Abstract
A decrease in the activity of proprotein convertase subtilisin/kexin type 9 (PCSK9) increases the amount of lowdensity lipoprotein (LDL) receptors on liver cells and, therefore, LDL clearance. The clearance of lipids from pathogens is related to endogenous lipid clearance; thus, PCSK9 may also regulate removal of pathogen lipids such as lipopolysaccharide (LPS). Compared to controls, Pcsk9 knockout mice displayed decreases in inflammatory cytokine production and in other physiological responses to LPS. In human liver cells, PCSK9 inhibited LPS uptake, a necessary step in systemic clearance and detoxification. Pharmacological inhibition of PCSK9 improved survival and inflammation in murine polymicrobial peritonitis. Human PCSK9 loss-of-function genetic variants were associated with improved survival in septic shock patients and a decrease in inflammatory cytokine response both in septic shock patients and in healthy volunteers after LPS administration. The PCSK9 effect was abrogated in LDL receptor (LDLR) knockout mice and in humans who are homozygous for an LDLR variant that is resistant to PCSK9. Together, our results show that reduced PCSK9 function is associated with increased pathogen lipid clearance via the LDLR, a decreased inflammatory response, and improved septic shock outcome.
INTRODUCTION
Microbial cell walls contain pathogenic lipid moieties such as lipopolysaccharide (LPS; Gram-negative bacteria), lipoteichoic acid (a structurally similar glycolipid found in Gram-positive bacteria) (1), and phospholipomannan (fungal pathogens) (2). These pathogen-associated lipids are major ligands for mammalian innate immune receptors [Tolllike receptors (TLRs)] and thus figure prominently in the septic inflammatory response (septic shock, or sepsis). Septic shock is an often fatal complication of a severe microbial infection (sepsis) that triggers an uncontrolled systemic inflammatory response (“cytokine storm”) and subsequent organ failure. Beyond antibiotic therapy, there are currently no effective treatments for sepsis or septic shock.
Free pathogen lipids in the circulation are quickly bound by transfer proteins (3). Microbial LPS-binding protein (LBP) and bactericidal permeability-increasing protein (BPI) are similar to the endogenous mammalian lipid transfer proteins phospholipid transfer protein (PLTP) and cholesterol ester transfer protein (CETP), all of which bind pathogen lipids (3). When they are either bound to transfer proteins or free in the circulation, pathogen lipids trigger an inflammatory response through TLRs (4). In addition, when bound to transfer proteins (5), pathogen lipids are incorporated initially into high-density lipoproteins (HDLs) and subsequently can be transferred to low- or very-low-density lipoproteins (LDLs and VLDLs, respectively) (6), cleared from the blood by the liver, detoxified in the liver, and ultimately secreted in the bile (7). Binding, sequestering, and clearance from the blood reduces the inflammatory response induced by pathogen lipids (5, 7, 8). Therefore, understanding mechanisms of clearance from the blood for microbial lipids might provide the insight into the development of new therapeutic strategies for sepsis.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a central regulatory molecule that inhibits clearance of endogenous cholesterol lipid from the blood. PCSK9 binds the LDL receptor (LDLR) on hepatocytes, stimulates its internalization, promotes LDLR lysosomal degradation, and prevents recycling of LDLR to the cell surface (9–11). Thus, PCSK9 decreases LDLR cell surface density and, in turn, decreases clearance of LDL particles and increases plasma LDL cholesterol. Loss-of-function (LOF) variants of the PCSK9 gene result in increase clearance of LDL cholesterol from the circulation (12–18), whereas PCSK9 gain-of-function (GOF) variants have the opposite effect (13, 19) with a concordant and marked impact on a person’s risk of developing cardiovascular disease (20), coronary artery disease (12), and myocardial infarction (20, 21). These genetic discoveries triggered a race to develop PCSK9 inhibitors (22), which now show great promise in increasing LDL clearance (9) and lowering blood LDL cholesterol as well as the risk of atherosclerosis and its sequelae (20). We hypothesized that pathogen lipid clearance may also be modulated by PCSK9 inhibition, particularly in view of the striking similarities between mechanisms of transport and clearance of cholesterol and pathogen lipids (3, 5).
RESULTS
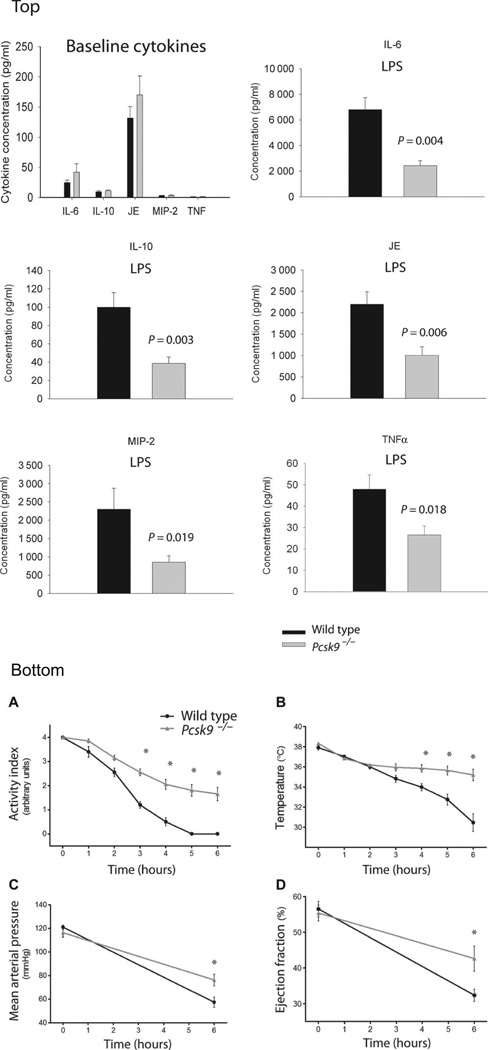
To test this hypothesis, we administered LPS intraperitoneally to Pcsk9 knockout (Pcsk9−/−) mice and genetic background control mice to measure LPS clearance, the cytokine inflammatory response, and clinically relevant physiological outcomes. Six hours after LPS administration, Pcsk9−/− mice had lower plasma concentrations of the early proinflammatory cytokine tumor necrosis factor α (TNFα) (P = 0.018); the integrative inflammatory cytokine interleukin-6 (IL-6), which is associated with survival in human sepsis (P= 0.004); the anti-inflammatory cytokine IL-10 (P = 0.003); the CC chemokine JE [also called murine monocyte chemotactic protein 1 (MCP-1)] (P = 0.006); and the CXC chemokine macrophage inflammatory protein 2 (MIP-2) (P = 0.019) (Fig. 1, top panel, LPS graphs). There were no differences in cytokine concentrations between Pcsk9−/− mice and controls after sham saline treatment (Fig. 1, top panel, baseline cytokines).
Fig. 1. Response to LPS in wild-type and Pcsk9∑/− mice.
Top: Plasma cytokine concentrations at baseline (top) and after LPS. A multiplex cytokine assay measured TNFα, IL-6, IL-10, JE, and MIP-2 at baseline and 6 hours after intraperitoneal injection of LPS (20 mg/kg). There were no significant differences at baseline shown in the bottom right frame (n = 9), whereas all cytokines were significantly (P < 0.05) lower in Pcsk9−/− mice (n = 9) than wild-type control mice (n = 9) after exposure to LPS. Bottom: (A) Activity phenotype. All 10 wild-type control mice had an activity index of 0 (no movement) by 6 hours after LPS administration compared to none of 10 Pcsk9−/− mice. Analysis of variance (ANOVA) demonstrates a difference between Pcsk9−/− and wild-type mice (P = 2.2 × 10−16) that was statistically significant (*P < 0.05) in hours 3 to 6. Typically, an activity index less than 0.5 represents a terminal state. (B) Body temperature. Wild-type control mice demonstrated a progressive loss of body temperature over the 6 hours after LPS administration such that 6 of the 10 mice had a body temperature <32°C, whereas none of 10 Pcsk9−/− mice had their temperature drop below 32°C. ANOVA demonstrates a difference between Pcsk9−/− and wild-type mice (P = 0.00035) that was statistically significant (*P < 0.05) in hours 4 to 6. Typically, a sustained temperature lower than 32°C represents a terminal state. (C) Blood pressure. Mean arterial pressure is preserved and was significantly higher in Pcsk9−/− mice than in wild-type control mice 6 hours after administration of LPS (P = 0.014 by ANOVA). (D) Left ventricular ejection fraction. Left ventricular function is preserved in Pcsk9−/− mice compared to wild-type control mice as evidenced by significantly higher left ventricular ejection fraction 6 hours after LPS infusion (P = 0.031 by ANOVA).
Pcsk9−/− mice displayed blunted systemic and cardiovascular responses to LPS treatment. By 6 hours after LPS injection, all 10 genetic background control mice exhibited continuously hunched posture and did not move despite strong stimulus (activity index of 0 of 4) (Fig. 1, bottom panel, A) and demonstrated a progressive loss of body temperature such that 6 of the 10 control mice had a final body temperature <32°C (mean, 30.5 ± 2.8°C) (Fig. 1, bottom panel, B). In contrast, the Pcsk9−/− mice had a mean activity index of 1.6 ± 0.9 (P = 2.2 × 10−16 versus control), which corresponded to decreased activity interspersed with normal activity. None of the 10 Pcsk9−/− mice had their temperature drop below 32°C (mean, 35.2 ± 1.8°C; P = 0.00035 versus control). LPS induced an acute decrease in mean arterial pressure and left ventricular ejection fraction (Fig. 1, bottom panel, C and D) with in hours in control mice (23, 24). Both the decrease in mean arterial pressure (P = 0.014) and that in left ventricular ejection fraction (P = 0.031) were significantly ameliorated in Pcsk9−/−mice.
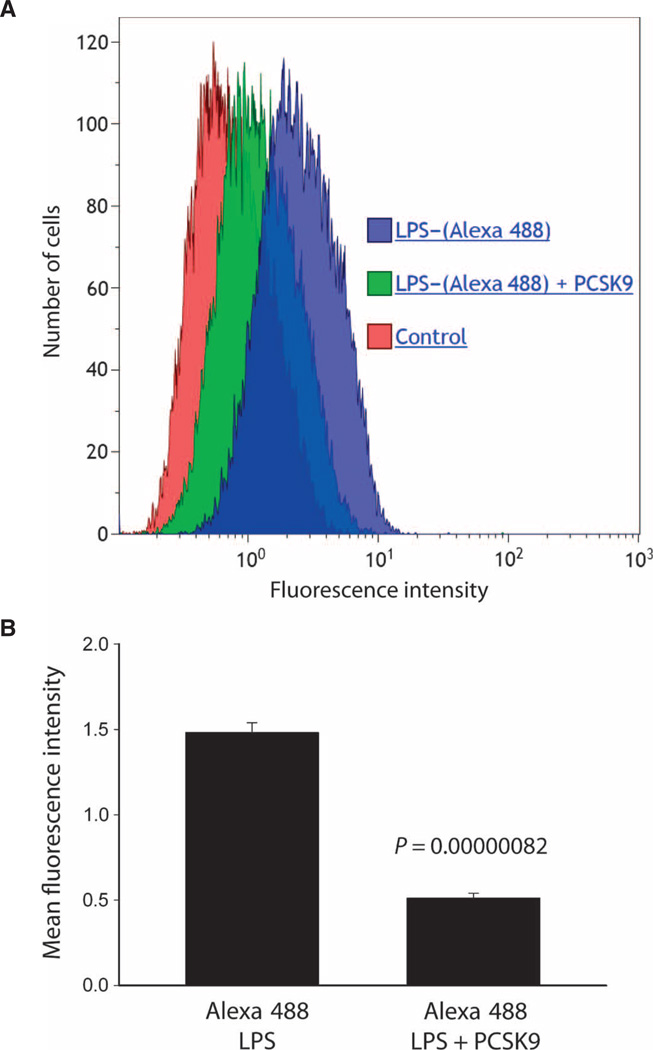
LPS clearance from the blood and detoxification are accomplished almost exclusively by the liver (25). Six hours after LPS administration, Pcsk9−/− mice had 55% lower plasma endotoxin concentrations compared to control mice (P = 0.021, Kruskal-Wallis test), suggesting that increased plasma LPS clearance had taken place. We used genetically identical human liver cells to confirm that the presence of extracellular PCSK9 altered the rate of LPS uptake into the liver and thus clearance from the circulation. After confirming by fluorescence-activated cell sorting analysis that the decline in LDLR expression reached a nadir by PCSK9 protein (3 µg/ml) (doses, 0, 1, 3, and 10 µg/ml) (26), we found that this dose resulted in a 65 ± 7.0% decrease in the amount fluorescent LPS [LPS–(Alexa 488)] detected in the cells (Fig. 2A), expressed as the mean fluorescence intensity (MFI). The MFI of cells exposed to Alexa 488–bound LPS was 1.49± 0.06, whereas the addition of PCSK9 (3 µg/ml) reduced the MFI to 0.52 ± 0.03. This effect is highly significant with a P value of 8.20 × 10−7 (Fig. 2B).
Fig. 2. Effect ofPCSK9on LPS uptake by HepG2 cells.
(A) Representative histogram of LPS uptake by HepG2 cells. HepG2 cells at 80% confluence were cultured in 80% Dulbecco’s modified Eagle’s medium (DMEM) and 20% human plasma from pooled healthy donors. Three hours before LPS treatment and at 4 and 19 hours after treatment, cells were treated with recombinant human PCSK9 (3 µg/ml) or vehicle control. Cells were then treated with Alexa Fluor 488–conjugated LPS. After 24 hours of LPS treatment, cells were analyzed by flow cytometry for MFI. (B) Group mean data from n = 6 flow cytometry runs. In HepG2 cells known to have only a trace of secreted PCSK9, the MFI of cells exposed to Alexa 488–bound LPS was 1.49 ± 0.06, whereas the addition of PCSK9 (3 µg/ml) reduced the MFI to 0.52 ± 0.03. This effect is highly significant with a P value of 8.20 × 10−7 by unpaired t test.
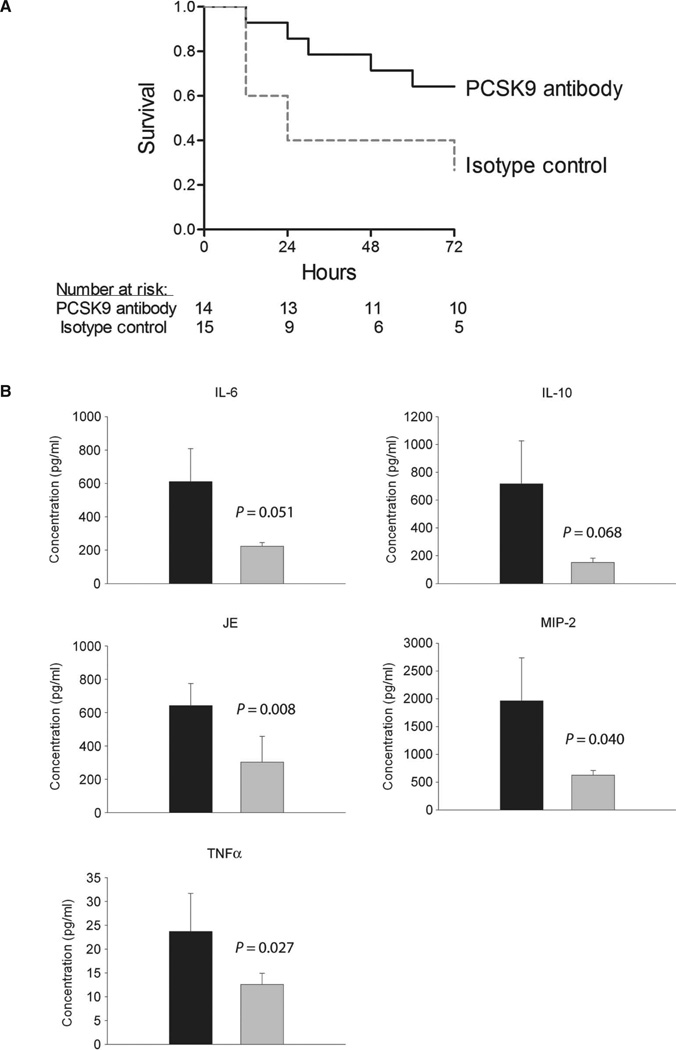
To determine whether pharmacological inhibition of PCSK9 would also have beneficial effects in a more clinically relevant model of sepsis, we induced polymicrobial sepsis in mice using cecal ligation and puncture (CLP). We administered resuscitation fluid immediately, and then 6 hours after CLP, we administered antibiotics, which is a typical treatment regimen for human sepsis. After randomization and while maintaining double-blind conditions, we also administered, at the 6-hour time point and daily thereafter, a PCSK9-blocking antibody or isotype control immunoglobulin G (IgG) subcutaneously and observed the mice for 72 hours. The PCSK9-blocking antibody significantly increased survival (P = 0.034) (Fig. 3A) and blunted the inflammatory cytokine response (Fig. 3B). By the third day after CLP, endotoxin activity was much lower in plasma from mice treated with the PCSK9-blocking antibody (0.05 ± 0.006) compared to controls (0.15 ± 0.50) (P = 0.05). In addition to the reduction in circulating endotoxin, we measured the density of bacteria seeded in mouse lungs through quantitative polymerase chain reaction (qPCR) assays designed to measure the bacterial 16S ribosomal RNA region of the genome. This technique is generally more sensitive than traditional in vitro culture methods and has been successfully performed previously in our laboratory (27, 28). In mice treated with anti-PCSK9, there was a nonsignificant trend toward fewer bacteria per milligram of lung tissue at both day 1 (4300 ± 1000 versus 13,000 ± 6400; P = 0.3) and day 3 (360 ± 180 versus 4000 ± 2700; P = 0.1) compared to control antibody–treated mice.
Fig. 3. Double-blind, randomized control study of PCSK9 inhibition in a murine model of polymicrobial peritonitis.
All study personnel were blinded as to group assignments (PCSK9-blocking antibody or isotype control IgG). C57BL/6 mice [n = 14 (PCSK9-blocking antibody) and n = 15 (isotype control)] were followed for 72 hours after CLP, and the time of death was recorded. Treatment with the antibiotic imipenem and the blinded study drug (PCSK9-blocking antibody of isotype control) began 6 hours after CLP, and both were administered daily thereafter. (A) There was a significant improvement in survival with PCSK9-blocking antibody (71%) versus isotype control treatment (40%) by log-rank test (P = 0.034). (B) Serial plasma inflammatory cytokine measurements beginning the first day (day 1) after CLP are shown. Using ANOVA, PCSK9-blocking antibody increases the clearance rate of TNFα (P = 0.027), IL-6 (P = 0.051), IL-10 (P = 0.068), JE (P = 0.0085), and MIP-2 (P = 0.040). Ab, antibody.
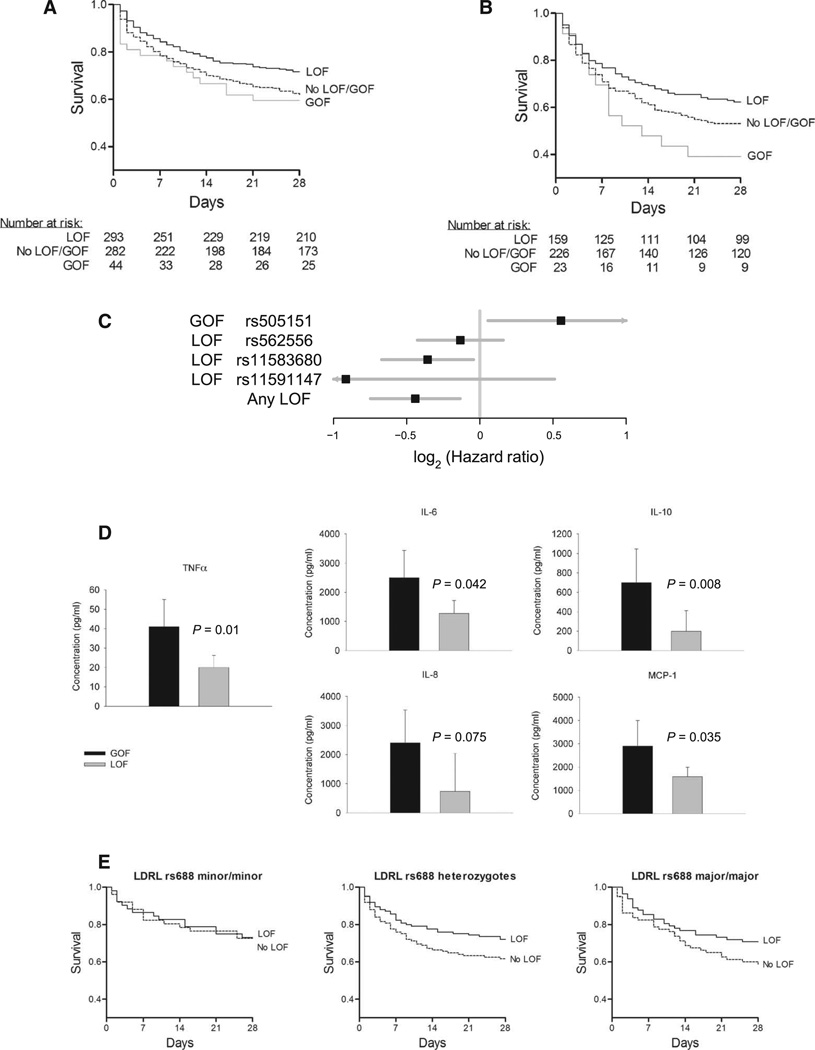
In humans, PCSK9 LOF genetic variants are associated with increased LDL clearance, whereas GOF mutations have the opposite effect (12, 14, 15, 19, 21). We reasoned that, because clearance of pathogen lipids intersects with pathways for clearance of LDL cholesterol, PCSK9 genetic variation could be used to test our PCSK9 hypothesis in humans. Accordingly, we genotyped the most common PCSK9 missense LOF variants (minor allele frequency, ≥0.5%), rs11591147 (R46L), rs11583680 (A53V), and rs562556 (V474I), and the most common missense GOF variant, rs505151 (G670E), in septic shock patients (29) enrolled in the Vasopressin and Septic Shock Trial (VASST) (30). DNA was available from 632 patients, and 13 patients who carried both LOF and GOF variants were excluded from analysis. All single-nucleotide polymorphisms (SNPs) were in Hardy-Weinberg equilibrium (table S2). Baseline characteristics were similar between genotypes when the participants were deemed to have met the study entry criteria (table S3). We found that septic patients in VASST who have at least one PCSK9 LOF allele showed increased survival over a 28-day period [71.7% survival at day 28 (28-day survival indicates 28 days after enrollment into the study)] compared to patients without a LOF or a GOF allele (61.0% survival at day 28); patients with a GOF allele showed the opposite effect (56.8% survival at day 28) (P = 0.0029; PCSK9 LOF, no LOF + no GOF, and GOF were compared by log-rank test stratified by ancestry) (Fig. 4A). Using logistic regression to identify and adjust for the potentially important covariates of age, gender, ancestry, and surgical versus medical diagnosis, we found that the PCSK9 LOF genotype remained significantly associated with increased survival, and GOF had the opposite effect (P = 0.0054) (table S4).
Fig. 4. Effect of PCSK9 genetic variants on human septic shock.
(A) LOF and GOF 28-day survival curves in VASST. Patients in VASST having at least one PCSK9 LOF allele (solid black line) had increased survival over 28 days compared to patients without a LOF or GOF allele (dashed black line) and those patients having a GOF allele (gray line) (P = 0.0029 by log-rank test). (B) Survival curve for SPH cohort by LOF and GOF genotype. Similarly, in the SPH replication cohort, patients carrying at least one LOF allele (solid black line) demonstrated increased survival compared to those who did not (dashed black line), and patients carrying a GOF allele (gray line) had decreased survival (P = 0.022 by log-rank test). (C) Plot of individual and pooled hazard ratios by SNP in a combined VASST + SPH sepsis cohort. (D) Plasma cytokines taken upon enrollment (median, 18 hours after the onset of septic shock) in VASST patients. Patients carrying a PCSK9 LOF allele had significantly (using ANOVA), or a trend toward, lower plasma cytokine concentrations compared to patients carrying a GOF allele (Fig. 3C) for TNFα (P = 0.01), IL-6 (P = 0.042), the anti-inflammatory cytokine IL-10 (P = 0.008), the human CC chemokine MCP-1 (P = 0.035), and the human CXC chemokine IL-8 (P = 0.075). (E) Survival in VASST of LOF and No LOF patients by LDLR rs688 genotype. In patients carrying two copies of the LDLR LOF allele of rs699, survival was not altered by PCSK9 LOF (P = 0.67 by log-rank test). In contrast, for patients carrying at least one wild-type allele of LDLR, PCSK9 LOF was significantly associated with improved survival (P = 0.009 by log-rank test).
These PCSK9 findings were replicated in a second independent cohort of 415 septic shock patients from St. Paul’s Hospital (SPH), Vancouver, Canada (Fig. 4B). Seven patients carried both PCSK9 LOF and GOF alleles and were excluded from analysis. PCSK9 LOF was associated with increased survival, whereas GOF was associated with decreased survival [P = 0.022 by log-rank test stratified by ancestry (Fig. 4B); P = 0.014 using logistic regression with above covariates (table S4)].
Although underpowered for such an experiment, we performed an exploratory analysis to determine which LOF mutation exerted the strongest effect. Figure 4C shows the adjusted hazard ratios for death for each of the LOF and the single GOF allele as well as their cumulative effect. This analysis suggests that rs11591147 may carry the greatest protective effect, and this LOF mutation is known to result in the greatest lowering of blood LDL cholesterol as well (31, 32). We went on to assess whether there was a dose effect of the sum of GOF and LOF alleles. Combining the SPH and VASST cohorts and using a linear model in which an individual could have aGOF (0, 1, or 2)and LOF (0, −1, or −2) variant with a resultant range of + 2 to −6, we observed a dose effect with an overall hazard ratio per “risk” allele (not having LOF or having GOF) of 1.19 (95% confidence interval, 1.05 to 1.35; P = 0.006) (Fig. 4C).
Inflammatory cytokines measured in plasma from a convenient sample of 152 patients carrying a PCSK9 LOF allele and 21 patients carrying a GOF allele from the VASST cohort mirrored the murine results; patients carrying a PCSK9 LOF allele had significantly, or a trend toward, lower plasma cytokine concentrations compared to patients carrying a GOF allele (Fig. 4D) for TNFa (P = 0.01), IL-6 (P = 0.042), the anti-inflammatory cytokine IL-10 (P = 0.008), the human CC chemokine MCP-1 (P = 0.035), and the human CXC chemokine IL-8 (P = 0.075).
To further test our PCSK9 hypothesis in humans while avoiding the heterogeneity of septic shock, we administered a uniform dose of LPS [Escherichia coli–derived LPS (1 ng/kg) delivered intravenously] to healthy human subjects who were genotyped for PCSK9 LOF SNPs, and we measured plasma IL-6 concentrations at baseline (pre-LPS) and at 1, 2, 4, and 12 hours after LPS administration. We found that plasma IL-6 peaked at 2 hours after LPS treatment, and subjects who carried a PCSK9 LOF allele (n = 110) displayed a significantly decreased peak IL-6 concentration [113.5 pg/ml; interquartile range (IQR), 46.5 to 201.5] compared to subjects who did not carry a LOF allele [n = 132; 146.7 pg/ml (IQR, 71.6 to 249.0)] [P = 0.019 by unadjusted rank sum test and P = 0.045 by regression of square root–transformed values with analysis adjusted for age, gender, body mass index (BMI), and the first three components of a principal components analysis]. Similarly, subjects who carried a PCSK9 LOF allele had a significantly decreased IL-6 area under the curve response (24.0 ng/ml; IQR, 11.9 to 36.2)to LPS administration compared to subjects who did not carry a LOF allele (28.1 ng/ml; IQR, 16.1 to 40.1; P = 0.048 adjusted as above). As in the Pcsk9−/− mice, this anti-inflammatory effect was demonstrated only with an inflammatory stimulus (LPS). At baseline, there was no difference in IL-6 concentrations between subjects who carried a PCSK9 LOF allele (2.21 pg/ml; IQR, 1.63 to 3.35) and those who carried a GOF (2.06 pg/ml; IQR, 1.38 to 4.11) (adjusted P value = 0.78 by unadjusted rank sum test).
The primary effect of PCSK9 inhibition on increased hepatic clearance of LDL cholesterol occurs via increased expression of the LDLR (9, 10, 33). To determine whether the LDLR was similarly causal for LPS clearance effects, we used pharmacological inhibition of PCSK9 in Ldlr knockout (Ldlr−/−) mice and compared them with genetic background control mice. Indeed, pretreatment with berberine (34, 35) for 72 hours inhibited hepatic PCSK9 mRNA expression by 65 ± 20% (P < 0.05). In the background control mice, we confirmed that pharmacological inhibition of PCSK9 using berberine blunted the effect of LPS injection on the activity index (P = 0.007) and body temperature (P = 0.046) (table S1). In contrast, in Ldlr−/− mice, we found that the effects of PCSK9 inhibition on activity and body temperature were abolished (table S1), indicating that PCSK9 altered these physiological responses to LPS injection via the LDLR. To validate and translate these results to human septic shock, we took advantage of the knowledge that the minor (less common) allele of an SNP within the LDLR gene, rs688, results in an altered EGF (epidermal growth factor) A domain/β propeller region (binding site of PCSK9) that renders the LDLR insensitive to changes in PCSK9 concentrations (36). Therefore, we further genotyped septic shock patients in VASST for LDLR rs688 and found that patients who were homozygous for the rs688 minor allele were indeed insensitive to changes in PCSK9 that arose from the PCSK9 genotype (P = 0.67; PCSK9 LOF, no LOF + no GOF, and GOF compared by log-rank test) (Fig. 4E). In contrast, patients carrying one or more major alleles of LDLR rs688 demonstrated clear differences in survival according to PCSK9 genotype (P = 0.009; PCSK9 LOF, no LOF + no GOF, and GOF compared by log-rank test).
DISCUSSION
Our results support the hypothesis that reduced PCSK9 function increases pathogen lipid clearance via the LDLR and thereby decreases the inflammatory response and improves outcomes in sepsis in both mice and humans. Binding, transport, and clearance of pathogen lipids are strikingly similar to analogous processes for cholesterol lipid clearance, suggesting a shared evolutionary origin (1, 3–8). Pathogen lipids play a particularly important role in triggering an innate immune inflammatory response via TLRs. Transfer proteins that bind pathogen lipids, including LBP and BPI, are similar in structure to endogenous lipid transfer proteins such as PLTP and CETP, and all of these proteins share the ability to bind pathogen lipids and facilitate incorporation into, and transfer between, lipoprotein particles (including HDL, LDL, VLDL, and chylomicrons) (1, 3–8). Our results demonstrate that pathogen lipid clearance and the downstream inflammatory cytokine and physiological consequences are affected by PCSK9, which binds and promotes lysosomal degradation of the LDLR. Genetic LOF of the LDLR in mice and a PCSK9-resistant variant of LDLR in humans reduced the effect of PCSK9, implicating the LDLR in this pathogen lipid clearance pathway. In the setting of intact LDLR function, PCSK9 LOF and GOF genetic variants had a statistically significant effect on patient survival from septic shock.
Three missense PCSK9 LOF variants are relatively common (minor allele frequency >0.5%). The missense variant rs11591147 results in an R46L substitution, which is consistently associated with low LDL cholesterol levels and, hence, is a LOF variant (12, 15, 16). More modest decreases in LDL cholesterol levels are reported for the LOF variants rs11583680 A53V (15) and rs562556, which result in decreased total cholesterol and LDL cholesterol (37). The GOF variant rs505151 G670E has a somewhat greater effect on increasing LDL cholesterol (38). The overall modest changes in LDL cholesterol observed in humans who carry these variations translate into much larger differences in clinical outcomes (20) associated with coronary artery disease (12) and myocardial infarction (14, 20). We similarly found substantial replicated differences in clinical outcomes in septic shock patients (Fig. 3, A and B). One possible explanation for the clinical impact we observed is that the effect of these PSCK9 genetic variants on pathogen lipid clearance was not fully reflected by their effects on static LDL cholesterol levels during health. Alternatively, a relatively modest increase in pathogen lipid clearance may have substantial clinical benefit, just as initiation of antibiotics only 1 hour earlier in septic shock patients results in a 7% decrease in mortality, which is considered to be a large clinical effect size (39). Similarly, the rs688 variant of LDLR has minimal impact on LDLR function but reduces susceptibility of LDLR to PCSK9 by ~25% (36). Taken alone, the LDLR rs688 results are not conclusive, but together with murine Ldlr−/− observations and with the knowledge that the primary effect of PCSK9 is on the LDLR with respect to cholesterol clearance (20), our observations suggest that the effect of PCSK9 in sepsis is mediated via the LDLR.
In humans, long-term statin therapy increases LDLR expression (40, 41) (which should therefore increase pathogen lipid clearance) and thus appears to be an alternative test of our hypothesis. However, statins have competing detrimental effects with respect to sepsis, including an increase in PCSK9 expression and a decrease in LDL cholesterol levels— LDL cholesterol may beneficially sequester pathogen lipids (42). Indeed, the effects of statins on human sepsis outcomes are mixed and inconclusive. Statin treatment modestly reduces the incidence of pneumonia (43) and reduces mortality of patients admitted to the hospital with pneumonia (44). However, continuation of statin therapy had no further effect on patients who were hospitalized with sepsis (45).
The strategy of modulation of the host response to sepsis has not been successful in altering mortality in severe sepsis and septic shock despite more than 50 clinical trials. In contrast, a strategy directed at reducing the inciting stimulus by early suppression of bacterial pathogens with antibiotics has been strikingly effective, reducing septic shock mortality by ~7% per hour (39). PCSK9 inhibition potentially supports this second strategy by enhancing pathogen lipid clearance. Thus, the current results suggest that mechanisms of clearance for pathogen lipids might offer a family of therapeutics targets for sepsis.
MATERIALS AND METHODS
Animal models
All animal studies were approved by the University of British Columbia animal ethics committee and conformed to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Animals
Male Pcsk9 knockout (Pcsk9−/−; B6;129S6-Pcsk9tm1Jdh/J) mice and genetic background control mice (body weight, 25 to 30 g; 10 to 14 weeks old) were obtained from Jackson Laboratories. Compared to wild-type control mice, Pcsk9−/− mice have reduced plasma cholesterol concentrations with nearly undetectable LDL cholesterol concentrations (33). In addition, PCSK9 protein is also undetectable in plasma (33). There are no other reported obvious phenotypic alterations.
LPS-induced systemic inflammation
We used LPS injection in mice to induce a well-characterized stereotypic systemic inflammatory response to maximize statistical power in detecting differences in the inflammatory response rather than as a model of highly variable human sepsis. We chose 6 hours after LPS treatment as the time point for blood collection because this time point was shown previously to be when cytokine levels peak after LPS infusion in C57BL/6 mice (24).
Healthy mice had activity level, body temperature, blood pressure, and left ventricular ejection fraction (echocardiography) assessed at baseline (time 0) (Supplementary Methods). Mice were then injected intraperitoneally with LPS (20 mg/kg, from E. coli strain 01 II: B4, Sigma). This dose was determined from previous experiments where, in the background strain of C57BL/6 mice, LPS (20 mg/kg) delivered intraperitoneally was the lowest dose that was lethal in all cases within 12 hours (24). Activity levels and body temperatures were then measured every hour for 6 hours. At 6 hours, left ventricular ejection fractions were assessed again, and blood pressure was measured by invasive arterial cannulation (Supplementary Methods). Inflammatory cytokine concentrations were measured in plasma, taken at this 6-hour time point, using a multiplex enzyme-linked immunosorbent assay–based assay, and plasma endotoxin concentrations were measured using a Limulus Amebocyte Lysate assay (Supplementary Methods).
Cecal ligation and puncture
CLP was performed as described in(46). Mice were anesthetized with 1 to 3% isoflurane and kept warm (>35°C) using a heating pad. A midline laparotomy (2 cm) was performed, and the cecum was exposed and ligated using a 6-0 suture anterior to the iliocecal valve, without causing intestinal obstruction. The cecum was perforated once through and through in the midsection using a 19-gauge needle. The incision was closed, and postoperatively, mice received 1 ml of normal saline subcutaneously. Beginning 6 hours postoperatively, mice received imipenem (25 mg/kg) subcutaneously twice daily. Sham operation included all of the above but did not puncture the cecum.
Bacterial copy number in lung tissue assessed with real-time qPCR
Lung tissue was rinsed in ice-cold phosphate-buffered saline (PBS) and snap-frozen in liquid nitrogen, followed by DNA extraction. Standard curves for reverse transcription qPCR (RT-qPCR) were generated as follows. Genomic DNA was extracted from E. coli DH5a bacteria grown in LB broth, using DNeasy kit (Qiagen, 69506). To construct a standard curve by qPCR, we compared cycle threshold versus copy number in which DNA is diluted 10-fold from 9.92 × 10−7 to 9.92 × 10−1 ng/µl (corresponding to 1,000,000 to 1 bacterial genomes).
Real-time qPCR was performed on the ViiA 7 system (Applied Biosystems) for a 466–base pair (bp) fragment of the bacterial 16S ribosomal DNA, which was amplified using the forward primer 5′-TCCTACGGGAGGCAGCAGT-3′ and the reverse primer 5′-GGAC-TACCAGGGTATCTAATCCTGTT-3′, as described in (47). Our published modifications to the published protocol included using a DNA-binding dye (SYBR Green) rather than the original TaqMan probe (28). The 466-bp product was amplified under the following conditions: 95°C for 5 min; 40 cycles of 95°C for 30 s, 60.5°C for 30 s, and 72°C for 60 s; and then a 10-min extension at 72°C. Bacterial copy number per milliliter was derived from the E. coli DH5α–derived standard curve.
Double-blind, randomized controlled trial design for CLP
A laboratory technician (M.S.C.) labeled packages of sterile 1-ml syringes (one package per mouse) with randomly generated numbers and filled them with 100 mg of PCSK9-blocking antibody (BPS Bioscience, 71207) or an IgG1 isotype control (R&D Systems, MAB002). An animal technician (Y. Wang) was blinded as to the identity of the syringes. Mice were injected once daily subcutaneously with the blinded solution beginning 6 hours after CLP. The team that analyzed the survival data was blinded to the identity of the syringes until assessment of the final mouse was completed.
Berberine pretreatment to inhibit hepatic PCSK9 mRNA expression
At concentrations of 1 to 10 µM, berberine inhibits transcription of PCSK9 within 24 hours (34, 48). With a volume of distribution of 1 ml and a molecular weight of 371, we targeted plasma levels of 10 µM in mice by administering a bolus intraperitoneal injection of berberine, followed by maintenance infusion. Specifically, mice (body weight, 25 g) were injected intraperitoneally 60 hours before exposure to LPS, with 100 µg of berberine (Sigma-Aldrich) in 100 µl of sterile saline or saline vehicle. The next morning, a 72-hour osmotic pump (ALZET) with an infusion rate of 1 µl/hour was implanted intraperitoneally. Pumps contained 100 mg of berberine or vehicle saline. We confirmed that berberine pretreatment decreased hepatic expression of Pcsk9 using RT-PCR (Supplementary Methods).
Ldlr knockout (Ldlr−/−) mice with and without inhibition of Pcsk9 expression
Twelve male Ldlr−/− mice (B6.129S7- Ldlrtm1Her/ J, Jackson Laboratories) 10 to 12 weeks of age and 25 g in weight were pretreated with berberine or sterile saline via a 72-hour infusion pump. Mice were injected intraperitoneally with LPS (100 mg/kg), and activity and body temperature were scored over the next 6 hours.
In vitro assay of hepatocyte LPS clearance
Immortalized human hepatocytes (HepG2 cell line, American Type Culture Collection) were seeded into a 24-well plate and grown to 80% confluence. The culture medium for the duration of the experiment was 80% DMEM (Invitrogen, 11965-065) and 20% human plasma from pooled healthy donors.
Addition of PCSK9
Three hours before LPS treatment, cells were treated with purified recombinant human PCSK9 protein (3 µg/ml) (ACROBiosystems, PC9-H5223) or a vehicle control. Four and 19 hours after, LPS cells were again treated with recombinant human PCSK9 (3 mg/ml) or a vehicle control. Cells were treated with Alexa Fluor 488– conjugated LPS (Invitrogen, L-23351) or with standard nonfluorescent LPS (Sigma, L2880) as a control. After 24 hours of LPS treatment, each well was rinsed two times with PBS) and detached from the wells with Accutase (BD 561527). Cells were collected, washed with PBS, and resuspended in 500 µl of PBS, then analyzed by flow cytometry (Beckman Coulter Gallios Flow Cytometer). Cells were gated by forward and side scatter for viability using previously determined parameters; 20,000 gated cells were counted per sample. The output of interest was MFI from the instrument’s FL1 laser (excitation wavelength, 488 nm, emission wavelength, 525/20 nm). Background autofluorescence of cells treated with nonfluorescent LPS was subtracted to determine the fluorescence level resulting from uptake of the LPS conjugate. Data analysis was performed using Kaluza Analysis 1.3 software (Beckman Coulter).
Human genetic association studies
VASST derivation cohort
VASST was a multicenter, randomized, double-blind, controlled trial evaluating the efficacy of vasopressin versus norepinephrine in 778 patients who had septic shock (29) and vasopressor infusion of at least 5 µg/min of norepinephrine or equivalent (30). Inclusion criteria and clinical phenotyping are described elsewhere (30). DNA was available from 632 patients. The research ethics boards of all participating institutions approved this trial, and written informed consent was obtained from all patients or their authorized representatives. The research ethics board at the coordinating center (University of British Columbia) approved the genetic analysis.
SPH validation cohort
All patients admitted to the intensive care unit (ICU) at SPH in Vancouver, Canada between July 2000 and January 2004 were screened, and of these, 415 patients were classified as having septic shock, had DNA available, and were successfully genotyped. Septic shock was defined by the presence of two or more diagnostic criteria for the systemic inflammatory response syndrome (29), proven or suspected infection, new dysfunction of at least one organ, and hypotension despite adequate fluid resuscitation (29). Inclusion criteria and clinical phenotyping are described elsewhere (8). The Institutional Review Board at SPH and the University of British Columbia approved the study.
GENE population
Healthy, nonobese subjects (n = 294) with self-reported European or African American racial background were recruited for participation in the GENE study (49). Major exclusion criteria included medication or supplement use or evidence of any disease. After overnight acclimatization, LPS [E. coli–derived LPS (1 ng/kg); U.S. standard reference, lot no. CCRE-LOT-1+2, Clinical Center, Pharmacy Department at NIH, Bethesda, MD] was administered as an intravenous bolus, and subjects were monitored for 24 hours (49). Serial blood draws were taken (5 min before and 1, 2, 4, 6, 12, and 24 hours after LPS injection). Serum and plasma were obtained for measurement of cytokines, proteins, and lipids, and buffy coat was stored for genetic analyses. Samples were stored at –80°C before analysis. The protocol was approved by the University of Pennsylvania Institutional Review Board, had regulatory oversight by the U.S. Food and Drug Administration (LPS: IND 5984), and was monitored by an NIH-appointed data safety and monitoring board. Genotyping and cytokine measurements from these human cohorts are described in Supplementary Methods.
Statistical analysis
We used repeated-measures ANOVA to test for differences in activity level, temperature, and ejection fraction between Pcsk9−/− and wild-type control mice over time. We used unpaired t tests to test for differences in mean arterial pressure because this variable was measured using two different instruments at baseline and at 6 hours after LPS administration. Cytokine concentrations were log-normally distributed, so we tested for differences in logarithms of concentrations for murine TNFa, IL-6, IL-10, JE, and MIP-2 and for replication of directionally similar changes in human homologs of TNFα, IL-6, IL-10, MCP-1, and IL-8 using a one-way ANOVA. To assess changes in cytokine concentrations for the murine CLP model, we tested for differences in change from day 1 in log-transformed and normalized values using the slope coefficient estimates and SEs for the two treatment groups [analysis of covariance (ANCOVA)].
We tested SNPs for Hardy-Weinberg equilibrium using a χ2 test. For human septic shock, our primary analysis of 28-day survival curves used a log-rank test stratified by Caucasian or non-Caucasian ancestry, and a secondary analysis used logistic regression to examine 28-day survival by PCSK9 genotype, including the covariates of age, gender, Caucasian ancestry, and a surgical versus medical primary diagnosis in the statistical model.
Subgroup SNP analysis
Survival analysis up to 28 days was performed used Cox proportional hazards models for SNPs using the covariates age, gender, ancestry, and surgical diagnosis. We required a minimum of three patients for each ethnicity in each cohort; therefore, we included only Caucasians, Asians, North American aboriginals, and Indians (patients of Latino or Middle Eastern ancestry were excluded because of low numbers). We performed the analysis for the VASST and SPHICU1 cohorts independently as well as merged (for the merged data set, a cohort factor was included as a covariate). All SNP alleles were present in more than 1% of the patients except for the homozygous 2 (GG) for rs505151 in only two patients in the ICU1 cohort; to improve robustness of modeling, homozygous 2 for rs505151 was replaced with heterozygous 1 and interpreted as one or more variant alleles. Each SNP was evaluated independently using the numeric values 0, 1, and 2 for wild-type and 1 or 2 SNP variant bases present. An additional LOF variable representing any LOF SNP present with values 0 or 1 was also evaluated. The method coxph (http://www.R-project.org) from the base stats package in the R statistical language was used.
To test LPS-evoked differences in plasma analytes, subjects were categorized as LOF carriers if they carried one or more minor alleles of the three LOF SNPs (rs562556, rs11583680, and rs11591147) and no copies of the minor allele for the GOF SNP rs505151. Peak IL-6 levels and area under the IL-6 curve, calculated using the trapezoidal rule, were compared between LOF and non-LOF by rank sum test. To allow adjustment for genetic ancestry and covariates shown to influence IL-6 measurements [gender, BMI, and age (49)], values were normalized, and linear regression including the first three components of the principal components analysis for genetic ancestry, gender, age, and BMI was performed. Differences were considered significant if P < 0.05. All analyses were performed using R (version 2.8.1, http://www.R-project.org), SPSS version 22.0 (SPSS Inc.), or Stata 12.0 statistical software packages.
Supplementary Material
Acknowledgments
Funding: This work was supported by a Canadian Institutes of Health Research grant to K.R.W. and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through grant UL1TR000003 as well as an NIH–National Heart, Lung, and Blood Institute Specialized Centers of Clinically Oriented Research Project grant (P50-HL-083799) to M.P.R.
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/6/258/258ra143/DC1
Methods
Table S1. Physiological response of mice to LPS.
Table S2. Allele frequency and Hardy-Weinberg equilibrium in VASST cohort.
Table S3. Baseline characteristics of patients by PCSK9 LOF and GOF genotype.
Table S4. Logistic regression. LOF and GOF effects on 28-day survival in septic shock cohorts.
Author contributions: K.R.W. contributed to study conception, experimental design, and analysis and wrote the manuscript. K.R.T. assisted with experiment design, data collection, and analysis and contributed to manuscript writing. J.A.R. contributed to project development and offered critical insight to manuscript content. M.P.R. conceived of and conducted all LPS administration experiments and contributed to manuscript writing. N.J.M. conducted the LPS administration analysis and contributed to manuscript writing. J.F.F. conducted the LPS administration experiments and reviewed the manuscript. J.D.C. contributed to LPS administration experiments and contributed to manuscript writing. T.N. contributed to human data analysis and manuscript critique. C.D.F. contributed to the statistical analysis. S.A.T. prepared the human samples for cytokine analysis and critiqued the manuscript. M.S.C. conducted cytokine and endotoxin assays and manuscript writing and editing. J.H.B. contributed to study conception, experimental design, analysis, and manuscript writing.
Competing interests: The University of British Columbia has filed a provisional patent application covering aspects of this manuscript. K.R.W., J.H.B., and J.A.R. are listed as inventors. K.R.W., J.H.B., and J.A.R. have founded Cyon Therapeutics, which has licensed this intellectual property. The other co-authors declare that they have no competing interests. K.R.W. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES AND NOTES
- 1.Levels JH, Abraham PR, van Barreveld EP, Meijers JC, van Deventer SJ. Distribution and kinetics of lipoprotein-bound lipoteichoic acid. Infect. Immun. 2003;71:3280–3284. doi: 10.1128/IAI.71.6.3280-3284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trinel PA, Plancke Y, Gerold P, Jouault T, Delplace F, Schwarz RT, Strecker G, Poulain D. The Candida albicans phospholipomannan is a family of glycolipids present in phosphoinositolmannosides with long linear chains of b-1,2-linked mannose residues. J. Biol. Chem. 1999;274:30520–30526. doi: 10.1074/jbc.274.43.30520. [DOI] [PubMed] [Google Scholar]
- 3.Azzam KM, Fessler MB. Crosstalk between reverse cholesterol transport and innate immunity. Trends Endocrinol. Metab. 2012;23:169–178. doi: 10.1016/j.tem.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallay P, Heumann D, Le Roy D, Barras C, Glauser MP. Mode of action of anti-lipopolysaccharide-binding protein antibodies for prevention of endotoxemic shock in mice. Proc. Natl. Acad. Sci. USA. 1994;91:7922–7926. doi: 10.1073/pnas.91.17.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gautier T, Lagrost L. Plasma PLTP (phospholipid-transfer protein): An emerging role in ‘reverse lipopolysaccharide transport’ and innate immunity. Biochem. Soc. Trans. 2011;39:984–988. doi: 10.1042/BST0390984. [DOI] [PubMed] [Google Scholar]
- 6.Levels JH, Marquart JA, Abraham PR, van den Ende AE, Molhuizen HO, van Deventer SJ, Meijers JC. Lipopolysaccharide is transferred from high-density to low- density lipoproteins by lipopolysaccharide-binding protein and phospholipid transfer protein. Infect. Immun. 2005;73:2321–2326. doi: 10.1128/IAI.73.4.2321-2326.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hailman E, Albers JJ, Wolfbauer G, Tu AY, Wright SD. Neutralization and transfer of lipopolysaccharide by phospholipid transfer protein. J. Biol. Chem. 1996;271:12172–12178. doi: 10.1074/jbc.271.21.12172. [DOI] [PubMed] [Google Scholar]
- 8.Gautier T, Klein A, Deckert V, Desrumaux C, Ogier N, Sberna AL, Paul C, Le Guern N, Athias A, Montange T, Monier S, Piard F, Jiang XC, Masson D, Lagrost L. Effect of plasma phospholipid transfer protein deficiency on lethal endotoxemia in mice. J. Biol. Chem. 2008;283:18702–18710. doi: 10.1074/jbc.M802802200. [DOI] [PubMed] [Google Scholar]
- 9.McNutt MC, Kwon HJ, Chen C, Chen JR, Horton JD, Lagace TA. Antagonism of secreted PCSK9 increases low density lipoprotein receptor expression in HepG2 cells. J. Biol. Chem. 2009;284:10561–10570. doi: 10.1074/jbc.M808802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tveten K, Strøm TB, Berge KE, Leren TP. PCSK9-mediated degradation of the LDL receptor generates a 17 kDa C-terminal LDL receptor fragment. J. Lipid Res. 2013;54:1560–1566. doi: 10.1194/jlr.M034371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, McCabe T, Condra JH, Ni YG, Peterson LB, Wang W, Strack AM, Wang F, Pandit S, Hammond H, Wood D, Lewis D, Rosa R, Mendoza V, Cumiskey AM, Johns DG, Hansen BC, Shen X, Geoghagen N, Jensen K, Zhu L, Wietecha K, Wisniewski D, Huang L, Zhao JZ, Ernst R, Hampton R, Haytko P, Ansbro F, Chilewski S, Chin J, Mitnaul LJ, Pellacani A, Sparrow CP, An Z, Strohl W, Hubbard B, Plump AS, Blom D, Sitlani A. An anti-PCSK9 antibody reduces LDL- cholesterol on top of a statin and suppresses hepatocyte SREBP-regulated genes. Int. J. Biol. Sci. 2012;8:310–327. doi: 10.7150/ijbs.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen JC, Boerwinkle E, Mosley HT, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 13.Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: Its role in LDL metabolism. Trends Biochem. Sci. 2007;32:71–77. doi: 10.1016/j.tibs.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kathiresan S. Myocardial Infarction Genetics Consortium, A PCSK9 missense variant associated with a reduced risk of early-onset myocardial infarction. N. Engl. J. Med. 2008;358:2299–2300. doi: 10.1056/NEJMc0707445. [DOI] [PubMed] [Google Scholar]
- 15.Kotowski IK, Pertsemlidis A, Luke A, Cooper RS, Vega GL, Cohen JC, Hobbs HH. A spectrum of PCSK9 alleles contributes to plasma levels of low-density lipoprotein cholesterol. Am. J. Hum. Genet. 2006;78:410–422. doi: 10.1086/500615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musunuru K, Lettre G, Young T, Farlow DN, Pirruccello JP, Ejebe KG, Keating BJ, Yang Q, Chen MH, Lapchyk N, Crenshaw A, Ziaugra L, Rachupka A, Benjamin EJ, Cupples LA, Fornage M, Fox ER, Heckbert SR, Hirschhorn JN, Newton-Cheh C, Nizzari MM, Paltoo DN, Papanicolaou GJ, Patel SR, Psaty BM, Rader DJ, Redline S, Rich SS, Rotter JI, Taylor HA, Jr, Tracy RP, Vasan RS, Wilson JG, Kathiresan S, Fabsitz RR, Boerwinkle E, Gabriel SB. NHLBI Candidate Gene Association Resource, Candidate gene association resource (CARe): Design, methods, and proof of concept. Circ. Cardiovasc. Genet. 2010;3:267–275. doi: 10.1161/CIRCGENETICS.109.882696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scartezini M, Hubbart C, Whittall RA, Cooper JA, Neil AH, Humphries SE. The PCSK9 gene R46L variant is associated with lower plasma lipid levels and cardiovascular risk in healthy U.K. men. Clin. Sci. 2007;113:435–441. doi: 10.1042/CS20070150. [DOI] [PubMed] [Google Scholar]
- 18.Talmud PJ, Drenos F, Shah S, Shah T, Palmen J, Verzilli C, Gaunt TR, Pallas J, Lovering R, Li K, Casas JP, Sofat R, Kumari M, Rodriguez S, Johnson T, Newhouse SJ, Dominiczak A, Samani NJ, Caulfield M, Sever P, Stanton A, Shields DC, Padmanabhan S, Melander O, Hastie C, Delles C, Ebrahim S, Marmot MG, Smith GD, Lawlor DA, Munroe PB, Day IN, Kivimaki M, Whittaker J, Humphries SE, Hingorani AD. ASCOT investigators, NORDIL investigators, BRIGHT Consortium, Gene-centric association signals for lipids and apolipoproteins identified via the HumanCVD BeadChip. Am. J. Hum. Genet. 2009;85:628–642. doi: 10.1016/j.ajhg.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham D, Danley DE, Geoghegan KF, Griffor MC, Hawkins JL, Subashi TA, Varghese AH, Ammirati MJ, Culp JS, Hoth LR, Mansour MN, McGrath KM, Seddon AP, Shenolikar S, Stutzman-Engwall KJ, Warren LC, Xia D, Qiu X. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat. Struct. Mol. Biol. 2007;14:413–419. doi: 10.1038/nsmb1235. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg D, Witztum JL. Inhibition of PCSK9: A powerful weapon for achieving ideal LDL cholesterol levels. Proc. Natl. Acad. Sci. U.S.A. 2009;106:9546–9547. doi: 10.1073/pnas.0904560106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myocardial Infarction Genetics Consortium. Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, Anand S, Engert JC, Samani NJ, Schunkert H, Erdmann J, Reilly MP, Rader DJ, Morgan T, Spertus JA, Stoll M, Girelli D, McKeown PP, Patterson CC, Siscovick DS, O’Donnell CJ, Elosua R, Peltonen L, Salomaa V, Schwartz SM, Melander O, Altshuler D, Ardissino D, Merlini PA, Berzuini C, Bernardinelli L, Peyvandi F, Tubaro M, Celli P, Ferrario M, Fetiveau R, Marziliano N, Casari G, Galli M, Ribichini F, Rossi M, Bernardi F, Zonzin P, Piazza A, Mannucci PM, Schwartz SM, Siscovick DS, Yee J, Friedlander Y, Elosua R, Marrugat J, Lucas G, Subirana I, Sala J, Ramos R, Kathiresan S, Meigs JB, Williams G, Nathan DM, MacRae CA, O’Donnell CJ, Salomaa V, Havulinna AS, Peltonen L, Melander O, Berglund G, Voight BF, Kathiresan S, Hirschhorn JN, Asselta R, Duga S, Spreafico M, Musunuru K, Daly MJ, Purcell S, Voight BF, Purcell S, Nemesh J, Korn JM, McCarroll SA, Schwartz SM, Yee J, Kathiresan S, Lucas G, Subirana I, Elosua R, Surti A, Guiducci C, Gianniny L, Mirel D, Parkin M, Burtt N, Gabriel SB, Samani NJ, Thompson JR, Braund PS, Wright BJ, Balmforth AJ, Ball SG, Hall AS, Wellcome Trust Case Control Consortium. Schunkert H, Erdmann J, Linsel-Nitschke P, Lieb W, Ziegler A, König I, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Schunkert H, Samani NJ, Erdmann J, Ouwehand W, Hengstenberg C, Deloukas P, Scholz M, Cambien F, Reilly MP, Li M, Chen Z, Wilensky R, Matthai W, Qasim A, Hakonarson HH, Devaney J, Burnett MS, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Knouff CW, Waterworth DM, Walker MC, Mooser V, Epstein SE, Rader DJ, Scheffold T, Berger K, Stoll M, Huge A, Girelli D, Martinelli N, Olivieri O, Corrocher R, Morgan T, Spertus JA, McKeown P, Patterson CC, Schunkert H, Erdmann E, Linsel-Nitschke P, Lieb W, Ziegler A, König IR, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Hólm H, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Engert JC, Do R, Xie C, Anand S, Kathiresan S, Ardissino D, Mannucci PM, Siscovick D, O’Donnell CJ, Samani NJ, Melander O, Elosua R, Peltonen L, Salomaa V, Schwartz SM, Altshuler D. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat. Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King A. Lipids. Antibodies against PCSK9—A new era of therapy. Nat. Rev. Cardiol. 2013;10:1. doi: 10.1038/nrcardio.2012.166. [DOI] [PubMed] [Google Scholar]
- 23.Boyd JH, Kan B, Roberts H, Wang Y, Walley KR. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ. Res. 2008;102:1239–1246. doi: 10.1161/CIRCRESAHA.107.167544. [DOI] [PubMed] [Google Scholar]
- 24.Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-κB dependent inflammatory response. Cardiovasc. Res. 2006;72:384–393. doi: 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Scott MJ, Liu S, Shapiro RA, Vodovotz Y, Billiar TR. Endotoxin uptake in mouse liver is blocked by endotoxin pretreatment through a suppressor of cytokine signaling-1-dependent mechanism. Hepatology. 2009;49:1695–1708. doi: 10.1002/hep.22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagace TA, Curtis DE, Garuti R, McNutt MC, Park SW, Prather HB, Anderson NN, Ho YK, Hammer RE, Horton JD. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J. Clin. Invest. 2006;116:2995–3005. doi: 10.1172/JCI29383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyle N, Boyd J. The potential for PCR based testing to improve diagnosis and treatment of sepsis. Curr. Infect. Dis. Rep. 2013;15:372–379. doi: 10.1007/s11908-013-0350-4. [DOI] [PubMed] [Google Scholar]
- 28.Boyd JH, Russell JA, Fjell CD. The meta-genome of sepsis: Host genetics, pathogens and the acute immune response. J. Innate Immun. 2014;6:272–283. doi: 10.1159/000358835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 30.Russell JA, Walley KR, Singer J, Gordon AC, Hébert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM, Cook DJ, Presneill JJ, Ayers D. VASST Investigators, Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl. J. Med. 2008;358:877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 31.Mayne J, Ooi TC, Raymond A, Cousins M, Bernier L, Dewpura T, Sirois F, Mbikay M, Davignon J, Chrétien M. Differential effects of PCSK9 loss of function variants on serum lipid and PCSK9 levels in Caucasian and African Canadian populations. Lipids Health Dis. 2013;12:70. doi: 10.1186/1476-511X-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soutar AK, Naoumova RP. Mechanisms of disease: Genetic causes of familial hypercholesterolemia. Nat. Clin. Pract. Cardiovasc. Med. 2007;4:214–225. doi: 10.1038/ncpcardio0836. [DOI] [PubMed] [Google Scholar]
- 33.Rashid S, Curtis DE, Garuti R, Anderson NN, Bashmakov Y, Ho YK, Hammer RE, Moon YA, Horton JD. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9 . Proc. Nat. Acad. Sci. U.S.A. 2005;102:5374–5379. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cameron J, Ranheim T, Kulseth MA, Leren TP, Berge KE. Berberine decreases PCSK9 expression in HepG2 cells. Atherosclerosis. 2008;201:266–273. doi: 10.1016/j.atherosclerosis.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Xiao HB, Sun ZL, Zhang HB, Zhang DS. Berberine inhibits dyslipidemia in C57BL/6 mice with lipopolysaccharide induced inflammation. Pharmacol. Rep. 2012;64:889–895. doi: 10.1016/s1734-1140(12)70883-6. [DOI] [PubMed] [Google Scholar]
- 36.Gao F, Ihn HE, Medina MW, Krauss RM. A common polymorphism in the LDL receptor gene has multiple effects on LDL receptor function. Hum. Mol. Genetics. 2013;22:1424–1431. doi: 10.1093/hmg/dds559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shioji K, Mannami T, Kokubo Y, Inamoto N, Takagi S, Goto Y, Nonogi H, Iwai N. Genetic variants in PCSK9 affect the cholesterol level in Japanese. J. Hum. Genet. 2004;49:109–114. doi: 10.1007/s10038-003-0114-3. [DOI] [PubMed] [Google Scholar]
- 38.Chen SN, Ballantyne CM, Gotto AM, Jr, Tan Y, Willerson JT, Marian AJ. A common PCSK9 haplotype, encompassing the E670G coding single nucleotide polymorphism, is a novel genetic marker for plasma low-density lipoprotein cholesterol levels and severity of coronary atherosclerosis. J. Am. Coll. Cardiol. 2005;45:1611–1619. doi: 10.1016/j.jacc.2005.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar A, Roberts D, E.Wood K, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 40.Kovanen PT, Bilheimer DW, Goldstein JL, Jaramillo JJ, Brown MS. Regulatory role for hepatic low density lipoprotein receptors in vivo in the dog. Proc. Nat. Acad. Sci. U.S.A. 1981;78:1194–1198. doi: 10.1073/pnas.78.2.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pocathikorn A, Taylor RR, Mamotte CD. Atorvastatin increases expression of low-density lipoprotein receptor mRNA in human circulating mononuclear cells. Clin. Exp. Pharmacol. Physiol. 2010;37:471–476. doi: 10.1111/j.1440-1681.2009.05337.x. [DOI] [PubMed] [Google Scholar]
- 42.Flegel WA, Baumstark MW, Weinstock C, Berg A, Northoff H. Prevention of endotoxin-induced monokine release by human low- and high-density lipoproteins and by apolipoprotein A-I. Infect. Immun. 1993;61:5140–5146. doi: 10.1128/iai.61.12.5140-5146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novack V, MacFadyen J, Malhotra A, Almog Y, Glynn RJ, Ridker PM. The effect of rosuvastatin on incident pneumonia: Results from the JUPITER trial. CMAJ. 2012;184:E367–E372. doi: 10.1503/cmaj.111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothberg MB, Bigelow C, Pekow PS, Lindenauer PK. Association between statins given in hospital and mortality in pneumonia patients. J. Gen. Intern. Med. 2012;27:280–286. doi: 10.1007/s11606-011-1826-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kruger PS, Harward ML, Jones MA, Joyce CJ, Kostner KM, Roberts MS, Venkatesh B. Continuation of statin therapy in patients with presumed infection: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2011;183:774–781. doi: 10.1164/rccm.201006-0955OC. [DOI] [PubMed] [Google Scholar]
- 46.Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect. Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148:257–266. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- 48.Li H, Dong B, Park SW, Lee HS, Chen W, Liu J. Hepatocyte nuclear factor 1α plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J. Biol. Chem. 2009;284:28885–28895. doi: 10.1074/jbc.M109.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferguson JF, Patel PN, Shah RY, Mulvey CK, Gadi R, Nijjar PS, Usman HM, Mehta NN, Shah R, Master SR, Propert KJ, Reilly MP. Race and gender variation in response to evoked inflammation. J. Transl. Med. 2013;11:63. doi: 10.1186/1479-5876-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.