Abstract
Fluconazole is widely used in the intensive care unit for prevention and treatment of fungal infections. Case reports have described an association between fluconazole and adrenal dysfunction, an important cause of morbidity and mortality in critically ill patients. We sought to determine whether 400 mg of fluconazole per day administered to critically ill surgical patients was associated with a reduction in cortisol levels. Cortisol levels were measured in stored plasma specimens drawn from 154 critically ill surgical patients randomized in 1998-1999 to receive fluconazole or placebo for the prevention of candidiasis. The primary outcome measure was the median plasma cortisol level ≥1 day after study drug initiation (MPCL). Secondary outcomes were adrenal dysfunction, defined as an MPCL of <15 μg/dl, changes in cortisol levels over time, and mortality. The median MPCL was 15.75 μg/dl (interquartile range [IQR], 11.65 to 21.33 μg/dl) in 79 patients randomized to fluconazole and 16.71 μg/dl (IQR, 11.67 to 23.00 μg/dl) in 75 patients randomized to placebo (P = 0.52). Patients randomized to fluconazole did not have significantly increased odds of adrenal dysfunction compared to patients randomized to placebo (odds ratio, 0.98; 95% confidence interval, 0.48 to 2.01). Randomization to fluconazole was not associated with a significant difference in cortisol level changes over time. Mortality was not different between patients with and without adrenal dysfunction, nor was it different between patients with adrenal dysfunction who were randomized to fluconazole and those randomized to placebo. Fluconazole prophylaxis in this population of critically ill surgical patients did not result in significant adrenal dysfunction.
Systemic candidiasis is an important cause of morbidity and mortality in the intensive care unit (ICU) (5, 12, 22, 43). Prophylaxis with fluconazole has been shown to reduce the occurrence of candidiasis in a variety of patient populations (15, 17, 39, 48), including select, high-risk, adult ICU patients (11, 13, 31). Fluconazole, an azole antifungal agent, exerts a fungistatic effect by inhibiting a fungal cytochrome P450 enzyme involved in ergosterol synthesis (14, 27). Side effects of other azole antifungals may arise from nonspecific inhibition of mammalian cytochrome P450 enzymes (14). Adrenal dysfunction is one of these side effects and has been shown to occur in patients treated with ketoconazole (18, 44, 47). While in vitro data show that fluconazole is not as potent an inhibitor of mammalian P450 enzymes as ketoconazole (10), adrenal insufficiency associated with fluconazole administration has recently been described (1, 38). In these case reports, adrenal insufficiency was diagnosed 1 to 3 days after fluconazole initiation (1, 38).
Adrenal dysfunction is an important cause of morbidity and mortality in critically ill patients (20, 25, 35, 40). Estimates of the prevalence of adrenal dysfunction among critically ill patients vary widely, from 0.66 to over 40%, depending on the definition of adrenal dysfunction and the specific patient population studied (3, 6, 9, 25, 35, 36, 40). Drugs administered to such patients may contribute to adrenal dysfunction by increasing the rate at which cortisol is metabolized (20) or, as in the case of etomidate (8, 46) or ketoconazole (26, 30, 33, 34), by inhibiting enzymes in the steroid biosynthetic pathway.
Because of the widespread use of fluconazole and the impact of adrenal dysfunction on outcomes in critically ill patients (2, 28), we explored the effect of fluconazole prophylaxis on plasma cortisol levels in critically ill surgical patients who participated in a randomized, double-blind, placebo-controlled trial of fluconazole for the prevention of candidiasis (31).
MATERIALS AND METHODS
Patients and specimens.
Patients participated in a randomized, double-blind, placebo-controlled trial of fluconazole prophylaxis in the Surgical ICU at Johns Hopkins Hospital from January 1998 to January 1999 (31). The Institutional Review Board of the Johns Hopkins Hospital approved the trial and the study described herein. Written, informed consent was obtained from patients or their surrogates prior to participation in the clinical trial. The Institutional Review Board determined that the present study met the requirements for exempt research.
Patients included in the clinical trial had anticipated ICU lengths of stay of ≥3 days and were at least 18 years old. Patients were excluded for the following reasons: ICU length of stay of <3 days; pregnancy; receipt of antifungal agents within the week prior to ICU admission; age <18 years; or an expectation of death within 24 h of ICU admission. After enrollment, patients were randomized to receive either daily, enteral fluconazole or placebo. The standard dose of fluconazole was an 800-mg loading dose followed by 400 mg each day thereafter. Dose adjustment was made for renal insufficiency (31).
Plasma samples and cortisol measurements.
Blood was collected for fluconazole measurements following the administration of the loading dose of the study drug and thrice weekly thereafter, until ICU discharge. Plasma remaining after fluconazole measurement was stored at −70οC from 1998-1999 until January 2002. Storage at −70οC was not expected to significantly affect cortisol levels in the specimens, based on data showing that plasma cortisol concentrations decreased 6 to 9% over a 3- to 4-year period when stored at −25οC (19).
Samples were included in the analyses if they were drawn from patients eligible for the clinical trial's intent-to-treat analysis, between 4:00 am and 11:59 am, before or at least 2 days after steroid administration, at least 1 day after study drug initiation, and during a patient's first ICU admission during the period of the trial.
Cortisol concentrations in the plasma samples were measured using the DSL-2100 ACTIVE cortisol coated-tube radioimmunoassay kit (Diagnostic Systems Laboratories, Inc., Webster, Tex.) according to the manufacturer's instructions. The Johns Hopkins University General Clinical Research Center core laboratory performed the cortisol assays and reported an intraassay coefficient of variation of 4.01% and an interassay coefficient of variation of 6.45%.
Statistical methods.
We based a power calculation on 79 patients randomized to fluconazole and 75 patients randomized to placebo. Our primary outcome measure was the median plasma cortisol level (MPCL), determined for each patient with ≥1 sample available at least 1 day after study drug initiation. With a mean MPCL of 18.68 μg/dl (standard deviation, 10.77 μg/dl) in patients randomized to placebo, we had 90% power to detect a difference in means of 30% (two-sided α = 0.05).
MPCL and interquartile ranges were determined for each treatment arm and compared using the Wilcoxon rank-sum test. Because case reports suggest that fluconazole's effect on adrenal function may be delayed (1, 38), we also evaluated MPCLs ≥3 and ≥7 days after study drug initiation.
A secondary outcome was adrenal dysfunction, defined as an MPCL of <15 μg/dl. A serum cortisol level of 15 μg/dl in patients with acute critical illness has recently been proposed as a threshold below which adrenal insufficiency is probable (7). We used the Z approximation and chi-square test of association to explore relationships between adrenal dysfunction and categorical variables. We used the Wilcoxon rank-sum test to assess associations between adrenal dysfunction and non-normally distributed continuous variables.
Univariate and multivariate logistic regressions were performed to assess the associations between demographic and clinical variables and adrenal dysfunction. Variables were selected for inclusion in the final, multivariate model if they were felt to be biologically important or if they were significantly associated (P < 0.05) with adrenal dysfunction in univariate analyses. Receipt of steroids, age category, and ICU length-of-stay category were investigated as potential confounders and effect modifiers. The final multiple logistic regression model was evaluated with goodness-of-fit testing and was found to be adequate.
To assess changes in patients' cortisol levels over time and the association with randomization to fluconazole, we incorporated an interaction term into a multiple linear regression model which used robust variance estimation and generalized estimating equations (23, 49) to account for within-individual correlations. We used a log transformation of cortisol levels to approximate a normal distribution. The time variable was days since study drug initiation. Age category and receipt of steroids were explored as possible confounders and effect modifiers of the relationship between randomization to fluconazole and cortisol levels. Other variables were selected for inclusion in the final model if they achieved significance (P < 0.05) in univariate analyses.
Proportions of patients who were alive at the time of ICU and hospital discharge were compared using chi-square and Fisher's exact tests. All analyses were done with Stata (versions 6.0 and 8.0; Stata Corporation, College Station, Tex.).
RESULTS
Patients and plasma samples.
Of 841 plasma samples from 260 patients enrolled in the randomized trial, 655 were available for study. Of the 655 available specimens, 5 were found to be duplicates of other specimens. Cortisol measurements from these duplicate specimens were averaged. Two hundred nine samples were excluded from analyses: 130 were from patients who had received steroids the day before or on the day the cortisol level was drawn; 61 samples were from patients readmitted to the ICU; 11 were from patients ineligible for the intent-to-treat analysis of the clinical trial; 6 samples were drawn on or before the first day of study drug administration; and 1 sample was drawn after 11:59 am. Therefore, a total of 441 serum samples from 154 patients (79 randomized to fluconazole, 75 to placebo) were included in the analyses.
Of the 441 samples included in the analyses, 276 were from patients randomized to fluconazole and 165 were from patients randomized to placebo. The median number of blood samples per patient was two in both the fluconazole and the placebo arms of the trial (ranges, 1 to 44 in the fluconazole arm and 1 to 9 in the placebo arm). Overall, the distribution of the number of blood samples drawn per patient was similar in the fluconazole and placebo arms, with 64 of 79 (81.0%) patients randomized to fluconazole and 67 of 75 (89.3%) patients randomized to placebo having between one and three blood samples obtained (chi-square test, P = 0.15). Five patients randomized to fluconazole had at least 10 blood samples obtained. No patients randomized to placebo had more than nine blood samples obtained. This difference approached but did not reach statistical significance (Fisher's exact test, P = 0.06).
Of the 154 patients included in this study, those randomized to fluconazole were similar demographically and clinically to those randomized to placebo (Table 1). Length of hospital stay was significantly greater in patients randomized to fluconazole (Table 1). The median length of hospital stay did not differ significantly between the treatment arms when the entire clinical trial population was considered (19 days in the placebo arm versus 20.5 days in the fluconazole arm; P = 0.15), nor when the subset of patients excluded from the present study was considered (19 days in the placebo arm versus 17 days in the fluconazole arm; P = 0.81).
TABLE 1.
Demographic and clinical characteristics
| Characteristic | Patients randomized to fluconazole (n = 79) | Patients randomized to placebo (n = 75) | P value |
|---|---|---|---|
| Demographic | |||
| Age, median yrs (range) | 64 (20-92) | 71 (20-88) | 0.09a |
| Male (%) | 44 (55.7) | 35 (46.7) | 0.26 |
| Race or ethnicity (%) | 0.28c | ||
| Caucasian (%) | 63 (79.8)b | 54 (72.0) | |
| African American (%) | 16 (20.3) | 19 (25.3) | |
| Hispanic (%) | 0 | 2 (2.7) | |
| Clinical severity | |||
| Vasopressor therapy on admission (%) | 11 (13.9) | 14 (18.7) | 0.43 |
| APACHE III, median (range) | 65.5 (18-119) | 68 (19-191) | 0.38a |
| Scheduled admission (%) | 33 (41.8) | 29 (38.7) | 0.69 |
| Systolic blood pressure <90 mm Hg on admission (%) | 8 (10.1) | 11 (14.7) | 0.39 |
| ICU length of stay,d median days (range) | 7 (3-113) | 6 (3-71) | 0.26a |
| Hospital length of stay,d median days (range) | 23 (7-271) | 19 (5-116) | 0.04a |
| Alive at ICU discharge (%) | 70 (88.6) | 63 (84.0) | 0.41 |
| Alive at hospital discharge (%) | 59 (74.7) | 56 (74.7) | 1.00 |
| Admission diagnosis | 0.66c | ||
| Vascular surgery (%) | 28 (35.4) | 25 (33.3) | |
| GI or biliary surgery (%) | 21 (26.6) | 21 (28.0) | |
| Liver transplantation (%) | 0 | 3 (4.0) | |
| Other surgery (%) | 10 (12.7) | 10 (13.3) | |
| Trauma (%) | 5 (6.3) | 5 (6.7) | |
| Complication of surgery or other (%) | 15 (19.0) | 11 (14.7) | |
| Comorbid illness | |||
| Malignancy (%) | 28 (35.4) | 24 (32.0) | 0.65 |
| Diabetes (%) | 13 (16.5) | 19 (25.3) | 0.18 |
| HIV (%) | 4 (5.1) | 1 (1.3) | 0.37c |
| Alcohol abuse (%) | 12 (15.2) | 6 (8.0) | 0.17 |
| NYHAe functional class III or IV heart failure (%) | 6 (7.6) | 9 (12.0) | 0.36 |
| Cirrhosis (%) | 5 (6.3) | 8 (10.7) | 0.33 |
| Dialysis (%) | 1 (1.3) | 4 (5.3) | 0.20c |
| Steroid therapy within 6 mos of admission (%) | 5 (6.3) | 4 (5.3) | 1.00c |
Wilcoxon rank-sum test
Percentages may not add up to 100 due to rounding.
Fisher's exact test.
Length of stay includes days of admission and discharge.
NYHA, New York Heart Association.
Among the 79 patients randomized to fluconazole, the median total dose of fluconazole was 2,000 mg (range, 800 to 42,200 mg). Patients randomized to fluconazole for whom weight data were available (n = 78) received a median daily dose of 5.77 mg/kg of body weight (range, 1.33 to 16 mg/kg).
Exogenous steroids were administered in the ICU to similar numbers of patients randomized to fluconazole and placebo (chi-square test, P = 0.19). Among the 79 patients randomized to fluconazole, 13 (16%) received ≥1 dose of hydrocortisone, hydrocortisone and prednisone, or methylprednisolone. Among the 75 patients randomized to placebo, 7 (9%) received ≥1 dose of dexamethasone, hydrocortisone, or methylprednisolone.
MPCLs.
The median MPCL for the 154 patients included in this study was 16.13 μg/dl (interquartile range [IQR], 11.67 to 22.25 μg/dl). MPCLs at least 1, 3, and 7 days after study drug initiation were not significantly different between the fluconazole and placebo groups (Table 2).
TABLE 2.
Median MPCL in patients randomized to fluconazole and placebo
| Days since study drug initiation | MPCL (μg/dl) in patients randomized to fluconazolea (IQR) | MPCL (μg/dl) in patients randomized to placebob (IQR) | P value |
|---|---|---|---|
| ≥1 | 15.75 (11.65-21.33) | 16.71 (11.67-23.00) | 0.52 |
| ≥3 | 13.60 (11.14-18.17) | 16.77 (13.13-24.00) | 0.08 |
| ≥7 | 14.94 (12.36-19.14) | 16.91 (11.85-21.85) | 0.53 |
Number of patients evaluable at ≥1 day = 79, at ≥3 days = 45, and at ≥7 days = 22.
Number of patients evaluable at ≥1 day = 75, at ≥3 days = 38, and at ≥7 days = 17.
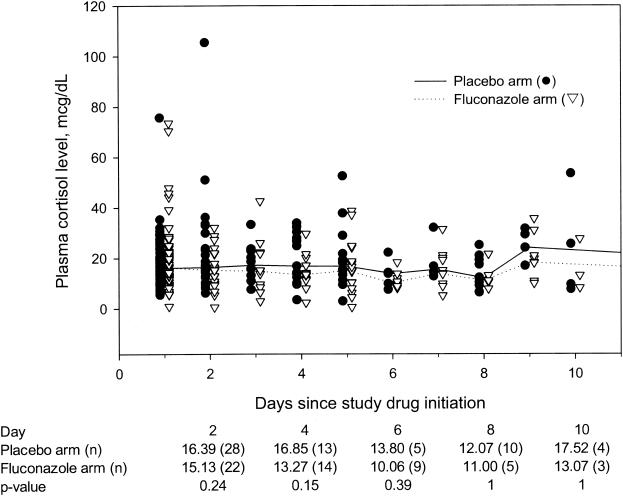
The MPCLs on days 1 to 10 after study drug initiation were also determined for patients in each treatment arm (Fig. 1). On day 1 after study drug initiation, the MPCL was 16.34 μg/dl in 68 patients randomized to fluconazole and 16.08 μg/dl in 63 patients randomized to placebo (P = 0.97). Although the medians of the cortisol levels on days 2 to 10 were lower in patients on fluconazole compared to patients on placebo, the differences were not statistically significant (Fig. 1). Cortisol levels beyond day 10 after study drug initiation are not shown due to the small number of patients in the placebo arm (Fig. 1).
FIG. 1.
Plasma cortisol levels (in micrograms per deciliter) and days since study drug initiation. Lines connect median levels on each day for each treatment group. Median cortisol levels after day 10 are not shown because of small numbers of patients.
Patients with adrenal dysfunction.
Sixty-nine of 154 patients (44.8%) had adrenal dysfunction, defined as an MPCL of <15 μg/dl. The proportion of patients in the fluconazole arm with adrenal dysfunction was not significantly different from the proportion of patients in the placebo arm (46.8 versus 42.7%; P = 0.60; difference in proportions, 4.1%; 95% confidence interval [95% CI], −11.5 to 19.9%). The proportions of patients in each arm of the study who ever had a cortisol level of <15, 15 to 34, and >34 μg/dl were also not significantly different (Table 3).
TABLE 3.
Patients who ever had a cortisol level of <15, 15 to 34, or >34 μg/dl
| Cortisol level (μg/dl) | Patients randomized to fluconazole (%) (n = 79) | Patients randomized to placebo (%) (n = 75) | P value |
|---|---|---|---|
| <15 | 51 (64.6) | 41 (54.7) | 0.21 |
| 15-34 | 49 (62.0) | 48 (64.0) | 0.80 |
| >34 | 10 (12.7) | 7 (9.33) | 0.51 |
When median cortisol levels ≥3 after study drug initiation were evaluated, 27 of 45 (60.0%) patients randomized to fluconazole and 16 of 38 (42.1%) patients randomized to placebo had adrenal dysfunction (P = 0.10; difference in proportions, 17.9% [95% CI, −3.35 to 39.1%]). When median cortisol levels of ≥7 after study drug initiation were evaluated, 11 of 22 (50.0%) patients randomized to fluconazole and 6 of 17 (35.3%) patients randomized to placebo had adrenal dysfunction (P = 0.36; difference in proportions, 14.7% [95% CI, −16.2 to 45.6%]). In a multiple logistic regression model adjusted for age category, ICU length of stay category, admitting diagnoses of vascular and other surgery, and African American race, randomization to fluconazole was not associated with a significantly increased odds of adrenal dysfunction (Table 4). In this multivariate model, no variables were significantly associated with adrenal dysfunction.
TABLE 4.
Unadjusted and adjusted odds of adrenal dysfunction associated with clinical and demographic variables
| Variable | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) |
|---|---|---|
| Randomization to fluconazole | 1.18 (0.63-2.24) | 0.98 (0.48-2.01) |
| Other surgery | 3.35 (1.21-9.26) | 2.50 (0.82-7.63) |
| Vascular surgery | 0.34 (0.17-0.70) | 0.48 (0.21-1.08) |
| African American race | 2.22 (1.03-4.79) | 1.40 (0.59-3.33) |
| Age | ||
| <55 yr (reference) | ||
| 55-64 yr | 1.30 (0.47-3.58) | 2.25 (0.74-6.83) |
| 65-74 | 0.56 (0.23-1.34) | 1.03 (0.38-2.79) |
| ≥75 | 0.28 (0.11-0.70) | 0.47 (0.16-1.34) |
| ICU length of stay >7 days | 1.57 (0.81-3.02) | 1.92 (0.91-4.05) |
Changes in cortisol levels over time.
Patients randomized to fluconazole and placebo did not have significantly different cortisol levels when assessed in a multiple linear regression model (Table 5). The possibility that randomization to fluconazole changed the evolution of cortisol levels over time was explored by incorporating an interaction term into the model. Randomization to fluconazole was not associated with a significant difference in the changes in cortisol levels over time, after adjusting for other clinical variables (coefficient, −0.004 [95% CI, −0.030 to 0.023]).
TABLE 5.
Multiple linear regression model using generalized estimating equationsa to assess the association between exposure variables and the natural logs of cortisol levels
| Variable | Coefficient (95% CI) | |
|---|---|---|
| Randomization to fluconazole | −0.088 (−0.253-0.078) | |
| Days since study drug initiation | −0.002 (−0.006-0.002) | |
| Age (yr) | ||
| <55 (reference) | ||
| 55-64 | 0.050 (−0.272-0.372) | |
| 65-74 | 0.127 (−0.159-0.413) | |
| ≥75 | 0.197 (−0.085-0.479) | |
| HIV disease | −0.080 (−0.507-0.346) | |
| Scheduled admission | 0.068 (−0.110-0.245) | |
| Vascular surgery | 0.200 (−0.002-0.402) | |
| Other surgery | −0.058 (−0.291-0.174) |
Mortality of patients with adrenal dysfunction.
ICU and hospital mortality were not different in the fluconazole and placebo arms (Table 1), nor were they different in patients with and without adrenal dysfunction. Fifty-nine of 69 (85.5%) patients with adrenal dysfunction were alive at ICU discharge, and 51 (73.9%) were alive at hospital discharge. Of 85 patients without adrenal dysfunction, 74 (87.1%) were alive at ICU discharge and 64 (75.3%) were alive at hospital discharge. These differences were not statistically significant. Of 37 patients randomized to fluconazole who had adrenal dysfunction, 32 (86.5%) were alive at ICU discharge and 29 (78.4%) were alive at hospital discharge. Of 32 patients randomized to placebo who had adrenal dysfunction, 27 (84.4%) were alive at ICU discharge and 22 (68.8%) were alive at hospital discharge. These differences were also not statistically significant.
DISCUSSION
We measured cortisol levels in banked plasma specimens from critically ill surgical patients who participated in a randomized, double-blind, placebo-controlled trial of fluconazole for the prevention of candidiasis. In this population of critically ill patients, those randomized to fluconazole did not have significantly lower cortisol levels than those randomized to placebo, nor did they have significantly increased odds of adrenal dysfunction. To our knowledge, this is the largest study to date evaluating the impact of fluconazole on cortisol levels in critically ill patients.
Like its predecessor ketoconazole, fluconazole inhibits the fungal cytochrome P450 enzyme 14α-demethylase in the ergosterol biosynthetic pathway. Ketoconazole is a potent inhibitor in vitro and in vivo of mammalian cytochrome P450 enzymes, including 11β-hydroxylase, which converts 11-deoxycortisol to cortisol (26, 30, 33, 34). The effects of ketoconazole on steroid synthesis may occur after a single dose (33, 34, 37).
The effects of fluconazole on steroid biosynthesis and metabolism have not been as well studied. Investigators examined the effects of ketoconazole and fluconazole on rat adrenal cells and found that the concentration of ketoconazole achieving 50% inhibition of corticosterone synthesis was 0.9 μM; 50% inhibition was not achieved with fluconazole concentrations as high as 100 μM (10). To our knowledge, only a single study of the adrenal effects of fluconazole in critically ill patients has been published previously (29). In this study, fluconazole had no effect on adrenocorticotropin (ACTH)-stimulated cortisol levels in 19 critically ill surgical patients (29). The first case report that we are aware of describing a possible association between fluconazole and adrenal dysfunction was published in 1990 (16); recently, two additional reports have appeared in the literature (1, 38).
Diagnostic guidelines for adrenal dysfunction in critically ill patients remain a subject of debate (4, 7, 28). The normal response to the stress of surgery or severe illness is a marked initial increase in ACTH and basal cortisol levels. Because of this, it has been postulated that for critically ill patients, levels that are within the accepted normal range for healthy, non-critically ill individuals may be inappropriately low (20). Investigators have used random cortisol levels as well as high-dose (250 μg) and low-dose (1 μg) ACTH stimulation testing (28, 41) to diagnose adrenal dysfunction in critically ill patients. We were not able to perform ACTH stimulation testing, nor were we able to confirm a laboratory diagnosis of adrenal dysfunction with a clinical response to a trial of corticosteroid therapy. The use of ACTH stimulation testing to diagnose adrenal dysfunction in critically ill patients is controversial (7, 24, 28). Because critical illness itself may result in maximal adrenal stimulation, such testing may reveal little about the sufficiency of the adrenal response (28).
Approximately 45% of patients in our study had adrenal dysfunction, which we defined as an MPCL of less than 15 μg/dl. A recent publication has supported the use of this threshold value (7). The prevalence in our study was similar to that reported by Rydvall and colleagues, who measured morning plasma cortisol levels in 55 general ICU patients (36). They found that 36% of patients had plasma cortisol levels of <14.5 μg/dl and 47% had levels of <18.1 μg/dl (36). By contrast, Barquist and Kirton studied surgical ICU patients with vasopressor-dependent hypotension and an unexplained systemic inflammatory response syndrome, 2 weeks of vasopressor dependency, or ≥2 failed attempts at weaning from mechanical ventilation (3). They defined adrenal dysfunction as a baseline cortisol level of <15 μg/dl or a 30-min post-ACTH stimulation level of ≤25 μg/dl if the baseline level was between 15 and 20 μg/dl (3). Less than 1% of all patients met this definition. Among patients hospitalized in the ICU for more than 14 days 6% had adrenal dysfunction, and among patients hospitalized for more than 14 days and over age 55, 11% had adrenal dysfunction (3).
Unlike the findings of Barquist and Kirton (3), neither age nor ICU length of stay was significantly associated with adrenal dysfunction in our study. In fact, none of the demographic or clinical variables included in our multivariate regression analyses were significantly associated with adrenal dysfunction. Mortality was also not significantly different in patients with adrenal dysfunction, compared to those without adrenal dysfunction, although this study had insufficient power to detect differences as small as those observed. Additional studies are needed to determine factors that identify critically ill patients at highest risk for adrenal dysfunction.
This study was not large enough to detect small differences in cortisol levels between patients randomized to fluconazole and those randomized to placebo. We cannot exclude the possibility that fluconazole may cause low-level adrenal suppression, or even clinically significant adrenal dysfunction in individual patients. However, based on our multiple analyses, the results of this study suggest that fluconazole prophylaxis does not contribute to widespread adrenal dysfunction in critically ill surgical patients who are not receiving daily steroid therapy. To minimize the confounding effects of exogenous steroid administration, we excluded those samples drawn less than 2 days after steroid administration. We did not have cortisol data available from patients after ICU discharge and fluconazole discontinuation to explore the impact of fluconazole discontinuation on the metabolism of exogenous steroids and adrenal function. A case report has described a liver transplant patient on prednisone therapy who developed Addisonian crisis after fluconazole was discontinued (42), presumably due to increased prednisone metabolism following the reversal of P450 enzyme suppression by fluconazole (42). Itraconazole, another triazole, has been shown to decrease the clearance of methylprednisolone and prolong methylprednisolone's inhibition of adrenal steroid synthesis (21, 45), although it does not itself appear to cause adrenal dysfunction (32). Fluconazole (and newer triazoles) may exert the same effects, given its similar structure and mechanism of action. Further studies are needed to determine the effect of fluconazole and the new triazoles on the pharmacokinetics of exogenously administered steroid compounds and whether critically ill patients receiving both triazole antifungal and steroid therapy are at increased risk for adrenal dysfunction after the triazole is discontinued.
In conclusion, randomization to fluconazole was not associated with significantly lower cortisol levels than randomization to placebo. Fluconazole prophylaxis in this group of critically ill surgical patients was not associated with significant adrenal dysfunction.
Acknowledgments
This work was supported by the Johns Hopkins University School of Medicine General Clinical Research Center grant no. M01-RR00052 from the National Center for Research Resources, National Institutes of Health. Pfizer Pharmaceuticals, Inc., provided an unrestricted educational grant for the 1998-1999 clinical trial that was the source of patients and specimens in this study. S.M. was supported by an Infectious Diseases Society of America/Pfizer Pharmaceuticals Fellowship in Medical Mycology. She is currently supported by a K23 grant from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (AI53601-01).
We thank the Johns Hopkins SICU nursing staff for their dedication to this research. We also thank the Johns Hopkins University School of Medicine GCRC Core Laboratory for processing the specimens in this study.
REFERENCES
- 1.Albert, S. G., M. J. DeLeon, and A. B. Silverberg. 2001. Possible association between high-dose fluconazole and adrenal insufficiency in critically ill patients. Crit. Care Med. 29:668-670. [DOI] [PubMed] [Google Scholar]
- 2.Annane, D., V. Sébille, C. Charpentier, P. E. Bollaert, B. François, J. M. Korach, G. Capellier, Y. Cohen, E. Azoulay, G. Troche, P. Chaumet-Riffaut, and E. Bellissant. 2002. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288:862-871. [DOI] [PubMed] [Google Scholar]
- 3.Barquist, E., and O. Kirton. 1997. Adrenal insufficiency in the surgical intensive care unit patient. J. Trauma 42:27-31. [DOI] [PubMed] [Google Scholar]
- 4.Beshuizen, A., and L. G. Thijs. 2001. Relative adrenal failure in intensive care: an identifiable problem requiring treatment? Best Practice Res. Clin. Endocrinol. Metab. 15:513-531. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg, H. M., W. R. Jarvis, J. M. Soucie, J. E. Edwards, J. E. Patterson, M. A. Pfaller, M. S. Rangel-Frausto, M. G. Rinaldi, L. Saiman, R. T. Wiblin, R. P. Wenzel, et al. 2001. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. Clin. Infect. Dis. 33:177-186. [DOI] [PubMed] [Google Scholar]
- 6.Chang, S. S., S. J. Liaw, M. J. Bullard, T. F. Chiu, J. C. Chen, and H. C. Liao. 2001. Adrenal insufficiency in critically ill emergency department patients: a Taiwan preliminary study. Acad. Emerg. Med. 8:761-764. [DOI] [PubMed] [Google Scholar]
- 7.Cooper, M. S., and P. M. Stewart. 2003. Corticosteroid insufficiency in acutely ill patients. N. Engl. J. Med. 348:727-734. [DOI] [PubMed] [Google Scholar]
- 8.de Jong, F. H., C. Mallios, C. Jansen, P. A. Scheck, and S. W. Lamberts. 1984. Etomidate suppresses adrenocortical function by inhibition of 11 beta-hydroxylation. J. Clin. Endocrinol. Metab. 59:1143-1147. [DOI] [PubMed] [Google Scholar]
- 9.Dimopoulou, I., I. Ilias, P. Roussou, A. Gavala, A. Malefaki, E. Milou, M. Pitaridis, and C. Roussos. 2002. Adrenal function in non-septic long-stay critically ill patients: evaluation with the low-dose (1 mcg) corticotropin stimulation test. Intensive Care Med. 28:1168-1171. [DOI] [PubMed] [Google Scholar]
- 10.Eckhoff, C., W. Oelkers, and V. Bahr. 1988. Effects of two oral antimycotics, ketoconazole and fluconazole, upon steroidogenesis in rat adrenal cells in vitro. J. Steroid Biochem. 31:819-823. [DOI] [PubMed] [Google Scholar]
- 11.Eggimann, P., P. Francioli, J. Bille, R. Schneider, M. M. Wu, G. Chapuis, R. Chiolero, A. Pannatier, J. Schilling, S. Geroulanos, M. P. Glauser, and T. Calandra. 1999. Fluconazole prophylaxis prevents intra-abdominal candidiasis in high-risk surgical patients. Crit. Care Med. 27:1066-1072. [DOI] [PubMed] [Google Scholar]
- 12.Fridkin, S. K., and R. P. Gaynes. 1999. Antimicrobial resistance in intensive care units. Clin. Chest Med. 20:303-316. [DOI] [PubMed] [Google Scholar]
- 13.Garbino, J., D. P. Lew, J. A. Romand, S. Hugonnet, R. Auckenthaler, and D. Pittet. 2002. Prevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: a randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontamination. Intensive Care Med. 28:1708-1717. [DOI] [PubMed] [Google Scholar]
- 14.Georgopapadakou, N. H. 1998. Antifungals: mechanism of action and resistance, established and novel drugs. Curr. Opin. Microbiol. 1:547-557. [DOI] [PubMed] [Google Scholar]
- 15.Goodman, J. L., D. J. Winston, R. A. Greenfield, P. H. Chandrasekar, B. Fox, H. Kaizer, R. K. Shadduck, T. C. Shea, P. Stiff, and D. J. Friedman. 1992. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N. Engl. J. Med. 326:845-851. [DOI] [PubMed] [Google Scholar]
- 16.Gradon, J. D., and D. V. Sepkowitz. 1991. Fluconazole-associated acute adrenal insufficiency. Postgrad. Med. J. 67:1084-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufman, D., R. Boyle, K. C. Hazen, J. T. Patrie, M. Robinson, and L. G. Donowitz. 2001. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N. Engl. J. Med. 345:1660-1666. [DOI] [PubMed] [Google Scholar]
- 18.Khosla, S., J. S. Wolfson, Z. Demerjian, and J. E. Godine. 1989. Adrenal crisis in the setting of high-dose ketoconazole therapy. Arch. Intern. Med. 149:802-804. [PubMed] [Google Scholar]
- 19.Kley, H. K., R. Schlaghecke, and H. L. Krüskemper. 1985. Stabilität von Steroiden im Plasma über einen zeitraum von 10 jahren. J. Clin. Chem. Clin. Biochem. 23:875-878. [PubMed] [Google Scholar]
- 20.Lamberts, S. W. J., H. A. Bruining, and F. H. de Jong. 1997. Corticosteroid therapy in severe illness. N. Engl. J. Med. 337:1285-1292. [DOI] [PubMed] [Google Scholar]
- 21.Lebrun-Vignes, B., V. C. Archer, B. Diquet, J. C. Levron, O. Chosidow, A. J. Puech, and D. Warot. 2001. Effect of itraconazole on the pharmacokinetics of prednisolone and methylprednisolone and cortisol secretion in healthy subjects. Br. J. Clin. Pharmacol. 51:443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leleu, G., P. Aegerter, B. Guidet, et al. 2002. Systemic candidiasis in intensive care units: a multicenter, matched-cohort study. J. Crit. Care 17:168-175. [DOI] [PubMed] [Google Scholar]
- 23.Liang, K. Y., and S. L. Zeger. 1993. Regression analysis for correlated data. Annu. Rev. Public Health 14:43-68. [DOI] [PubMed] [Google Scholar]
- 24.Ligtenberg, J. J. M., A. R. J. Girbes, J. A. M. Beentjes, J. E. Tulleken, T. S. van der Werf, and J. G. Zijlstra. 2001. Hormones in the critically ill patient: to intervene or not to intervene? Intensive Care Med. 27:1567-1577. [DOI] [PubMed] [Google Scholar]
- 25.Loisa, P., T. Rinne, and S. Kaukinen. 2002. Adrenocortical function and multiple organ failure in severe sepsis. Acta Anaesthesiol. Scand. 46:145-151. [DOI] [PubMed] [Google Scholar]
- 26.Loose, D. S., P. B. Kan, M. A. Hirst, R. A. Marcus, and D. Feldman. 1983. Ketoconazole blocks adrenal steroidogenesis by inhibiting cytochrome P450-dependent enzymes. J. Clin. Investig. 71:1495-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lupetti, A., R. Danesi, M. Campa, M. Del Tacca, and S. Kelly. 2002. Molecular basis of resistance to azole antifungals. Trends Mol. Med. 8:76-81. [DOI] [PubMed] [Google Scholar]
- 28.Marik, P. E., and G. P. Zaloga. 2002. Adrenal insufficiency in the critically ill: a new look at an old problem. Chest 122:1784-1796. [DOI] [PubMed] [Google Scholar]
- 29.Michaelis, G., D. Zeiler, J. Biscoping, R. Füssle, and G. Hempelmann. 1993. Function of the adrenal cortex during therapy with fluconazole in intensive care patients. Mycoses 36:117-123. [DOI] [PubMed] [Google Scholar]
- 30.Nagai, K., I. Miyamori, M. Ikeda, H. Koshida, R. Takeda, K. Suhara, and M. Katagiri. 1986. Effect of ketoconazole (an imidazole antimycotic agent) and other inhibitors of steroidogenesis on cytochrome P450-catalyzed reactions. J. Steroid Biochem. 24:321-323. [DOI] [PubMed] [Google Scholar]
- 31.Pelz, R. K., C. W. Hendrix, S. M. Swoboda, M. Diener-West, W. G. Merz, J. Hammond, and P. A. Lipsett. 2001. Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann. Surg. 233:542-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips, P., J. R. Graybill, R. Fetchick, and J. F. Dunn. 1987. Adrenal response to corticotropin during therapy with itraconazole. Antimicrob. Agents Chemother. 31:647-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pont, A., J. R. Graybill, P. C. Craven, J. N. Galgiani, W. E. Dismukes, R. E. Reitz, and D. A. Stevens. 1984. High-dose ketoconazole therapy and adrenal and testicular function in humans. Arch. Intern. Med. 144:2150-2153. [PubMed] [Google Scholar]
- 34.Pont, A., P. L. Williams, D. S. Loose, D. Feldman, R. E. Reitz, C. Bochra, and D. A. Stevens. 1982. Ketoconazole blocks adrenal steroid synthesis. Ann. Intern. Med. 97:370-372. [DOI] [PubMed] [Google Scholar]
- 35.Rivers, E. P., M. Gaspari, G. Abi Saad, M. Mlynarek, J. Fath, H. M. Horst, and J. Wortsman. 2001. Adrenal insufficiency in high-risk surgical ICU patients. Chest 119:889-896. [DOI] [PubMed] [Google Scholar]
- 36.Rydvall, A., A. K. Brändström, R. Banga, K. Asplund, U. Bäcklund, and B. G. Stegmayr. 2000. Plasma cortisol is often decreased in patients treated in an intensive care unit. Intensive Care Med. 26:545-551. [DOI] [PubMed] [Google Scholar]
- 37.Schurmeyer, T., and E. Nieschlag. 1984. Effect of ketoconazole and other imidazole fungicides on testosterone biosynthesis. Acta Endocrinol. (Copenhagen) 105:275-280. [DOI] [PubMed] [Google Scholar]
- 38.Shibata, S., M. Kami, Y. Kanda, U. Machida, H. Iwata, Y. Kishi, A. Takeshita, S. Miyakoshi, J. Ueyama, S. Morinaga, and Y. Mutou. 2001. Acute adrenal failure associated with fluconazole after administration of high-dose cyclophosphamide. Am. J. Hematol. 66:303-305. [DOI] [PubMed] [Google Scholar]
- 39.Slavin, M. A., B. Osborne, R. Adams, M. J. Levenstein, H. G. Schoch, A. R. Feldman, J. D. Meyers, and R. A. Bowden. 1995. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation—a prospective, randomized, double-blind study. J. Infect. Dis. 171:1545-1552. [DOI] [PubMed] [Google Scholar]
- 40.Soni, A., G. M. Pepper, P. M. Wyrwinski, N. E. Ramirez, R. Simon, T. Pina, H. Gruenspan, and C. E. Vaca. 1995. Adrenal insufficiency occurring during septic shock: incidence, outcome, and relationship to peripheral cytokine levels. Am. J. Med. 98:266-271. [DOI] [PubMed] [Google Scholar]
- 41.Streeten, D. H. 1999. What test for hypothalamic-pituitary-adrenocortical insufficiency? Lancet 354:179-180. [DOI] [PubMed] [Google Scholar]
- 42.Tiao, G. M., J. Martin, F. L. Weber, R. M. Cohen, and D. W. Hanto. 1999. Addisonian crisis in a liver transplant patient due to fluconazole withdrawal. Clin. Transplant. 13:62-64. [DOI] [PubMed] [Google Scholar]
- 43.Trick, W. E., S. K. Fridkin, J. R. Edwards, R. A. Hajjeh, R. P. Gaynes, et al. 2002. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin. Infect. Dis. 35:627-630. [DOI] [PubMed] [Google Scholar]
- 44.Tucker, W. S., B. B. Snell, D. P. Island, and C. R. Gregg. 1985. Reversible adrenal insufficiency induced by ketoconazole. JAMA 253:2413-2414. [PubMed] [Google Scholar]
- 45.Varis, T., K. T. Kivistö, J. T. Backman, and P. J. Neuvonen. 1999. Itraconazole decreases the clearance and enhances the effects of intravenously administered methylprednisolone in healthy volunteers. Pharmacol. Toxicol. 85:29-32. [DOI] [PubMed] [Google Scholar]
- 46.Wagner, R. L., P. F. White, P. B. Kan, M. H. Rosenthal, and D. Feldman. 1984. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N. Engl. J. Med. 310:1415-1421. [DOI] [PubMed] [Google Scholar]
- 47.White, M. C., and P. Kendall-Taylor. 1985. Adrenal hypofunction in patients taking ketoconazole. Lancet 1:44-45. [DOI] [PubMed] [Google Scholar]
- 48.Winston, D. J., A. Pakrasi, and R. W. Busuttil. 1999. Prophylactic fluconazole in liver transplant recipients: a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 131:729-737. [DOI] [PubMed] [Google Scholar]
- 49.Zeger, S. L., and K. Y. Liang. 1992. An overview of methods for the analysis of longitudinal data. Stat. Med. 11:1825-1839. [DOI] [PubMed] [Google Scholar]