Abstract
The increased use of sentinel lymph node (SLN) excision for staging the axilla in women with breast cancer has benefited women by lowering morbidity and at the same time has raised issues related to the extent of treatment needed to the nodal basin. This is of particular concern when micrometastases or isolated tumor cells are found in the sentinel nodes on the final pathology. The probability of finding metastatic disease in non-sentinel lymph nodes (NSLN) ranges from 0 to 20% with only micrometastatic deposits in the SLN. Very low rates (0–3.7%) of axillary recurrence have been reported in selected patients with micrometastases tumor in sentinel nodes who have not had a completion axillary node dissection (ALND). Risk factors for additional positive NSLN include primary tumor size, the presence of lymphovascular invasion and the size of the SN metastatic deposit. Currently, the decision to not complete the ALND when micrometastic disease is found in the SLN should be made on a case-by-case basis. One should consider the tumor characteristics, findings within the SLN, and a multidisciplinary treatment plan. Clinical trial results may help to resolve the dilemma. There appears to be a low risk for axillary recurrence.
INTRODUCTION
The acceptance of sentinel lymph node biopsy (SLNB) to stage the axilla in patients with early stage breast cancer has created new issues for patient care. Even though there is a 5% to 10% false negative rate when SLN does not detect metastases [1, 2], a SLN excision without completion ALND is now the recommended method to stage clinically negative axilla according to the American Society of Clinical Oncologists (ASCO) [3] and the National Comprehensive Cancer Network (NCCN) [4]. If SLNs are positive by multi-level hematoxylin and eosin (H&E) evaluation, a level I and II axillary dissection is recommended.
However, the finding of minimal disease (micrometastases or less) in the sentinel nodes raises questions about the significance of this technique and about how to treat the remaining axillary nodes. This point of discussion continues to stimulate debate at meetings as well as in clinical settings.
DEFINING MICROMETASTASES
According to the American Joint Committee on Cancer (AJCC) 7th edition of staging manual, micrometastatic deposits of tumor cells in the axillary lymph nodes are defined as > 0.2 mm and ≤ 2.0 mm and are classified as N1mi for staging purposes.
Isolated tumor cells (ITC) are distinct from the remainder of micrometastases in that they are usually no detected on H & E staining. ITCs are small clusters of cells ≤ 0.2 mm or non-confluent 200 cells in a single lymph node. H&E, immunohistochemical (IHC), or reverse-transcriptase polymer chain reaction (RT-PRC) may find these deposits. The nodes containing ITC are considered N0 but with notations (i+) and (sn) if found in a SLN by IHC and N0 (mo1+)(sn) if by RT-PRC [5]. The staging guide now lists Stage 1b for T1N1mi breast cancer rather than classifying it as Stage 2 [5].
DETECTING ITC AND MICROMETASTASES
With the advent of the removal of SLNs, a pathologist is now able to more thoroughly evaluate these nodes for metastatic cells. Many surgeons ask for an intra-operative examination. The patient and surgeon are prepared to proceed directly to an ALND if a positive node is found. The accuracy of the intra-operative exam is variable. In the NSABP B-32 trial, the SLN intra-operative evaluation used imprint or scrape- and- smear cytology with subsequent H&E permanent section. The false negative rate for the intra-operative cytology was 38.6%, and the false positive rate was 0.5% [2]. The sensitivity rate for finding cancer cells with intra-operative cytology is less if the primary is an invasive lobular (39.7%) rather than an invasive ductal (55.5%) [6]. Intraoperative cytology is also less sensitive for micrometastases [7]. When touch imprint cytology, frozen section (FS), and rapid cytokeratin immunostaining (RCI) were compared to the final pathology, 20 of 100 patients had a metastatic deposit found with 12 macrometastases and 8 micrometastases. The combination of FS and RCI was statistically superior to touch imprint cytology and was comparable to the final pathology. Intra-operative cytology had a 13% sensitivity for detecting micrometastases. The immunostain was completed in 25 minutes [8].
Frozen section techniques detected 66% to 73% of positive SLNs in patients were used to develop the Memorial Sloan Kettering Cancer Center (MSKCC) nomogram [9]. Veronesi et al. [1] has reported using frozen section examination of the entire node as the final and only pathology examination of the SLN.
A real-time RT-PCR assay set to detect metastatic deposits > 0.2 mm demonstrated sensitivity 10% higher than FS evaluation. Sensitivity was still lower for patients with micrometastases versus those with macromestastases. Only a portion of any node was used for the RT-PCR assay and may not have contained the tiny deposit. Tissue used in this assay cannot be evaluated secondarily using light microscopy [10]. The need for a quick, cost-effective, intra-operative answer places limitations on the sensitivity of the examination. It is when an initial negative examination is followed by confirmation of a sentinel node metastatic lesion that the appropriateness of completing an ALND is most often debated.
Regardless of the technique used to examine the sentinel node, the incidence of micrometastases has increased beyond what it was before the SLN procedure was used. SEER cancer registry data between 1991 and 2003 show an increase in the diagnosis of N1mi disease from 2.3% to 7%. When only the SLN group is considered, the frequency of N1mi becomes 8.5% [11]. Information from the National Cancer Center Data Bank from 1998 to 2005 from 97,314 patients with a positive SLN showed that 10.5% contained metastatic foci 0.2 to 2.0 mm in size [12].
When micrometastases are found in the SLN and the ALND is completed, recent individual studies report a wide range of positive non-sentinel lymph nodes (NSLN) between 6% and 27% for patients classified with N1mi and 0% to 18% for those with ITC by IHC (Table 1).
Table 1.
Rate of Positive NSLN with N1mi or N0(i+) SLN
| Author(year) | N1mi with + NSLN/ALND (%) | N0(i+) with + NSLN/ALND (%) |
|---|---|---|
| Hwang (2003) [13] | 5/30 (17%) | |
| Cox(2008) [14] | 15/97 (15.5%) | 10/107 (9.3%) |
| Pugliesi (2010) [15] | 17/95 (18%) | |
| Reed (2009) [16] | 11/41 (27%) | 0/13 (0%) |
| Fan (2005) [17] | 3/18 (16.7%) | |
| Fournier (2004) [18] | 1/16 (6%) | |
| Calhoun (2005) [19] | 3/61 (4.9%) | |
| Cserni (2007) [20] | 0/26 (0%) | |
| Van Deurzen (2007) [21] | 20/101 (19.8%) | 3/23 (13%) |
| Houvenaeghel (2006) [22] | 43/301 (14.3%) | 30/187 (16%) |
NSLN: non-sentinel lymph nodes.
N1mi: micrometastasis (> 0.2 mm and/or more than 200 cells, but none ≤ 2.0 mm).
N0(i+): malignant cells in regional lymph node(s) < 0.2 mm.
SLN: sentinel lymph node.
ALND: axillary lymph node dissection.
Houvenaeghel et al. [22] reviewed published reports of a total of almost 6,000 patients. They found that when the SLN contained micrometastases, positive NSLN were found in 0 to 57% of the ALND specimens; with IHC positive foci in the SLN, 0% to 25% was found. A meta-analysis of 25 series published from 1998 to 2003 showed that after an ALND 9% of IHC-only positive SLN had additional involvement in NSLN; for H&E-detected micrometastases, the rate was 15% [23].
AXILLARY RECURRENCE WHEN ALND IS NOT PERFORMED
With the increasing use of SLN technology, a small portion of SLN-positive patients have not had a completion ALND. Recently that proportion appears to be increasing for patients with micrometastases and, to a lesser extent, for patients with macrometastases. From 1998 to 2005, the rate of SLN removal only when a patient was known to have micrometastases in- creased from 24.7% to 45.3%, while for patients with macrometastases this rate decreased from 24.2% to 16.7% [12]. An early NSABP trial provides information on the safety of not treating the positive axilla. In the NSABP B-04 study, women with clinical node negative status were randomly assigned to have their axilla treated by ALND, radiation, or observation alone with delayed ALND for recurrence. Forty percent of the women in the ALND arm had metastases; this was representative of the two other arms of the study as well. Only 18% of those who did not undergo direct treatment of the axilla manifested metastasis with 78% of the recurrences evident in the first 24 months. Over-all survival (OS) was equal in each group; the radiation group suffered a 12% axillary node recurrence, versus 1% in the ALND arm [24].
Reports that include data on axillary recurrence when the ALND is not completed for micrometastases are mostly retrospective single institution series. These reports often discuss the significance of minimal disease in the axilla and involve small numbers of patients (Table 2). Very few isolated axillary recurrences are reported by these authors. The reasons given for not completing the ALND include patient preference, the character of the primary tumor, and perceived low risk for additional positive NSLN. There is no uniformity of systemic therapy administered.
Table 2.
Axillary Recurrence Rate Without an ALND
| Author (year) | Micromets | ITC or IHC |
Axillary recurrence rate |
Systemic therapy received |
Radiation therapy | F/U Time | Reason for no ALND |
|---|---|---|---|---|---|---|---|
| Fant (2003) [25] | 27 | 0 | All | Breast or chest wall | 30 mo median | ACOSOG trial/patient refusal | |
| Guenther (2003) [26] | 16 | 23 | 0 | Per medical oncology | Breast | 32 mo | Pt refusal or co-morbidities |
| Fan (2004) [17] | 27 | 1 (3.7%) | Most without | 34.7 mo | Pt refusal | ||
| Calhoun (2005) [19] | 17 | 0 | 88% | 80.5 mo | Pt refusal | ||
| Chagpar (2005) [27] | 12 | 2 | 0 | 5-chemo | 40.2 mo | Found on re-staging SLN with IHC | |
| Haid (2006) [28] | 6 | 2 | 0 | ” | Pt refusal | ||
| Hwang (2007) [13] | 90 | 67 | 0 | 55% chemo, 27% hormonal only | 30% "Comprehensive" RT | 29.5 mo | ACOSOG trial / patient preference |
| Van Deurzen (2007) [21] | 6 | 17 | 0 | 12.5 mo | Unknown | ||
| Cox(2008) [14] | 25 | 44 | 1(2.29%) in ITC group | 48% chemo if N1mi, 42% if ITC | 20 mo | ||
| Zakaria (2008) [29] | 69 | 0 | 87% ER+ hormonal and 53% chemo recommended | 40% no dal fields along with breast 12% with mastectomy | 30 mo | Judged low risk for +NSLN | |
| Bilimoria (2009) [12] | 802 | 16(0.4%) | 64 mo | Unknown | |||
| Bulte (2009) [30] | 20 | 0 | Individually assessed | 46 mo | Not given | ||
| Reed (2009) [16] | 16 | 12 | 0 | 78% chemo if N1mi, 88% if ITC | 58 mo | ||
| Pugliese (2010) [15] | 76 | 0 | 63% chemo, 89% hormonal | 4.6% axillary | 76 mo | Older, ER+, lower MSKCC nomogram score | |
| Langer (2009) [31] | 27 | 0 | Same as for N0-88% systemic | No axillary | 77 mo | Prospective decision | |
| Pernas (2009) [32] | 35 | 0 | N1mi same as N1 | No axillary | 60 mo | Prospective decision |
ALND: axillary lymph node dissection; ITC: isolated tumor cells; IHC: immunohistochemistry; F/U: follow-up; NSLN: non-sentinel lymph nodes; MSKCC: Memorial Sloan-Kettering cancer center.
In reports by Langer, et al. [31] and Pernas, et al. [32] the decision to omit an ALND for N1mi (sn) was a prospective one. In a large data set from Bilimoria, et al. [12] the axillary recurrence rate was 0.4% without ALND and 0.2% if the ALND had been completed. There was no significant difference in the axillary recurrence rate or in OS with or without an ALND for micrometastases in the SLN. The reasons for treatment decisions were not available.
The widespread application of the observations from these small, usually single-institution studies, is confounded by a lack of randomized trials, differing systemic therapies, and a varied extent of regional radiation therapy, which that may have sterilized the NSLN. The recently reported outcome from NSABP B-32 indicated that there was no difference in OS and disease-free survival (DFS) for patients after removal of negative SLNs or removal of negative SLNs and ALND. The false negative SLN rate was 9.8% but nodal recurrence rate was < 1% [2, 33].
The American College of Surgeons Oncology Group (ACOSOG) Z11 trial randomly assigned patients with 1 or 2 histologically positive SLNs to no further alary treatment versus ALND. They found no difference in local or regional recurrence at a mean of 5.9 years follow-up between the treatment groups [34]. Further analysis determined that there was no significant difference in OS or DFS between the two groups. Forty-five percent of the patients in the SLN without ALND group had micrometastases only. Equal numbers of each treatment arm received chemotherapy and/or hormonal therapy [35].
The recent Dutch MIRROR trial evaluated, in a retrospective manner, the use of adjuvant systemic treatment for women with ITC or micrometastatic axillary node involvement. In contrast to the ACOSOG Z11 study, the Dutch mirror study found that after correcting for tumor characteristics and adjuvant treatment, the rate for axillary recurrence in the subgroup of 141 patients with N1mi (sn) who had not had an ALND or axillary radiation therapy was 5.0%. There was a significant hazard risk for axillary recurrence at 4.39 compared to that in the 887 women whose axilla was treated. In the N0 (i+) population, the axillary recurrence rate was 2% if the axilla was not otherwise directly treated; this was not significantly different from the rate in those who had had ALND or axillary radiation [36].
INFLUENCING THE DECISION FOR ALND
Women who do not have an ALND after the finding of a positive SLN are a select group. Multiple factors are considered in deciding whether to proceed to the ALND, and the surgeon may turn to prediction models to calculate the chance of finding positive NSLN. The amount of disease in the SLN may be taken into account in these models as either size of deposit [37] or method of detection [9]. Coutant et al. [38] evaluated 9 models for estimating NSLN disease, and the MSKCC and Tenon models outperformed the others, even with N1mi and ITC-positive nodes. No model has been shown to be able to predict a group with zero chance for additional nodal disease beyond the SLN.
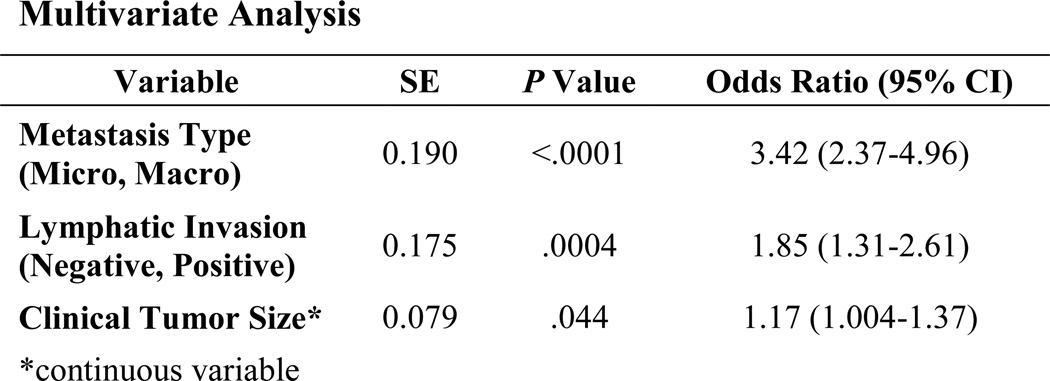
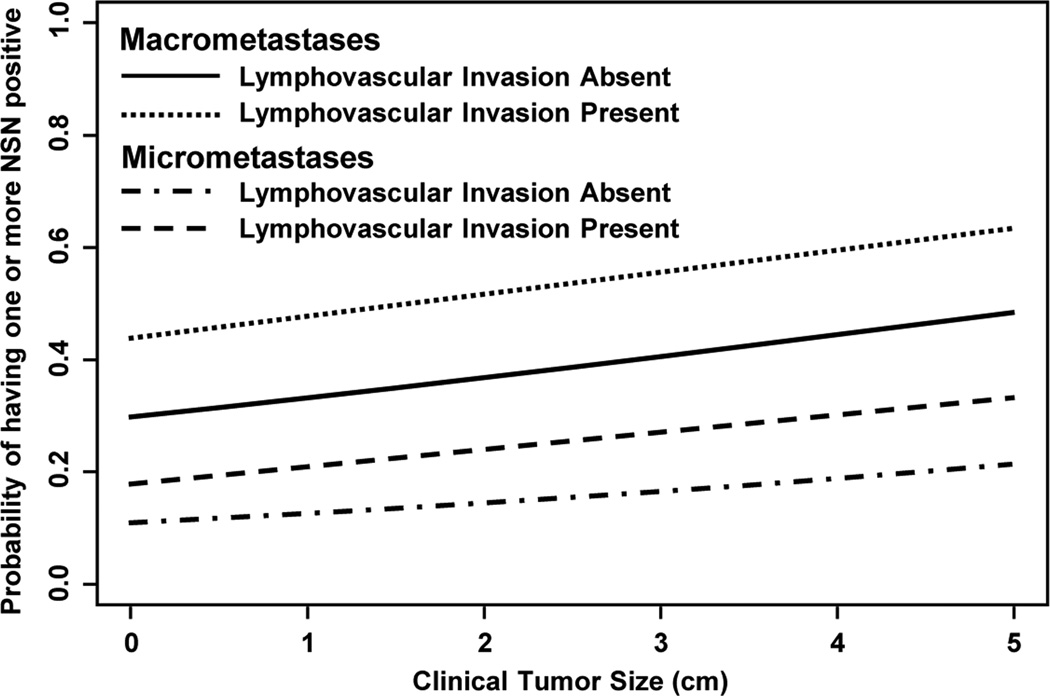
A recent abstract in which 1,361 SLN-positive patients were reviewed (NSABP B-32) showed positive NSLN in 19% of patients with SLN micrometastases. Type of metastases, lymphovascular invasion (LVI), and primary tumor size predicted for finding involved NSLN (Fig. 1). These data may suggest a group of patients in which completion axillary dissection is not needed [39]. These factors, as well as micrometastases versus macrometastases, result in a continuum of probability of finding positive NSLN (Fig. 2).
Fig. 1.
Predictive Factors for Non-SLN Metastases After Positive SLN Biopsy in NSABP B-32 [39]
Fig. 2.
Houvenaeghel et al. [22] reported a multivariant analysis in which tumor size, detection of micrometastases by H&E versus IHC, and LVI predicted for additional axillary disease. A minority of patients will have positive NSLN. According to current data, the rate of axillary recurrence without ALND appears to be low. The decision to not complete the ALND with small volume disease in the SLN may be multifactorial. Patient fear of the morbidity of the ALND may be an issue.
In the SLN-versus-ALND trials for node-negative axilla, edema, either as objective measure or as subjective complaint, is documented in 2% to 8% of the SLN-only recipients versus 13% to 19% of those who have undergone an ALND. The variability of these results may be secondary to the definitions and methods used to measure lymphedema. Other complications such as seroma, wound infection, paresthesia, decreased mobility, numbness, and pain, are increased in the ALND group [40–42]. In the NSABP B-32 study, measured water displacement was used to determine the amount of edema between the ipsilateral and contralateral arms. At 36 months, a 10% arm volume difference was seen in 7 to 9% of SLN patients and in 13% to 14% of the ALND patients. There was a consistently lower rate of morbidity in the SLN patients than in the ALND patients [43].
The surgeon and the patient consider the risk/benefit ratio of additional axillary treatment. For women with positive SLN and a high probability of having no additional axillary nodal disease, the risk of morbidity may begin to outweigh the benefit of completing the ALND.
CONCLUSION
For women with minimal metastatic disease in the axilla, the risk of axillary recurrence appears to be low without further treatment directed at the axilla beyond the SLNB. Tumor size, LVI, tumor markers, and grade, may influence the recommendation to complete an ALND because of the concern for associated positive NSLN. ACOSOG Z11 data show that older patient age, estrogen receptor-negative tumors, and lack of systemic therapy are associated on multivariant analysis with worse OS, but the operation performed for the axillary nodes is not [35]. The individual tumor characteristics and the knowledge of the presence of a micrometastatic focus in the SLN may be enough to formulate treatment plans.
In the event of an axillary recurrence noted on follow-up, an ALND can be performed to address the recurrences.
In the future, additional data from the NSABP B-32 trial may clarify the risk for axillary recurrence when there is minimal disease in the SLN. A randomized trial of women with micrometastases could also be considered, but, given the small numbers of patients who experience an axillary recurrence, a large number of participants would be needed. The After Mapping of the Axilla: Radiotherapy or Surgery (AMAROS) trial is evaluating the use of radiation rather than axillary dissection to treat the axilla and may provide another option for these patients. If an ALND continues to be recommended for breast cancer patients with micrometastases, a quick, reliable, intra-operative evaluation of SLNs is needed. RT-PCR could be set at a limit to define N1mi disease reliably intra-operatively. The goal would be to decrease false negatives and to allow for immediate ALND, avoiding the need for a second axillary operation.
For the present, it is recommended that ALND for N1mi disease be completed. However, in selected patients, particularly those with T1a primaries, the absence of LVI, and favorable tumor characteristics, it appears that the ALND could be omitted with a low risk for an axillary recurrence.
A multi-disciplinary team consultation is appropriate to evaluate the need for additional axillary operations and to recommend therapy. Systemic therapy or radiation to the intact breast may also decrease the rate of axillary recurrence.
The need to further identify patients who do not require or who will not benefit from an axillary dissection is a work in progress that can be addressed by clinical trials and with a more thorough understanding of primary tumor biology through the application of molecular taxonomy. The retreat from the Halstedian era of breast cancer treatment continues.
Acknowledgments
This work was supported by: Public Health Service grants U10-CA-69651, U10-CA-12027, U10-CA-37377, U10-CA-69974, and NCI P30-CA-14599 from the U.S. National Cancer Institute.
References
- 1.Veronesi U, Galimberti V, Paganelli G, et al. Axillary metastases in breast cancer patients with negative sentinel nodes: a follow-up of 3548 cases. EJC. 2009;45:1381–1388. doi: 10.1016/j.ejca.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 2.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph node resection and conventional axillary-lymph node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 3.American Society of Clinical Oncology (ASCO) Website www.asco.org.
- 4.NCCN Clinical Practice Guidelines in Oncology Breast Cancer V.2.2.2010. www.nccn.org.
- 5.AJCC Cancer Staging Manual. 7th Edition. New York: Springer; 2010. pp. 364–365. [Google Scholar]
- 6.Cox C, Centeno B, Dickson D, et al. Accuracy of intraoperative imprint cytology for sentinel lymph node evaluation in the treatment of breast carcinoma a 6-year study. Cancer. 2005;105:13–20. doi: 10.1002/cncr.20738. [DOI] [PubMed] [Google Scholar]
- 7.Motomura K, Nagumo S, Yoshifumi K, et al. Intraoperative imprint cytology for the diagnosis of sentinel node metastases in breast cancer. Breast Cancer. 2007;14:350–353. doi: 10.2325/jbcs.14.350. [DOI] [PubMed] [Google Scholar]
- 8.Krishnamurthy S, Meric-Bernstam F, Lucci A, et al. A prospective study comparing touch imprint cytology, frozen section analysis, and rapid cytokeratin immunostain for interoperative evaluation of axillary sentinel lymph nodes in breast cancer. Cancer. 2009;115:1555–1562. doi: 10.1002/cncr.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Zee KJ, Manasseh DME, Bevilacqua JLB, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–1151. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Julian TB, Blumencranz P, Deck K, et al. Novel intraoperative molecular test for sentinel lymph node metastases in patients with early-stage breast cancer. J Clin Oncol. 2008;26:3338–3345. doi: 10.1200/JCO.2007.14.0665. [DOI] [PubMed] [Google Scholar]
- 11.Chen SL, Hoehne FM, Giuliano AE. The prognostic significant of micrometastases in breast cancer: a SEER population-based analysis. Ann Surg Oncol. 2007;14:3378–3384. doi: 10.1245/s10434-007-9513-6. [DOI] [PubMed] [Google Scholar]
- 12.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27:2946–2953. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 13.Hwang RF, Gonzalez-Angulo AM, Yi M, et al. Low locoregional failure rates in selected breast cancer patients with tumor-positive sentinel lymph nodes who do not undergo completion axillary dissection. Cancer. 2007;110:723–730. doi: 10.1002/cncr.22847. [DOI] [PubMed] [Google Scholar]
- 14.Cox CE, Kiluk JV, Riker AI, et al. Significance of sentinel lymph node micrometastases in human breast cancer. J Am Coll Surg. 2008;206:261–268. doi: 10.1016/j.jamcollsurg.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Pugliese MS, Karam AK, Hsu M, et al. Predictors of completion axillary lymph node dissection in patients with immunohistochemical metastases to the sentinel lymph node in breast cancer. Ann Surg Oncol. 2010;17:1063–1068. doi: 10.1245/s10434-009-0834-5. [DOI] [PubMed] [Google Scholar]
- 16.Reed J, Rosman M, Verbanac KM, et al. Prognostic implications of isolated tumor cells and micrometastases in sentinel nodes of patients with invasive breast cancer: 10-year analysis of patients enrolled in the prospective East Carolina University/Anne Arundel Medical Center sentinel node multicenter study. J Am Coll Surg. 2009;208:333–340. doi: 10.1016/j.jamcollsurg.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Fan YG, Tan YY, Wu CT, et al. The effect of sentinel node tumor burden on non-sentinel node status and recurrence rates in breast cancer. Ann Surg Onc. 2005;12:705–711. doi: 10.1245/ASO.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Fournier K, Schiller A, Perry RR, et al. Micrometastasis in the sentinel lymph node of breast cancer does not mandate completion axillary dissection. Ann Surg. 2004;239:859–865. doi: 10.1097/01.sla.0000128302.05898.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calhoun KE, Hansen NM, Turner RR, Giuliano AE. Nonsentinel node metastases in breast cancer patients with isolated tumor cells in the sentinel node: implications for completion axillary node dissection. Am J Surg. 2005;190:588–591. doi: 10.1016/j.amjsurg.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Cserni G, Bianchi S, Vezzosi V, et al. Validation of clinical prediction rules for a low probability of nonsentinel and extensive lymph node involvement in breast cancer patients. Am J Surg. 2007;194:288–293. doi: 10.1016/j.amjsurg.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 21.van Deurzen CHM, van Hillegersberg R, Hobbelink MGG, et al. Predictive value of tumor load in breast cancer sentinel lymph nodes for second echelon lymph node metastases. Cellular Oncology. 2007;29:497–505. doi: 10.1155/2007/570683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houvenaeghel G, Nos C, Mignotte H, et al. Micrometastases in sentinel lymph node in a multicentric study: predictive factors of nonsentinel lymph node involvement – Groupe Des Chirurgiens De La Federation Des Centres De Lutte Contre Le Cancer. J Clin Oncol. 2006;24:1814–1822. doi: 10.1200/JCO.2005.03.3225. [DOI] [PubMed] [Google Scholar]
- 23.Cserni G, Gregori D, Merletti F, et al. Meta-analysis of non-sentinel node metastases associated with micrometastatic sentinel nodes in breast cancer. Br J Surg. 2004;91:1245–1252. doi: 10.1002/bjs.4725. [DOI] [PubMed] [Google Scholar]
- 24.Fisher B, Redmond C, Fisher ER, et al. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med. 1985;312:674–681. doi: 10.1056/NEJM198503143121102. [DOI] [PubMed] [Google Scholar]
- 25.Fant JS, Grant MD, Knox SM, et al. Preliminary outcomes analysis in patients with breast cancer and a positive sentinel lymph node who declined axillary dissection. Ann Surg Oncol. 2003;10:126–130. doi: 10.1245/aso.2003.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Guenther JM, Hansen NM, DiFronzo LA, et al. Axillary dissection is not required for all patients with breast cancer and positive sentinel nodes. Arch Surg. 2003;138:52–56. doi: 10.1001/archsurg.138.1.52. [DOI] [PubMed] [Google Scholar]
- 27.Chagpar A, Middleton LP, Sahin AA, et al. Clinical outcome of patients with lymph node-negative breast carcinoma who have sentinel lymph node micrometastases detected by immunohistochemistry. Cancer. 2005;103:1581–1586. doi: 10.1002/cncr.20934. [DOI] [PubMed] [Google Scholar]
- 28.Haid A, Knauer M, Koberle-Wuhrer R, et al. Medium-term follow-up data after sentinel node biopsy alone for breast cancer. Eur J Surg Oncol. 2006;32:1180–1185. doi: 10.1016/j.ejso.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Zakaria S, Pantvaidya G, Reynolds CA, et al. Sentinel node positive breast cancer patients who do not undergo axillary dissection: Are they different? Surgery. 2008;143:641–647. doi: 10.1016/j.surg.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Bulte CSE, van der Heiden-van der Loo M, Hennipman A. Axillary recurrence rate after tumour negative and micrometastatic positive sentinel node procedures in breast cancer patients, a population based multicenter study. Eur J Surg Oncol. 2009;35:25–31. doi: 10.1016/j.ejso.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Langer I, Guller U, Viehl CT, et al. Axillary lymph node dissection for sentinel lymph node micrometastses may be safely omitted in early-stage breast cancer patients: long-term outcomes of a prospective study. Ann Surg Oncol. 2009;16:3366–3374. doi: 10.1245/s10434-009-0660-9. [DOI] [PubMed] [Google Scholar]
- 32.Pernas S, Gil M, Benitez A, et al. Avoiding axillary treatment in sentinel lymph node micrometastases of breast cancer: a prospective analysis of axillary or distant recurrence. Ann Surg Oncol. 2010;17:772–777. doi: 10.1245/s10434-009-0804-y. [DOI] [PubMed] [Google Scholar]
- 33.Krag DN, Anderson SJ, Julian TB, et al. Primary outcome results of NSABP B-32, a randomized phase III clinical trial to compare sentinel node resection (SNR) to conventional axillary dissection (AD) in clinically node – negative breast cancer patients. J Clin Oncol. 2010;28(18s) suppl abstr LBA505. [Google Scholar]
- 34.Giuliano AE, McCall L, Beitsch P, et al. Local and regional control in breast cancer after sentinel node biopsy without axillary lymph node dissection: results from a randomized trial. Am Surgical Assoc 130th Annual Meeting Abstract. 2010 Apr 1;:8–10. [Google Scholar]
- 35.Giuliano AE, McCall LM, Beitsch PD, et al. ACOSOG Z0011: A randomized trial of axillary node dissection in women with clinical T1-2 N0 M0 breast cancer who have a positive sentinel node. J Clin Oncol. 2010;28(18S) (suppl; abstr CRA506) [Google Scholar]
- 36.Tjan-Heijnen VC, Pepels MJ, de Boer M, et al. Impact of omission of completion axillary lymph node dissection (cALND) or axillary radiotherapy (ax RT) in breast cancer patients with micrometastases (pN1mi) or isolated tumor cells (pN0i+]) in the sentinel lymph node (SN), Results from the MIRROR study. Abstract Presentation at ASCO Annual Meeting. 2009 Jun [Google Scholar]
- 37.Hwang RF, Krishnamurthy S, Hunt KK, et al. Clinicopathologic factors predicting involvement of nonsentinel axillary nodes in women with breast cancer. Ann Surg Onc. 2003;10:248–254. doi: 10.1245/aso.2003.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Coutant C, Olivier C, Lambaudie E, et al. Comparison of models to predict nonsentinel lymph node status in breast cancer patients with metastatic sentinel lymph nodes: a prospective multicenter study. J Clin Oncol. 2009;27:2800–2808. doi: 10.1200/JCO.2008.19.7418. [DOI] [PubMed] [Google Scholar]
- 39.Julian TB, Anderson SJ, Golesorkhi N, et al. Predictive factors for positive non-sentinel nodes following a positive sentinel node biopsy: NSABP B-32. San Antonio Breast Cancer Symposium. 2009 Dec; Abstract 301. [Google Scholar]
- 40.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–3663. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 41.Langer I, Guller U, Berclaz G, et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery. A prospective Swiss multicenter study of 659 patients. Ann Surg. 2007;245:452–461. doi: 10.1097/01.sla.0000245472.47748.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 43.Ashikaga T, Krag DN, Land SR, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010:111–118. doi: 10.1002/jso.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]