Abstract
The purpose of this study was to examine the effect of sampling epoch on total time spent walking and number of walking bouts/day in persons with stroke. 98 persons post-stroke with average age of 63.8±10.3 years and 43.6±58.1 months post-stroke participated in this study. Participants wore a StepWatch Activity Monitor (SAM) for 3–5 consecutive days. The number of strides taken was collected in consecutive 5 second epochs and down sampled into 10, 20, 30, and 60 second epochs. Total time walking and total number of walking bouts were determined for each day. Low and high activity days were determined as days below and above the 25th and 75th percentile of total steps/day, respectively. Total time walking and total number of bouts were different for each sampling epoch (p<0.001 for all). The 5 second sampling epoch resulted in calculation of ~40% of the walking time and ~6 times as many bouts as a 60 second sampling epoch. Differences were greater for low activity days (p<0.001 for all). Sampling epoch affects the calculation of daily step activity variables whose calculation depends on time, especially during low activity days. Sampling epoch must be carefully considered when designing studies aimed at understanding patterns of daily walking activity.
Keywords: Ambulation, CVA, Hemiparesis, Hemiplegia, Locomotor activity, Neurological Disorders, Physical activity, Physical exercise, Stroke, Walking
Introduction
Approximately 80% of the 5.5 million people living with stroke have some level of disability1. This disability is a consequence of, and a risk factor for, physical inactivity2, 3. Lack of physical activity has serious health and functional consequences for people post stroke4. Therefore, a thorough and accurate measurement of inactivity after stroke is necessary to develop optimal interventions to improve activity.
To obtain objective measurement of walking activity after stroke, many studies utilize an accelerometry-based system5. Several studies have established the reliability and accuracy of these devices in persons with stroke6–8 and have emphasized the importance of the ability to adjust measurement properties of the device (e.g.-sensitivity to motion) to enhance accurate measurement6, 7. One measurement property, however, that has not received systematic investigation is the sampling epoch. While the length of the sampling epoch will not affect the calculation of steps per day, as studies begin to analyze step activity data in a more detailed way9, 10, the length of the sampling epoch becomes critical11, 12. For example, the calculation of the amount of time spent walking is highly dependent upon sampling epoch. Consider two studies of walking activity after stroke. In one study, the sampling interval is set to 60 seconds13 and in the other it is set to 15 seconds14. Now imagine that a participant in each study took several steps in a row, amounting to 7 seconds of continuous walking, and then sat down. In the study where the sampling epoch was set to 60 seconds, the subject would be given credit for 60 seconds of walking, since the system cannot detect time intervals shorter than 60 seconds. In contrast, in the study where the sampling epoch was set to 15 seconds, the subject would be given credit for 15 seconds of walking, much closer to the actual time spent walking.
This overestimation of time spent walking when using a longer sampling epoch is particularly problematic in calculations aimed at comparing different groups of subjects. The purpose of this study was to systematically examine the effect of sampling epoch on total time spent walking and number of walking bouts per day in persons with stroke. We hypothesized that the use of longer sampling epochs would result in an overestimate of the time spent walking and an underestimate of the number of discrete walking bouts and that the magnitude of this over/underestimation due to longer sampling epochs would be greater when the number of steps per day was low.
Methods
Participants
Individuals post-stroke living in the community over the age of 18 who had experienced one or more strokes participated. Individuals were excluded if they had additional neurologic diagnoses, were unable to walk without assistance from another person (orthotics and assistive devices allowed), or were unable to follow instruction or communicate with investigators. All included individuals signed informed consent approved by the Human Subjects Review Board at University of Delaware prior to participation.
Procedures
A StepWatch Activity Monitor (SAM) (Orthocare Innovations, Seattle, Washington) was calibrated to a subject’s height and weight and placed above the subject’s ankle on the non-paretic leg. Calibration was checked with 30 strides at a subject’s self-selected walking speed and 10 strides at a faster speed. The SAM was recalibrated if the stride count differed by >2 between the SAM and manual counting. Participants wore the SAM during all waking hours (except bathing and swimming) for 3–5 consecutive days. The number of strides taken was collected in consecutive 5 second epochs.
Data analysis
Prior to data analysis, all data from the SAM was reviewed to eliminate partial days (e.g.- days when the subject did not wear the SAM during all waking hours). The SAM data were processed using a custom-written MATLAB program (MathWorks, Natick, MA). Data recorded by the SAM in strides was converted to steps for analysis within the program by multiplying by a factor of 2. Data collected in 5 second epochs were down sampled into 10, 20, 30, and 60 second sampling epochs to investigate the effect of sampling frequency. For each day collected, the total time walking and the total number of walking bouts were calculated at each of the 5 sampling rates. Total time walking was calculated as the total number of sampling epochs during which at least one stride was recorded multiplied by the length of the sampling epoch. The total number of walking bouts was calculated as the sum of discrete walking bouts, with the start of a walking bout defined as a sampling epoch with activity (non-zero) and the end of a bout defined as a sampling epoch with no activity (zero strides).
Data were normalized to the 60 second sampling epoch. A repeated measures ANOVA was used to assess differences between the five sampling rates for both the normalized time walking and normalized number of bouts. Post-hoc paired t-tests were performed to assess significance between individual sampling epochs.
To analyze the influence of amount of walking activity on the relationship between sampling rates, days in which the total number of steps fell below the 25th percentile were classified as low activity days. Similarly, days in which the total number of steps was greater than the 75th percentile were classified as high activity days. Post-hoc paired t-tests were run comparing the time walking and number of bouts between low and high activity days for all sampling epochs. The significance level for the repeated measures ANOVA was set at 0.05. A Bonferroni correction was applied for the post-hoc paired t-test with 10 comparisons, yielding a significance level of 0.005.
Results
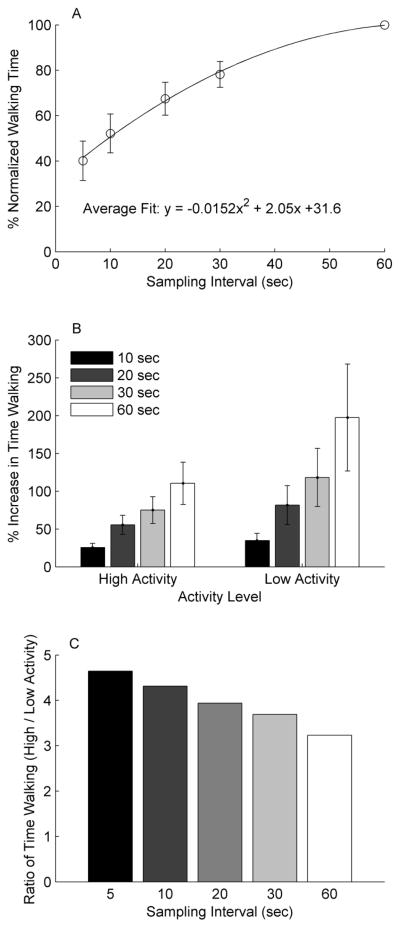
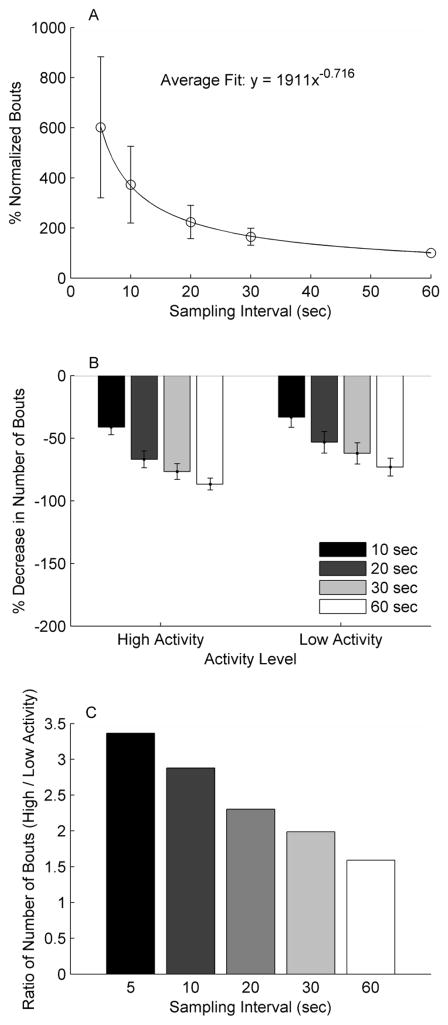
Ninety-eight subjects (Male: n=59) with the average age of 63.8 ±10.3 years, average gait speed of 0.67 ± 0.29 meters per second, and 43.6 ± 58.1 months post stroke participated in this study, resulting in a total of 366 days sampled. Subjects walked an average of 5432 ± 3727 steps per day. Total time walking and total number of bouts were different for each sampling epoch (p<0.001 for all cases). The 5 second sampling epoch resulted in calculation of ~40% of the walking time of a 60 second sampling epoch (Figure 1a, Table 1) and ~6 times as many bouts as a 60 second sampling epoch (Figure 2a).
Figure 1.
(A) Time spent walking per day normalized to the time calculated using 60 second sampling epochs. Significant differences were found between all sampling epochs. (B) Percent increase in time walking per day compared to the 5 second sampling epoch in the high (>7500 steps/day) and low (<2500 steps/day) activity days. (C) Ratio of time walking between high and low activity days at all sampling epochs. Notice that the difference between high and low activity days is reduced with each subsequently larger sampling epoch.
Table 1.
Calculated time spent walking and total number of bouts per day for the 5 epoch lengths analyzed.
| Bout Length | Time Walking/Day (hrs) | Number of Bouts/Day | ||
|---|---|---|---|---|
| Average | SD | Average | SD | |
| 5 sec | 1.65 | 0.97 | 356.25 | 196.40 |
| 10 sec | 2.14 | 1.21 | 223.77 | 119.55 |
| 20 sec | 2.76 | 1.50 | 135.31 | 67.63 |
| 30 sec | 3.18 | 1.68 | 100.08 | 46.92 |
| 60 sec | 4.02 | 2.00 | 60.22 | 25.51 |
Figure 2.
(A) Number of walking bouts per day normalized to the 60 second sampling epoch. Significant differences were found between all sampling epochs. (B) Percent decrease in number of bouts per day compared to the 5 second sampling epoch in the high (>7500 steps/day) and low (<2500 steps/day) activity days. (C) Ratio of the number of bouts between high and low activity days at all sampling epochs. Notice that the difference between high and low activity days is reduced with each subsequently larger sampling epoch.
When comparing low activity (<2500 steps, n=93) and high activity (>7500 steps, n=86) days, the percent change in walking time and number of bouts was greater for the low activity days from the 5 second sampling epoch to each subsequent higher sampling epoch (all comparisons p<0.001, Figure 1b, 2b). This indicates that the over/underestimation that occurs with the longer sampling epochs was worse for the low activity days.
Discussion
The results of this study show that the effect of the length of the sampling epoch differed based on the amount of walking activity performed. The impact of sampling epoch was greater for the days with low activity (<2500 steps) compared to those with high activity (>7500 steps). The result of this is that differences between groups with largely different number of steps/day would be compressed when larger sampling epochs are chosen (Figures 1c and 2c). This has potential implications for the interpretation of step activity data comparisons between groups of subjects with largely different number of steps/day, such as sedentary and active older adults15 or adults with stroke compared to neurologically intact adults9.
The selection of epoch size is also important when comparing subjects who take a similar number of steps per day, but have different activity patterns. As an example, the analysis of two subjects that walked a similar number of steps in a day is shown in Table 2. When using 60 second epochs to analyze their walking patterns, it is calculated that subject Y walked for 1 hour longer than subject X, in only 9 greater bouts (20% more bouts). In contrast, when analyzing the data with 5 second bouts, it is calculated that subject Y walked only ~8 mins (0.13 hrs.) longer than subject X, while participating in 110 more bouts (35% more bouts). For these subjects, the selection of epoch duration has a meaningful influence on the subjects’ calculated activity level, despite walking a similar number of steps.
Table 2.
Example day of activity for two subjects with similar steps within the day. Steps, time walking, and number of bouts for the day across the 5 epoch lengths analyzed.
| Bout Length | Steps | Time Walking (hrs) | Number of Bouts | |||
|---|---|---|---|---|---|---|
| Subject | X | Y | X | Y | X | Y |
| 5 sec | 4778 | 4858 | 1.45 | 1.58 | 308 | 418 |
| 10 sec | 4778 | 4858 | 1.87 | 2.16 | 179 | 266 |
| 20 sec | 4778 | 4858 | 2.34 | 2.92 | 106 | 150 |
| 30 sec | 4778 | 4858 | 2.72 | 3.43 | 74 | 114 |
| 60 sec | 4778 | 4858 | 3.35 | 4.35 | 53 | 64 |
Many studies that examine daily step activity using an accelerometry-based device in persons with stroke do not report the sampling epoch used7, 16, 17, and may use the default epoch set by the company (e.g.- 60 sec epoch for the SAM device). While this choice may be appropriate for measurement of steps/day, the results of this study suggest that it may not be optimal when examining the time spent walking or number of bouts per day. Moreover, it is likely that other measurements, such as step intensity10, where the time interval is important in the calculation, are also impacted by the choice of sampling epoch. A measurement such as cadence, which describes the rate of steps taken, is highly dependent on sampling epoch. For example, if an individual takes 5 steps in a 5 second epoch and rests for a minute, they would have a cadence of 1 step per second during the 5 second epoch of activity. If the data was collected in 60 second epochs, however, those same 5 steps would result in a cadence of 5 steps per 60 seconds, or 0.083 steps per second, a much lower cadence for the same activity. For some devices, longer sampling epochs result in the storage of a fewer number of days of walking11, 12, so it is necessary to strike a balance between recording an adequate number of days of walking18 with the need to use an appropriate sampling frequency.
Conclusions
The length of the sampling epoch affects the calculation of daily step activity variables whose calculation depends implicitly or explicitly on time. The size of this effect varies depending on the amount of daily step activity. Therefore, the sampling epoch must be carefully considered when designing studies aimed at understanding patterns of daily walking activity.
JRRD at a Glance.
Lack of physical activity has serious health and functional consequences for people post stroke. Therefore, interventions to improve post-stroke activity and accurate methods to measure activity are needed. The purpose of this study was to examine how the length of the interval over which activity data is sampled affects the measurement of activity. The results showed that the length of the sampling interval results in over or underestimation of activity and this is worse when activity is low, which could impact the interpretation of comparisons between groups of people with largely different amounts of activity.
Acknowledgments
Research reported in this article was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P30GM103333-01, and National Institutes of Health grants NR010786 and R21 HD071042.
Abbreviations
- SAM
Step Activity Monitor
Footnotes
Author Contributions
BK processed and analyzed the data and prepared the manuscript. MR collected and analyzed the data and edited the manuscript. DR conceived the study, analyzed the data, and prepared the manuscript.
References
- 1.Lees KR, Zivin JA, Ashwood T, et al. NXY-059 for acute ischemic stroke. N Engl J Med. 2006 Feb 9;354(6):588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- 2.Mayo NE, Wood-Dauphinee S, Cote R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months poststroke. Arch Phys Med Rehabil. 2002 Aug;83(8):1035–1042. doi: 10.1053/apmr.2002.33984. [DOI] [PubMed] [Google Scholar]
- 3.Rimmer JH, Wang E. Aerobic exercise training in stroke survivors. Top Stroke Rehabil. 2005 Winter;12(1):17–30. doi: 10.1310/L6HG-8X8N-QC9Q-HHM8. [DOI] [PubMed] [Google Scholar]
- 4.Hornnes N, Larsen K, Boysen G. Little change of modifiable risk factors 1 year after stroke: a pilot study. Int J Stroke. 2010;5(3):157–162. doi: 10.1111/j.1747-4949.2010.00424.x. [DOI] [PubMed] [Google Scholar]
- 5.Gebruers N, Vanroy C, Truijen S, Engelborghs S, De Deyn PP. Monitoring of physical activity after stroke: a systematic review of accelerometry-based measures. Arch Phys Med Rehabil. 2012 Feb;91(2):288–297. doi: 10.1016/j.apmr.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Macko RF, Haeuber E, Shaughnessy M, et al. Microprocessor-based ambulatory activity monitoring in stroke patients. Med Sci Sports Exerc. 2002 Mar;34(3):394–399. doi: 10.1097/00005768-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Mudge S, Stott NS. Test--retest reliability of the StepWatch Activity Monitor outputs in individuals with chronic stroke. Clin Rehabil. 2008 Oct-Nov;22(10–11):871–877. doi: 10.1177/0269215508092822. [DOI] [PubMed] [Google Scholar]
- 8.Mudge S, Stott NS, Walt SE. Criterion validity of the StepWatch Activity Monitor as a measure of walking activity in patients after stroke. Arch Phys Med Rehabil. 2007 Dec;88(12):1710–1715. doi: 10.1016/j.apmr.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 9.Roos MA, Rudolph KS, Reisman DS. The structure of walking activity in people after stroke compared with older adults without disability: a cross-sectional study. Phys Ther. 2012 Sep;92(9):1141–1147. doi: 10.2522/ptj.20120034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manns PJ, Baldwin E. Ambulatory activity of stroke survivors: measurement options for dose, intensity, and variability of activity. Stroke. 2009 Mar;40(3):864–867. doi: 10.1161/STROKEAHA.108.531590. [DOI] [PubMed] [Google Scholar]
- 11.Coleman KL, Smith DG, Boone DA, Joseph AW, del Aguila MA. Step activity monitor: long-term, continuous recording of ambulatory function. J Rehabil Res Dev. 1999 Jan;36(1):8–18. [PubMed] [Google Scholar]
- 12.Matthews CE, Hagstromer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012 Jan;44(1 Suppl 1):S68–76. doi: 10.1249/MSS.0b013e3182399e5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hale LA, Pal J, Becker I. Measuring free-living physical activity in adults with and without neurologic dysfunction with a triaxial accelerometer. Arch Phys Med Rehabil. 2008 Sep;89(9):1765–1771. doi: 10.1016/j.apmr.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Rand D, Eng JJ, Tang PF, Jeng JS, Hung C. How active are people with stroke?: use of accelerometers to assess physical activity. Stroke. 2009 Jan;40(1):163–168. doi: 10.1161/STROKEAHA.108.523621. [DOI] [PubMed] [Google Scholar]
- 15.Cavanaugh JT, Coleman KL, Gaines JM, Laing L, Morey MC. Using step activity monitoring to characterize ambulatory activity in community-dwelling older adults. J Am Geriatr Soc. 2007 Jan;55(1):120–124. doi: 10.1111/j.1532-5415.2006.00997.x. [DOI] [PubMed] [Google Scholar]
- 16.Bowden MG, Balasubramanian CK, Behrman AL, Kautz SA. Validation of a speed-based classification system using quantitative measures of walking performance poststroke. Neurorehabil Neural Repair. 2008 Nov-Dec;22(6):672–675. doi: 10.1177/1545968308318837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mudge S, Stott NS. Timed walking tests correlate with daily step activity in persons with stroke. Arch Phys Med Rehabil. 2009 Feb;90(2):296–301. doi: 10.1016/j.apmr.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 18.Tudor-Locke C, Burkett L, Reis JP, Ainsworth BE, Macera CA, Wilson DK. How many days of pedometer monitoring predict weekly physical activity in adults? Prev Med. 2005 Mar;40(3):293–298. doi: 10.1016/j.ypmed.2004.06.003. [DOI] [PubMed] [Google Scholar]