Abstract
Human immunodeficiency virus (HIV), tuberculosis (TB), and helminthic infections are among the commonest public health problems in the sub-Saharan African countries like Ethiopia. Multiple micronutrient deficiencies also known as the “hidden hunger” are common in people living in these countries either playing a role in their pathogenesis or as consequences. This results in a vicious cycle of multiple micronutrient deficiencies and infection/disease progression. As infection is profoundly associated with nutritional status resulting from decreased nutrient intake, decreased nutrient absorption, and nutrient losses, micronutrient deficiencies affect immune system and impact infection and diseases progression. As a result, micronutrients, immunity, and infection are interrelated. The goal of this review is therefore to provide a summary of available findings regarding the “quadruple burden trouble” of HIV, TB, intestinal parasitic infections, and multiple micronutrient deficiencies to describe immune-modulating effects related to disorders.
1. Introduction
Human immunodeficiency virus (HIV), tuberculosis (TB), and helminthic infections are among the commonest public health problems in the sub-Saharan African countries. Micronutrient deficiencies are an additional burden for these groups of population either playing a role in their pathogenesisor as a consequence ending up in a vicious cycle.
It is estimated that one-third of the world's population is latently infected with Mycobacterium tuberculosis (M. tb) and that each year about three million people die of TB [1, 2]. The emergence of drug-resistant strains is further worsening the threat [1]. Despite global research efforts, mechanisms underlying pathogenesis, virulence, and persistence of M. tb infection remain poorly understood [2]. In 1993, the World Health Organization (WHO) declared TB a global public health emergency [2]. The WHO global reports on TB showed that Ethiopia is among the top ten high burden countries in terms of prevalence or incidence cases of TB [1, 3]. Tuberculosis is the second leading cause of death from an infectious disease worldwide, only second to HIV. The HIV/AIDS pandemic, on the other hand, has had its most profound impact to date in sub-Saharan Africa. The majority of people living with HIV/AIDS (67%), new HIV infections (70%), and AIDS-related deaths (75%) are in this region, which only accounts for about 11-12% of the world's population [4]. With a national adult HIV prevalence of 2.1%, Ethiopia is one of the sub-Saharan countries most severely hit by the epidemic. The dominant mode of transmission of the virus among adults is heterosexual transmission while mother-to-child transmission accounts for more than 90% of pediatric HIV infections [5, 6].
About three billions of people are infected with one or more species of intestinal parasites which are distributed virtually throughout the world, with higher prevalence rates in many tropical and subtropical regions [7, 8]. These parasites release multitude of antigens into the circulation which would lead to chronic activation of the immune system [9–11]. In sub-Saharan Africa, where the prevalence of parasitic infections is very high, a dominant type-2 T helper polarized immune response has been reported [12] and suggested to increase susceptibility to both M. tb and HIV. Coinfection also hastens progression of their respective diseases [13–15].
Along with these infections, single or multiple micronutrient deficiencies have been shown to influence host resistance mechanisms, thus altering the susceptibility to infectious diseases [9–15]. Knowledge of the immune-modulating effects of micronutrients and their interactions with HIV, TB, and chronic intestinal parasitic infection which cause major public health problem in Ethiopia (Table 1), is of great importance in planning comprehensive strategies to promote health through nutrition and to augment specific therapy. The goal of this review is to provide a summary of available findings and summarize current state of knowledge regarding the “quadruple burden,” multiple micronutrient deficiency, HIV, TB, and intestinal parasitic infection, and to describe immune-modulating effects of these disorders.
Table 1.
Selected micronutrient levels in serum of blood donors (apparently healthy controls), pregnant women, and tuberculosis patients by HIV serostatus in Ethiopia.
| Trace elements | Controls (blood donors) [16–20] | Pregnant women [20] | Tuberculosis patients [16, 17] | Diarrheic patients [18, 19, 21] | ||||
|---|---|---|---|---|---|---|---|---|
| HIV− (n = 68) |
HIV+ (n = 32) |
HIV− (n = 327) |
HIV+ (n = 42) |
HIV− (n = 81) |
HIV+ (n = 71) |
HIV− (n = 97) |
HIV+ (n = 109) |
|
| Mg (mg/dl) | 2.85 ± 0.61* | — | 2.43 ± 0.82 | 2.14 ± 0.86 | — | — | 1.76 ± 0.34 | 1.68 ± 0.26 |
| Ca (mg/dl) | 14.41 ± 3.61 | 11.11 ± 1.46 | 14.39 ± 4.69 | 13.41 ± 5.22 | — | — | 8.38 ± 1.97 | 7.82 ± 1.23 |
| Fe (μg/dl) | 480.9 ± 449.0 | 288.3 ± 194.8 | 561.97 ± 415.23 | 485.86 ± 275.23 | 280.82 ± 314.31 | 265.99 ± 369.91 | 352.06 ± 351.23 | 420.82 ± 665.14 |
| Cu (μg/dl) | 140.3 ± 47.95 | 166.2 ± 45.4 | 240.19 ± 73.55 | 239.59 ± 81.47 | 188.19 ± 58.65 | 176.59 ± 63.19 | 113.51 ± 38.28 | 126.83 ± 34.91 |
| Zn (μg/dl) | 88.1 ± 4.02 | 77.2 ± 25.3 | 75.19 ± 44.79 | 76.30 ± 125.43 | 81.14 ± 14.16 | 73.65 ± 37.66 | 62.39 ± 43.64 | 68.13 ± 44.53 |
| Se (μg/dl) | 9.6 ± 4.37 | 10.2 ± 4.5 | 10.49 ± 4.24 | 8.0 ± 4.71 | 8.86 ± 3.93 | 7.55 ± 2.63 | 6.99 ± 4.26 | 5.90 ± 2.79 |
| Vitamin A (μg/dl) | 42.83 ± 20.37 | 25.83 ± 14.28 | 31.57 ± 12.79 | 27.56 ± 12.01 | 21.57 ± 13.81 | 19.98 ± 13.28 | 24.18 ± 15.68 | 23.57 ± 16.77 |
| N = 92 | N = 30 | N = 379 | N = 44 | N = 107 | N = 115 | N = 101 | N = 110 | |
*Mean ± standard deviation.
Trace elements were measured by ICP-MS (inductively coupled plasma mass spectroscopy) and vitamin A was measured by HPLC (high performance liquid chromatography).
2. Methods
This review was on paper after reviewing the relevant information available about the burden of HIV, TB, intestinal helminthes, and micronutrient deficiencies in Ethiopia and current evidences on their interactions from Hinari (http://www.who.int/hinari/en/) and PubMed (http://www.ncbi.nlm.nih.gov/pubmed). Although much has been published in the last 10 years regarding our topic, we still need more information so as to understand the issues that will help us develop effective programs in Ethiopia and other African countries with similar conditions. Therefore, we have also used more literatures which are less than ten years old.
3. Interactions between Micronutrient Deficiency and Infection
Micronutrients, immunity, and infections are interrelated [22]. Undernourished persons show immune dysfunction, which predisposes them to infections [23, 24]. Micronutrient deficiencies, also known as “hidden hunger,” disturb the normal function of the immune system components, weakening immune defenses, and increasing susceptibility to various infectious diseases [24–29]. Infection, in turn, is associated with profound effects on nutritional status resulting from decreased nutrient intake due to loss of appetite, decreased nutrient absorption as a result of intestinal damage and malabsorption, and nutrient losses arising from diarrhea and increased urinary excretion. A number of micronutrient deficiencies have been reported in persons with TB [16, 17, 30–36] and HIV infection [21, 37–44] and among those with intestinal parasitic infections [18, 19, 45–49]. The risk of multiple micronutrient deficiencies is high in developing countries, due to monotonous diets based on staple foods of low nutrient density [50].
4. Factors Contributing to Micronutrient Deficiencies during Infections
Malnutrition can lead to expression of overt disease among individuals with latent infection by weakening the immune system. Malnutrition can make a person more susceptible to infectious diseases, and infection also contributes to malnutrition (Figure 1). An inadequate dietary intake results in stunting, lowered immunity, mucosal damage, invasion by pathogens, and impaired growth and development in children [27–29]. The interaction between micronutrient deficiency, infection, and immunity has been well documented. Infection may lead to micronutrient deficiencies and micronutrient deficiencies may affect the risk of infectious disease morbidity [22, 27–29, 45, 51, 52], which causes a vicious cycle. As seen from the conceptual framework presented in Figure 1, the effects of an infection are mediated via the acute phase response and localized lesions, leading to reduced intake and absorption which results in an increased utilization and loss of micronutrients. A micronutrient deficiency may affect the risk of infection with a specific infectious agent as well as the severity of the infectious disease morbidity. These effects are mediated through pathogenicity of the infectious agent and hosts immunity [53].
Figure 1.
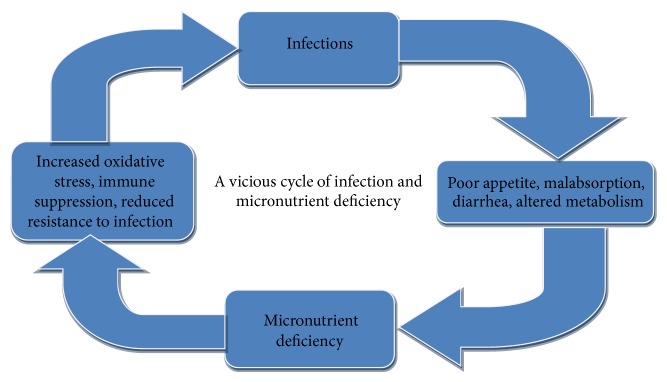
A vicious cycle of infection and micronutrient deficiencies.
5. The Influence of Micronutrient Deficiency on the Progression/Mother to Child Transmission and Treatment Outcomes of HIV/AIDS
5.1. Micronutrients on the Progression of HIV/AIDS
The progression time of HIV infection to AIDS and from AIDS to death is of highly variable length. The examinations on the relationship between micronutrient deficiencies and HIV disease progression began in 1990s [54]. An inverse correlation between serum selenium concentrations and HIV disease progression including CD4 cell counts, opportunistic infections, and viral load has been reported by numerous authors [55, 56]. Low plasma or serum selenium concentrations were reported among symptomatic HIV patients as compared to symptom-free HIV-positive subjects [55]. Similarly, lower serum levels of selenium were reported in patients with a CD4 count less than 400 cells/mm3 of blood [57]. Another study reported that the occurrence of opportunistic infections was more frequent among patients with lower serum selenium concentration [17, 19, 58]. Moreover, it has been reported that mean serum selenium levels were significantly lower in patients at CDC HIV stage B and C as compared to healthy subjects and to HIV stage I patients [59]. In one study, low serum selenium levels increased the risk of HIV-related mortality by more than ten times [60]. Likewise, vitamin A status as an important cofactor in HIV progression has been reported. Low vitamin A concentrations were significantly associated with CD4 T-cell counts and increased progression to AIDS and as a result increased risk of mortality in HIV infected people [61, 62]. In Ethiopia, vitamin A deficiency has been reported as a severe public health problem among HIV infected patients [20, 21]. Low serum zinc levels in HIV patients are also reported in Ethiopia [17, 18, 63].
5.2. Micronutrients Deficiency on Mother to Child Transmission and Pregnancy Outcome
In Sub-Saharan countries, only 50% of women living with HIV were receiving antiretroviral medicine for PMTCT in the year 2010 [64]. Transmission of HIV from mother to infant can occur in utero, during delivery, or through breastfeeding [65]. Vertical transmission rates of HIV without any preventive measures are estimated to be 25–35% in developing countries [66]. Both maternal and child factors affect vertical transmission, and many of these factors relate to nutritional status. There has been concern of increased risk of HIV transmission from mother to child, with particular micronutrient deficiencies.
Vitamin A deficiency which is high among Ethiopian HIV-positive pregnant women [20] was first correlated with increased vertical transmission of HIV in Africa [67]. This has implications for potential clinical importance particularly in African regions where accesses to other forms of treatment are virtually impossible. Observational studies in sub-Saharan Africa have shown significantly increased rates of mother to child transmission of HIV [67, 68] and infant mortality [68, 69] among HIV-infected women with low serum vitamin A levels.
On the other hand, a study in African women showed that vitamin A supplementation was not associated with decreased HIV transmission [70]. However, it had positive effects on pregnancy outcomes such as decreasing preterm births, lowering the transmission rate in preterm babies, and reducing the incidence of low birth weight deliveries [70]. In addition, a study in Tanzania on pregnancy outcomes found that multivitamins decreased the risk of low birth weight, severe preterm birth, and fetal death while increasing CD4, CD8, and CD3 lymphocytes [71]. These results has important public health implications because preterm delivery rates of HIV-1 infected mothers can reach up to 42% in African countries and are associated with increased mortality and morbidity [71].
5.3. Micronutrients Deficiency and Oxidative Stress during HAART
HIV infection is accompanied by severe metabolic and immune dysfunctions. Oxidative stress is one of the dysfunctions which results from the imbalance between reactive oxygen species (ROS) production and antioxidants concentration [72]. Exposure to oxidants challenges cellular systems and their responses may create conditions that are favorable for the replication of HIV which is an increasing cause of morbidity and mortality among HIV/AIDS patients [73]. Currently, however, introduction of HAART has led to a decrement of viral load and a quantitative and qualitative improvement of the immune functions in patients, especially CD4+ count. This results in a decrement of infectious complications and global clinical improvement [74, 75]. But HAART also plays a role in oxidative damage to DNA and membrane polyunsaturated fatty acids, which later on generates more free radicals potentiating the cellular damage [76]. Therefore, an HIV infected individual on antiretroviral therapy is exposed to two courses of free radical injury: one is from the virus itself and the other from the antiretroviral drugs. Hence, in areas where multiple antioxidant micronutrient deficiency is common, an increased oxidative stress is expected among those on HAART. However, it remains to be determined whether multiple antioxidant micronutrient supplementations will have any effect on oxidative stress or viral replication and disease progression.
6. The Influence of Micronutrient Deficiency on the Transmission, Drug Resistance Development, and Treatment Outcome of TB
Malnutrition is more common in patients with active tuberculosis than in people without TB [77]. Weight loss, including loss of lean body mass, is a well-recognized symptom of the disease. A study conducted in Ethiopian TB patients showed that low body mass index (BMI < 18.5 kg/m2) was common and it was observed among 65.4% of TB patients and 71.6% of TB/HIV coinfected patients. Severe malnutrition (BMI < 165 kg/m2) was observed to be more common among TB/HIV coinfected patients [17]. Although generalized malnutrition has been commonly described during active TB, less is known about micronutrient status and TB disease pathogenesis [78]. However, the concentration of vitamins, minerals, and trace elements all have key roles in metabolic pathways, cellular function, and defense against TB [32, 79].
In the era before the introduction of TB chemotherapy, vitamin D rich cod liver oil and exposure to sunlight were once a part of regular therapy for TB [80]. Vitamin D plays a role in macrophage activation and was shown to be a key factor in host resistance to tuberculosis [81]. In addition, vitamin D downregulates the transcription of virulence factors that are important for the intracellular survival of M. tb in macrophages [82, 83].
Susceptibility to M. tb infection and progression to active TB may be increased by vitamin D deficiency [82, 84]. Abnormalities in vitamin D status are influenced by dietary, genetic, and exposure to sunlight. In addition, genetic variations in vitamin D receptor were identified as a major determinant of the risk for TB among Africans [85].
In Ethiopia, in spite of abundant availability of UV radiation, it has been reported that the population from Addis Ababa situated in tropics had a high rate of biochemical vitamin D deficiency [86]. Increased risk of vitamin D deficiency in darker skinned individuals is due in part to decreased dermal synthesis of vitamin D as a result of the absorption of UV radiation by the increased melanin pigmentation [87]. Vitamin D deficiency helps the disease to progress rapidly to the active form.
In recent years, rates of drug-resistant TB have been spreading fast across the world, causing alarm among public health officials and prompting calls for more research into new and more effective treatments. The emergence of multidrug-resistant TB (MDR-TB), where the bacteria are resistant to both rifampicin and isoniazid, extensively drug-resistant (XDR-TB), where the bacillus is additionally resistant to fluoroquinolones and at least to one injectable agent (such as amikacin, capreomycin, or kanamycin), and the more recent form which is resistant to all anti-TB drugs represents an emerging problem in the struggle to contain TB [88–90].
Vitamin A is an important immune enhancer that has been shown to increase lymphocyte proliferation in response to antigens and to potentiate antibody responses to T-cell-dependent antigens and inhibit apoptosis. Vitamin A is also important in maintaining the integrity of epithelial surfaces. Deficiency in vitamin A leads to reduced levels of secretory immunoglobulin A in mucous and, therefore, to a weakening of the local barriers to infection [91–93]. However, in Ethiopia, vitamin A deficiency among TB patients is extremely high, occurring in about 60% of patients with TB [16, 94].
Numerous studies have reported decreased antioxidants levels, disturbed glutathione metabolism, and enhanced spontaneous generations of reactive oxygen species (ROS) in TB patients [95, 96]. For that reason, an increased level of ROS is the main factor to lower concentrations of antioxidants in TB patients. To make matters worse, inadequate dietary intakes of antioxidant compounds that are capable of reacting with and inactivating ROS result in further ROS generation which leads to an increased utilization of endogenous antioxidants. Therefore, these oxidant-antioxidant imbalances (oxidative stress) may represent a pathogenic loop that results in markedly enhanced oxidative stress during TB infection [97, 98].
In Ethiopia, it was reported that levels of the antioxidant vitamins C, E, and A were considerably lower in TB patients than in healthy controls; particularly high concentrations of lipid peroxidation products were seen among those who were coinfected with HIV [94]. In another study conducted in northwest Ethiopia, low concentrations of trace elements such as zinc, iron and selenium were also reported [17]. Whether single or multiple antioxidant supplementations will improve TB treatment outcome or are of importance for its prevention requires in depth future prospective studies.
7. The Relationship between Intestinal Parasitic Infections and Micronutrient Deficiency
Malnutrition and intestinal parasitic infections are common public health problems in developing countries. Malnutrition and parasitic diseases have a strikingly similar geographical distribution with the same people experiencing both diseases together for much of their lives [94]. In Ethiopia, intestinal parasitic infection and malnutrition still constitute a major health challenge with the resultant clinical and social impact on the people [99–102].
As micronutrient deficiencies disrupt the function of various immune system components that increase vulnerability to various infectious diseases [24–29], intestinal parasitic infections affect the micronutrient status by decreasing nutrient intake due to loss of appetite, decreased nutrient absorption as a result of intestinal damage and malabsorption, and nutrient losses arising from diarrhea and increased urinary excretion [18, 19, 45–49]. The consequences of such coexistence deleteriously affect the immune mechanisms of the host [103].
Basically, immune responses to infectious agents engage two antagonistic, reciprocally cross-regulated classes of T helper cells: type-1 and type-2 T helper cells. Type-1 T helper immune cells are responsible for cell-mediated immunity against bacterial, protozoal, viral, and intracellular parasitic infections whereas type-2 T helper cells mediate antibody-dependent immunity against extracellular parasites including intestinal helminthes [104].
Intestinal helminth infection leads to micronutrient deficiency [18, 19, 45–49]. In turn, micronutrient deficiency decreases immunological response against intestinal helminthes [24–29]. Evidence suggests that type-2 immune response may play a crucial role in reducing the severity of acute disease upon helminth infection [105] resulting in chronic helminth infection. In this case, type-2 T helper cells produces a dominant pattern of cytokine immune effectors capable of downregulating type-1 T helper cells response [14, 104, 106–109], increasing vulnerability to other intracellular infections like HIV and TB [14, 108, 109].
Other studies proposed that undernutrition may prevent the expression of the dominant type-2 phenotypes and that energy deficiency [110], vitamin A deficiency [111], and protein deficiency [110] cause overexpression of type-1 T helper cells cytokine IFN-γ and consequently downregulation of essential type-2 T helper cell cytokines. The absence of type-2 T helper cell cytokines and their effectors results in prolonged survival of helminthes. In addition, current evidence shows that zinc deficiency is characterized by declines in several type-2 T helper immune effectors in mice [112]. In Ethiopia, several studies reported multiple micronutrient deficiencies in different segments of the population [16–19, 21, 66, 100, 107].
8. Conclusion
From the extensive literature, it can be concluded that effect of single and multiple micronutrient deficiency on pathogenesis of HIV, TB, and intestinal parasitic infections is of immense clinical and public health importance in Ethiopia where these diseases often coexist. Furthermore, the bidirectional interactions between multiple micronutrient deficiencies and infectious diseases may have potentially enormous long term developmental and societal impacts in the country. Therefore, it is needless to point out that coinfection with two or more pathogens may even make the problem worse. Thus, the authors hope that this information will fuel the development of new ideas and research studies focused on investigating the effect of single or multiple micronutrient supplementations on infection transmission, immune status, diseases progression, morbidity, mortality, and treatment/vaccine outcome. Further investigation is also needed to evaluate the prophylactic and therapeutic potential of micronutrient interventions in augmenting chemotherapy during coinfection.
Abbreviations
- HIV:
Human immunodeficiency virus
- TB:
Tuberculosis
- WHO:
World Health Organization
- AIDS:
Acquired immunodeficiency syndrome
- HAART:
Highly active antiretroviral therapy
- BMI:
Body mass index
- MDR-TB:
Multidrug-resistant tuberculosis
- XDR-TB:
Extensively drug-resistant tuberculosis
- ROS:
Reactive oxygen species.
Conflict of Interests
The authors declare that they have no competing interests.
References
- 1.WHO. Global Tuberculosis Control, Epidemiology, Strategy, Financing. Geneva, Switzerland: WHO; 2009. (WHO Report, WHO/HTM/TB/2009.411). [Google Scholar]
- 2.World Health Organisation (WHO) WHO Report. WHO/HTM/TB 2011.16. Geneva, Switzerland: WHO; 2011. Global tuberculosis control. [Google Scholar]
- 3.WHO. WHO Report. WHO/HTM/TB/2009.411. Geneva, Switzerland: WHO; 2010. Global tuberculosis control, epidemiology, strategy, financing. [Google Scholar]
- 4.UNAIDS/WHO Joint United Nations Programme on HIV/AIDS (UNAIDS) Status of the global HIV epidemic. Report on the Global AIDS Epidemic. 2008
- 5.Federal HIV/AIDS Prevention and Control Office. Prevention of Mother to Child Transmission. 2010. http://www.etharc.org/ [Google Scholar]
- 6.Federal HIV/AIDS Prevention and Control Office. Multi-Sectoral HIV/AIDS Response Annual Monitoring and Evaluation Report 2001. 4th. Addis Ababa, Ethiopia: EFY; 2009. [Google Scholar]
- 7.Awasthi S., Bundy D. A. P., Savioli L. Helminthic infections. British Medical Journal. 2003;327(7412):431–433. doi: 10.1136/bmj.327.7412.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Control of Tropical Diseases. Geneva, Switzerland: WHO; 1998. [Google Scholar]
- 9.Kassu A., Tsegaye A., Wolday D., et al. Role of incidental and/or cured intestinal parasitic infections on profile of CD4+ and CD8+ T cell subsets and activation status in HIV-1 infected and uninfected adult Ethiopians. Clinical and Experimental Immunology. 2003;132(1):113–119. doi: 10.1046/j.1365-2249.2003.02106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borkow G., Leng Q., Weisman Z., et al. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. Journal of Clinical Investigation. 2000;106(8):1053–1060. doi: 10.1172/JCI10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borkow G., Leng Q., Weisman Z., et al. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. The Journal of Clinical Investigation. 2000;106(8):1053–1060. doi: 10.1172/jci10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bement A., Yeshambel B., Beyene M. Serum IgE levels of diarrheic patients in Northwest Ethiopia with high prevalence of HIV and intestinal parasitoses. Journal of AIDS & Clinical Research. 2012;3(1):136–140. [Google Scholar]
- 13.Bentwich Z., Kalinkovich A., Weisman Z., Borkow G., Beyers N., Beyers A. D. Can eradication of helminthic infections change the face of AIDS and tuberculosis? Immunology Today. 1999;20(11):485–487. doi: 10.1016/s0167-5699(99)01499-1. [DOI] [PubMed] [Google Scholar]
- 14.Borkow G., Weisman Z., Leng Q., et al. Helminths, human immunodeficiency virus and tuberculosis. Scandinavian Journal of Infectious Diseases. 2001;33(8):568–571. doi: 10.1080/00365540110026656. [DOI] [PubMed] [Google Scholar]
- 15.Fincham J. E., Markus M. B., Adams V. J. Could control of soil-transmitted helminthic infection influence the HIV/AIDS pandemic. Acta Tropica. 2003;86(2-3):315–333. doi: 10.1016/S0001-706X(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 16.Kassu A., van Nhien N., Nakamori M., et al. Deficient serum retinol levels in HIV-infected and uninfected patients with tuberculosis in Gondar, Ethiopia. Nutrition Research. 2007;27(2):86–91. doi: 10.1016/j.nutres.2006.12.009. [DOI] [Google Scholar]
- 17.Kassu A., Yabutani T., Mahmud Z. H., et al. Alterations in serum levels of trace elements in tuberculosis and HIV infections. European Journal of Clinical Nutrition. 2006;60(5):580–586. doi: 10.1038/sj.ejcn.1602352. [DOI] [PubMed] [Google Scholar]
- 18.Bemnet A., Ketema T., Feleke M., et al. Levels of serum zinc, copper and copper/zinc ratio in patients with Diarrhea and HIV infection in Ethiopia. Journal of Vitamin and Trace Elements. 2011;1:101–106. [Google Scholar]
- 19.Amare B., Tafess K., Ota F., et al. Serum concentration of selenium in diarrheic patients with and without HIV/AIDS in Gondar, Northwest Ethiopia. Journal of AIDS and Clinical Research. 2011;2(6, article 128) doi: 10.4172/2155-6113.1000128. [DOI] [Google Scholar]
- 20.Mulu A., Kassu A., Huruy K., et al. Vitamin A deficiency during pregnancy of HIV infected and non-infected women in tropical settings of Northwest Ethiopia. BMC Public Health. 2011;11, article 569 doi: 10.1186/1471-2458-11-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassu A., Andualem B., Van Nhien N., et al. Vitamin A deficiency in patients with diarrhea and HIV infection in Ethiopia. Asia Pacific Journal of Clinical Nutrition. 2007;16(1):323–328. [PubMed] [Google Scholar]
- 22.Bhaskaram P. Micronutrient malnutrition, infection, and immunity: an overview. Nutrition Reviews. 2002;60(5):S40–S45. doi: 10.1301/00296640260130722. [DOI] [PubMed] [Google Scholar]
- 23.Black R. E., Morris S. S., Bryce J. Where and why are 10 million children dying every year? The Lancet. 2003;361(9376):2226–2234. doi: 10.1016/s0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- 24.Katona P., Katona-Apte J. The interaction between nutrition and infection. Clinical Infectious Diseases. 2008;46(10):1582–1588. doi: 10.1086/587658. [DOI] [PubMed] [Google Scholar]
- 25.Glennie S. J., Nyirenda M., Williams N. A., Heyderman R. S. Do multiple concurrent infections in African children cause irreversible immunological damage? Immunology. 2012;135(2):125–132. doi: 10.1111/j.1365-2567.2011.03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMurray D. N. Cell-mediated immunity in nutritional deficiency. Progress in Food and Nutrition Science. 1984;8(3-4):193–228. [PubMed] [Google Scholar]
- 27.Keusch G. T. The history of nutrition: malnutrition, infection and immunity. Journal of Nutrition. 2003;133(1):336S–340S. doi: 10.1093/jn/133.1.336S. [DOI] [PubMed] [Google Scholar]
- 28.Keusch G. T. Symposium: nutrition and infection, prologue and progress since 1968, the history of nutrition—malnutrition, infection and immunity. Journal of Nutrition. 2003;133:336S–340S. doi: 10.1093/jn/133.1.336S. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez L., Cervantes E., Ortiz R. Malnutrition and gastrointestinal and respiratory infections in children: a public health problem. International Journal of Environmental Research and Public Health. 2011;8(4):1174–1205. doi: 10.3390/ijerph8041174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irfan A., Srivastava V. K., Prasad R., Mohd Y., Saleem M., Wahid A. Deficiency of micronutrient status in pulmonary tuberculosis patients in North India. Biomedical Research. 2011;22(4):449–454. [Google Scholar]
- 31.Gupta K., Gupta R., Atreja A., Verma M., Vishvkarma S. Tuberculosis and nutrition. Lung India. 2009;26(1):9–16. doi: 10.4103/0970-2113.45198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karyadi E., Schultink W., Nelwan R. H. H., et al. Poor micronutrient status of active pulmonary tuberculosis patients in Indonesia. Journal of Nutrition. 2000;130(12):2953–2958. doi: 10.1093/jn/130.12.2953. [DOI] [PubMed] [Google Scholar]
- 33.Koyanagi A., Kuffó D., Gresely L., Shenkin A., Cuevas L. E. Relationships between serum concentrations of C-reactive protein and micronutrients, in patients with tuberculosis. Annals of Tropical Medicine and Parasitology. 2004;98(4):391–399. doi: 10.1179/000349804225003424. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee A., Saini S., Kabra S. K., et al. Effect of micronutrient deficiency on QuantiFERON-TB Gold In-Tube test and tuberculin skin test in diagnosis of childhood intrathoracic tuberculosis. European Journal of Clinical Nutrition. 2014;68(1):38–42. doi: 10.1038/ejcn.2013.216. [DOI] [PubMed] [Google Scholar]
- 35.Karyadi E., West C., Schultink W., et al. A double-blind, placebo-controlled study of vitamin A and zinc supplementation in persons with tuberculosis in Indonesia: effects on clinical response and nutritional status. The American Journal of Clinical Nutrition. 2002;75(4):720–727. doi: 10.1093/ajcn/75.4.720. [DOI] [PubMed] [Google Scholar]
- 36.Irfan A., Srivastava V. K., Prasad R., Yusuf M., Safia M. S., Wahid A. Deficiency of micronutrient status in pulmonary tuberculosis patients in North India. Biomedical Research. 2011;22(4):449–454. [Google Scholar]
- 37.Drain P. K., Kupka R., Mugusi F., Fawzi W. W. Micronutrients in HIV-positive persons receiving highly active antiretroviral therapy. The American Journal of Clinical Nutrition. 2007;85(2):333–345. doi: 10.1093/ajcn/85.2.333. [DOI] [PubMed] [Google Scholar]
- 38.Baylin A., Villamor E., Rifai N., Msamanga G., Fawzi W. W. Effect of vitamin supplementation to HIV-infected pregnant women on the micronutrient status of their infants. European Journal of Clinical Nutrition. 2005;59(8):960–968. doi: 10.1038/sj.ejcn.1602201. [DOI] [PubMed] [Google Scholar]
- 39.Mehta S., Fawzi W. W. Micronutrient supplementation as adjunct treatment for HIV-infected patients. Clinical Infectious Diseases. 2010;50(12):1661–1663. doi: 10.1086/652865. [DOI] [PubMed] [Google Scholar]
- 40.Coodley G., Girard D. E. Vitamins and minerals in HIV infection. Journal of General Internal Medicine. 1991;6(5):p. 472. doi: 10.1007/BF02598176. [DOI] [PubMed] [Google Scholar]
- 41.Irlam J. H., Siegfried N., Visser M. E., Rollins N. C. Micronutrient supplementation for children with HIV infection. Cochrane Database of Systematic Reviews. 2013;10 doi: 10.1002/14651858.CD010666.CD010666 [DOI] [PubMed] [Google Scholar]
- 42.Papathakis P. C., Rollins N. C., Chantry C. J., Bennish M. L., Brown K. H. Micronutrient status during lactation in HIV-infected and HIV-uninfected South African women during the first 6 mo after delivery. The American Journal of Clinical Nutrition. 2007;85(1):182–192. doi: 10.1093/ajcn/85.1.182. [DOI] [PubMed] [Google Scholar]
- 43.Campa A., Baum M. K. Micronutrients and HIV infection. HIV Therapy. 2010;4(4):437–469. doi: 10.2217/hiv.10.36. [DOI] [Google Scholar]
- 44.Semba R. D. Iron-deficiency anemia and the cycle of poverty among human immunodeficiency virus-infected women in the inner city. Clinical Infectious Diseases. 2003;37(2):S105–S111. doi: 10.1086/375892. [DOI] [PubMed] [Google Scholar]
- 45.Hughes S., Kelly P. Interactions of malnutrition and immune impairment, with specific reference to immunity against parasites. Parasite Immunology. 2006;28(11):577–588. doi: 10.1111/j.1365-3024.2006.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casey G. J., Montresor A., Cavalli-Sforza L. T., et al. Elimination of iron deficiency anemia and soil transmitted helminth infection: evidence from a fifty-four month iron-folic acid and de-worming program. PLoS Neglected Tropical Diseases. 2013;7(4) doi: 10.1371/journal.pntd.0002146.e2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hesham M. S., Edariah A. B., Norhayati M. Intestinal parasitic infections and micronutrient deficiency: a review. Medical Journal of Malaysia. 2004;59(2):284–293. [PubMed] [Google Scholar]
- 48.Crompton D. W. T., Nesheim M. C. Nutritional impact of intestinal helminthiasis during the human life cycle. Annual Review of Nutrition. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- 49.Ahmed A., Al-Mekhlafi H. M., Al-Adhroey A. H., Ithoi I., Abdulsalam A. M., Surin J. The nutritional impacts of soil-transmitted helminths infections among Orang Asli schoolchildren in rural Malaysia. Parasites & Vectors. 2012;5(1, article 119) doi: 10.1186/1756-3305-5-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.WHO. Iron Deficiency Anemia Assessment Prevention and Control, A Guide for Program Managers. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 51.Bhaskaram P. Immunobiology of mild micronutrient deficiencies. British Journal of Nutrition. 2001;85(2):S75–S80. doi: 10.1079/bjn2000297. [DOI] [PubMed] [Google Scholar]
- 52.Rivera M. T., de Souza A. P., Araujo-Jorge T. C., de Castro S. L., Vanderpas J. Trace elements, innate immune response and parasites. Clinical Chemistry and Laboratory Medicine. 2003;41(8):1020–1025. doi: 10.1515/cclm.2003.156. [DOI] [PubMed] [Google Scholar]
- 53.Friis H. Micronutrients and infections: an introduction. In: Friis H., editor. Micronutrients and HIV Infection. Boca Raton, Fla, USA: CRC Press; 2001. pp. 1–21. [Google Scholar]
- 54.Alice M. T., Jane L., Kristy H., et al. Micronutrients: current issues for HIV care providers. AIDS. 2005;19(9):847–861. doi: 10.1097/01.aids.0000171398.77500.a9. [DOI] [PubMed] [Google Scholar]
- 55.Stone C. A., Kawai K., Kupka R., Fawzi W. W. Role of selenium in HIV infection. Nutrition Reviews. 2010;68(11):671–681. doi: 10.1111/j.1753-4887.2010.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kupka R., Msamanga G. I., Spiegelman D., et al. Selenium status is associated with accelerated HIV disease progression among HIV-1-infected pregnant women in Tanzania. Journal of Nutrition. 2004;134(10):2556–2560. doi: 10.1093/jn/134.10.2556. [DOI] [PubMed] [Google Scholar]
- 57.Constans J., Pellegrin J. L., Peuchant E., et al. Membranous fatty acid and antioxidant plasma in 77 HIV infected patients. La Revue de Médecine Interne. 1993;14(10):p. 1003. doi: 10.1016/s0248-8663(05)80121-3. [DOI] [PubMed] [Google Scholar]
- 58.Constans J., Pellegrin J. L., Sergeant C., et al. Serum selenium predicts outcome in HIV infection. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1995;10(3):p. 392. doi: 10.1097/00042560-199511000-00015. [DOI] [PubMed] [Google Scholar]
- 59.Look M. P., Rockstroh J. K., Rao G. S., et al. Serum selenium, plasma glutathione (GSH) and erythrocyte glutathione peroxidase GSH-Px)-levels in asymptomatic versus symptomatic human immunodeficiency virus-1 (HIV-1)-infection. European Journal of Clinical Nutrition. 1997;51(4):266–272. doi: 10.1038/sj.ejcn.1600401. [DOI] [PubMed] [Google Scholar]
- 60.Baum M. K., Shor-Posner G., Lai S., et al. High risk of HIV-related mortality is associated with selenium deficiency. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1997;15(5):370–374. doi: 10.1097/00042560-199708150-00007. [DOI] [PubMed] [Google Scholar]
- 61.Semba R. D., Caiaffa W. T., Graham N. M. H., Cohn S., Vlahov D. Vitamin A deficiency and wasting as predictors of mortality in human immunodeficiency virus-infected injection drug users. Journal of Infectious Diseases. 1995;171(5):1196–1202. doi: 10.1093/infdis/171.5.1196. [DOI] [PubMed] [Google Scholar]
- 62.Semba R. D., Graham N. M. H., Caiaffa W. T., Margolick J. B., Clement L., Vlahov D. Increased mortality associated with vitamin A deficiency during human immunodeficiency virus type 1 infection. Archives of Internal Medicine. 1993;153(18):2149–2154. doi: 10.1001/archinte.1993.00410180103012. [DOI] [PubMed] [Google Scholar]
- 63.Fufa H., Umeta M., Taffesse S., Mokhtar N., Aguenaou H. Nutritional and immunological status and their associations among HIV-infected adults in Addis Ababa, Ethiopia. Food and Nutrition Bulletin. 2009;30(3):227–232. doi: 10.1177/156482650903000303. [DOI] [PubMed] [Google Scholar]
- 64.UNAIDS. Progress Report. 2011. Geneva, Switzerland: UNAIDS; 2011. Global HIV/AIDS response—epidemic update and health sector progress toward univeral access. [Google Scholar]
- 65.Dreyfuss M. L., Fawzi W. W. Micronutrients and vertical transmission of HIV-1. The American Journal of Clinical Nutrition. 2002;75(6):959–970. doi: 10.1093/ajcn/75.6.959. [DOI] [PubMed] [Google Scholar]
- 66.UNAIDS. Mother-to-Child Transmission of HIV. Geneva, Switzerland: UNAIDS; 1998. (UNAIDS Technical Update). [Google Scholar]
- 67.Semba R. D., Miotti P. G., Chiphangwi J. D., et al. Maternal vitamin A deficiency and mother-to-child transmission of HIV-1. The Lancet. 1994;343(8913):1593–1597. doi: 10.1016/s0140-6736(94)93056-2. [DOI] [PubMed] [Google Scholar]
- 68.Dushimimana A., Graham M. N., Humphrey J. H., et al. Maternal vitamin A levels and HIV-related birth outcome in Rwanda. VIII International Conference on AIDS; 1992; Amsterdam, The Netherlands. Abstract no POC 4221. [Google Scholar]
- 69.Semba R. D., Miotti P. G., Chiphangwi J. D., et al. Infant mortality and maternal vitamin A deficiency during human immunodeficiency virus infection. Clinical Infectious Diseases. 1995;21(4):966–972. doi: 10.1093/clinids/21.4.966. [DOI] [PubMed] [Google Scholar]
- 70.Coutsoudis A., Pillay K., Spooner E., Kuhn L., Coovadia H. M. Randomized trial testing the effect of vitamin A supplementation on pregnancy outcomes and early mother-to-child HIV-1 transmission in Durban, South Africa. AIDS. 1999;13(12):1517–1524. doi: 10.1097/00002030-199908200-00012. [DOI] [PubMed] [Google Scholar]
- 71.Fawzi W. W., Msamanga G. I., Spiegelman D., et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. The Lancet. 1998;351(9114):1477–1482. doi: 10.1016/s0140-6736(98)04197-x. [DOI] [PubMed] [Google Scholar]
- 72.Gil L., Martínez G., González I., et al. Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacological Research. 2003;47(3):217–224. doi: 10.1016/s1043-6618(02)00320-1. [DOI] [PubMed] [Google Scholar]
- 73.Anthony H. K., Ashok A. Oxidants and antioxidants in the pathogenesis of HIV/AIDS. The Open Reproductive Science Journal. 2011;3(1):154–161. doi: 10.2174/1874255601103010154. [DOI] [Google Scholar]
- 74.UNAIDS. AIDS epidemic update 2008. http://www.unaids.org/
- 75.Centres for Disease Control and Prevention. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. MMWR—Recommendations and Reports. 2009;58(RR-4):1–207. http://www.aidsinfo.nih.gov/Guidelines. [PubMed] [Google Scholar]
- 76.Masiá M., Padilla S., Bernal E., et al. Influence of antiretroviral therapy on oxidative stress and cardiovascular risk: a prospective cross-sectional study in HIV-infected patients. Clinical Therapeutics. 2007;29(7):1448–1455. doi: 10.1016/j.clinthera.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 77.Semba R. D., Kumwenda J., Zijlstra E., et al. Micronutrient supplements and mortality of HIV-infected adults with pulmonary TB: a controlled clinical trial. International Journal of Tuberculosis and Lung Disease. 2007;11(8):854–859. [PubMed] [Google Scholar]
- 78.van Lettow M., Fawzi W. W., Semba R. D. Triple trouble: the role of malnutrition in tuberculosis and human immunodeficiency virus co-infection. Nutrition Reviews. 2003;61(3):81–90. doi: 10.1301/nr.2003.marr.81-90. [DOI] [PubMed] [Google Scholar]
- 79.PrayGod G., Range N., Faurholt-Jepsen D., et al. Daily multi-micronutrient supplementation during tuberculosis treatment increases weight and grip strength among HIV-uninfected but not HIV-infected patients in Mwanza, Tanzania. Journal of Nutrition. 2011;141(4):685–691. doi: 10.3945/jn.110.131672. [DOI] [PubMed] [Google Scholar]
- 80.Davies P., Grange J. The genetics of host resistance and susceptibility to tuberculosis. Annals of the New York Academy of Sciences. 2001;953:151–156. doi: 10.1111/j.1749-6632.2001.tb11373.x. [DOI] [PubMed] [Google Scholar]
- 81.Liu P. T., Stenger S., Tang D. H., Modlin R. L. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. Journal of Immunology. 2007;179(4):2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 82.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? British Journal of Nutrition. 2003;89(5):552–572. doi: 10.1079/bjn2003837. [DOI] [PubMed] [Google Scholar]
- 83.Ustianowski A., Shaffer R., Collin S., Wilkinson R. J., Davidson R. N. Prevalence and associations of vitamin D deficiency in foreign-born persons with tuberculosis in London. Journal of Infection. 2005;50(5):432–437. doi: 10.1016/j.jinf.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 84.Roth D. E., Soto G., Arenas F., et al. Association between vitamin D receptor gene polymorphisms and response to treatment of pulmonary tuberculosis. The Journal of Infectious Diseases. 2004;190(5):920–927. doi: 10.1086/423212. [DOI] [PubMed] [Google Scholar]
- 85.Bellamy R., Ruwende C., Corrah T., et al. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. Journal of Infectious Diseases. 1999;179(3):721–724. doi: 10.1086/314614. [DOI] [PubMed] [Google Scholar]
- 86.Feleke Y., Abdulkadir J., Mshana R., et al. Low levels of serum calcidiol in an African population compared to a North European population. European Journal of Endocrinology. 1999;141(4):358–360. doi: 10.1530/eje.0.1410358. [DOI] [PubMed] [Google Scholar]
- 87.Gilchrest B. A. Sun exposure and vit D sufficiency. Clinical Nutrition. 2008;88:570S–577S. doi: 10.1093/ajcn/88.2.570S. [DOI] [PubMed] [Google Scholar]
- 88.Gandhi N. R., Moll A., Sturm A. W., et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. The Lancet. 2006;368(9547):1575–1580. doi: 10.1016/s0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 89.Migliori G. B., de Iaco G., Besozzi G., Centis R., Cirillo D. M. First tuberculosis cases in Italy resistant to all tested drugs. Euro Surveillance. 2007;12(5) doi: 10.2807/esw.12.20.03194-en.E070517.1 [DOI] [PubMed] [Google Scholar]
- 90.Velayati A. A., Masjedi M. R., Farnia P., et al. Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran. Chest. 2009;136(2):420–425. doi: 10.1378/chest.08-2427. [DOI] [PubMed] [Google Scholar]
- 91.Sommer A., West KP. Vitamin A Deficiency: Health, Survival and Vision. New York, NY, USA: Oxford University Press; 1996. [Google Scholar]
- 92.Ross A. C. Vitamin A status: relationship to immunity and the antibody response. Proceedings of the Society for Experimental Biology and Medicine. 1992;200(3):303–320. doi: 10.3181/00379727-200-43436a. [DOI] [PubMed] [Google Scholar]
- 93.Semba R. D. Vitamin A immunity and infection. Clinical Infectious Diseases. 1994;19(3):489–499. doi: 10.1093/clinids/19.3.489. [DOI] [PubMed] [Google Scholar]
- 94.Madebo T., Lindtjørn B., Aukrust P., Berge R. K. Circulating antioxidants and lipid peroxidation products in untreated tuberculosis patients in Ethiopia. The American Journal of Clinical Nutrition. 2003;78(1):117–122. doi: 10.1093/ajcn/78.1.117. [DOI] [PubMed] [Google Scholar]
- 95.Palanisamy G. S., Kirk N. M., Ackart D. F., Shanley C. A., Orme I. M., Basaraba R. J. Evidence for oxidative stress and defective antioxidant response in guinea pigs with tuberculosis. PLoS ONE. 2011;6(10) doi: 10.1371/journal.pone.0026254.e26254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suresh D. R., Annam V., Pratibha K., Hamsaveena Immunological correlation of oxidative stress markers in tuberculosis patients. International Journal of Biological and Medical Research. 2010;1(4):185–187. [Google Scholar]
- 97.Kuo H. P., Ho T. C., Wang C. H., Yu C. T., Lin H. C. Increased production of hydrogen peroxide and expression of CD11b/CD18 on alveolar macrophages in patients with active pulmonary tuberculosis. Tubercle and Lung Disease. 1996;77(5):468–475. doi: 10.1016/s0962-8479(96)90122-7. [DOI] [PubMed] [Google Scholar]
- 98.Sies H., Stahl W. Vitamins E and C, β-carotene, and other carotenoids as antioxidants. The American Journal of Clinical Nutrition. 1995;62(6):1315–1321. doi: 10.1093/ajcn/62.6.1315S. [DOI] [PubMed] [Google Scholar]
- 99.Amare B., Ali J., Moges B., et al. Nutritional status, intestinal parasite infection and allergy among school children in Northwest Ethiopia. BMC Pediatrics. 2013;13(1, article 7) doi: 10.1186/1471-2431-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amare B., Moges B., Fantahun B., et al. Micronutrient levels and nutritional status of school children living in Northwest Ethiopia. Nutrition Journal. 2012;11(1, article 108) doi: 10.1186/1475-2891-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Belay G., Reji P., Erko B., Legesse M., Belay M. Intestinal parasitic infections and malnutrition amongst first-cycle primary schoolchildren in Adama, Ethiopia. African Journal of Primary Health Care and Family Medicine. 2011;3(1, article 198) doi: 10.4102/phcfm.v3i1.198. [DOI] [Google Scholar]
- 102.Asfaw S. T., Giotom L. Malnutrition and enteric parasitoses among under-five children in Aynalem Village, Tigray. Ethiopian Journal of Health Development. 2000;14(1):67–75. doi: 10.4314/ejhd.v14i1.9931. [DOI] [Google Scholar]
- 103.Koski K. G., Scott M. E. Gastrointestinal nematodes, nutrition and immunity: breaking the negative spiral. Annual Review of Nutrition. 2001;21:297–321. doi: 10.1146/annurev.nutr.21.1.297. [DOI] [PubMed] [Google Scholar]
- 104.Rafi W., Ribeiro-Rodrigues R., Ellner J. J., Salgame P. ‘Coinfection-helminthes and tuberculosis’. Current Opinion in HIV and AIDS. 2012;7(3):239–244. doi: 10.1097/coh.0b013e3283524dc5. [DOI] [PubMed] [Google Scholar]
- 105.MacDonald A. S., Araujo M. I., Pearce E. J. Immunology of parasitic helminth infections. Infection and Immunity. 2002;70(2):427–433. doi: 10.1128/iai.70.2.427-433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Diniz L. M., Magalhães E. F. L., Pereira F. E. L., Dietze R., Ribeiro-Rodrigues R. Presence of intestinal helminths decreases T helper type 1 responses in tuberculoid leprosy patients and may increase the risk for multi-bacillary leprosy. Clinical & Experimental Immunology. 2010;161(1):142–150. doi: 10.1111/j.1365-2249.2010.04164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bentwich Z., Weisman Z., Moroz C., Bar-Yehuda S., Kalinkovich A. Immune dysregulation in Ethiopian immigrants in Israel: relevance to helminth infections? Clinical and Experimental Immunology. 1996;103(2):239–243. doi: 10.1046/j.1365-2249.1996.d01-612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Elias D., Wolday D., Akuffo H., Petros B., Bronner U., Britton S. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guérin (BCG) vaccination. Clinical & Experimental Immunology. 2001;123(2):219–225. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tristão-Sá R., Ribeiro-Rodrigues R., Johnson L. T., Pereira F. E. L., Dietze R. Intestinal nematodes and pulmonary tuberculosis. Revista da Sociedade Brasileira de Medicina Tropical. 2002;35(5):533–535. doi: 10.1590/S0037-86822002000500020. [DOI] [PubMed] [Google Scholar]
- 110.Ing R., Su Z., Scott M. E., Koski K. G. Suppressed T helper 2 immunity and prolonged survival of a nematode parasite in protein-malnourished mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(13):7078–7083. doi: 10.1073/pnas.97.13.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carman J. A., Pond L., Nashold F., Wassom D. L., Hayes C. E. Immunity to Trichinella spiralis infection in vitamin A-deficient mice. The Journal of Experimental Medicine. 1992;175(1):111–120. doi: 10.1084/jem.175.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scott M. E., Koski K. G. Zinc deficiency impairs immune responses against parasitic nematode infections at intestinal and systemic sites. Journal of Nutrition. 2000;130(5):1412–1420. doi: 10.1093/jn/130.5.1412S. [DOI] [PubMed] [Google Scholar]


