Abstract
Chagas' disease is a serious public health problem in Latin America, and no treatment is available for the prevalent chronic stage. Its causative agent, Trypanosoma cruzi, requires specific endogenous sterols for survival, and we have recently demonstrated that squalene synthase (SQS) is a promising target for antiparasitic chemotherapy. E5700 and ER-119884 are quinuclidine-based inhibitors of mammalian SQS that are currently in development as cholesterol- and triglyceride-lowering agents in humans. These compounds were found to be potent noncompetitive or mixed-type inhibitors of T. cruzi SQS with Ki values in the low nanomolar to subnanomolar range in the absence or presence of 20 μM inorganic pyrophosphate. The antiproliferative 50% inhibitory concentrations of the compounds against extracellular epimastigotes and intracellular amastigotes were ca. 10 nM and 0.4 to 1.6 nM, respectively, with no effects on host cells. When treated with these compounds at the MIC, all of the parasite's sterols disappeared from the parasite cells. In vivo studies indicated that E5700 was able to provide full protection against death and completely arrested the development of parasitemia when given at a concentration of 50 mg/kg of body weight/day for 30 days, while ER-119884 provided only partial protection. This is the first report of an orally active SQS inhibitor that is capable of providing complete protection against fulminant, acute Chagas' disease.
Chagas' disease, a parasitic disease caused by the kinetoplastid protozoan Trypanosoma (Schizotrypanum) cruzi, is still one of the most serious public health problems in Latin America, despite recent advances in the control of the vectorial and transfusional transmission of the parasite (36). There are ca. 18 million people currently infected with T. cruzi, most of them in the chronic phase, which can lead to severe heart and gastrointestinal lesions (36). Current chemotherapy, based on the nitrofuran nifurtimox (Bayer) and the nitroimidazole benznidazole (Roche), is unsatisfactory as these compounds are effective only for recent infections and have frequent toxic side effects (21, 24, 28). Studies have shown that T. cruzi has a strict requirement for specific endogenous sterols (ergosterol and analogs) for survival and growth and cannot use the abundant supply of cholesterol present in its mammalian host (21-23, 25). Ergosterol biosynthesis inhibitors with potent in vitro activity and special pharmacokinetic properties in mammals (large volumes of distribution and long half-lives) can induce the radical cure of parasitic infection in experimental animal models of both acute and chronic Chagas' disease (21-23, 25). Squalene synthase (SQS; EC 2.5.1.21) catalyzes a head-to-head reductive dimerization of two molecules of farnesyl pyrophosphate (FPP) in a two-step reaction to form squalene (Fig. 1) (14), this reaction being the first committed step in sterol biosynthesis. This enzyme is currently under intense study as a possible target for cholesterol-lowering agents in humans (1, 12, 35), but it has been shown only recently as a promising target for antiparasitic chemotherapy (26, 27). For the present work, we investigated the in vitro and in vivo activities of E5700 and ER-119884, two novel quinuclidine SQS inhibitors currently in development by Eisai Company, Ltd. (Ibaraki, Japan) as cholesterol- and triglyceride-lowering agents in humans (T. Okada, D. Shinmyo, K. Tanaka, N. Kurusu, K. Miyazaki, H. Sugumi, H. Ikuta, M. Ito, M. Yanagimachi, H. Hiyoshi, T. Saeki, and S. Abe, Abstr. 226th Am. Chem. Soc. Natl. Meet., abstr. MEDI-293, 2003; M. Yanagimachi, H. Hiyoshi, M. Ito, N. Yasuda, T. Okada, H. Ikuta, and T. Saeki, Abstr. 13th Int. Symp. Atherosclerosis, Atherosclerosis Suppl. 4, p. 226, 2003) against T. cruzi and found that they also have potent and specific antiparasitic activity.
FIG. 1.
Chemical reaction catalyzed by SQS.
(A short version of this work has been presented previously [J. A. Urbina, J. L. Concepcion, G. Visbal, S. Rangel, R. Lira, and A. Caldera, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 493, 2001].)
MATERIALS AND METHODS
Parasite.
The EP (5) and Y stocks of T. cruzi were used in this study. The handling of live T. cruzi organisms was done according to established guidelines (7).
SQS purification and assay.
SQS activity was assayed using highly purified T. cruzi glycosomes and microsomes, which were isolated as described previously (27), as enzyme sources. The assay was carried out by the spot-wash method originally developed by Tait (18).
Studies of in vitro antiproliferative activity.
The epimastigote form of the parasite was cultivated in liver infusion tryptose medium (5) supplemented with 10% newborn calf serum (Gibco) at 28°C with strong agitation (120 rpm). The cultures were initiated with a cell density of 2 × 106 epimastigotes per ml, and the drug was added when the cell density was 0.5 × 107 to 1 × 107 epimastigotes per ml. Cell densities were measured with an electronic particle counter (model ZBI; Coulter Electronics Inc., Hielah, Fla.) and by direct counting with a hemocytometer. Cell viability was monitored by trypan blue exclusion analysis using light microscopy. Amastigotes were cultured in Vero cells maintained in minimal essential medium supplemented with 1% fetal calf serum in a humidified 95% air-5% CO2 atmosphere at 37°C as previously described (30-33). Briefly, the cells were infected with 10 tissue culture-derived trypomastigotes per cell for 2 h and then washed three times with phosphate-buffered saline to remove nonadherent parasites; fresh medium with and without drugs was added, and the cells were incubated for 96 h with a medium change at 48 h. Quantification of the infected cells and the parasites per cell was done by use of light microscopy, and statistical analysis of the results was carried out as described previously (30-33).
Studies of lipid composition.
For the analysis of the effects of drugs on the lipid composition of the epimastigotes, total lipids from control and drug-treated cells were extracted and fractionated into neutral and polar lipid fractions by silicic acid column chromatography and gas-liquid chromatography (32, 33). The neutral lipid fractions were first analyzed by thin-layer chromatography (on Merck 5721 silica gel plates with heptane-isopropyl ether-glacial acetic acid [60:40:4] as the developing solvent) and conventional gas-liquid chromatography (isothermal separation in a 4-m glass column packed with 3% OV-1 on Chromosorb 100/200 mesh with nitrogen as the carrier gas at a flow rate of 24 ml/min and flame ionization detection in a Varian 3700 gas chromatograph). For quantitative analysis and structural assignments, the neutral lipids were separated in a capillary high-resolution column (25-m by 0.20-mm [inside diameter] Ultra-2 column, 5% phenyl-methyl-siloxane, 0.33-μm film thickness) in a Hewlett-Packard 6890 Plus gas chromatograph equipped with an HP5973A mass-sensitive detector. The lipids were dissolved in chloroform and injected; the column was kept at 50°C for 1 min, and then the temperature was increased to 270°C at a rate of 25°C min−1 and finally to 300°C at a rate of 1°C min−1. The carrier gas (He) flow was kept constant at 0.5 ml min−1. The injector temperature was 250°C, and the detector was kept at 280°C.
In vivo studies.
The in vivo studies were carried out using a murine model of acute Chagas' disease as previously described (30-33). Briefly, groups of 10 female NMRI-IVIC (20- to 25-g) mice were infected with 105 T. cruzi bloodstream trypomastigotes per mouse, and oral treatment with E5700 or ER-119884 at a concentration of 25 or 50 mg/kg of body weight/day was started 24 h later and was given for 30 consecutive days. Negative (untreated) controls received only the vehicle, 1% methylcellulose plus 0.5% Tween 80. As positive controls, a group of animals received the reference drug nifurtimox at 50 mg/kg/day. The animals were monitored for 40 days postinoculation (p.i.). The survival rate was monitored daily, and parasitemia was checked weekly by direct microscopic examination as described previously (30-33).
Statistical analysis.
The Kaplan-Meier nonparametric method was used to estimate the survival functions of the different experimental groups. Rank tests (log rank, which gives equal weight to all observations, and the Peto-Peto-Wilcoxon test, which uses an estimate of the survival functions for its weightings) were used to compare them. The analyses were done with the Survival Tools package for StatView version 4.5 (Abacus Concepts Inc., Berkeley, Calif.) run on a Power Macintosh G4 Cube computer.
Drugs.
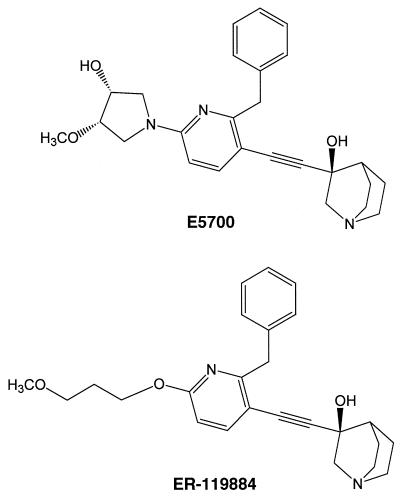
E5700 {(3R)-3-[[2-benzyl-6-[(3R,4S)-3-hydroxy-4-methoxypyrrolidin-1-yl]pyridin-3-yl]ethynyl]quinuclidin-3-ol monohydrate} (Fig. 2, top) and ER-119884 {(3R)-3-[[2-benzyl-6-(3-methoxypropyloxy)pyridin-3-yl]ethynyl]quinuclidin-3-ol} (Fig. 2, bottom) were provided by Tsukuba Research Laboratories, Eisai Co. For in vitro studies the compounds were dissolved in dimethyl sulfoxide and added directly to the growth medium; the final concentration of dimethyl sulfoxide in the medium never exceeded 1% (vol/vol) and had no effect on the proliferation of extracellular or intracellular parasites.
FIG. 2.
Chemical structures of E5700 and ER-119884.
RESULTS
Inhibitory activities against purified T. cruzi SQS.
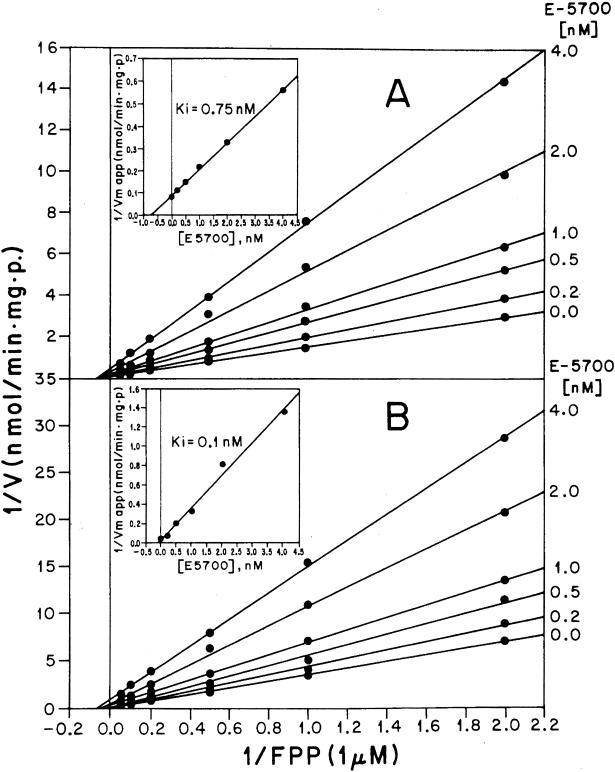
SQS has a dual subcellular localization in T. cruzi, being associated with both glycosomes (peroxisome-like organelles typical of trypanosomatid parasites) and microsomes (27). E5700 and ER-119884 were extremely potent inhibitors of both forms of the enzyme, with 50% inhibitory concentrations (IC50s) (at saturating concentrations of the enzyme substrates FPP and NADPH) in the low nanomolar to subnanomolar range in the absence or presence of 20 μM inorganic pyrophosphate (PPi) (Table 1). The compounds were shown to be noncompetitive (E5700) (Fig. 3) or mixed-type (ER-119884) (Fig. 4) inhibitors, with Ki values mostly in the subnanomolar range (Table 2).
TABLE 1.
Inhibitory activities of E5700 and ER-119884 against SQS from T. cruzi epimastigotesa
| Compound | IC50 (nM)
|
|||
|---|---|---|---|---|
| Glycosomal SQS
|
Microsomal and mitochondrial SQS
|
|||
| Without PPi | With 20 μM PPi | Without PPi | With 20 μM PPi | |
| E5700 | 5.4 | 0.42 | 15 | 0.49 |
| ER-119884 | 7.2 | 0.63 | 5.5 | 0.99 |
Highly purified glycosomes and microsomal and mitochondrial vesicles were obtained from cell homogenates prepared by grinding with silicon carbide followed by differential and isopycnic centrifugation as described in reference 27. SQS was assayed in the presence of 0.1% Triton X-100, 20 μM FPP, and 1 mM NADPH as described by Tait (18).
FIG. 3.
Kinetics of T. cruzi glycosomal SQS inhibition by E5700. Double reciprocal plots of the enzyme activity as a function of various concentrations of FPP, in the presence of the indicated fixed concentrations of E5700 in the absence (A) and presence (B) of 20 μM PPi, are presented. Insets, secondary plots of the intercepts as functions of E5700. SQS activity was measured in the presence of 0.1% Triton X-100. 1/V, inverse of the reaction rate; 1/Vm app, inverse of the maximum apparent reaction rate; p, protein content of the sample.
FIG. 4.
Kinetics of T. cruzi glycosomal SQS inhibition by ER-119884. Double reciprocal plots of the enzyme activity as a function of various concentrations of FPP, in the presence of the indicated fixed concentrations of ER-119884 in the absence (A) and presence (B) of 20 μM PPi, are presented. Insets, secondary plots of the slopes as functions of E5700. SQS activity was measured in the presence of 0.1% Triton X-100. 1/V, inverse of the reaction rate; 1/Vm app, inverse of the maximum apparent reaction rate; p, protein content of the sample.
TABLE 2.
Inhibition kinetic constants for E5700 and ER-119884 against SQS from T. cruzi epimastigotesa
| Compound |
Ki (nM)
|
Inhibition type | |||
|---|---|---|---|---|---|
| Glycosomal SQS
|
Microsomal and mitochondrial SQS
|
||||
| Without PPi | With 20 μM PPi | Without PPi | With 20 μM PPi | ||
| E5700 | 0.75 | 0.1 | 0.4 | 0.1 | Noncompetitive |
| ER-119884 | 1.48 | 0.8 | 1.4 | 0.7 | Mixed |
Highly purified glycosomes and microsomal and mitochondrial vesicles were obtained from cell homogenates prepared by grinding with silicon carbide followed by differential and isopycnic centrifugation as described in reference 27. SQS was assayed in the presence of 0.1% Triton X-100 as described by Tait (18).
In vitro antiproliferative activities.
Both quinuclidine derivatives were potent growth inhibitors against the extracellular epimastigote form of the parasite, with an IC50 and a MIC (defined as the minimal concentration required to induce complete growth arrest) of 8 and 30 nM, respectively, for E5700 and 11 and 100 nM, respectively, for ER-119884 (Fig. 5). Growth arrest and cell lysis induced by both compounds were associated with the disappearance of the parasite's endogenous sterols, no accumulation of lanosterol or squalene, and a concomitant increase in the content of externally derived cholesterol (5) (Tables 3 and 4). Against the clinically relevant intracellular amastigote form, the compounds were even more active: the IC50 and the minimum concentration required to reduce the intracellular parasite load by >99% (MICa) were 1.7 and 100 nM, respectively, for E5700 and 0.38 and 30 nM, respectively, for ER-119884 (Fig. 6). No deleterious effects of any of the compounds against host cells were observed at concentrations up to 10-fold higher than the MIC against amastigotes, indicating a selective antiparasitic effect.
FIG. 5.
Effects of E5700 (A) and ER-119884 (B) on the proliferation of T. cruzi epimastigotes. Epimastigotes were cultured in liver infusion-tryptose medium at 28°C, with agitation, as described in Materials and Methods. Arrows indicate the times of addition of the drug at the indicated concentrations. Experiments were carried out in triplicate, and each error bar represents 1 standard deviation.
TABLE 3.
Free sterols and precursors present in T. cruzi epimastigotes (EP stock) grown in the presence or absence of E5700a
ND, not detected.
TABLE 4.
Free sterols and precursors present in T. cruzi epimastigotes (EP stock) grown in the presence or absence of ER-119884a
ND, not detected
FIG. 6.
Concentration dependence of the effects of E5700 (A) and ER-119884 (B) on the proliferation of T. cruzi amastigotes cultured in Vero cells at 37°C. The percentage of infected cells, the number of amastigotes per cell, and the number of Vero cells per field after 96 h of incubation are shown as functions of the drug concentration. Vero cells were infected with T. cruzi as described in Materials and Methods. Experiments were carried out in quadruplicate, and each error bar represents 1 standard deviation.
In vivo antiparasitic activities.
Using a murine model of acute Chagas' disease, in which animals were infected with 105 T. cruzi bloodstream trypomastigotes per mouse and oral treatment was started 24 h p.i., it was found that E5700 given for 30 days had a dose-dependent effect on parasitemia and survival and at 50 mg/kg/day suppressed development of parasitemia (Fig. 7) and provided full protection against death, while all of the control (untreated) animals were dead 35 days p.i. (Fig. 8). The results were comparable with those obtained with the reference drug, nifurtimox, also given at 50 mg/kg/day. In contrast, ER-119884 was able to induce only 50% survival at 50 mg/kg/day, while no effects on parasitemia or survival were seen when it was given at 25 mg/kg/day (Fig. 6 and 7). However, even in animals treated with 50 mg of E5700/kg/day for 30 days, no cures of the parasitic infections were obtained, as indicated by the presence of low but detectable levels of circulating parasites after the discontinuation of treatment. No discernible toxic side effects to the mice were associated with any of the quinuclidine derivatives.
FIG. 7.
Effects of E5700, ER-119884, and nifurtimox on parasitemia in a murine model of acute Chagas' disease. NMRI-IVIC mice were inoculated with 105 blood trypomastigotes of the Y strain, and oral treatment was started 24 h later. All drugs were given orally at the indicated doses for 30 consecutive days. For details, see Materials and Methods.
FIG. 8.
Effects of E5700, ER-119884, and nifurtimox on survival in a murine model of acute Chagas' disease. NMRI-IVIC mice were inoculated with 105 blood trypomastigotes of the Y strain, and oral treatment was started 24 h later. All drugs were given orally at the indicated doses for 30 consecutive days. Statistical analysis using the log rank (Mantel-Cox) test indicated very significant (P < 0.0001) differences in survival rates between the control (untreated) animals and those that received E5700 or nifurtimox at 50 mg/kg/day and significant (P = 0.04) differences between the controls and those receiving E5700 at 25 mg/kg/day or ER-119884 at 50 mg/kg/day but no difference (P > 0.4) between the controls and those treated with ER-119884 at 25 mg/kg/day. For details, see Materials and Methods.
DISCUSSION
Quinuclidine derivatives are well-known inhibitors of mammalian SQS (4, 8, 9, 11, 19, 20, 34), and it has been shown previously that the prototype compound BPQ-OH [3-(biphenyl-4-yl)-3-hydroxyquinuclidine] (4, 11) is also a potent inhibitor of T. cruzi SQS and has selective anti-T. cruzi and anti-Leishmania activity in vitro (27). The novel quinuclidine derivatives studied in the present work, E5700 and ER-119884 (Fig. 2), were 1 order of magnitude more potent than BPQ-OH as inhibitors of the parasite enzyme, and their activity was further enhanced by the presence of micromolar levels of PPi (Fig. 3 and 4 and Tables 1 and 2). The compounds were noncompetitive or mixed-type inhibitors, and their Ki values (Table 2) were 3 to 4 orders of magnitude lower than the Km values for the substrate FPP and 4 to 5 orders of magnitude lower than the corresponding values for NADPH (27). Taken together, these facts strongly support the notion that quinuclidine derivatives, with positively charged aza groups in the centers of relatively hydrophobic molecules (Fig. 2), could act by mimicking the carbocationic transition state of the reaction catalyzed by SQS (Fig. 1), which also involves an ion pair with the leaving pyrophosphate group (2, 10, 14, 15). In this context it is interesting that we have recently discovered that T. cruzi and other trypanosomatid and apicomplexan parasites contain high levels of PPi and other short-chain polyphosphates that are distributed throughout the cells, although they are mostly stored in specialized organelles called acidocalcisomes (13, 16, 29). It is an intriguing possibility that the presence of significant levels of PPi in the intracellular milieu of these parasites may make them more susceptible than other eukaryotes to the effects of quinuclidine derivatives on SQS, including that in their mammalian host cells, where PPi levels are kept very low due to the presence of highly active soluble pyrophosphatases.
Both quinuclidine derivatives were potent growth inhibitors in vitro, particularly against the clinically relevant forms of the parasite, the intracellular amastigotes, with IC50s in the low nanomolar to subnanomolar range (Fig. 6); they are among the most active anti-T. cruzi compounds ever tested (21, 24, 28). In epimastigotes, the growth arrest and loss of cell viability induced by these compounds were associated with an almost complete disappearance of the parasite's endogenous sterols (Fig. 5 and Tables 3 and 4), as seen previously with other SQS inhibitors acting against this parasite (26, 27). This altered chemical composition should lead to drastic modifications of the physical properties of the cell's membranes, such as passive permeability and sterol-mediated lipid-protein interactions in lipid rafts (3, 17). In contrast, there were no effects on the viability and proliferation of the mammalian host cells, even at concentrations more than 10 times higher than those required to eradicate the intracellular parasites (Fig. 6), despite the fact that these compounds are also very potent inhibitors of mammalian SQS (Okada et al., Abstr. 226th Am. Chem. Soc. Natl. Meet., abstr. MEDI-293). As discussed elsewhere (27), the selective antiparasitic activity of these compounds is probably explained by the capacity of the host cells to compensate for the blockade of de novo cholesterol synthesis by up-regulating the expression low-density lipoprotein receptors and taking this sterol from the growth medium (6). In contrast, there is no way for the parasite to compensate in this manner for the quinuclidine-induced blockade of ergosterol biosynthesis, as there are no appreciable amounts of ergosterol in the host cells or growth medium. It is also possible, as discussed above, that whole mammalian cells are intrinsically less susceptible to SQS inhibition by quinuclidine compounds, due to the absence of intracellular PPi in them. In this context, it is noteworthy that E5700, in contrast to its potent anti-T. cruzi action, has very low in vitro antifungal activity (MIC, >200 μg/ml or 440 μM for Candida albicans, Aspergillus fumigatus, and Saccharomyces cerevisiae) [Eisai Co., unpublished data], a fact most probably explained by poor penetration by the compound due to the presence of cell walls.
Although the two quinuclidine derivatives had comparable in vitro anti-T. cruzi activities, E5700 was clearly more potent in vivo, as it was able to completely suppress the development of parasitemia and provide full protection against death when given at 50 mg/kg/day, while ER-119884 at the same dose provided only partial protection, comparable to that seen with 25 mg of E5700/kg/day (Fig. 7 and 8). When the effects of these compounds on cholesterol and triglyceride synthesis and on plasma lipid levels were investigated, the same patterns of in vitro and in vivo activities emerged: the compounds were of comparable potencies against purified rat SQS and in reducing cholesterol and triglyceride synthesis in primary cultured rat hepatocytes, but E5700 was much more active in reducing plasma lipid levels in rhesus monkeys after a 7-day course of treatment (Okada et al., Abstr. 226th Am. Chem. Soc. Natl. Meet., abstr. MEDI-293; Yanagimachi et al., Abstr. 13th Int. Symp. Atherosclerosis, Atherosclerosis Suppl. 4, p. 226). The higher in vivo activity of E5700 as a cholesterol-lowering and antiparasitic agent is most probably due to better pharmacokinetic properties. Studies of rats (Eisai Co., unpublished data) have shown that ER-119884 has a clearance rate threefold higher than that of E5700 after intravenous injection at 3 mg/kg and is associated with four- to sixfold-lower maximum concentrations in plasma and five- to sixfold-lower values for the area under the plasma concentration-time curve from 0 h to infinity after oral doses of 3 to 10 mg/kg are given; a similar pattern was observed for rhesus monkeys. Studies of mice (Eisai Co., unpublished data) found that the maximum concentrations of E5700 in plasma attained after oral doses of 30 to 100 mg/kg/day were 10- to 70-fold higher than the MIC for intracellular T. cruzi amastigotes determined in the present work (MICa, 100 nM) (Fig. 6); furthermore, in the rat model, this compound was found to have a wide tissue distribution, attaining levels in the tissue of relevant organs such as heart, spleen, liver, and small and large intestines that were 1 to 2 orders of magnitude higher than those found in plasma (Eisai Co., unpublished data). Thus, assuming a similar distribution in mice, intracellular amastigotes in animals treated with 50 mg of E5700/kg/day are expected to be exposed to steady-state drug levels that are 2 to 3 orders of magnitude higher than the MICa, a fact which could explain the complete blockade of parasite proliferation induced by this compound. Nevertheless, no cures of parasitic infection were obtained in these animals even after 30 days of treatment, a fact probably related to the short mean residence time (MRT = AUMC/AUC, where AUMC is the area under the first moment of the plasma concentration-time curve and AUC is the area under the curve) of E5700 in mice (2.5 to 3.8 h [Eisai Co., unpublished data]). New experiments exploring the effects of more frequent dosing as well as combinations with other sterol biosynthesis inhibitors such as antifungal azoles (inhibitors of sterol C14-α-demethylase) are under way (23-25). It also possible that, to attain full cures of parasitic infections, compounds with longer terminal half-lives than that of E5700 will be required, and this could be a key element in lead optimization for this application.
In conclusion, our results have shown that the new quinuclidine-based SQS inhibitors E5700 and ER-119884 have outstanding and selective activities against T. cruzi in vitro. It was also found that E5700 was capable of providing complete protection against T. cruzi infection in a murine model of fulminant acute Chagas' disease. This is the first demonstration of such antiparasitic activity for an orally active SQS inhibitor. Thus, these quinuclidine derivatives are promising leads for the development of more effective and safe anti-T. cruzi agents.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute (grant number 55000620) and the Venezuelan Institute for Scientific Research.
We thank Luis Alvarez for help in the preparation of figures.
REFERENCES
- 1.Bergstrom, J. D., C. Dufresne, G. F. Bills, M. Nallin-Omstead, and K. Byrne. 1995. Discovery, synthesis and mechanism of action of the zaragozic acids: potent inhibitors of squalene synthase. Annu. Rev. Microbiol. 49:607-639. [DOI] [PubMed] [Google Scholar]
- 2.Blagg, B. S., M. B. Jarstfer, D. H. Rogers, and C. D. Poulter. 2002. Recombinant squalene synthase. A mechanism for the rearrangement of presqualene diphosphate to squalene. J. Am. Chem. Soc. 124:8846-8853. [DOI] [PubMed] [Google Scholar]
- 3.Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221-17224. [DOI] [PubMed] [Google Scholar]
- 4.Brown, G. R., A. J. Foubister, S. Freeman, P. J. Harrison, M. C. Johnson, K. B. Mallion, J. McCormick, F. McTaggart, A. C. Reid, G. J. Smith, and M. J. Taylor. 1996. Synthesis and activity of a novel series of 3-biarylquinuclidine squalene synthase inhibitors. J. Med. Chem. 39:2971-2979. [DOI] [PubMed] [Google Scholar]
- 5.De Maio, A., and J. A. Urbina. 1984. Trypanosoma (Schizotrypanum) cruzi: terminal oxidases in two growth phases in vitro. Acta Cient. Venez. 35:136-141. [PubMed] [Google Scholar]
- 6.Goldstein, J. L., and M. S. Brown. 2001. The cholesterol quartet. Science 292:1310-1312. [DOI] [PubMed] [Google Scholar]
- 7.Hudson, L., F. Grover, W. E. Gutteridge, R. A. Klein, W. Peters, R. A. Neal, M. A. Miles, M. T. Scott, R. Nourish, and B. P. Ager. 1983. Suggested guidelines for work with live Trypanosoma cruzi. Trans. R. Soc. Trop. Med. Hyg. 77:416-419. [DOI] [PubMed] [Google Scholar]
- 8.Ishihara, T., H. Kakuta, H. Moritani, T. Ugawa, S. Sakamoto, S. Tsukamoto, and I. Yanagisawa. 2003. Syntheses and biological evaluation of novel quinuclidine derivatives as squalene synthase inhibitors. Bioorg. Med. Chem. 11:2403-2414. [DOI] [PubMed] [Google Scholar]
- 9.Ishihara, T., H. Kakuta, H. Moritani, T. Ugawa, S. Sakamoto, S. Tsukamoto, and I. Yanagisawa. 2003. Syntheses of 3-ethylidenequinuclidine derivatives as squalene synthase inhibitors. Part 2: enzyme inhibition and effects on plasma lipid levels. Bioorg. Med. Chem. 11:3735-3745. [DOI] [PubMed] [Google Scholar]
- 10.Jarstfer, M. B., D. L. Zhang, and C. D. Poulter. 2002. Recombinant squalene synthase. Synthesis of non-head-to-tail isoprenoids in the absence of NADPH. J. Am. Chem. Soc. 124:8834-8845. [DOI] [PubMed] [Google Scholar]
- 11.McTaggart, F., G. R. Brown, R. G. Davidson, S. Freeman, G. A. Holdgate, K. B. Mallion, D. J. Mirrlees, G. J. Smith, and W. H. J. Ward. 1996. Inhibition of squalene synthase of rat liver by novel 3′ substituted quinuclidines. Biochem. Pharmacol. 51:1477-1487. [DOI] [PubMed] [Google Scholar]
- 12.Menys, V. C., and P. N. Durrington. 2003. Squalene synthase inhibitors. Br. J. Pharmacol. 139:881-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno, B., J. A. Urbina, E. Oldfield, B. N. Bailey, C. O. Rodrigues, and R. Docampo. 2000. 31P NMR spectroscopy of Trypanosoma brucei, Trypanosoma cruzi and Leishmania major: evidence for high levels of condensed inorganic phosphates. J. Biol. Chem. 275:28356-28362. [DOI] [PubMed] [Google Scholar]
- 14.Poulter, C. D. 1990. Biosynthesis of non-head-to tail terpenes. Formation of 1′-1 and 1′-3 linkages. Acc. Chem. Res. 23:70-77. [Google Scholar]
- 15.Radisky, E. S., and C. D. Poulter. 2000. Squalene synthase: steady-state, pre-steady state and isotope-trapping studies. Biochemistry 39:1748-1760. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz, F. A., C. O. Rodrigues, and R. Docampo. 2001. Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation and environmental stress in Trypanosoma cruzi. J. Biol. Chem. 276:26114-26121. [DOI] [PubMed] [Google Scholar]
- 17.Simons, K., and E. Ikonen. 1997. Functional lipid rafts in cell membranes. Nature 387:569-571. [DOI] [PubMed] [Google Scholar]
- 18.Tait, R. M. 1992. Development of a radiometric spot-wash assay for squalene synthase. Anal. Biochem. 203:310-316. [DOI] [PubMed] [Google Scholar]
- 19.Ugawa, T., H. Kakuta, H. Moritani, O. Inagaki, and H. Shikama. 2003. YM-53601, a novel squalene synthase inhibitor, suppresses lipogenic biosynthesis and lipid secretion in rodents. Br. J. Pharmacol. 139:140-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ugawa, T., H. Kakuta, H. Moritani, K. Matsuda, T. Ishihara, M. Yamaguchi, S. Naganuma, Y. Iizumi, and H. Shikama. 2000. YM-53601, a novel squalene synthase inhibitor, reduces plasma cholesterol and triglycerides in several animal species. Br. J. Pharmacol. 131:63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urbina, J. A. 2002. Chemotherapy of Chagas disease. Curr. Pharm. Des. 8:287-295. [DOI] [PubMed] [Google Scholar]
- 22.Urbina, J. A. 1997. Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology 117:S91-S99. [PubMed] [Google Scholar]
- 23.Urbina, J. A. 2003. Rational approaches to specific chemotherapy of Chagas disease, p. 127-135. In K. M. Tyler and M. A. Miles (ed.), World class parasites, vol. 7. American trypanosomiasis. Kluwer Academic Publishers, Boston, Mass.
- 24.Urbina, J. A. 2001. Specific treatment of Chagas disease: current status and new developments. Curr. Opin. Infect. Dis. 14:733-741. [DOI] [PubMed] [Google Scholar]
- 25.Urbina, J. A. 2000. Sterol biosynthesis inhibitors for Chagas' disease. Curr. Opin. Anti-infect. Investig. Drugs 2:40-46. [Google Scholar]
- 26.Urbina, J. A., J. L. Concepcion, A. Montalvetti, J. B. Rodriguez, and R. Docampo. 2003. Mechanism of action of 4-phenoxyphenoxyethyl thiocyanate (WC-9) against Trypanosoma cruzi, the causative agent of Chagas' disease. Antimicrob. Agents Chemother. 47:2047-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urbina, J. A., J. L. Concepcion, S. Rangel, G. Visbal, and R. Lira. 2002. Squalene synthase as a chemotherapeutic target in Trypanosoma cruzi and Leishmania mexicana. Mol. Biochem. Parasitol. 125:35-45. [DOI] [PubMed] [Google Scholar]
- 28.Urbina, J. A., and R. Docampo. 2003. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol. 19:495-501. [DOI] [PubMed] [Google Scholar]
- 29.Urbina, J. A., B. Moreno, S. Vierkotter, E. Oldfield, G. Payares, C. Sanoja, B. N. Bailey, W. Yan, D. A. Scott, S. N. J. Moreno, and R. Docampo. 1999. Trypanosoma cruzi contains major pyrophosphate stores and its growth in vitro and in vivo is blocked by pyrophosphate analogs. J. Biol. Chem. 274:33609-33615. [DOI] [PubMed] [Google Scholar]
- 30.Urbina, J. A., G. Payares, L. M. Contreras, A. Liendo, C. Sanoja, J. Molina, M. Piras, R. Piras, N. Perez, P. Wincker, and D. Loebenberg. 1998. Antiproliferative effects and mechanism of action of SCH 56592 against Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Antimicrob. Agents Chemother. 42:1771-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbina, J. A., G. Payares, J. Molina, C. Sanoja, A. Liendo, K. Lazardi, M. M. Piras, R. Piras, N. Perez, P. Wincker, and J. F. Ryley. 1996. Cure of short- and long-term experimental Chagas disease using D0870. Science 273:969-971. [DOI] [PubMed] [Google Scholar]
- 32.Urbina, J. A., G. Payares, C. Sanoja, R. Lira, and A. J. Romanha. 2003. In vitro and in vivo activities of ravuconazole on Trypanosoma cruzi, the causative agent of Chagas disease. Int. J. Antimicrob. Agents 21:27-38. [DOI] [PubMed] [Google Scholar]
- 33.Urbina, J. A., G. Payares, C. Sanoja, J. Molina, R. Lira, Z. Brener, and A. J. Romanha. 2003. Parasitological cure of acute and chronic experimental Chagas disease using the long-acting experimental triazole TAK-187. Activity against drug-resistant Trypanosoma cruzi strains. Int. J. Antimicrob. Agents. 21:39-48. [DOI] [PubMed] [Google Scholar]
- 34.Ward, W. H. J., G. A. Holdgate, S. Freeman, F. McTaggart, P. A. Girdwood, R. G. Davidson, K. B. Mallion, G. R. Brown, and M. A. Eakin. 1996. Inhibition of squalene synthase in vitro by 3-(biphenyl-4-yl)-quinuclidine. Biochem. Pharmacol. 51:1489-1501. [DOI] [PubMed] [Google Scholar]
- 35.Watson, N. S., and P. A. Procopiou. 1996. Squalene synthase inhibitors: their potential as hypocholesterolemic agents. Prog. Med. Chem. 33:331-378. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. 2002. Control of Chagas disease, p. 1-109. In Technical Report Series no. 905. World Health Organization, Geneva, Switzerland. [PubMed]