Abstract
The gram-positive bacterium Staphylococcus epidermidis is the most common cause of infections associated with catheters and other indwelling medical devices. S. epidermidis produces an extracellular slime that enables it to form adherent biofilms on plastic surfaces. We found that a biofilm-releasing enzyme produced by the gram-negative periodontal pathogen Actinobacillus actinomycetemcomitans rapidly and efficiently removed S. epidermidis biofilms from plastic surfaces. The enzyme worked by releasing extracellular slime from S. epidermidis cells. Precoating surfaces with the enzyme prevented S. epidermidis biofilm formation. Our findings demonstrate that biofilm-releasing enzymes can exhibit broad-spectrum activity and that these enzymes may be useful as antibiofilm agents.
Staphylococcus epidermidis is the primary cause of infections of indwelling medical devices such as intravascular catheters, cerebrospinal fluid shunts, peritoneal dialysis catheters, aortofemoral grafts, intraocular lenses, prosthetic cardiac valves, cardiac pacemakers, and prosthetic joints (6). S. epidermidis is a numerically important member of the human skin and mucous membrane microflora and can be transmitted to the surfaces of these devices when they are implanted or manipulated (13). S. epidermidis grows on medical devices as an adherent biofilm consisting of cells enmeshed in a sticky, extracellular slime that is firmly attached to the underlying surface (3). The slime matrix makes S. epidermidis biofilms highly resistant to antibiotics and host defenses and nearly impossible to eradicate (4). Chronic infection of an indwelling device by S. epidermidis acts as a septic focus that can lead to osteomyelitis, acute sepsis, and death, particularly in immunocompromised patients. S. epidermidis is the leading cause of hospital-acquired bloodstream, cardiovascular, eye, ear, nose, and throat infections (19) and is a major pathogen in catheterized AIDS patients (18) and premature newborns (14).
We have been studying biofilm growth and detachment of the gram-negative, oral bacterium Actinobacillus actinomycetemcomitans, the causative agent of a highly destructive form of periodontal disease that affects adolescents (21). Like S. epidermidis, fresh clinical isolates of A. actinomycetemcomitans form tightly adherent biofilms on plastic surfaces in vitro (5, 7). We recently identified a family 20 glycosyl hydrolase produced by A. actinomycetemcomitans that causes the detachment of cells from A. actinomycetemcomitans biofilms grown attached to plastic and the disaggregation of highly autoaggregated clumps of A. actinomycetemcomitans cells in solution (10). This enzyme, named dispersin B (formerly DspB), is an N-acetylglucosaminidase (10) which degrades an N-acetylglucosamine-containing extracellular polysaccharide that mediates A. actinomycetemcomitans intercellular adhesion (J. B. Kaplan and K. Velliyagounder, submitted for publication). Because S. epidermidis slime is also a polysaccharide that contains primarily N-acetylglucosamine residues (12), we decided to test whether A. actinomycetemcomitans dispersin B could cause the detachment of S. epidermidis biofilms from plastic surfaces. In this report we show that A. actinomycetemcomitans dispersin B exhibits potent biofilm-releasing activity against slime-producing, clinical strains of S. epidermidis.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Four S. epidermidis strains (designated NJ9709, NJ9710, NJ9711, and NJ9712) were isolated from the surfaces of infected intravenous catheters removed from patients at University Hospital, Newark, N.J. Strains were identified by using the Api-Staph biochemical identification kit (bioMérieux, Durham, N.C.). All four strains contained the ica genetic locus (1) and produced black colonies on Congo red agar (2), both of which are indicative of slime production. Strains were streaked onto blood agar plates and incubated for 24 h at 37°C in air. Plates were stored at 4°C, and bacteria were passaged weekly. Biofilms were grown in Trypticase soy broth (Becton-Dickinson) supplemented with 6 g of yeast extract and 8 g of glucose per liter. Inoculated culture vessels were incubated statically in air at 37°C.
Preparation of inocula.
A loopful of colonies scraped from the surface of an agar plate was transferred to a microcentrifuge tube containing 200 μl of fresh medium. The tube was vortexed for 30 s at high speed, and the cells were allowed to settle for 5 min. One hundred microliters of the upper layer was transferred to a 100-mm-diameter polystyrene petri dish (model 3003; Falcon) containing 20 ml of fresh medium, and the dish was incubated for 16 h. The biofilm that formed on the surface of the dish was rinsed with phosphate-buffered saline (PBS) and then scraped from the surface of the dish into 3 ml of PBS by using a cell scraper. The cell aggregate was transferred to a 15-ml conical centrifuge tube, vortexed for 30 s, and allowed to settle to the bottom of the tube for 10 min. A 0.5-ml aliquot of the top layer was transferred to a tube containing 5 ml of fresh broth, and the tube was vortexed briefly. The resulting inoculum contained 109 to 1010 CFU ml−1. Serial decimal dilutions were made with fresh broth.
Enzymes.
A. actinomycetemcomitans dispersin B (formerly DspB) was purified as previously described (10). Protein concentration was determined by using a Bio-Rad protein assay kit. The purified enzyme had a specific activity of 970 units per mg of protein, where 1 unit of enzyme activity was defined as the amount of enzyme needed to hydrolyze 1 μmol of 4-nitrophenyl-β-d-N-acetylglucosaminide to 4-nitrophenol and N-acetylglucosamine per min at pH 4.5 at 25°C in 50 mM sodium phosphate buffer -100 mM NaCl. Heat-inactivation of dispersin B was carried out at 100°C for 3 min. Serratia marcescens chitinase and Jack bean N-acetylglucosaminidase were purchased from Sigma Chemical Company.
Growth of biofilms in 96-well polystyrene microtiter plates.
The wells of a 96-well polystyrene microtiter plate (model 3595; Corning) were filled with 100-μl aliquots of inoculum, and the plate was incubated for 16 h. The wells were rinsed with either three 200-μl aliquots of PBS or by submerging the entire plate in a tub of cold, running tap water. Biofilms were stained with crystal violet as previously described (8). The optical densities (OD) of the wells were determined by using a Bio-Rad Benchmark microplate reader set to 590 nm. To assay biofilm detachment, 1 μl of enzyme was added directly into the well of the microtiter plate 30 min prior to rinsing. Unless otherwise indicated, all enzyme treatments were carried out for 30 min at 30°C with a final enzyme concentration of 40 μg ml−1. In some experiments, the wells were washed with water and then filled with 100 μl of PBS prior to addition of the enzyme.
Growth of biofilms on polystyrene rods.
Polystyrene rods (1.5-mm-diameter; Plastruct Corp., City of Industry, Calif.) were cut to 35-mm lengths, sterilized in 70% ethanol for 30 min, and air dried in a biological safety cabinet. Rods were placed into 1.5-ml microcentrifuge tubes containing 0.5 ml of a 10−3 dilution of S. epidermidis strain NJ9709 and incubated for 16 h. The rods were then rinsed with water and placed into fresh microcentrifuge tubes containing 0.75 ml of PBS or PBS containing 40 μg ml−1 of dispersin B. After 15 min, the rods were rinsed with water and stained with crystal violet as previously described (8). For sonication, rods were placed in 15-ml conical centrifuge tubes containing 3 ml of fresh broth and then sonicated for 30 s at 40% duty cycle and 70% capacity in a Branson model 200 sonicator equipped with a cup horn. For quantitation of detached cells, sonicates were serially diluted in fresh broth and spread on agar medium. Colonies were enumerated after 24 h.
Growth of biofilms on intravenous catheters.
Polyurethane catheters (20 gauge, 1.1-mm diameter, model 381434; Becton-Dickinson) and Teflon catheters (18 gauge, 1.2-mm diameter, model 3055; Critikon) were employed. The tips of the catheters were plugged with sterile high-vacuum grease to prevent media and dye from entering the lumen. Catheters were inoculated and treated as described above for polystyrene rods. Catheters were precoated by soaking in PBS containing 40 μg ml−1 of dispersin B for 24 h at 4°C and then in PBS for 5 min at room temperature. Coated catheters were dried at 37°C for 1 h prior to use. Teflon catheters were stained with crystal violet as previously described (8). Polyurethane catheters were stained in a solution of 1% methylene blue in water for 2 min and then rinsed with water.
Carbohydrate techniques.
Four milliliters of a 10−3 dilution of S. epidermidis strain NJ9709 was transferred to a 35-mm-diameter polystyrene petri dish (model 340165; Corning), and the dish was incubated for 16 h. The biofilm that formed on the surface of the dish was rinsed with water, scraped from the surface into 1 ml of PBS, and transferred to a 1.5-ml microcentrifuge tube. Biofilm cells were treated with 40 μg ml−1 of dispersin B or mock treated for 5 min at room temperature with gentle rocking. The tube was then centrifuged for 2 min at 15,000 rpm in a Sorvall MC12V microcentrifuge, and the supernatant was transferred to a new tube.
Supernatants were analyzed for the presence of glycosaminoglycans by using the quantitative measurement of Alcian Blue-glycosaminoglycan complexes described by Whiteman (20). Total hexosamine was determined after acid hydrolysis (4 M HCl, 16 h, 100°C) by using the Morgan-Elson assay (17).
Polysaccharide was purified from cell supernatants as described by Steinmetz et al. (16), except that samples were phenol extracted prior to precipitation of polysaccharide with ethanol. Polysaccharide samples were electrophoresed through a 12% acrylamide-0.17% bisacrylamide gel by using the buffer system of Laemmli (15). Silver staining was carried out by use of a Bio-Rad Silver Stain kit.
RESULTS
Detachment of S. epidermidis biofilms by A. actinomycetemcomitans dispersin B.
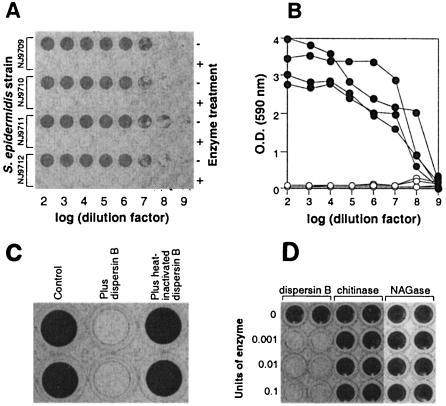
Four strains of S. epidermidis isolated from infected intravenous catheters were tested for their ability to form biofilms by growing serial dilutions of overnight cultures in the wells of a 96-well polystyrene microtiter plate. After 16 h of incubation, the wells were washed to remove loosely adherent cells and the bacteria remaining attached to the bottoms of the wells were stained with crystal violet (Fig. 1A). All four strains produced adherent biofilms as indicated by the presence of dark-staining material on the bottoms of the wells. The amount of biofilm could be quantitated by measuring the OD of the wells at 590 nm with a microtiter plate reader (Fig. 1B). When dispersin B was added to the wells 30 min prior to washing (final concentration, 40 μg ml−1), little or no biofilm was evident (Fig. 1A and B). Heat-inactivated dispersin B had no effect on S. epidermidis biofilms (Fig. 1C). Two other family 20 glycosyl hydrolases, S. marcescens chitinase and Jack bean N-acetylglucosaminidase, also had no effect on S. epidermidis biofilms (Fig. 1D). Dispersin B had no bactericidal effect on S. epidermidis cells (data not shown). These results indicate that the enzymatic activity of A. actinomycetemcomitans dispersin B caused the detachment of S. epidermidis biofilm cells from the surfaces of the wells.
FIG. 1.
S. epidermidis biofilm formation in the wells of 96-well polystyrene microtiter plates. (A) Serial decimal dilutions (bottom axis) of four S. epidermidis strains (left axis) were grown in duplicate horizontal rows of wells and then rinsed with water and stained with crystal violet. Thirty minutes prior to rinsing, dispersin B was added to alternate horizontal rows of wells (right axis). (B) OD of the wells in panel A. Filled circles correspond to values for untreated rows. Open circles correspond to values for dispersin B-treated rows. (C) S. epidermidis biofilms were mock treated (Control) or treated with dispersin B or heat-inactivated dispersin B and then stained with crystal violet. (D) S. epidermidis biofilms were treated with increasing amounts of A. actinomycetemcomitans dispersin B, S. marcescens chitinase, or Jack bean N-acetylglucosaminidase (NAGase) and then rinsed with water and stained with crystal violet.
Kinetics of biofilm detachment.
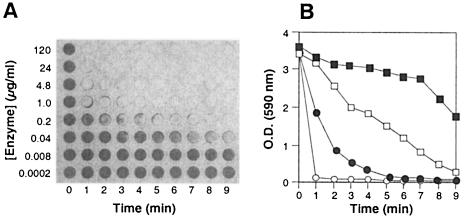
S. epidermidis biofilms were grown for 16 h in microtiter plate wells and then treated with increasing amounts of dispersin B (final concentrations, 200 pg to 120 μg ml−1) for 0 to 9 min (Fig. 2A and B). A concentration of 4.8 μg ml−1 of dispersin B resulted in a decrease in OD to background levels (ca. 0.09 OD units) after 2 min, and 40 ng ml−1 of dispersin B resulted in a >50% reduction in OD (from 3.63 to 1.74 OD units) after 9 min. These data demonstrate that dispersin B-mediated detachment of S. epidermidis biofilms was rapid and efficient and occurred at clinically achievable concentrations of enzyme.
FIG. 2.
Time course and dose-response curves for dispersin B-mediated detachment of S. epidermidis biofilms from the wells of a 96-well microtiter plate. (A) Biofilms were treated with increasing amounts of dispersin B (vertical axis) for increasing amounts of time (horizontal axis). The wells were then rinsed with water and stained with crystal violet. (B) OD of the rows of wells in panel A corresponding to 0.04 μg ml−1 (filled squares), 0.2 μg ml−1 (open squares), 1.0 μg ml−1 (filled circles), and 4.8 μg ml−1 (open circles).
Quantitation of biofilm detachment.
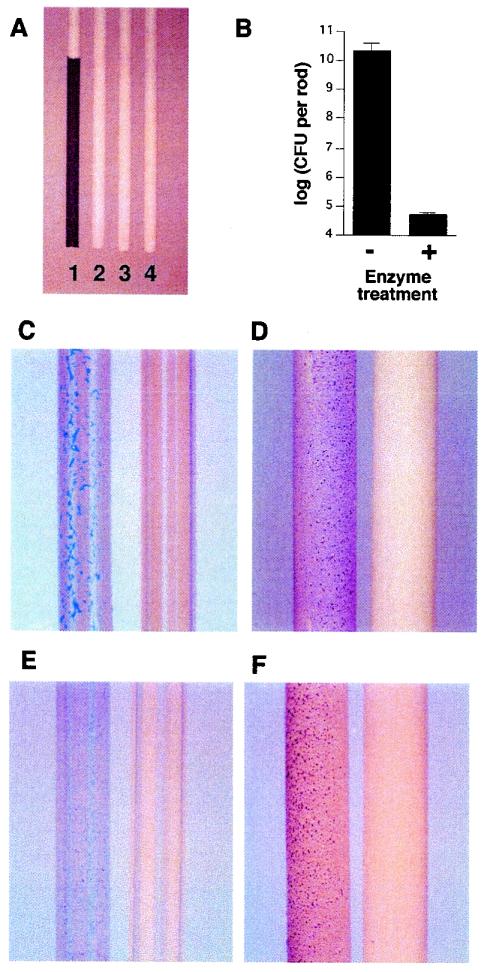
S. epidermidis biofilms were grown attached to the surfaces of polystyrene rods and then treated with 40 μg ml−1 of dispersin B for 15 min. The bacteria remaining attached to the rods after treatment were removed by sonication and then quantitated by plating on agar. Figure 3A shows mock-treated and dispersin B-treated rods after staining with crystal violet. The mock-treated control rod (rod 1) contained a layer of dark-staining material corresponding to the thick biofilm that formed on its surface. The dispersin B-treated rod showed no trace of dark-staining material and was similar in appearance to a rod which had been sonicated prior to staining (rod 3) and to an uninoculated rod (rod 4). Quantitation of cells remaining attached to the rods revealed that dispersin B treatment resulted in a 5.8 log reduction in the number of surface-associated bacteria (Fig. 3B).
FIG. 3.
Growth of S. epidermidis biofilms on the surfaces of polystyrene rods and polyurethane and Teflon intravenous catheters. (A) Polystyrene rods (1.5-mm diameter) stained with crystal violet. Rods were incubated in broth containing S. epidermidis strain NJ9709 (rods 1 to 3) or in uninoculated broth (rod 4). Prior to staining, rod 1 was mock treated, rod 2 was treated with dispersin B, and rod 3 was sonicated. (B) The bacteria remaining attached to the polystyrene rods after mock treatment and treatment with dispersin B were removed by sonication and enumerated by plating of serial dilutions on agar. Values indicate mean numbers of bacteria per rod (± standard error) for triplicate rods. (C) Polyurethane catheters were incubated in broth containing S. epidermidis strain NJ9709 and then mock treated (left catheter) or treated with dispersin B (right catheter). Catheters were then rinsed and stained with methylene blue. (D) Teflon catheters (1.2-mm diameter) were treated as described for panel C, except that bacteria were stained with crystal violet. (E) Polyurethane catheters were precoated with PBS (left catheter) or PBS plus dispersin B (right catheter), rinsed with water, and then incubated in broth containing S. epidermidis strain NJ9709 for an additional 6 h. Catheters were then rinsed and stained with methylene blue. (F) Teflon catheters were treated as described for panel E, except that bacteria were stained with crystal violet.
Dispersin B displayed similar biofilm-releasing activity against S. epidermidis biofilms grown attached to the surfaces of polyurethane and Teflon catheters (Fig. 3C and D). Precoating catheters with dispersin B prevented S. epidermidis biofilm formation (Fig. 3E and F). Precoated polyurethane catheters retained enzyme activity for at least 30 days when stored at room temperature (data not shown).
Dispersin B degrades S. epidermidis slime.
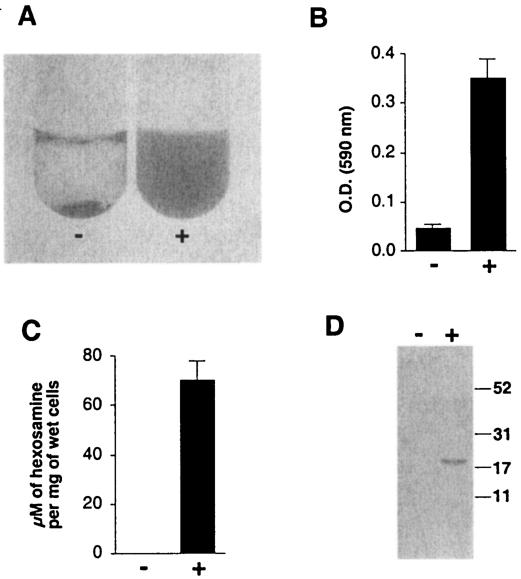
To demonstrate that dispersin B degraded the S. epidermidis intercellular slime matrix, we scraped biofilm cells from the surface of a culture vessel and transferred them to a tube. Under these conditions, the cells formed a sticky aggregate that rapidly settled to the bottom of the tube (Fig. 4A, left). Treatment of cell aggregates with dispersin B resulted in uniformly turbid cell suspensions (Fig. 4A, right), indicating that treatment with dispersin B interfered with intercellular adhesion. Treatment with dispersin B also resulted in increased amounts of glycosaminoglycans (Fig. 4B) and total hexosamine (Fig. 4C) in cell supernatants. When polysaccharide was purified from supernatants of mock-treated and dispersin B-treated cells and then analyzed by polyacrylamide gel electrophoresis, a single band with an apparent molecular mass of 20 kDa was observed in the supernatant of dispersin B-treated cells (Fig. 4D). This band may correspond to a 20-kDa species of low-sulfated polysaccharide that has been shown to be a component of S. epidermidis exopolysaccharide slime (11). These data are consistent with the hypothesis that dispersin B is disrupting the S. epidermidis slime matrix, either by degrading it or by detaching it from the cell surface.
FIG. 4.
Dispersin B releases extracellular slime from S. epidermidis biofilm cells. Minus sign, mock-treated cells; plus sign, dispersin B-treated cells. (A) Biofilm cells that were rinsed and scraped from the surface of a culture vessel formed an aggregate that settled to the bottom of the tube (left). Treatment of the cell aggregate with dispersin B for 5 min resulted in complete dispersion of the aggregate (right). (B) Quantitation of glycosaminoglycans in cell culture supernatants. The OD is proportional to the amount of glycosaminoglycans in the supernatants. Values show means (± standard error) for triplicate samples. (C) Quantitation of total hexosamine in cell culture supernatants. Values show means (± standard error) for triplicate samples. (D) Polysaccharides purified from cell culture supernatants were analyzed by polyacrylamide gel electrophoresis and stained with silver. The sizes (in kilodaltons) of molecular mass standards electrophoresed in an adjacent lane are indicated on the right.
DISCUSSION
Our findings demonstrate that A. actinomycetemcomitans dispersin B exhibits high biofilm-releasing activity against S. epidermidis biofilms. The enzyme was active at physiologically achievable concentrations and prevented biofilm formation when used to coat plastic biomaterials. These properties suggest that dispersin B could be used as an agent to prevent or treat S. epidermidis infections of catheters and other medical devices.
We previously showed that purified dispersin B hydrolyzes β-substituted N-acetylglucosamine (10). S. epidermidis slime consists of a polymer of β(1→6)-linked N-acetyl-d-glucosamine residues (12), suggesting that the natural substrate for dispersin B is a β(1→6)-linked glycosamine polymer.
Biological role of dispersin B.
We originally proposed that the function of dispersin B was to cause the detachment of cells from biofilm colonies during the dispersal phase of the A. actinomycetemcomitans life cycle (8-10). Our present findings raise the possibility that dispersin B may act as a colonization factor that is capable of detaching biofilms produced by other species of bacteria, thereby clearing a surface which can subsequently be colonized by A. actinomycetemcomitans. It is unlikely that S. epidermidis is a natural target for dispersin B, because S. epidermidis and A. actinomycetemcomitans colonize different surfaces of the human body and are not likely to compete in nature. It is possible that dispersin B can detach biofilms produced by other bacterial species that are present in the oral cavity. It is also possible that the observed interspecific biofilm-detaching activity of dispersin B plays no ecological role but instead reflects a common evolutionary origin of the exopolysaccharide genes of S. epidermidis and A. actinomycetemcomitans.
Acknowledgments
We thank David Furgang, Frances Devonshire, and Darshini Shah for technical assistance and Gill Diamond, David Figurski, Peter Frederikse, David Furgang, Paul Goncharoff, Scott Kachlany, Ken Markowitz, and Helen Schreiner for helpful comments.
This study was supported in part by Public Health Service award DE12585 (to N.R.).
REFERENCES
- 1.Aricola, C. R., L. Baldassarri, and L. Montanaro. 2001. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J. Clin. Microbiol. 39:2151-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aricola, C. R., D. Campoccia, S. Gamberini, M. Cervellati, E. Donati, and L. Montanaro. 2002. Detection of slime production by means of an optimised Congo red agar plate test on a colourimetric scale in Staphylococcus epidermidis clinical isolates genotyped for ica locus. Biomaterials 23:4233-4239. [DOI] [PubMed] [Google Scholar]
- 3.Christensen, G. D., W. A. Simpson, A. L. Bisno, and E. H. Beachey. 1982. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 37:318-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 5.Fine, D. H., D. Furgang, J. Kaplan, J. Charlesworth, and D. H. Figurski. 1999. Tenacious adhesion of Actinobacillus actinomycetemcomitans strain CU1000 to salivary-coated hydroxyapatite. Arch. Oral Biol. 44:1063-1076. [DOI] [PubMed] [Google Scholar]
- 6.Huebner, J., and D. A. Goldman. 1999. Coagulase-negative staphylococci: role as pathogens. Annu. Rev. Med. 50:223-236. [DOI] [PubMed] [Google Scholar]
- 7.Kachlany, S. C., P. J. Planet, R. DeSalle, D. H. Fine, D. H. Figurski, and J. B. Kaplan. 2001. flp-1, first representative of a new pilin gene subfamily, is required for nonspecific adherence of Actinobacillus actinomycetemcomitans. Mol. Microbiol. 40:542-554. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan, J. B., and D. H. Fine. 2002. Biofilm dispersal of Neisseria subflava and other phylogenetically diverse oral bacteria. Appl. Environ. Microbiol. 68:4943-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan, J. B., M. F. Meyenhofer, and D. H. Fine. 2003. Biofilm growth and detachment of Actinobacillus actinomycetemcomitans. J. Bacteriol. 185:1399-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan, J. B., C. Ragunath, N. Ramasubbu, and D. H. Fine. 2003. Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous β-hexosaminidase activity. J. Bacteriol. 185:4693-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karamanos, N. K., H. S. Panagiotopoulou, A. Syrokou, C. Frangides, A. Hjerpe, G. Dimitracopoulos, and E. D. Anastassiou. 1997. Identity of macromolecules present in the extracellular slime layer of Staphylococcus epidermidis. Biochimie 77:217-224. [DOI] [PubMed] [Google Scholar]
- 12.Mack, D., W. Fisher, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mermel, L. A. 2000. Prevention of intravascular catheter-related infections. Ann. Intern. Med. 132:391-402. [DOI] [PubMed] [Google Scholar]
- 14.Ohlsson, A., and M. Vearncombe. 1987. Congenital and nosocomial sepsis in infants born in a regional perinatal unit: cause, outcome, and white blood cell response. Am. J. Obstet. Gynecol. 156:407-413. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 16.Steinmetz, I., M. Rohde, and B. Brenneke. 1995. Purification and characterization of an exopolysaccharide of Burkholderia (Pseudomonas) pseudomallei. Infect. Immun. 63:3959-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strominger, J. L., J. T. Park, and R. E. Thompson. 1959. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J. Biol. Chem. 234:3263-3268. [PubMed] [Google Scholar]
- 18.Tacconelli, E., M. Tumbarello, M. Pittiruti, F. Leone, M. B. Lucia, R. Cauda, and L. Ortona. 1997. Central venous catheter-related sepsis in a cohort of 366 hospitalised patients. Eur. J. Clin. Microbiol. Infect. Dis. 16:203-209. [DOI] [PubMed] [Google Scholar]
- 19.Vuong, C., and M. Otto. 2002. Staphylococcus epidermidis infections. Microbes Infect. 4:481-489. [DOI] [PubMed] [Google Scholar]
- 20.Whiteman, P. 1973. The quantitative measurement of Alcian Blue-glycosaminoglycan complexes. Biochem. J. 131:343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zambon, J. J. 1985. Actinobacillus actinomycetemcomitans in human periodontal disease. J. Clin. Periodontol. 12:1-20. [DOI] [PubMed] [Google Scholar]