets were run on SDS-polyacrylamide gels, and the hemoglobin levels in the parasites were determined by Western blotting with antihemoglobin antiserum (Fig. 1A). The results confirm that chloroquine treatment leads to a striking increase in hemoglobin levels compared to those in the control parasites. Artemisinin treatment also reproducibly increased the hemoglobin levels, although to a much lesser extent than chloroquine. By contrast, mefloquine appeared to decrease parasite hemoglobin levels compared to those in the controls, which is in agreement with previously published results (8).
FIG. 1.
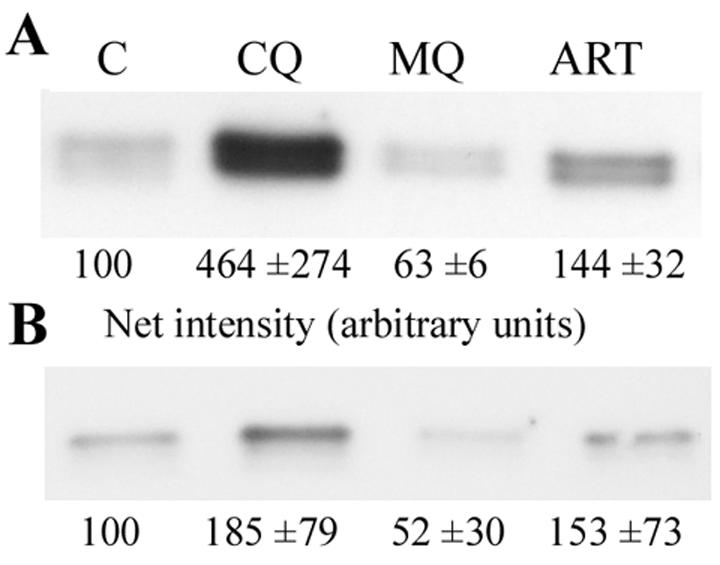
Hemoglobin accumulation in parasites. Parasite cultures were left untreated (control; C) or were incubated with chloroquine (CQ), mefloquine (MQ), or artemisinin (ART) for 12 h (A) or 5 h (B). Parasites were released from RBCs by saponin treatment and were analyzed for their hemoglobin contents by Western blotting with an antihemoglobin antiserum. The net intensity of the individual hemoglobin bands was determined with Kodak 1D image analysis software. The intensity values indicated are normalized values ± SD obtained from four different experiments in each case.
Chloroquine treatment results in an accumulation of hemoglobin in transport vesicles.
Ultrastructural studies have suggested that hemoglobin accumulation in chloroquine-treated parasites may result from the presence of undigested hemoglobin-filled vesicles in the parasite food vacuole (51). To determine the subcellular location of hemoglobin in parasites, control parasites and parasites cultured with chloroquine for 12 h were fixed and reacted with antihemoglobin antiserum in an immunofluorescence assay (Fig. 2). In control parasites, hemoglobin was located prominently in the food vacuole (Fig. 2A, arrow). The latter compartment is easily located by phase-contrast microscopy of fixed parasites, due to the presence of a prominent, shiny hemozoin crystal formed there from heme moieties released during hemoglobin digestion (39) (Fig. 2, right panels, arrows). Note that the RBC surrounding the parasite is not visible in these images, due to a brief saponin lysis which is carried out prior to parasite fixation in order to remove extraparasitic hemoglobin, reduce background levels, and improve the quality of the immunofluorescence signals. In contrast to the controls, chloroquine-treated parasites displayed little or no fluorescence in the food vacuole (Fig. 2B, arrow), and hemoglobin was found to be concentrated in separate punctate structures. This suggests that the accumulation of undigested hemoglobin during chloroquine treatment (Fig. 1A) may be due to a failure to correctly deliver endocytosed hemoglobin to the food vacuole.
FIG. 2.
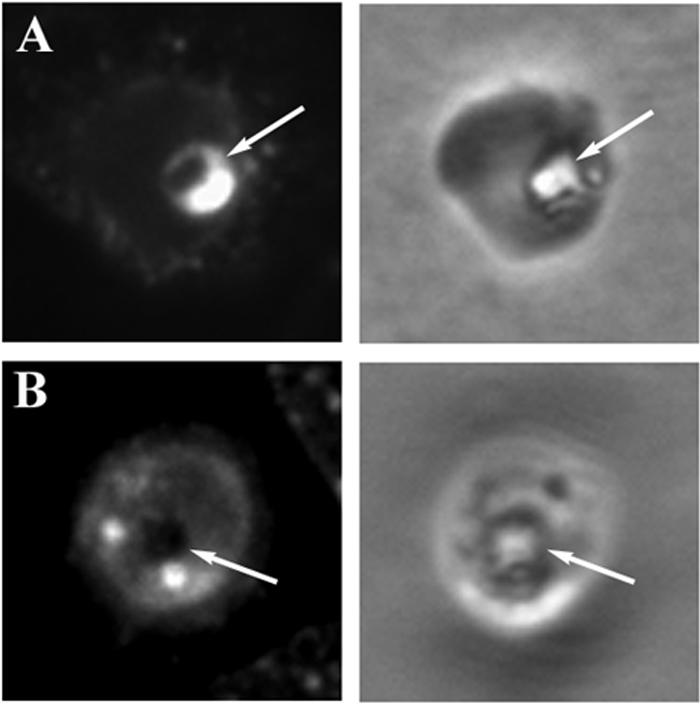
Subcellular localization of hemoglobin by immunofluorescence. pRBCs from a control culture (A) and one treated with chloroquine for 12 h (B) were immobilized on glass coverslips, rinsed with saponin to release extraparasitic hemoglobin, fixed, permeabilized, and incubated with antihemoglobin antiserum and fluorescent secondary antibodies. Left-hand panels, fluorescence images; right-hand panels, the corresponding phase-contrast images. A single parasite can be seen in each phase-contrast image. The surrounding RBC is not visible due to saponin lysis, but the position of the food vacuole is denoted by the easily identifiable hemozoin crystal (arrows).
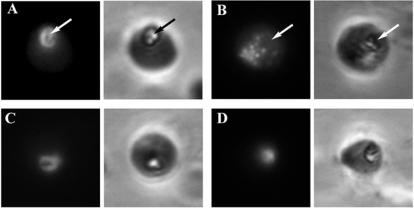
To further investigate the effect of chloroquine on the trafficking of macromolecules ingested by the parasite from the RBC cytosol, RBCs were preloaded with FITC-dextran and infected by parasites, and the location of the dextran following a 12-h drug treatment was determined by fluorescence microscopy (Fig. 3). In control parasites, the fluorescent dextran collected in the parasite food vacuole (Fig. 3A, arrow), while chloroquine treatment again led to the accumulation of punctate structures containing fluorescent tracer clearly distinct from the food vacuole (Fig. 3B, arrow). This effect appeared to be specific to chloroquine, since treatment of parasites with artemisinin (Fig. 3D) and the other quinoline antimalarials, mefloquine (Fig. 3C), primaquine, and quinine (data not shown), did not divert the endocytosed material from the vacuole and resulted in fluorescence images qualitatively indistinct from those for the controls.
FIG. 3.
Parasite endocytosis of FITC-dextran from preloaded RBCs. RBCs preloaded with FITC-dextran and infected with parasites were left untreated (A) or were treated with chloroquine (B), artemisinin (C), or mefloquine (D) for 12 h. The pRBCs were subsequently immobilized on glass coverslips, rinsed in saponin, fixed, and viewed by fluorescence microscopy. Left panels, fluorescence images; right panels, corresponding phase-contrast images. Arrows denote the locations of the food vacuole, as indicated by the presence of the prominent hemozoin crystal.
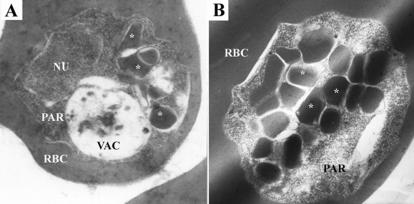
To confirm ultrastructurally that the punctate structures found in chloroquine-treated parasites represented hemoglobin transport vesicles that had failed to deliver their contents to the food vacuole, control and treated parasites were examined by electron microscopy. The chloroquine-exposed parasites clearly contained large numbers of typical double-membrane transport vesicles filled with ingested RBC cytosol (Fig. 4A, asterisks), distinct from the food vacuole. Although these transport vesicles are also found in normal, control parasites (data not shown), they are present in much larger numbers after chloroquine treatment, a more extreme case of which can be seen in Fig. 4B. By counting the number of transport vesicles present in random cross sections of parasites, it was found that control parasites contained an average of 1.85 (standard deviation [SD], ±0.70; n = 80) vesicles, while chloroquine-treated parasites contained an average of 5.5 (SD, ±1.91; n = 80) of these structures.
FIG. 4.
Transmission electron micrographs of chloroquine-treated parasites. Labeled structures are the infected RBC, parasite (PAR), parasite nucleus (NU), food vacuole (VAC), and transport vesicles (asterisks).
Mefloquine and artemisinin markedly inhibit endocytosis.
Although the increase in hemoglobin levels in chloroquine-treated parasites probably reflects a block in hemoglobin digestion, an alternative explanation could be a stimulation of endocytosis or uptake of hemoglobin from the infected RBC. Conversely, the fact that mefloquine exposure results in a reduction in parasite hemoglobin levels (Fig. 1A) could be interpreted as either a stimulation of hemoglobin digestion or a block in hemoglobin endocytosis. To investigate the effects of the antimalarials on the endocytosis of RBC cytoplasmic macromolecules, RBCs were preloaded with biotinylated dextran and infected with parasites. Following a 12-h incubation in the presence of antimalarials, the parasites were removed from the preloaded RBCs by saponin treatment, washed to remove excess dextran, and lysed in detergent; and the amount of parasite-associated biotin-dextran was quantitated by a novel streptavidin-dependent microtiter plate assay. Chloroquine treatment resulted in a 44% reduction in parasite-associated biotinylated dextran compared to the amount in the untreated controls, while mefloquine and artemisinin had a more pronounced effect, reducing biotin-dextran levels in the parasite by 85 and 76%, respectively (Fig. 5A).
FIG. 5.
Quantitative endocytosis assays. (A and B) RBCs were preloaded with biotinylated dextran; infected with parasites; and treated with chloroquine (CQ), mefloquine (MEF), or artemisinin (ART) or were left untreated as controls (CON) for 12 h (A) or 5 h (B). Subsequently, the parasites were released from their host RBCs by saponin treatment, and their biotin-dextran contents were determined by incubating parasite lysates with streptavidin-coated microtiter plates, followed by incubation with streptavidin-horseradish peroxidase and colorimetric peroxidase substrate. (C) Parasites released from RBCs by saponin lysis were incubated with FITC-dextran in the presence of the indicated antimalarials, fixed, and viewed by fluorescence microscopy. Images of individual parasites were captured with a cooled charge-coupled device camera, and the mean fluorescence intensity in the food vacuoles was determined with Adobe Photoshop software. *, P < 0.01; **, P < 0.05. Error bars indicate SDs.
Chloroquine-dependent accumulation of transport vesicles does not occur during shorter treatment times.
To minimize the nonspecific effects of prolonged incubation on endocytosis of parasites with antimalarial drugs, the compounds were added to parasite cultures in the early to mid-trophozoite stage, as opposed to the late ring stage, and treatment was carried out for 5 h instead of 12 h prior to analysis. Under these conditions, the effects of the antimalarials on biotin-dextran uptake (Fig. 5B) followed the same trend observed before with longer incubation times (Fig. 5A). Both mefloquine and artemisinin inhibited biotin-dextran endocytosis by 26%, while chloroquine reduced the parasite-associated biotin-dextran by a small but statistically significant (P < 0.01) amount of 5%. In addition, the undigested hemoglobin levels assessed by Western blotting (Fig. 1B) mirrored those obtained earlier (Fig. 1A): mefloquine reduced parasite-associated hemoglobin, artemisinin-treated parasites contained slightly increased levels of hemoglobin, and chloroquine strongly increased the undigested hemoglobin content.
Surprisingly, however, despite the increase in hemoglobin levels in parasites treated with chloroquine for 5 h, there was no evident accumulation of extravacuolar transport vesicles in these parasites, as assessed by immunofluorescence microscopy with antihemoglobin antiserum or by fluorescence microscopy of parasitized RBCs preloaded with FITC-dextran (data not shown). The fluorescence was concentrated in the food vacuole, and parasites were indistinguishable from controls.
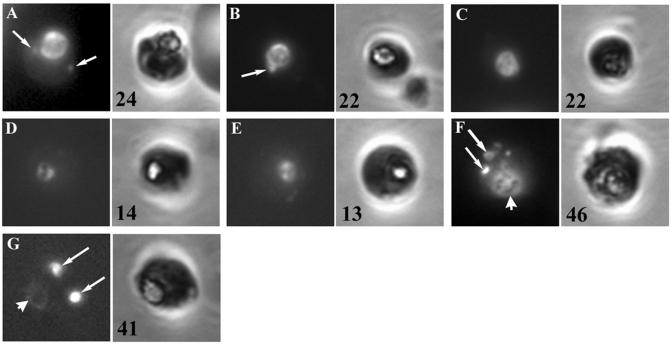
To further examine the effect of chloroquine on FITC-dextran endocytosis during shorter incubation times, we developed an RBC-free endocytosis assay in which trophozoites were released from infected RBCs by saponin lysis and incubated in an RBC lysate containing fluorescent tracer and the respective drugs (Fig. 6). The advantage of this approach is that drug and tracer are added to the parasites at the same time, and hence, the distribution of the tracer at the end of the treatment period more accurately reflects the effect of the drug. In control, untreated parasites, the FITC-dextran was faithfully endocytosed and delivered to the food vacuole, where it became concentrated, yielding an intense fluorescence in that area (Fig. 6A). Often, punctate fluorescent structures could be observed in the parasites (Fig. 6A, arrows). These structures presumably represent transport vesicles en route from sites of endocytosis at the plasma membrane to the food vacuole. By randomly sampling a hundred parasites and counting the amount of extravacuolar puncta, it was estimated that 24% of the control parasites contain two or more discernible transport vesicles (Fig. 6; percentages are indicated in bottom left-hand corner of the phase-contrast images). Chloroquine-treated parasites appeared indistinguishable from the controls and also contained intense fluorescence in the food vacuole and occasional discernible transport vesicles (Fig. 6B, arrow). The result therefore agrees with that found with the 5-h incubation by the RBC preloading approach, as discussed above. Furthermore, a buildup of transport vesicles was not observed in the parasites when chloroquine levels were increased from 120 nM to 1.5 μM (Fig. 6C). Among the latter parasites, 22% contained two or more transport vesicles, whereas 22% of parasites treated with the lower chloroquine concentration and 24% of the control parasites contained two or more transport vesicles. In the presence of 1.5 μM chloroquine, parasites endocytosed amounts of FITC-dextran comparable to that endocytosed by the controls, as determined by digitally quantitating the mean fluorescence intensity in the vacuoles of randomly photographed parasites (Fig. 5C).
FIG. 6.
RBC-free endocytosis assay. Parasites released from cultured pRBCs by saponin treatment were incubated for 5 h in a diluted RBC lysate containing FITC-dextran and no drug (A), 120 nM chloroquine (B), 1.5 μM chloroquine (C), mefloquine (D), artemisinin (E), ammonium chloride (F), or monensin (G). The parasites were washed, fixed, and viewed by fluorescence microscopy. Left panels, fluorescence images of individual parasites; right panels, the corresponding phase-contrast images. Arrows, transport vesicles; arrowheads, positions of the food vacuole. The percentages of parasites containing two or more transport vesicles are indicated in the bottom left corner of the phase-contrast panels.
RBC-free parasites incubated in the presence of artemisinin and mefloquine also accumulated FITC-dextran in their food vacuoles (Fig. 6D and E, respectively), as was found previously with the FITC-dextran assay with preloaded RBCs (Fig. 3). However, both treatments resulted in a decrease in the amount of transport vesicles discernible in the parasites: 14 and 13% of the artemisinin- and mefloquine-treated parasites contained two or more transport vesicles, respectively, whereas 24% of the controls and 22% of the two chloroquine-treated samples contained two or more transport vesicles. In conjunction with an apparent qualitative decrease in the fluorescence intensities in the food vacuoles of these parasites, this suggested that both mefloquine and artemisinin inhibit endocytosis. This was further confirmed by digitally quantitating the mean fluorescence in the vacuoles of the drug-treated parasites (Fig. 5C) and agrees with the results obtained by the biotin-dextran uptake assay (Fig. 5B) and the reduction in the levels of hemoglobin associated with the parasites (in the case of mefloquine; Fig. 1B).
Ammonium chloride and monensin cause transport vesicle accumulation in RBC-free parasites.
Even though treatment of parasites with ammonium chloride and monensin appeared to result in a buildup of transport vesicles during a 12-h incubation with infected preloaded RBCs by the FITC-dextran assay, similar to that observed with chloroquine (Fig. 3B), by phase-contrast microscopy the treated parasites appeared to be highly abnormal and stunted, probably reflecting the severe cytotoxic effects of these compounds. Their effects on the parasite endocytic pathway were therefore assessed by the RBC-free endocytosis assay with shorter incubation times described above. Ammonium chloride treatment resulted in parasites containing distended vacuoles filled with FITC-dextran (Fig. 6F, arrowhead), as well as an accumulation of enlarged transport vesicles (Fig. 6F, arrows). In the case of monensin-treated parasites, there appeared to be an almost complete block in the delivery of FITC-dextran to the food vacuole (Fig. 6G, arrowhead), in conjunction with an accumulation of fluorescent transport vesicles (Fig. 6G, arrows): 41 and 46% of the monensin- and ammonium chloride-treated parasites, respectively, contained two or more discernible transport vesicle structures, whereas 24% of the controls contained two or more discernible transport vesicle structures.
DISCUSSION
Several studies have reported an increase in undigested hemoglobin levels in malaria parasites treated with chloroquine (8, 9, 51, 52). Due to concerns over possible strain and species differences in the detailed effects of antimalarials, we sought to confirm the inhibition of hemoglobin digestion by chloroquine in the chloroquine-sensitive D10 strain of P. falciparum. Using a Western blotting assay, we found a striking increase in hemoglobin levels in treated parasites, which is in agreement with published studies. We further found that chloroquine added to late ring stage parasites significantly inhibits endocytosis of biotinylated tracers from preloaded RBCs. This endocytosis block may conceivably exacerbate parasite starvation caused by levels of reduced hemoglobin digestion. Although parasites appear to require only a fraction of the amino acids released during hemoglobin digestion for protein synthesis (26), the parasiticidal properties of hemoglobin protease inhibitors argues that hemoglobin digestion is vital for parasite survival (3). Alternative roles for hemoglobin digestion include the provision of extra volume into which the growing parasite can expand and osmotic stabilization of the infected RBC to prevent premature hemolysis (30). It remains to be seen if the hemoglobin endocytosis and digestion block contributes significantly to the antimalarial effects of chloroquine or is, rather, a secondary by-product of other, more lethal effects.
The chloroquine-mediated block in hemoglobin digestion has been reported to manifest itself subcellularly as an accumulation of hemoglobin-filled endocytic transport vesicles inside the parasite food vacuole (51), possibly due to inhibition of the phospholipases responsible for digesting the delimiting membrane of the vesicles after their delivery to the vacuole (27). By contrast, we found no evidence of transport vesicle accumulation in the food vacuoles of chloroquine-treated parasites at the electron microscopic level and noticed only a change in the amount and appearance of the hemozoin crystals (J. Egan, unpublished results). Furthermore, after chloroquine treatment we could detect no hemoglobin (by immunofluorescence) or fluorescent dextran tracer in the food vacuole but, instead, found these macromolecules in cytoplasmic transport vesicles that appear morphologically identical to the transport vesicles found in untreated controls, except that they are present in significantly larger numbers. A recent report (9) also showed evidence of transport vesicle accumulation in the cytoplasm of chloroquine-treated P. berghei parasites, and the investigators argued that this might be due to a redistribution of neutral aminopeptidase from transport vesicles to the cytoplasm and a consequent block in hemoglobin degradation. We favor the interpretation that the addition of chloroquine to parasites in the late ring stage results in a block in transport vesicle-vacuole fusion. The accumulation of transport vesicles in the parasite cytoplasm as opposed to undigested vesicles in the food vacuole, as reported previously, suggests that the cell biological responses of malaria parasites to chloroquine intoxication may be strain dependent.
The mechanism by which chloroquine causes a block in transport vesicle-vacuole fusion requires exploration. Chloroquine is a weak base which accumulates in acidic compartments due to protonation and consequent pH trapping (13, 25, 50). In mammalian cells the resultant increase in luminal pH has significant effects on trafficking in the endocytic pathway and on lysosome function (17, 24, 41, 45). Although this occurs at chloroquine concentrations higher than the therapeutic range for malaria parasites, the high-affinity binding of chloroquine to hemozoin crystals (43, 44) or to heme moieties generated by hemoglobin digestion (1) could markedly increase the effective concentration of chloroquine in the food vacuole to levels where it may overcome the acidic pH in that compartment necessary for proteolytic activity and, possibly, fusion competence. In support of this notion, treatment of parasites with the lysosomotropic alkalinizing agents ammonium chloride and monensin also leads to the accumulation of transport vesicles containing fluorescent dextran and, by inference, a block in vacuole-vesicle fusion.
The interpretation provided above is hampered by the fact that the measurement of vacuolar pH in malaria parasites has proved fraught with difficulties and controversy, and hence, uncertainty about the ability of chloroquine to modulate the vacuolar pH remains (2, 3, 42, 46). Earlier studies proposed that an alkalinization of the food vacuole by chloroquine might play an important role in its parasiticidal effects (24, 25). However, other reports suggest that the drug concentrations reached in the vacuole are insufficient to overcome the buffering capacity of this compartment to a significant extent, pH changes do not correlate with antimalarial effects, and parasites are surprisingly tolerant to changes in vacuolar pH (15, 42, 46, 50). An alternative explanation for the vacuole-transport vesicle fusion block observed here may therefore be required. It is widely held that chloroquine mainly exerts its parasiticidal effects by binding to heme moieties released in the food vacuole by hemoglobin digestion, thus preventing sequestration of the heme in inert hemozoin crystals and producing toxic levels of free heme or chloroquine-heme complexes (5, 23, 38). The latter molecules are thought to be lipophilic and have detergent-like qualities which could cause significant vacuole membrane damage (14). Conceivably, such an alteration in the physical characteristics of the lipid bilayer of the vacuole or the fusion machinery associated with it could significantly inhibit the ability of this compartment to fuse with incoming endocytic transport vesicles. Consequently, the similarities of the effects of chloroquine, ammonium chloride, and monensin on the trafficking of endocytic cargo in the parasite could be coincidental and not indicative of a common pH-related mechanism. In this regard, it should be borne in mind that the intracellular effects of ammonium chloride and monensin may be multiple; e.g., the compounds may elevate parasite cytoplasmic Ca2+ levels due to a disruption in acidic calcium pools (12), while the latter is also a K+ and H+ ionophore. Their effects on the endocytic pathway may therefore be unrelated to changes in vacuole or transport vesicle pH. A possible experimental approach that could be used to determine whether the fusion block as well as the inhibition of endocytosis caused by chloroquine is due to a direct effect of the drug on the pertinent protein machinery or a more secondary effect of chloroquine's action on heme detoxification and possibly pH in the vacuole could be to repeat the relevant experiments with chloroquine-resistant parasites. The latter should fail to accumulate chloroquine in the vacuole to a similar extent, while protein trafficking events outside the vacuole should remain chloroquine sensitive.
The results obtained by treating parasites with chloroquine for a shorter, 5-h period rather than 12 h suggest that a block in transport vesicle-vacuole fusion alone cannot fully account for the observed inhibition of hemoglobin digestion. During the 5-h treatment, parasites accumulated endocytosed fluorescent dextran in the food vacuole to levels comparable to those in the controls, and no buildup of transport vesicles was observed, even when chloroquine concentrations were increased by more than an order of magnitude. Despite this, a significant increase in hemoglobin levels was still seen by Western blotting. This suggests that the chloroquine-dependent increase in undigested hemoglobin levels is unrelated to aberrations in transport vesicle behavior alone and possibly results from an inhibition of hemoglobin-degrading proteases by chloroquine or chloroquine-heme complexes, as has been suggested before (47). In addition to the absence of a vesicle-vacuole fusion block during 5-h experiments (when drug is added to trophozoites), the level of biotin-dextran uptake was similar to that for control parasites and the block in endocytosis observed in the previous 12-h experiments (when drug is added to late ring stage parasites) was marginal at best. This is in agreement with previous reports which proposed that ring stages may be more sensitive to the effects of chloroquine than the later trophozoite stage (53).
Famin and Ginsburg (8) reported a reduction of hemoglobin levels in mefloquine-treated FCR3 strain parasites relative to those in control parasites and have argued that this is indicative of a block in endocytosis. Here we show directly that mefloquine strongly inhibits endocytosis in the D10 strain of P. falciparum using several lines of evidence: a reduction in hemoglobin levels in the parasite as assessed by Western blotting, decreased levels of accumulation of biotinylated dextran by the parasite in preloaded RBCs, significantly lower concentrations of fluorescent dextran in the food vacuole, and a reduced percentage of parasites with multiple transport vesicles. Although our data indicate that chloroquine also inhibits endocytosis during longer treatment times, the extent of the block is considerably less than that obtained with mefloquine and is hardly noticeable with the shorter incubation times. Moreover, the residual fluorescent tracer that is taken up during long mefloquine treatments is efficiently delivered to the food vacuole, and there is no evidence of vacuole-transport vesicle fusion inhibition like that which occurs with chloroquine. Although it has been suggested that mefloquine may also interfere with the heme detoxification process in the food vacuole as its principal mode of action (44), the evidence supports the notion that the intracellular targets and the effects of chloroquine and mefloquine are likely to be quite different.
Artemisinin treatment results in a small increase in hemoglobin levels, as assessed by Western blotting, which, taken alone, could suggest that it has little or no effect on the endocytic and digestive systems of the parasite. However, we found that artemisinin strongly inhibits endocytosis over long (12-h) and short (5-h) treatment periods, as assessed by biotin-dextran uptake, the intensity of FITC-dextran in the vacuole, and the percentage of parasites with multiple transport vesicles. Seen in this light, the Western blotting data indicate a considerable block in hemoglobin digestion by artemisinin. A significant increase in hemoglobin levels in artemisinin-treated P. yoelii has also been reported. It was proposed to be the result of protease inhibition and vacuole damage by increased free heme or artemisinin-heme adducts (34). Although both chloroquine and artemisinin may therefore act by increasing free heme levels and inhibiting hemoglobin digestion, they also differ in their effects on the endocytic pathway: artemisinin blocks endocytosis to a much greater extent and does not produce a vacuole-transport vesicle fusion inhibition. A recent report indicates that artemisinin may act by binding the parasite SERCA homologue by a nonheme Fe2+-dependent mechanism (6). Although a direct link between SERCA activity and endocytosis has not been reported, it is conceivable that perturbations of the cytosolic Ca2+ concentration due to SERCA inhibition (35) could have a significant regulatory effect on endocytosis, as has been found in neuronal synapses (4).
In evaluating the cell biological consequences of antimalarial drug treatment, as presented here, it is difficult to distinguish between effects that are the result of a primary mode of action of the drug (e.g., a direct interaction of the drugs or their adducts with the endocytosis or fusion machinery of the cell), an important secondary effect of a more general perturbation (e.g., pH changes or membrane damage), or a nonspecific manifestation of a cell that is stressed and dying. Resolution of these questions would require a detailed characterization at the protein level of the parasite endocytosis and vesicular trafficking machinery, an area which remains unexplored. In addition, the matter is complicated significantly by the apparent pleiotropic effects of all the drugs tested. Irrespective of the precise modes of action of the drugs, however, we propose that their effects on the parasite, as described here, should make them useful reagents for manipulating and studying endocytosis and the endocytic pathway. Consequently, antimalarials could acquire a utility in malaria cell biology research supplemental to the study of drug action, mechanisms of resistance, and structure-function relationships and the development of more efficacious derivatives.
Acknowledgments
The work presented here was funded by a Wellcome Trust Senior International Research Fellowship and a self-initiated research award from the Medical Research Council of South Africa to H.C.H.
We thank T. J. Egan and P. J. Smith for critically reading the manuscript.
REFERENCES
- 1.Bray, P. G., O. Janneh, K. J. Raynes, M. Mungthin, H. Ginsburg, and S. A. Ward. 1999. Cellular uptake of chloroquine is dependent on binding to ferriprotoporphyrin IX and is independent of NHE activity in Plasmodium falciparum. J. Cell Biol. 145:363-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray, P. G., K. J. Saliba, J. D. Davies, D. G. Spiller, M. R. H. White, K. Kirk, and S. A. Ward. 2002. Distribution of acridine orange fluorescence in Plasmodium falciparum-infected erythrocytes and its implications for the evaluation of digestive vacuole pH. Mol. Biochem. Parasitol. 119:301-304. [DOI] [PubMed] [Google Scholar]
- 3.Coombs, G. H., D. E. Goldberg, M. Klemba, C. Berry, J. Kay, and J. C. Mottram. 2001. Aspartic proteases of Plasmodium falciparum and other parasitic protozoa as drug targets. Trends Parasitol. 17:532-537. [DOI] [PubMed] [Google Scholar]
- 4.Cousin, M. A. 2000. Synaptic vesicle endocytosis. Mol. Neurobiol. 22:115-128. [DOI] [PubMed] [Google Scholar]
- 5.Dorn, A., S. R. Vippagunta, H. Matile, C. Jaquet, J. L. Vennerstrom, and R. G. Ridley. 1998. An assessment of drug-haematin binding as a mechanism for inhibition of haematin polymerisation by quinoline antimalarials. Biochem. Pharmacol. 55:727-736. [DOI] [PubMed] [Google Scholar]
- 6.Eckstein-Ludwig, U., R. J. Webb, I. D. A. van Goethem, J. M. East, A. G. Lee, M. Kimura, P. M. O'Neill, P. G. Bray, S. A. Ward, and S. Krishna. 2003. Artemisinins target the SERCA of Plasmodium falciparum. Nature 424:957-961. [DOI] [PubMed] [Google Scholar]
- 7.Egan, T. J., D. C. Ross, and P. A. Adams. 1994. Quinoline antimalarial drugs inhibit spontaneous formation of β-haematin (malaria pigment). FEBS Lett. 352:54-57. [DOI] [PubMed] [Google Scholar]
- 8.Famin, O., and H. Ginsburg. 2002. Differential effects of 4-aminoquinoline-containing antimalarial drugs on hemoglobin digestion in Plasmodium falciparum-infected erythrocytes. Biochem. Pharmacol. 63:393-398. [DOI] [PubMed] [Google Scholar]
- 9.Fitch, C. D., G. Cai, Y. Chen, and J. S. Reyerse. 2003. Relationship of chloroquine-induced redistribution of a neutral aminopeptidase to hemoglobin accumulation in malaria parasites. Arch. Biochem. Biophys. 410:296-306. [DOI] [PubMed] [Google Scholar]
- 10.Fivelman, Q. L., J. C. Walden, P. J. Smith, P. I. Folb, and K. I. Branes. 1999. The effect of artesunate combined with standard antimalarials against chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 93:429-432. [DOI] [PubMed] [Google Scholar]
- 11.Foley, M., and L. Tilley. 1998. Quinoline antimalarials: mechanism of action and resistance and prospects for new agents. Pharmacol. Ther. 79:55-87. [DOI] [PubMed] [Google Scholar]
- 12.Garcia, C. R., S. E. Ann, E. S. Tavares, A. R. Dluzewski, W. T. Mason, and F. B. Paiva. 1998. Acidic calcium pools in intraerythrocytic malaria parasites. Eur. J. Cell Biol. 76:133-138. [DOI] [PubMed] [Google Scholar]
- 13.Geary, T. G., J. B. Jensen, and H. Ginsburg. 1986. Uptake of H-3 chloroquine by drug sensitive and drug resistant strains of the human malaria parasite Plasmodium falciparum. Biochem. Pharmacol. 35:3805-3812. [DOI] [PubMed] [Google Scholar]
- 14.Ginsburg, H., and R. A. Demel. 1984. Interactions of hemin, antimalarial drugs and hemin-antimalarial complexes with phospholipid monolayers. Chem. Phys. Lipids 35:331-347. [DOI] [PubMed] [Google Scholar]
- 15.Ginsburg, H., E. Nissani, and M. Krugliak. 1989. Alkalinization of the food vacuole of malaria parasites by quinoline drugs and alkylamines is not correlated with their antimalarial activity. Biochem. Pharmacol. 38:2645-2654. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg, D. E. 1993. Hemoglobin degradation in Plasmodium-infected red blood cells. Semin. Cell Biol. 4:355-361. [DOI] [PubMed] [Google Scholar]
- 17.Helenius, A., M. Marsh, and J. White. 1982. Inhibition of Semliki forest virus penetration by lysosomotropic weak bases. J. Gen. Virol. 58:47-61. [DOI] [PubMed] [Google Scholar]
- 18.Hempelmann, E., C. Motta, R. Hughes, S. A. Ward, and P. G. Bray. 2003. Plasmodium falciparum: sacrificing membrane to grow crystals? Trends Parasitol. 19:23-26. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs, G. H., M. Aikawa, W. K. Milhous, and J. R. Rabbege. 1987. An ultrastructural study of the effects of mefloquine on malaria parasites. Am. J. Trop. Med. Hyg. 36:9-14. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs, G. H., A. M. Oduola, D. E. Kyle, W. K. Milhous, S. K. Martin, and M. Aikawa. 1988. Ultrastructural study of the effects of chloroquine and verapamil on Plasmodium falciparum. Am. J. Trop. Med. Hyg. 39:15-20. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, J. B., G. Jacobs, D. S. Liang, and M. Aikawa. 1985. Qinghaosu-induced changes in the morphology of Plasmodium inui. Am. J. Trop. Med. Hyg. 34:424-428. [DOI] [PubMed] [Google Scholar]
- 22.Kamchonwongpaisan, S., and S. R. Meshnick. 1996. The mode of action of the antimalarial artemisinin and its derivatives. Gen. Pharamacol. 27:587-592. [DOI] [PubMed] [Google Scholar]
- 23.Kaschula, C. H., T. J. Egan, R. Hunter, N. Basilico, S. Parapini, D. Taramelli, E. Pasini, and D. Monti. 2002. Structure-activity relationships in 4-aminoquinoline antiplasmodials. The role of the 7-position. J. Med. Chem. 45:3531-3539. [DOI] [PubMed] [Google Scholar]
- 24.Krogstad, D. J., and P. H. Schlesinger. 1986. A perspective on antimalarial action: effects of weak bases on Plasmodium falciparum. Biochem. Pharmacol. 35:547-552. [DOI] [PubMed] [Google Scholar]
- 25.Krogstad, D. J., P. H. Schlesinger, and L. Y. Gluzman. 1985. Antimalarials increase vesicle pH in Plasmodium falciparum. J. Cell Biol. 101:2302-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krugliak, M., J. Zhang, and H. Ginsburg. 2002. Intraerythrocytic Plasmodium falciparum utilizes only a fraction of the amino acids derived from the digestion of host cell cytosol for the biosynthesis of its proteins. Mol. Biochem. Parasitol. 119:249-256. [DOI] [PubMed] [Google Scholar]
- 27.Kubo, M., and K. Y. Hostetler. 1985. Mechanism of cationic amphiphilic drug inhibition of purified lysosomal phospholipase A1. Biochemistry 24:6515-6520. [DOI] [PubMed] [Google Scholar]
- 28.Labro, M. T., and C. Babin-Chevaye. 1988. Effects of amodiaquine, chloroquine, and mefloquine on human polymorphonuclear neutrophil function in vitro. Antimicrob. Agents Chemother. 32:1124-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambros, C., and J. P. Vanderberg. 1979. Synchronisation of P. falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 30.Lew, V. L., T. Tiffert, and H. Ginsburg. 2003. Excess hemoglobin digestion and the osmotic stability of Plasmodium falciparum-infected red blood cells. Blood 101:4189-4194. [DOI] [PubMed] [Google Scholar]
- 31.Meshnick, S. R., T. F. Taylor, and S. Kamchonwongpaisan. 1996. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol. Rev. 60:301-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore, H. P., B. Gumbiner, and R. B. Kelly. 1983. Chloroquine diverts ACTH from a regulated to a constitutive secretory pathway in AtT-20 cells. Nature 302:434-436. [DOI] [PubMed] [Google Scholar]
- 33.Mungthin, M., P. G. Bray, R. G. Ridley, and S. A. Ward. 1998. Central role of hemoglobin degradation in mechanisms of action of 4-aminoquinolines, quinoline methanols, and phenanthrene methanols. Antimicrob. Agents Chemother. 42:2973-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandey, A. V., B. L. Tekwani, R. L. Singh, and V. S. Chauhan. 1999. Artemisinin, an endoperoxide antimalarial, disrupts the hemoglobin catabolism and heme detoxification systems in malarial parasite. J. Biol. Chem. 274:19383-19388. [DOI] [PubMed] [Google Scholar]
- 35.Periasamy, M., and S. Huke. 2001. SERCA pump level is a critical determinant of Ca2+ homeostasis and cardiac contractility. J. Mol. Cell. Cardiol. 33:1053-1063. [DOI] [PubMed] [Google Scholar]
- 36.Rose, M. Y., R. A. Thompson, W. R. Light, and J. S. Olson. 1985. Heme transfer between phospholipid membranes and uptake by apohemoglobin. J. Biol. Chem. 260:6632-6640. [PubMed] [Google Scholar]
- 37.Schwartz, A. L., A. Bolognesi, and S. E. Fridovich. 1984. Recycling of the asialoglycoprotein receptor and the effect of lysosomotropic amines in hepatoma cells. J. Cell Biol. 98:732-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slater, A. F. G., and A. Cerami. 1992. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature 355:167-169. [DOI] [PubMed] [Google Scholar]
- 39.Slater, A. F. G., W. J. Swiggard, B. R. Orton, W. D. Flitter, D. E. Goldberg, A. Cerami, and G. B. Henderson. 1991. An iron-carboxylate bond links the heme units of malaria pigment. Proc. Natl. Acad. Sci. USA 88:325-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slomianny, C. 1990. Three-dimensional reconstruction of the feeding process of the malaria parasite. Blood Cells 16:369-378. [PubMed] [Google Scholar]
- 41.Smith, R. M., and L. Jarett. 1982. Ultrastructural basis for chloroquine-induced increase in intracellular insulin in adipocytes: alteration of lysosomal function. Proc. Natl. Acad. Sci. USA 79:7302-7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiller, D. G., P. G. Bray, R. H. Hughes, S. A. Ward, and M. R. H. White. 2002. The pH of the Plasmodium falciparum digestive vacuole: holy grail or dead-end trail? Trends Parasitol. 18:441-444. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan, D. J., I. Y. Gluzman, D. G. Russell, and D. E. Goldberg. 1996. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. USA 93:11865-11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan, D. J., H. Matile, R. G. Ridley, and D. E. Goldberg. 1998. A common mechanism for blockade of heme polymerization by antimalarial quinolines. J. Biol. Chem. 273:31103-31107. [DOI] [PubMed] [Google Scholar]
- 45.Tietze, C., P. Schlesinger, and P. Stahl. 1980. Chloroquine and ammonium ion inhibit receptor-mediated endocytosis of mannose-glycoconjugates by macrophages: Apparent inhibition of receptor recycling. Biochem. Biophys. Res. Commun. 93:1-8. [DOI] [PubMed] [Google Scholar]
- 46.Ursos, L. M. B., S. M. Dzekunov, and P. D. Roepe. 2000. The effects of chloroquine and verapamil on digestive vacuolar pH of P. falciparum either sensitive or resistant to chloroquine. Mol. Biochem. Parasitol. 110:124-134. [DOI] [PubMed] [Google Scholar]
- 47.Vander Jagt, D. L., L. A. Hunsaker, and N. M. Campos. 1986. Characterisation of hemoglobin degrading, low molecular weight protease from Plasmodium falciparum. Mol. Biochem. Parasitol. 18:389-400. [DOI] [PubMed] [Google Scholar]
- 48.Van Weert, A. W. M., H. J. Geuze, B. Groothuis, and W. Stoorvogel. 2000. Primaquine interferes with membrane recycling from endosomes to the plasma membrane through a direct interaction with endosomes which does not involve neutralisation of endosomal pH nor osmotic swelling of endosomes. Eur. J. Cell Biol. 79:394-399. [DOI] [PubMed] [Google Scholar]
- 49.Wenisch, C., B. Parschalk, K. Zedwitz-Liebenstein, W. Wernsdorfer, and W. Graninger. 1997. The effect of artemisinin on granulocyte function assessed by flow cytometry. J. Antimicrob. Chemother. 39:99-101. [DOI] [PubMed] [Google Scholar]
- 50.Yayon, A., Z. I. Cabantchik, and H. Ginsburg. 1984. Identification of the acidic compartment of Plasmodium falciparum-infected human erythrocytes as the target of the antimalarial drug chloroquine. EMBO J. 11:2695-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yayon, A., R. Timberg, S. Friedman, and H. Ginsburg. 1984. Effects of chloroquine on the feeding mechanism of the intraerythrocytic human malarial parasite Plasmodium falciparum. J. Protozool. 31:367-372. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, Y. 1987. Inhibition of hemoglobin degradation in Plasmodium falciparum by chloroquine and ammonium chloride. Exp. Parasitol. 64:322-327. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, Y., K. S. Asante, and A. Jung. 1986. A stage-dependent inhibition of chloroquine on Plasmodium falciparum in vitro. J. Parasitol. 72:830-836. [PubMed] [Google Scholar]