Abstract
Background
Clear cell sarcoma (CCS), initially named malignant melanoma of soft parts, is an aggressive soft tissue sarcoma (STS) that, due to MITF activation, shares with melanoma the expression of melanocyte differentiation antigens. CCS is poorly sensitive to chemotherapy. Multi-kinase inhibitors have been used as therapeutic agents. In the case we report here, treatment with sunitinib induced a long-lasting clinical response that was associated with an immune activation directed against Melan-A/MART-1 antigen.
Case presentation
A 28 years old female patient with an advanced molecularly confirmed CCS resistant to conventional chemotherapy was started in January 2012 on sunitinib, 37.5 mg/day, with evidence of radiologic and metabolic response at the primary and metastatic sites of disease. Pathologic response and loss of the Melan-A/MART-1 antigen were evidenced on residual tumor removed in April 2012. Immunological monitoring performed on patient’s blood during pharmacological treatment revealed a systemic, Melan-A/MART-1 specific immunity and a low frequency of immunosuppressive cells. Sunitinib was restarted in May 2012, with a new response, and continued for 11 months although with repeatedly interruptions due to toxicity. Disease progression and new responses were documented at each treatment interruption and restart. Sunitinib was definitively interrupted in April 2013 for disease progression.
Conclusion
The analysis of this case proves that antigens expressed by CCS, as for melanoma, can be immunogenic in vivo and that tumor-antigen specific T cells may exert anti-tumor activity in CCS patient. Thus, manipulation of the immune response may have therapeutic potential for this STS subtype and immunotherapy approaches, can be promising therapeutic options for these patients.
Keywords: Sarcoma, Sunitinib, Clear cell sarcoma, Tumor-specific T cell, Immunotherapy, Chemotherapy
Background
Clear cell sarcoma (CCS) is a very rare and aggressive soft tissue sarcoma (STS), usually arising from deep soft tissue or viscera [1], and marked by a very high metastatic risk resulting in a 5-year overall survival of about 50% [2-4]. In contrast with other STS, and similarly to melanoma, its metastatic sites include lymph nodes (LNs). CCS, initially named malignant melanoma of soft parts [5], are molecularly characterized in most cases by a specific translocation, t(12;22)(q13;q12), which results in fusion of the Ewing’s sarcoma gene, EWS, with the cyclic AMP (cAMP) regulated transcription factor, ATF1, a member of the cAMP-responsive element binding protein (CREB) family [6]. The EWS-ATF1 chimeric fusion protein interacts with the MITF (melanocyte master transcription factor) promoter, thus it directly and aberrantly activates MITF expression. Consequently, CCS is characterized by the expression of the melanocytic differentiation markers HMB-45/gp100 and Melan-A/MART-1 [7]. Overall, several immunophenotypic and molecular features are shared between CCS and malignant melanoma. Importantly, a proportion of CCS cases lack specific translocation and thus, clinical presentation as well as fluorescence in situ hybridization (FISH) analysis and reverse transcription polymerase chain reaction (RT-PCR) for the specific translocation are crucial to distinguish the two entities. Receptor tyrosine kinase expression/activation [8] and gene expression analysis [9] indicate that MITF drives the same down-stream pathways in CCS and in melanoma, and that PDGFRβ and c-Met are expressed by CCS [10,11]. Moreover, BRAF activating mutations have been occasionally detected in both EWS-ATF1 positive and negative CCS [8,12,13]. CCS is poorly sensitive to chemotherapy and anecdotal responses to regimens containing dacarbazine, vincristine, anthracycline, cyclophosphamide and to interferon-alpha-2b [14] have been reported. Based on the molecular features described above, multi-kinase inhibitors have been used as therapeutic agents in this STS and objective responses to sunitinib, and sorafenib treatments have been recently reported [15,16]. Here we describe a case of a 28 years old female patient with a metastatic, translocated CCS who experienced a prolonged, objective response to sunitinib malate (hereafter reported as sunitinib). We consider this case of interest as objective response to sunitinib correlated with a low frequency of immunosuppressive cells in the periphery, the presence of a systemic immunity directed against the CCS associated antigen Melan-A/MART-1 and the in vivo immune selection of post-sunitinib, MART-1 negative tumor. The analysis of this case proves that antigens expressed by CCS, as for the melanoma, can be immunogenic in vivo and that tumor-antigen specific T cells may exert anti-tumor activity in vivo.
Case presentation
A female patient aged 28 years presented in 2007 with a lesion arising from the deep soft tissue of the left foot, covered by a healthy skin. Prior clinical history was negative for melanoma. TNM classification at presentation was: stage 3, e.g. primary lesion arising from soft tissue of the left foot with positivity of the ipsilateral inguinal sentinel LNs. On whole body CT scan there was no evidence of secondary lesions. Initial treatment consisted of wide excision of the primary tumor (surgery 2007) with diagnosis of clear cell sarcoma (CCS), and confirmed by the positivity of the FISH analysis for EWS-ATF1. The patient received sequential hyperthermic limb perfusion with melphalan and TNF and the surgical dissection of the left inguinal-iliac-obturator LNs. Three of the LNs removed were positive for disease. Local recurrence and inguinal LN relapse was detected in July 2011 and treated with doxorubicin plus dacarbazine for 5 cycles with response. Given the evidence of a new disease progression (a loco-regional and inguinal LN relapse, as confirmed by CT scan and PET) and based on preliminary evidence of sunitinib possible activity in CCS [15], in January 2012 sunitinib was started at the dose of 37.5 mg/day, with a tumor partial response (classified according to RECIST, version 1.1 [17]) to the lesion located on left foot and a complete response to metastasis on upper left leg. The response was confirmed by PET and CT scan (Figure 1). In April 2012, patient underwent left leg amputation, with evidence of pathologic response to sunitinib in the surgical specimen. In May 2012, sunitinib was restarted and maintained at the same dosage till January 2013. During these months of treatment, sunitinib was repeatedly stopped due to toxicity, with evidence of rapid disease progression following treatment interruption and of a new response after restoring treatment. From January 2013, sunitinib was reduced to 12.5 mg/day due to grade 3 cardiac toxicity. After initial disease stabilization, disease progression occurred marked by a re-growth of previously responsive tumor lesions and by the evidence of new lesions to the soft tissues of the left leg and pelvic LNs. Sunitinib was definitively interrupted in April 2013. Patient died of disease in February 2014.
Figure 1.
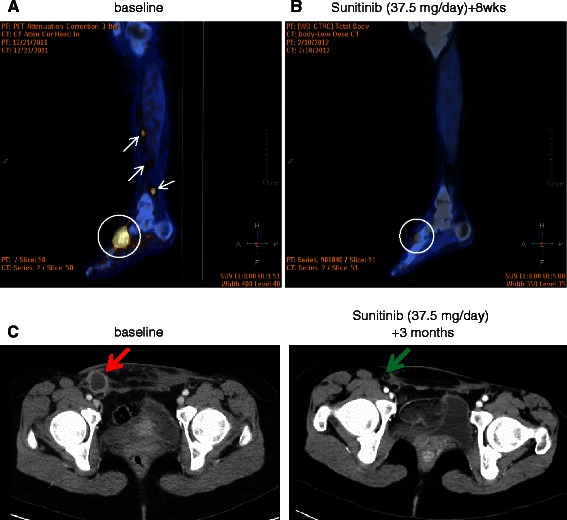
Response to sunitinib: FDG-PET and CT scan evaluation. (A) Baseline FDG PET/CT study: sagittal fused PET/CT image showing abnormal FDG uptakes in the left foot tumor (circle; SUVmax 12.0) and in soft tissue metastatic nodules in the ankle and leg (arrows); (B) After 8 weeks of treatment with SM 37.5 mg/day, PET/CT re-evaluation shows a complete metabolic response of the foot lesion (circle; SUVmax 2.5; ΔSUV −79%) and the disappearance of the soft tissue nodules. (C) CT scan (venous phase after contrast medium) shows a complete response to a right inguinal lymph node after 3 months of treatment with sunitinib (green arrow), compared to baseline (red arrow).
Immune-related analysis were performed at the tumor site and in the peripheral blood of this patient (Table 1). The expression of the MITF regulated melanocytic antigens (HMB-45/gp100 and Melan-A/MART-1, Figure 2A) and S-100 (data not shown) was assessed by immunohistochemistry on pre- (surgery December-2010 and November-2011) and post-sunitinib tumor specimens (surgery April-2012). Pre-treatment tumor lesions displayed a clear positivity for all of the analyzed antigens. Conversely, tumor specimen removed after treatment with sunitinib displayed a selective loss of MART-1 expression, while it retained the positivity for HMB-45 and S-100 (Figure 2A). Post-sunitinib tumor was heavily infiltrated by CD3+ T cells that contained a significant proportion of CD8+ T cells. Areas of pathological regression were clearly evident in association with lymphocyte infiltration (Figure 2B). No T cells infiltration were detected in the pre-treated lesion (data not shown). The in vivo generation of the MART-1 loss antigen variant paralleled the presence of anti-MART-1 systemic immunity in the blood of this CCS patient. Patient’s peripheral blood mononuclear cells (PBMCs) isolated in the course of sunitinib treatment and before surgery (surgery April-2012), sensitized in vitro with the immunogenic HLA-A*0201 restricted peptide Melan-A/MART-1[27L] displayed the presence of a remarkable frequency of MART-1 specific CD8+T cells (7,72%), as monitored by pentamer staining (Figure 3). These anti-MART-1 specific T cells were functionally active. MART-1 sensitized PBMC released IFNγ when stimulated with the target cells loaded with Melan-A/MART-1-epitope (modified and native) and, importantly, they recognized in a MHC restricted fashion HLA-A*0201+MART1+, but not HLA-A*0201+MART1− and HLA-A*0201−MART1+ tumor cells as evaluated by ELIspot assay (Figure 3). Conversely, no T cells specific for the HLA-A*0201- gp100[210M] peptide were detected in post-sunitinib PBMCs of the patient applying the same procedure. All together these evidences strongly support the hypothesis that the post-sunitinib MART-1 negative tumor variant is the in vivo outcome of a T cell-mediated immune selection occurring in CCS patient during sunitinib treatment. The anti-MART-1 systemic immunity in post-sunitinib CCS patients was associated with low frequency of circulating immunosuppressive CD14+CD11b+HLADRneg/low monocytic myeloid-derived suppressor cells (mMDSCs), a population expanded in cancer patients, including melanoma [18-21]. Multi-parametric flow cytometry indicate that PBMCs collected during sunitinib treatment displayed a frequency of mMDSCs, comparable to that of healthy donors (HD) (Figure 4). Moreover, this low percentage of mMDSCs correlates with functional active convenctional T lymphocytes measured ex vivo as IL-2 and IFN-γ produced by CD3+ cells upon TCR stimulation (Figure 4). A strong increase in the number of circulating mMDSC and functionally impaired T cells was detected at the time of disease progression. Conversely, reduced frequency of CD3+CD4+CD25hiFoxp3hi regulatory T cells (Tregs) comparable to that of HD persisted all along the drug treatment (data not shown).
Table 1.
Summary of the immune-related analysis
| Date | Immune-related analysis | ||
|---|---|---|---|
| December 2010 (Dec-2010) | biopsy | IHC: MART-1/Melan-A +; S-100 +; gp100/HMB-45 + | |
| November 2011 | surgery | IHC: MART-1/Melan-A +; S-100 +; gp100/HMB-45 + | |
| January 2012 (Jan-2012) | sunitinib | Immunological monitoring: | |
| April 2012 | surgery | IHC: MART-1/Melan-A -; S-100 +; gp100/HMB-45 +; CD3 +; CD8 + | • Frequency of immunosuppressive cells and CD3+ T cell function (Figure 4) |
| May 2012/April 2013 | sunitinib* | • Presence of Melan-A/MART-1 specific CD8+ T cells (Figure 3) | |
Abbreviations: IHC, immunohistochemistry.
Note: *stopped many times due to toxicity with evidence of disease progression following treatment interruption and new response after restoring treatment.
Figure 2.
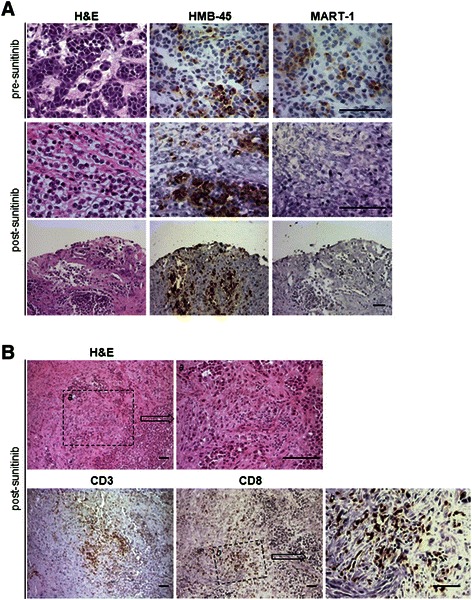
Immunohistochemical analysis of tumor antigens expression and T cell infiltration. (A) Hematoxylin and eosin (H&E), Melan-A/MART-1 and HMB-45/gp100 stainings in pre- (November 2011) and post- (April 2012) sunitinib tumor lesions. (B) Analysis of infiltrating immune T cells (CD3 and CD8) in sunitinib-treated tumor. Higher magnification in a shows area of pathologic tumor regression associated with lymphocyte infiltration; Bottom panels show CD3 and CD8 stainings; square b reports the high magnification of area infiltrated by CD8+ T cells. All scale bars indicate 50 μm.
Figure 3.
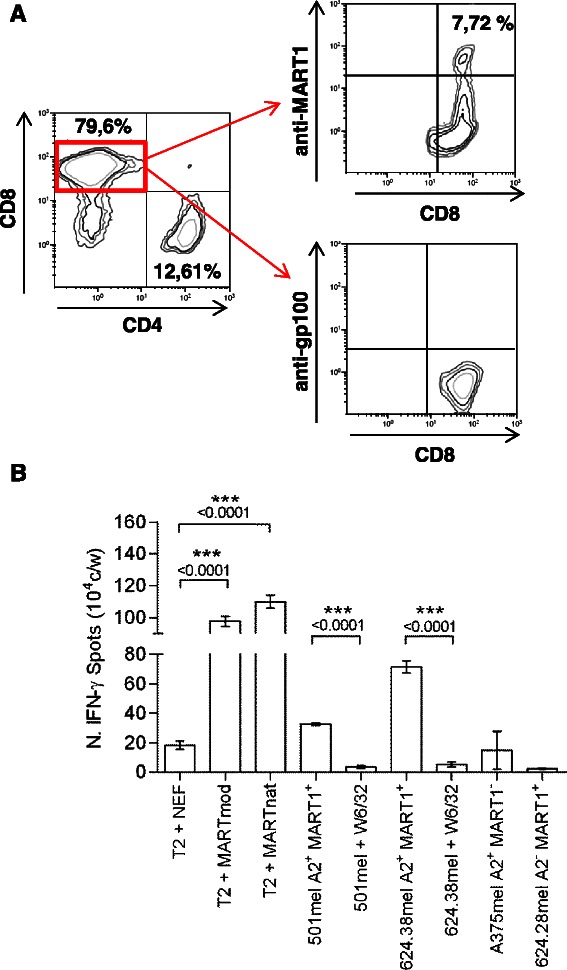
Phenotypic and functional analysis of tumor antigen-specific CD8 T cells. (A) Phenotypic analysis of pentamer+ CD8+ T cells after sensitization with the HLA2-A*0201 restricted-modified peptides (Melan-A/MART-1[27L] or gp100[210M]). (B) The tumor specificity of peptide sensitized T cells was assessed by measuring IFN-γ secretion (Enzyme-Linked ImmunoSpot (ELISpot) assay) upon stimulation with HLA-A*0201-restricted Melan A/MART-1 (modified or native)-pulsed (2 μmol/L) lymphoblastoid T2 cell line or HLA-matched HLA-A*0201+MART1+ tumor cells (#501mel and #624.38mel) pretreated or not with the anti-HLA class I (W6/32) mAb. Moreover, T cells were also incubated with HLA-mismatched allogeneic HLA-A*0201−MART1+ (#624.28mel) or HLA-A*0201+MART1− melanoma cells (#A375mel). The irrelevant peptide NEF[180–189] was used as negative control. P values were calculated by two-tailed t test.
Figure 4.
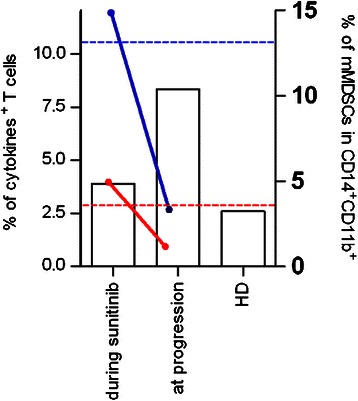
Frequency of circulating mMDSCs and T cell function during sunitinib treatment. Histograms show the frequencies of CD14+HLADRneg/low (mMDSCs) in live gated CD14+CD11b+ cells of patient’s PBMCs. Average level of mMDSC frequency of healthy donors (HD) is reported. Patient’s PBMCs were assayed for IFN-γ (red line) and IL-2 (blue line) production in response to overnight activation with anti-CD3/CD28-coated beads. Dotted horizontal lines (IFN-γ (red) and IL-2 (blue)) indicate the average level of cytokine-producing T cells in HD.
Conclusions
We described herein the case of a CCS (HLA-A*0201) patient with advanced disease that displayed a long-lasting response to treatment with the anti-angiogenic drug sunitinib. Based on the expression and the activation status of PDGFRβ in CSC, documented by our and other groups [8,15], sunitinib likely exerts a direct inhibition of the PDGFRβ-driven pathway in the tumor cells of the patient here studied. However, along with the effect on the tumor cells, this case report documented that in this patient, objective response during sunitinib treatment was associated to traits of tumor-specific immunity. The study of this clinical case shows that antigen expresses by CCS can be immunogenic and indicates that manipulation of the immune response may have therapeutic potential in this STS subtype. As melanoma, CCS expresses the MITF-regulated genes, including genes encoding for the melanoma differentiation antigens. Thus, we look at the presence of antigen-specific response in this CCS patient. Interestingly, we observed that tumor specimen resected after treatment with sunitinib had lost the expression of MART-1 antigen. The in vivo generation of MART-1 loss variant was associated to a CD3 + CD8+ T cell infiltration and to the presence of areas of pathologic regression, thus suggesting the in vivo occurrence of MART1-specific response. This immune contexture at the tumor site was paralleled by the finding that functionally active anti-MART-1 T cells were detectable in the blood of this patients collected during sunitinib treatment. To our knowledge this is the first report documenting the in vivo immunogenicity of CCS tumor. The immune response in the CSC patient studied in this report was directed toward Melan-A/MART-1. No specific immunity directed against the less immunogenic differentiation antigen gp100 was developed and, as expected, reactivity for HMB-45/gp100 was maintained in post-sunitinib surgical specimen. These findings are in line with the observation that Melan-A/MART-1/HLA-A*0201 restricted peptide behaves as immune-dominant epitope in melanoma patients and a high proportion (about 70%) of advanced stage III-IV melanoma patients display a natural anti-Melan-A/MART-1 immunity [22]. In the peripheral blood of this patient, we observed that sunitinib treatment sustained a down-modulation in the frequency of immune suppressive cells, Tregs and mMDSCs, and a parallel activation of T cell functions evaluated by the capacity of CD3+ T cells to release Th1 cytokines in response to a polyclonal stimulation. The immunomodulatory function of sunitinib has been clearly documented in other human tumors and we confirmed this activity in the setting of CCS [23,24]. However, our observations also suggest that the release in the immune suppression induced by sunitinib may have unleashed anti-tumor immunity in this CCS patient. Indeed, this hypothesis is in agreement with the observation that, in melanoma patients, antigen-specific responses are prevented by the presence of high frequency of circulating mMDSCs [25]. By contrast a decrease of their number favors the clinical response in patients treated with immunotherapy [26].
In conclusion, this case shed light on immune-similarities between CCS and melanoma, and indicates that manipulation of the immune response in this STS subtype likely evokes antigen-specific response. In addition to T cells specific for MITF-regulated antigens, anti-tumor immunity may potentially include also T cells recognizing unique, mutation-specific determinants. As previously shown by in vitro immunological assays [27], the chimeric protein encoded by the specific chromosome translocation of CCS is certainly a source for these type of antigens and it is well known that immune response directed to mutated antigens plays a crucial role in determining tumor rejection and clinical response in cancer patients under immunotherapy regimens [28,29]. Although generalized conclusion cannot be depicted from a single case, these findings suggest that immunotherapy approaches, which include tumor-specific vaccine and antibodies directed to immunological checkpoints, such as ipilimumab (anti-CTLA4) or nivolumab (anti-PD1), may offer, alone or in association with targeted-therapies, a new therapeutic option for advanced CCS patients, for which no successful therapies are currently available.
Materials and methods
PBMCs and cell lines
PBMCs were obtained by Ficoll density gradient centrifugation followed by cryopreservation. 501mel cell line was generated as previously described [30], 624.38mel and 624.28mel were cloned as previously described [31]. A375mel and the lymphoblastoid cell line T2 were obtained from the American Type Cell Culture (ATCC). All these cell lines were cultured in RPMI 1640 (Lonza) supplemented with 10% FCS (Lonza), Hepes and antibiotics. For tumor cell line immuno-phenotyping, the FITC–labeled BB7.2 monoclonal antibody (BD Bioscences, San Diego, CA) was used.
Immunohistochemical analysis of antigen expression in tumor biopsies
5-μm thick formalin-fixed, paraffin-embedded tissue sections were processed for IHC staining. The monoclonal antibodies used were directed against the following antigens: anti-S100, anti-Melan-A/MART-1, anti-HMB-45/gp100, anti-CD8 (DAKO) and anti-CD3 (Novocastra).
Lymphocyte stimulation and Enzyme-Linked ImmunoSpot (ELISpot) assay
PBMCs isolated from the patient were thawed and cultured in the presence of the HLA2-A*0201 restricted-modified peptides (Melan-A/MART-1[27L] or gp100[210M]) (2 μmol/L) plus 60 IU/mL IL-2 (Proleukin). The cells were tested every 10 to 14 days by flow cytometry analysis for the enrichment of CD8+pentamer+ T cells. To assess their reactivity against tumor cells, IFN-γ release was determined by ELISpot assay (Mabtech) in the presence of MART1 (modified or native)-pulsed (2 μmol/L) lymphoblastoid T2 cell line or HLA-A*0201+/− (MART+/−) melanoma cell lines. HLA class I-blocking experiments required preincubation of target cells with the W6/32 mAb.
Flow cytometry analysis of antigen specific T cells and immunosuppressive cells
Phenotypic characterization of T cell cultures was done by the multiparametric flow cytometry analysis using the following mAbs: anti-CD8-Krome Orange (Beckman Coulter, Brea, CA), anti-CD4-APC (BD Bioscences), the HLA-A*0201 multimers were provided by Proimmune Ltd. Tregs and MDSCs frequencies were determined by multi-colour immunofluorescence staining of thawed PBMCs, excluding dead cells using the LIVE-DEAD® Fixable Violet Dead Cell Stain Kit (Life Technologies, Carlsbad, CA). For surface staining, after treatment with FcR Blocking Reagent (Miltenyi, Bergisch-Gladbach, Germany), cells were incubated with the following antibodies for 30 minutes at 4°C :APCH7-conjugated anti-CD4, PE-Cy7-conjugated anti-CD25 (for detecting Treg); APCH7 conjugated anti-CD14, PE-Cy7-conjugated anti-CD11b, PE-conjugated anti-HLADR (for detecting mMDSC). All antibodies were from BD Bioscences except PE-Cy7-conjugated anti-CD11b (from Beckman Coulter). For Treg analysis, intracellular staining with APC-conjugated anti-Foxp3 (eBioscience) or the proper isotype control (rat IgG2a) was performed. Lymphocytes activated overnight with anti-CD3/CD28 beads (DynaBeads® CD3/CD28 T cell Expander, Invitrogen Dynal AS, Oslo, Norway) in the presence of 1 μl/ml Golgi Plug (BD Biosciences) were stained for the cell surface marker CD3. The cells were then washed, fixed and permeabilized with Cytofix/Cytoperm buffer (BD Biosciences) and stained with a 488-labelled anti-IFN-γ (BioLegend), PE-labelled anti-IL-2 (BD Biosciences). Data acquisition was performed using a Gallios™ (Beckman Coulter) flow cytometer, and the Kaluza® software (Tree Star Inc, Ashland, OR) was used for data analysis.
Consent
Written informed consent was obtained from the patient. A copy of the written consent is available for review by the Editor of this journal.
Acknowledgements
The authors thank Dr. Paola Frati and Mrs. Felicetta Giardino (Unit of Immunotherapy of Human Tumors, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy) for their precious help in the clinical data management. We are grateful to Dr. Valeria Beretta (Unit of Immunotherapy of Human Tumors, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy) for expert technical help. This study was supported by AIRC (Associazione Italiana Ricerca sul Cancro) (IG grants: CC (10615), SP (10300)). MT is supported by a fellowship from FIRC (Fondazione Italiana Ricerca sul Cancro).
Footnotes
Competing interests
SS — Pfizer: travel coverage for medical meetings, research funding. Glaxo Smith Kline: research funding. PGC — Pfizer: advisory, honoraria, research funding. Glaxo Smith Kline: advisory, honoraria, research funding. All of the remaining authors have no conflicts of interest.
Authors’ contributions
MT and FR conducted the experiments. BV performed the immunohistochemical analysis. MT, BV, AV, FR, TN, FC, CM, SS, SP and CC made substantial contributions to analysis and interpretation of data; MT, SS, LR, SP and CC have been involved in drafting the manuscript or revising it critically for important intellectual content. All authors read and approved the final manuscript.
Contributor Information
Marcella Tazzari, Email: marcella.tazzari@istitutotumori.mi.it.
Elena Palassini, Email: elena.palassini@istitutotumori.mi.it.
Barbara Vergani, Email: barbara.vergani@unimib.it.
Antonello Villa, Email: antonello.villa@unimib.it.
Francesca Rini, Email: francesca.rini@istitutotumori.mi.it.
Tiziana Negri, Email: tiziana.negri2@istitutotumori.mi.it.
Chiara Colombo, Email: chiara.colombo@istitutotumori.mi.it.
Flavio Crippa, Email: flavio.crippa@istitutotumori.mi.it.
Carlo Morosi, Email: carlo.morosi@istitutotumori.mi.it.
Paolo G Casali, Email: paolo.casali@istitutotumori.mi.it.
Silvana Pilotti, Email: silvana.pilotti@istitutotumori.mi.it.
Silvia Stacchiotti, Email: silvia.stacchiotti@istitutotumori.mi.it.
Licia Rivoltini, Email: licia.rivoltini@istitutotumori.mi.it.
Chiara Castelli, Email: chiara.castelli@istitutotumori.mi.it.
References
- 1.Chung EB, Enzinger FM. Malignant melanoma of soft parts. A reassessment of clear cell sarcoma. Am J Surg Pathol. 1983;7(5):405–413. doi: 10.1097/00000478-198307000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Deenik W, Mooi WJ, Rutgers EJ, Peterse JL, Hart AA, Kroon BB. Clear cell sarcoma (malignant melanoma) of soft parts: A clinicopathologic study of 30 cases. Cancer. 1999;86(6):969–975. doi: 10.1002/(SICI)1097-0142(19990915)86:6<969::AID-CNCR11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 3.Eckardt JJ, Pritchard DJ, Soule EH. Clear cell sarcoma. A clinicopathologic study of 27 cases. Cancer. 1983;52(8):1482–1488. doi: 10.1002/1097-0142(19831015)52:8<1482::AID-CNCR2820520825>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Kawai A, Hosono A, Nakayama R, Matsumine A, Matsumoto S, Ueda T, et al. Clear cell sarcoma of tendons and aponeuroses: a study of 75 patients. Cancer. 2007;109(1):109–116. doi: 10.1002/cncr.22380. [DOI] [PubMed] [Google Scholar]
- 5.Weis S, Goldblum J. Malignant Tumors of Uncertain Type. St-Louis, MO: Mosby: Enzinger and Weiss’s Soft Tissue Tumors; 2001. [Google Scholar]
- 6.Zucman J, Delattre O, Desmaze C, Epstein AL, Stenman G, Speleman F, et al. EWS and ATF-1 gene fusion induced by t(12;22) translocation in malignant melanoma of soft parts. Nat Genet. 1993;4(4):341–345. doi: 10.1038/ng0893-341. [DOI] [PubMed] [Google Scholar]
- 7.Davis IJ, Kim JJ, Ozsolak F, Widlund HR, Rozenblatt-Rosen O, Granter SR, et al. Oncogenic MITF dysregulation in clear cell sarcoma: defining the MiT family of human cancers. Cancer Cell. 2006;9(6):473–484. doi: 10.1016/j.ccr.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Negri T, Brich S, Conca E, Bozzi F, Orsenigo M, Stacchiotti S, et al. Receptor tyrosine kinase pathway analysis sheds light on similarities between clear-cell sarcoma and metastatic melanoma. Genes Chromosomes Cancer. 2012;51(2):111–126. doi: 10.1002/gcc.20933. [DOI] [PubMed] [Google Scholar]
- 9.Segal NH, Pavlidis P, Noble WS, Antonescu CR, Viale A, Wesley UV, et al. Classification of clear-cell sarcoma as a subtype of melanoma by genomic profiling. J Clin Oncol. 2003;21(9):1775–1781. doi: 10.1200/JCO.2003.10.108. [DOI] [PubMed] [Google Scholar]
- 10.Davis IJ, McFadden AW, Zhang Y, Coxon A, Burgess TL, Wagner AJ, et al. Identification of the receptor tyrosine kinase c-Met and its ligand, hepatocyte growth factor, as therapeutic targets in clear cell sarcoma. Cancer Res. 2010;70(2):639–645. doi: 10.1158/0008-5472.CAN-09-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuda M, Davis IJ, Argani P, Shukla N, McGill GG, Nagai M, et al. TFE3 fusions activate MET signaling by transcriptional up-regulation, defining another class of tumors as candidates for therapeutic MET inhibition. Cancer Res. 2007;67(3):919–929. doi: 10.1158/0008-5472.CAN-06-2855. [DOI] [PubMed] [Google Scholar]
- 12.Hocar O, Le Cesne A, Berissi S, Terrier P, Bonvalot S, Vanel D, et al. Clear cell sarcoma (malignant melanoma) of soft parts: a clinicopathologic study of 52 cases. Dermatol Res Pract. 2012;2012:984096. doi: 10.1155/2012/984096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park BM, Jin SA, Choi YD, Shin SH, Jung ST, Lee JB, et al. Two cases of clear cell sarcoma with different clinical and genetic features: cutaneous type with BRAF mutation and subcutaneous type with KIT mutation. Br J Dermatol. 2013;169(6):1346–1352. doi: 10.1111/bjd.12480. [DOI] [PubMed] [Google Scholar]
- 14.Steger W, Steger GG, Wrba F, Mader R, Schlappack O, Dittrich C, et al. Complete remission of metastasised clear cell sarcoma of tendons and aponeuroses. Eur J Cancer. 1991;27(3):254–256. doi: 10.1016/0277-5379(91)90509-C. [DOI] [PubMed] [Google Scholar]
- 15.Stacchiotti S, Grosso F, Negri T, Palassini E, Morosi C, Pilotti S, et al. Tumor response to sunitinib malate observed in clear-cell sarcoma. Ann Oncol. 2010;21(5):1130–1131. doi: 10.1093/annonc/mdp611. [DOI] [PubMed] [Google Scholar]
- 16.Mir O, Boudou-Rouquette P, Larousserie F, Babinet A, Dumaine V, Anract P, et al. Objective response to sorafenib in advanced clear-cell sarcoma. Ann Oncol. 2012;23(3):807–809. doi: 10.1093/annonc/mds005. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 19.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70(11):4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 20.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135(1):234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamideassociates with longer patient survival. Nat Med. 2012;18(8):1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 22.Romero P, Valmori D, Pittet MJ, Zippelius A, Rimoldi D, Levy F, et al. Antigenicity and immunogenicity of Melan-A/MART-1 derived peptides as targets for tumor reactive CTL in human melanoma. Immunol Rev. 2002;188:81–96. doi: 10.1034/j.1600-065X.2002.18808.x. [DOI] [PubMed] [Google Scholar]
- 23.Bose A, Taylor JL, Alber S, Watkins SC, Garcia JA, Rini BI, et al. Sunitinib facilitates the activation and recruitment of therapeutic anti-tumor immunity in concert with specific vaccination. Int J Cancer. 2011;129(9):2158–2170. doi: 10.1002/ijc.25863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15(6):2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 25.Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, et al. Myeloid-Derived Suppressor Cells Predict Survival of Patients with Advanced Melanoma: Comparison with Regulatory T Cells and NY-ESO-1- or Melan-A-Specific T Cells. Clin Cancer Res. 2014;20(6):1601–1609. doi: 10.1158/1078-0432.CCR-13-2508. [DOI] [PubMed] [Google Scholar]
- 26.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worley BS, van den Broeke LT, Goletz TJ, Pendleton CD, Daschbach EM, Thomas EK, et al. Antigenicity of fusion proteins from sarcoma-associated chromosomal translocations. Cancer Res. 2001;61(18):6868–6875. [PubMed] [Google Scholar]
- 28.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344(6184):641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Rooij N, Van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31(32):e439–e442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci U S A. 1994;91(9):3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivoltini L, Barracchini KC, Viggiano V, Kawakami Y, Smith A, Mixon A, et al. Quantitative correlation between HLA class I allele expression and recognition of melanoma cells by antigen-specific cytotoxic T lymphocytes. Cancer Res. 1995;55(14):3149–3157. [PMC free article] [PubMed] [Google Scholar]


